Ionic Gelatin-Based Flexible Thermoelectric Generator with Scalability for Human Body Heat Harvesting
Abstract
:1. Introduction
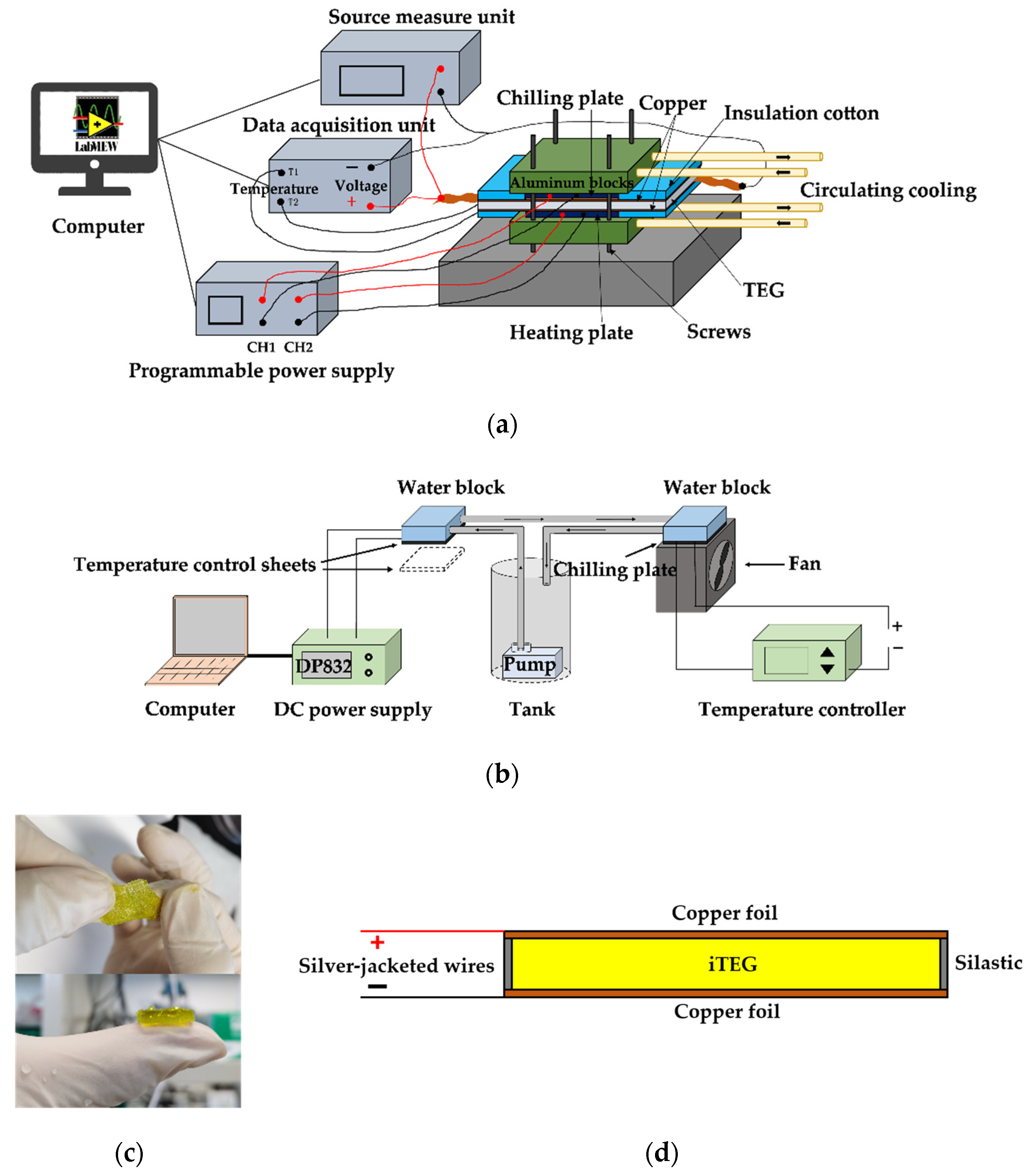
2. Materials and Methods
3. Results and Discussion
3.1. Improvement of iTEG
3.1.1. Determination of SiO2 NPs Dispersion Quantity
3.1.2. Microstructure of iTEG
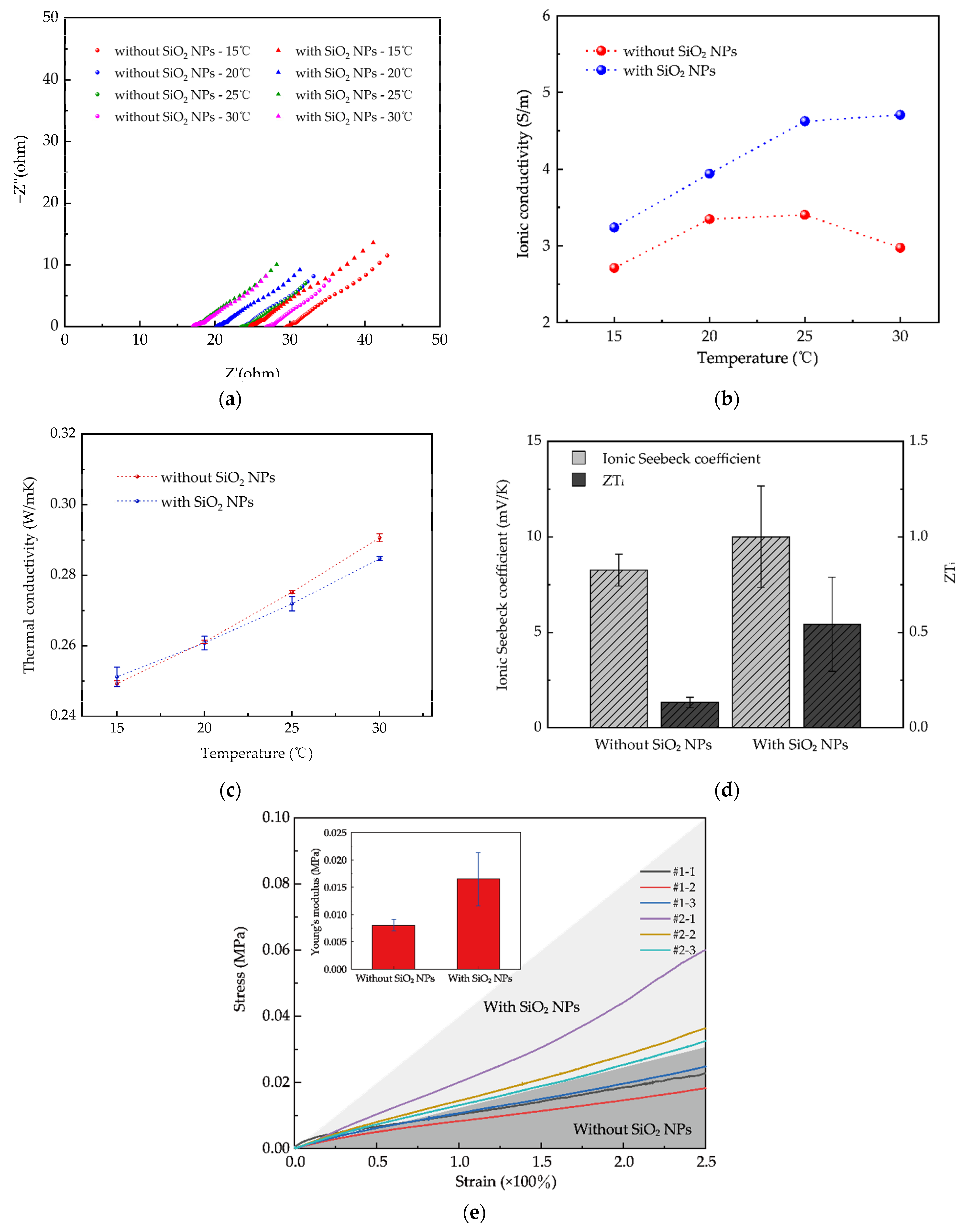
3.1.3. Ionic Conductivity and Thermal Conductivity of iTEG
3.1.4. Ionic Seebeck Coefficient and Thermoelectric Figure of Merit (ZTi) of iTEG
3.1.5. Mechanical Properties of iTEG
3.1.6. FTIR and Raman Spectra of iTEG
3.2. Performance of Flexible Wearable TEG
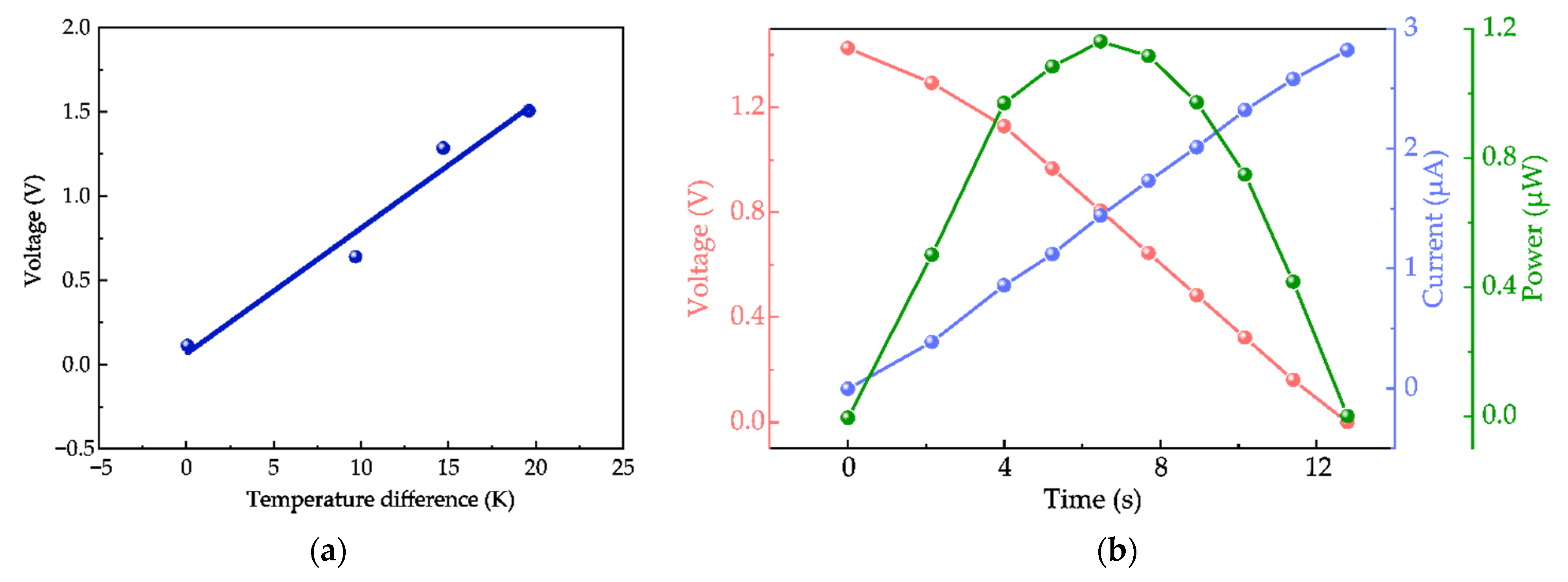
3.2.1. Seebeck Coefficient and V-I-P Curves Tests
3.2.2. Bending and Stretching Tests
3.2.3. Impedance Matching Test
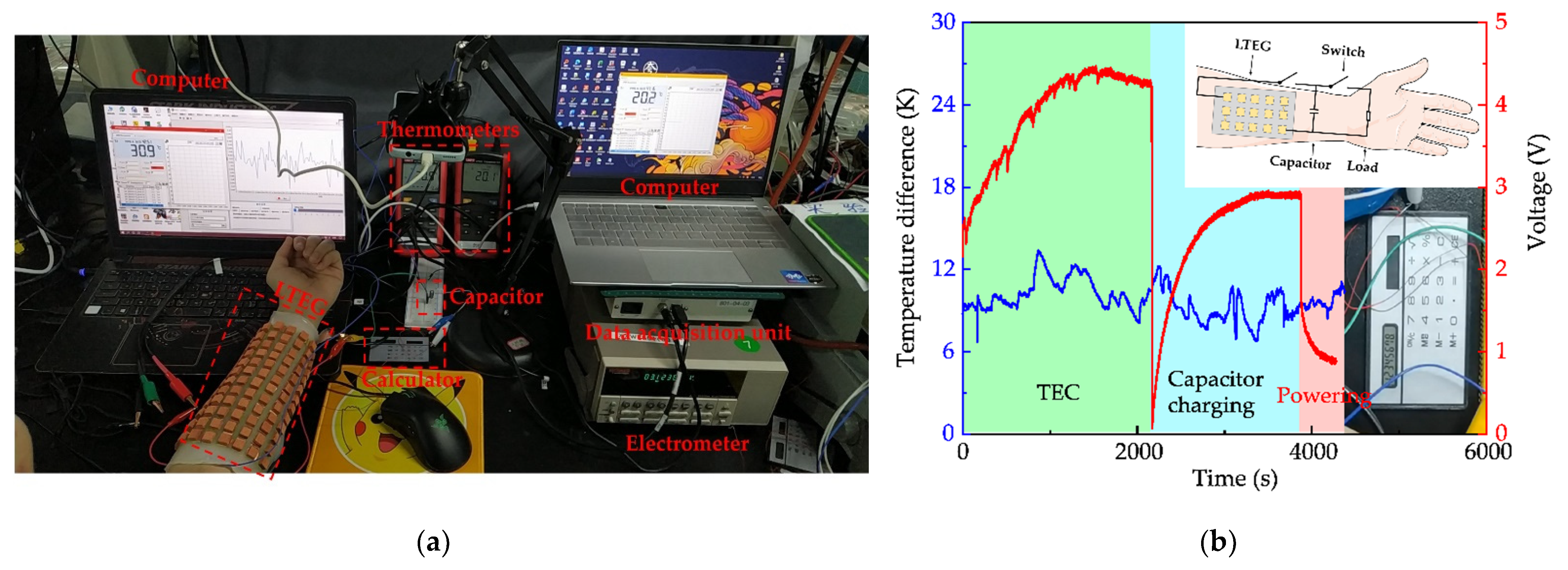
3.2.4. Capacitor Charging Test
3.2.5. Scalability of TEG and Optimization of Heat Dissipation Structure
3.3. Wearing Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WEH | Wearable energy harvester |
| AT | Atmospheric temperature |
| RF | Radio Frequency |
| △T | Temperature difference |
| TEC | Thermoelectric conversion |
| TEG | Thermoelectric generator |
| TEM | Thermoelectric material |
| PVA | Poly(vinyl alcohol) |
| SiO2 NPs | Silica nanoparticles |
| iTEG | Ionic thermoelectric gel |
| SMU | Source measure unit |
| DAU | Data acquisition unit |
| STEG | Small wristband TEG (3 rows × 8 columns iTEG units) |
| LTEG | Large extended oversleeve TEG (9 rows × 12 columns iTEG units) |
| TIMPD | Temperature-insensitive maximum power density |
| SEM | Scanning electron microscope |
| V-I-P | Voltage–current–power |
| Si | Ionic Seebeck coefficient |
| VOC | Open-circuit voltage |
| Pmax | The maximum output power |
| A | Effective electrode area |
| σi | Ionic conductivity |
| R | Intrinsic resistance |
| d | Sample thickness |
| ZTi | Thermoelectric figure of merit of iTEG |
| λ | Thermal conductivity |
| T | Temperature |
References
- Huawei Consumer Business Official Website. Available online: https://consumer.huawei.com/cn/ (accessed on 24 March 2022).
- Trung, T.Q.; Lee, N.-E. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoringand personal healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef]
- Kim, W.-G.; Kim, D.-W.; Tcho, I.-W.; Kim, J.-K.; Kim, M.-S.; Choi, Y.-K. Triboelectric nanogenerator: Structure, mechanism, and applications. ACS Nano 2021, 15, 258–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, Y.; Xu, F.; Duan, Y.; Yin, Z. Energy harvesters for wearable and stretchable electronics: From flexibility to stretchability. Adv. Mater. 2016, 28, 9881–9919. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Chung, W.-Y. High-efficient energy harvester with flexible solar panel for a wearable sensor device. IEEE Sens. J. 2016, 16, 9021–9028. [Google Scholar] [CrossRef]
- Kartsch, V.; Benatti, S.; Mancini, M.; Magno, M.; Benini, L. Smart wearable wristband for EMG based gesture recognition powered by solar energy harvester. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018; pp. 1–5. [Google Scholar]
- Delnavaz, A.; Voix, J. Electromagnetic micro-power generator for energy harvesting from breathing. In Proceedings of the IECON 2012-38th Annual Conference on IEEE Industrial Electronics Society, Montreal, QC, Canada, 25–28 October 2012; pp. 984–988. [Google Scholar]
- Padasdao, B.; Boric-Lubecke, O. Respiratory rate detection using a wearable electromagnetic generator. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 3217–3220. [Google Scholar]
- Hamid, R.; Yuce, M.R. A wearable energy harvester unit using piezoelectric–electromagnetic hybrid technique. Sens. Actuators A Phys. 2017, 257, 198–207. [Google Scholar] [CrossRef]
- Zhao, J.; You, Z. A shoe-embedded piezoelectric energy harvester for wearable sensors. Sensors 2014, 14, 12497–12510. [Google Scholar] [CrossRef]
- Jung, W.-S.; Lee, M.-J.; Kang, M.-G.; Moon, H.G.; Yoon, S.-J.; Baek, S.-H.; Kang, C.-Y. Powerful curved piezoelectric generator for wearable applications. Nano Energy 2015, 13, 174–181. [Google Scholar] [CrossRef]
- Qian, F.; Xu, T.-B.; Zuo, L. Design, optimization, modeling and testing of a piezoelectric footwear energy harvester. Energy Convers. Manag. 2018, 171, 1352–1364. [Google Scholar] [CrossRef]
- Lee, S.; Ko, W.; Oh, Y.; Lee, J.; Baek, G.; Lee, Y.; Sohn, J.; Cha, S.; Kim, J.; Park, J.; et al. Triboelectric energy harvester based on wearable textile platforms employing various surface morphologies. Nano Energy 2015, 12, 410–418. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, Y.; Sun, N.; Li, G.; Liu, Y.; Chen, C.; Shi, J.; Xie, L.; Jiang, H.; Bao, D.; et al. A wrinkled PEDOT: PSS film based stretchable and transparent triboelectric nanogenerator for wearable energy harvesters and active motion sensors. Adv. Funct. Mater. 2018, 28, 1803684. [Google Scholar] [CrossRef]
- Masotti, D.; Costanzo, A.; Adami, S. Design and realization of a wearable multi-frequency RF energy harvesting system. In Proceedings of the 5th European Conference on Antennas and Propagation (EUCAP), Rome, Italy, 11–15 April 2011; pp. 517–520. [Google Scholar]
- Nguyen, S.; Amirtharajah, R. A hybrid RF and vibration energy harvester for wearable devices. In Proceedings of the 2018 IEEE Applied Power Electronics Conference and Exposition (APEC), San Antonio, TX, USA, 4–8 March 2018; pp. 1060–1064. [Google Scholar]
- Wu, X.; Gao, N.; Jia, H.; Wang, Y. Thermoelectric converters based on ionic conductors. Chem. Asian J. 2021, 16, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, B.; Huang, L.; Hu, B.; Xu, M.; Feng, G.; Zhou, J. Liquid-state thermocells: Opportunities and challenges for low-grade heat harvesting. Joule 2021, 5, 768–779. [Google Scholar] [CrossRef]
- Tamura, T.; Huang, M.; Togawa, T. Body temperature, heat flow, and evaporation. In Seamless Healthcare Monitoring: Advancements in Wearable, Attachable, and Invisible Devices; Tamura, T., Chen, W., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 281–307. [Google Scholar]
- Park, T.; Lim, H.; Hwang, J.U.; Na, J.; Lee, H.; Kim, E. Roll type conducting polymer legs for rigid-flexible thermoelectric generator. APL Mater. 2017, 5, 074106. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.; Tang, F.; Zhang, N.; Wang, X. Flexible, high-power density, wearable thermoelectric nanogenerator and self-powered temperature sensor. ACS Appl. Mater. Interfaces 2019, 11, 38616–38624. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.; Alpuim, P.; Correia, J. Fabrication of thermoelectric devices by applying microsystems technology. J. Electron. Mater. 2010, 39, 1516–1521. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, D.; Pedrycz, W.; Fan, K.; Guo, Y. Human body heat based thermoelectric harvester with ultra-low input power management system for wireless sensors powering. Energies 2019, 12, 3942. [Google Scholar] [CrossRef] [Green Version]
- Thielen, M.; Sigrist, L.; Magno, M.; Hierold, C.; Benini, L. Human body heat for powering wearable devices: From thermal energy to application. Energy Convers. Manag. 2017, 131, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Leonov, V.; Fiorini, P.; Van Hoof, C. Realization of a wearable miniaturized thermoelectric generator for human body applications. Sens. Actuators A Phys. 2009, 156, 95–102. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Shi, X.-L.; Shi, X.; Chen, L.; Dargusch, M.S.; Zou, J.; Chen, Z.-G. Flexible thermoelectric materials and generators: Challenges and innovations. Adv. Mater. 2019, 31, 1807916. [Google Scholar] [CrossRef]
- Wu, J.; Black, J.J.; Aldous, L. Thermoelectrochemistry using conventional and novel gelled electrolytes in heat-to-current thermocells. Electrochim. Acta 2017, 225, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Han, C.-G.; Qian, X.; Li, Q.; Deng, B.; Zhu, Y.; Han, Z.; Zhang, W.; Wang, W.; Feng, S.-P.; Chen, G. Giant thermopower of ionic gelatin near room temperature. Science 2020, 368, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; He, X.; Fan, Z.; Ouyang, J. Flexible quasi-solid state ionogels with remarkable seebeck coefficient and high thermoelectric properties. Adv. Energy Mater. 2019, 9, 1901085. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Lacey, S.D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat. Mater. 2019, 18, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, K.; Chen, Q.; Mo, X.; Zhou, Y.; Li, S.; Feng, G.; Zhou, J. Wearable thermocells based on gel electrolytes for the utilization of body heat. Angew. Chem. 2016, 128, 12229–12232. [Google Scholar] [CrossRef]
- Duan, J.; Yu, B.; Liu, K.; Li, J.; Yang, P.; Xie, W.; Xue, G.; Liu, R.; Wang, H.; Zhou, J. PN conversion in thermogalvanic cells induced by thermo-sensitive nanogels for body heat harvesting. Nano Energy 2019, 57, 473–479. [Google Scholar] [CrossRef]
- Yu, B.; Duan, J.; Cong, H.; Xie, W.; Liu, R.; Zhuang, X.; Wang, H.; Qi, B.; Xu, M.; Wang, Z.L. Thermosensitive crystallization–boosted liquid thermocells for low-grade heat harvesting. Science 2020, 370, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Qian, X.; Han, C.-G.; Li, Q.; Chen, G. Ionic thermoelectric materials for near ambient temperature energy harvesting. Appl. Phys. Lett. 2021, 118, 020501. [Google Scholar] [CrossRef]
- He, X.; Cheng, H.; Yue, S.; Ouyang, J. Quasi-solid state nanoparticle/(ionic liquid) gels with significantly high ionic thermoelectric properties. J. Mater. Chem. A 2020, 8, 10813–10821. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, T.; Li, N.; Kang, T.J.; Chen, J.; Pringle, J.M.; Zhang, M.; Kazim, A.H.; Fang, S.; Haines, C. High power density electrochemical thermocells for inexpensively harvesting low-grade thermal energy. Adv. Mater. 2017, 29, 1605652. [Google Scholar] [CrossRef]
- Romano, M.S.; Li, N.; Antiohos, D.; Razal, J.M.; Nattestad, A.; Beirne, S.; Fang, S.; Chen, Y.; Jalili, R.; Wallace, G.G.; et al. Carbon nanotube-reduced graphene oxide composites for thermal energy harvesting applications. Adv. Mater. 2013, 25, 6602–6606. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Cola, B.A.; Haram, N.; Barisci, J.N.; Lee, S.; Stoughton, S.; Wallace, G.; Too, C.; Thomas, M.; Gestos, A. Harvesting waste thermal energy using a carbon-nanotube-based thermo-electrochemical cell. Nano Lett. 2010, 10, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Smooth-On, Inc. Official Website. Available online: https://www.smooth-on.com/ (accessed on 24 March 2022).
- Duan, J.; Feng, G.; Yu, B.; Li, J.; Chen, M.; Yang, P.; Feng, J.; Liu, K.; Zhou, J. Aqueous thermogalvanic cells with a high Seebeck coefficient for low-grade heat harvest. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Wang, Y.; Mei, D.; Chen, Z. Wearable thermoelectric generator with copper foam as the heat sink for body heat harvesting. IEEE Access 2018, 6, 43602–43611. [Google Scholar] [CrossRef]
- Im, H.; Kim, T.; Song, H.; Choi, J.; Park, J.S.; Ovalle-Robles, R.; Yang, H.D.; Kihm, K.D.; Baughman, R.H.; Lee, H.H.; et al. High-efficiency electrochemical thermal energy harvester using carbon nanotube aerogel sheet electrodes. Nat. Commun. 2016, 7, 10600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smitha, S.; Mukundan, P.; Krishna Pillai, P.; Warrier, K.G.K. Silica–gelatin bio-hybrid and transparent nano-coatings through sol–gel technique. Mater. Chem. Phys. 2007, 103, 318–322. [Google Scholar] [CrossRef]
- Vila, J.; Ginés, P.; Pico, J.; Franjo, C.; Jiménez, E.; Varela, L.; Cabeza, O. Temperature dependence of the electrical conductivity in EMIM-based ionic liquids: Evidence of Vogel–Tamman–Fulcher behavior. Fluid Phase Equilibria 2006, 242, 141–146. [Google Scholar] [CrossRef]
- Wieczorek, W.; Such, K.; Wyciślik, H.; Płocharski, J. Modifications of crystalline structure of PEO polymer electrolytes with ceramic additives. Solid State Ion. 1989, 36, 255–257. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.-C.; Li, Y.; Lee, H.-W.; Cui, Y. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Lett. 2015, 15, 2740–2745. [Google Scholar] [CrossRef]
- Liu, W.; Lee, S.W.; Lin, D.; Shi, F.; Wang, S.; Sendek, A.D.; Cui, Y. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat. Energy 2017, 2, 17035. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Q.X.; Ye, Y.S.; Xue, Y.; Wang, Y.; Peng, H.Y.; Xie, X.L.; Mai, Y.W. Constructing desirable ion-conducting channels within ionic liquid-based composite polymer electrolytes by using polymeric ionic liquid-functionalized 2D mesoporous silica nanoplates. Nano Energy 2017, 33, 110–123. [Google Scholar] [CrossRef]
- Properties: Silica-Silicon Dioxide (SiO2). Available online: https://www.azom.com/properties.aspx?ArticleID=1114 (accessed on 24 March 2022).
- Yoon, J.; Lee, J.; Hur, J. Stretchable supercapacitors based on carbon nanotubes-deposited rubber polymer nanofibers electrodes with high tolerance against strain. Nanomaterials 2018, 8, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pailler-Mattei, C.; Bec, S.; Zahouani, H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng. Phys. 2008, 30, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Kariminejad, M.; Sadeghi, E.; Rouhi, M.; Mohammadi, R.; Askari, F.; Taghizadeh, M.; Moradi, S. The effect of nano-SiO2 on the physicochemical and structural properties of gelatin-polyvinyl alcohol composite films. J. Food Process Eng. 2018, 41, e12817. [Google Scholar] [CrossRef]
- Bartlett, M.D.; Kazem, N.; Powell-Palm, M.J.; Huang, X.; Sun, W.; Malen, J.A.; Majidi, C. High thermal conductivity in soft elastomers with elongated liquid metal inclusions. Proc. Natl. Acad. Sci. USA 2017, 114, 2143–2148. [Google Scholar] [CrossRef] [Green Version]
- Jacquemoud, C.; Bruyere-Garnier, K.; Coret, M. Methodology to determine failure characteristics of planar soft tissues using a dynamic tensile test. J. Biomech. 2007, 40, 468–475. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Han, L.; Liu, H.; Dong, Y.; Wang, X. Ionic Gelatin-Based Flexible Thermoelectric Generator with Scalability for Human Body Heat Harvesting. Energies 2022, 15, 3441. https://doi.org/10.3390/en15093441
Wang S, Han L, Liu H, Dong Y, Wang X. Ionic Gelatin-Based Flexible Thermoelectric Generator with Scalability for Human Body Heat Harvesting. Energies. 2022; 15(9):3441. https://doi.org/10.3390/en15093441
Chicago/Turabian StyleWang, Shucheng, Liuyang Han, Hanxiao Liu, Ying Dong, and Xiaohao Wang. 2022. "Ionic Gelatin-Based Flexible Thermoelectric Generator with Scalability for Human Body Heat Harvesting" Energies 15, no. 9: 3441. https://doi.org/10.3390/en15093441
APA StyleWang, S., Han, L., Liu, H., Dong, Y., & Wang, X. (2022). Ionic Gelatin-Based Flexible Thermoelectric Generator with Scalability for Human Body Heat Harvesting. Energies, 15(9), 3441. https://doi.org/10.3390/en15093441




