Minimum Emissions Configuration of a Green Energy–Steel System: An Analytical Model
Abstract
:1. Introduction
2. The Analytical Model for Identifying the Minimum Emissions Configuration of a Green Energy–Steel System
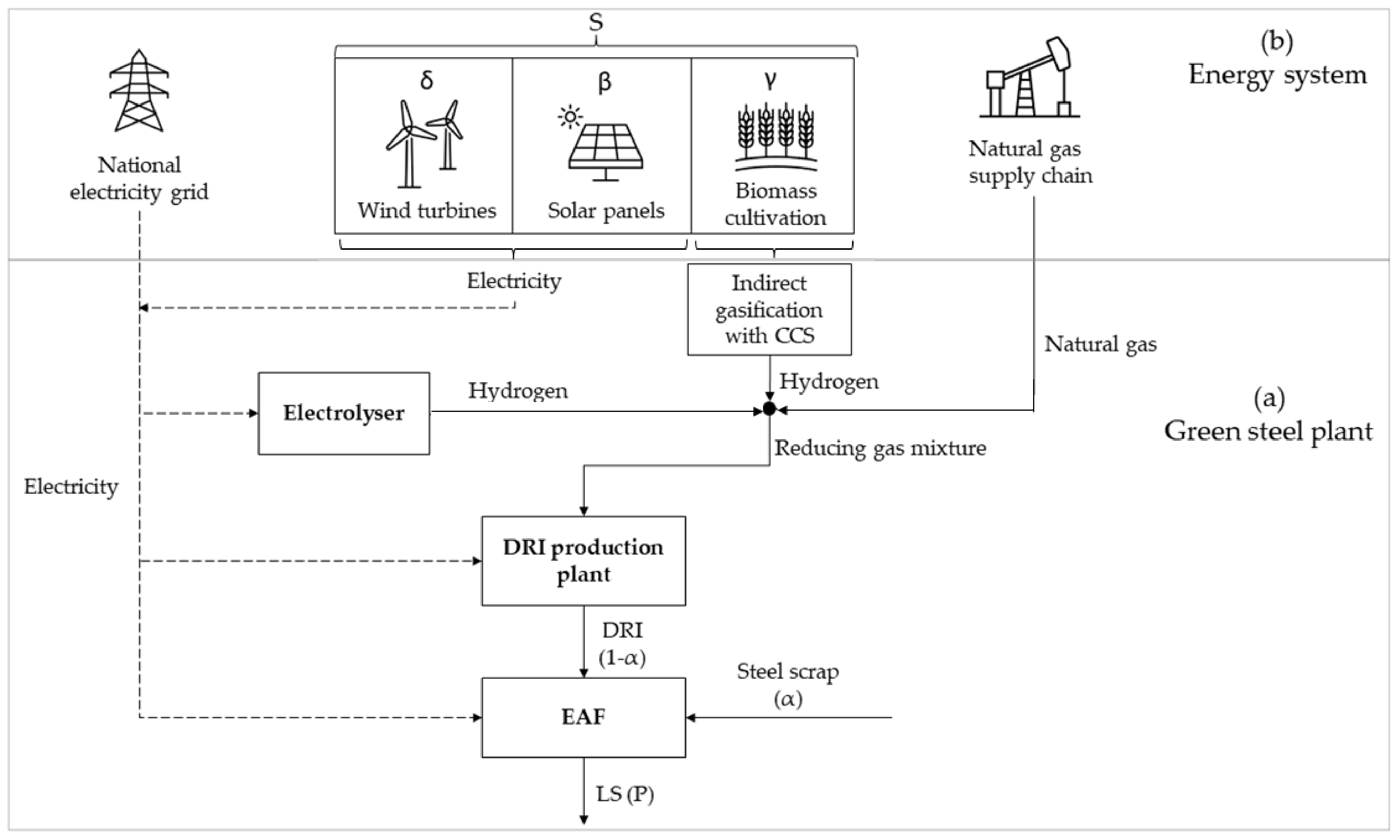
2.1. The Energy System and the Green Steel Plant
2.1.1. The Green Steel Plant
2.1.2. The Energy System
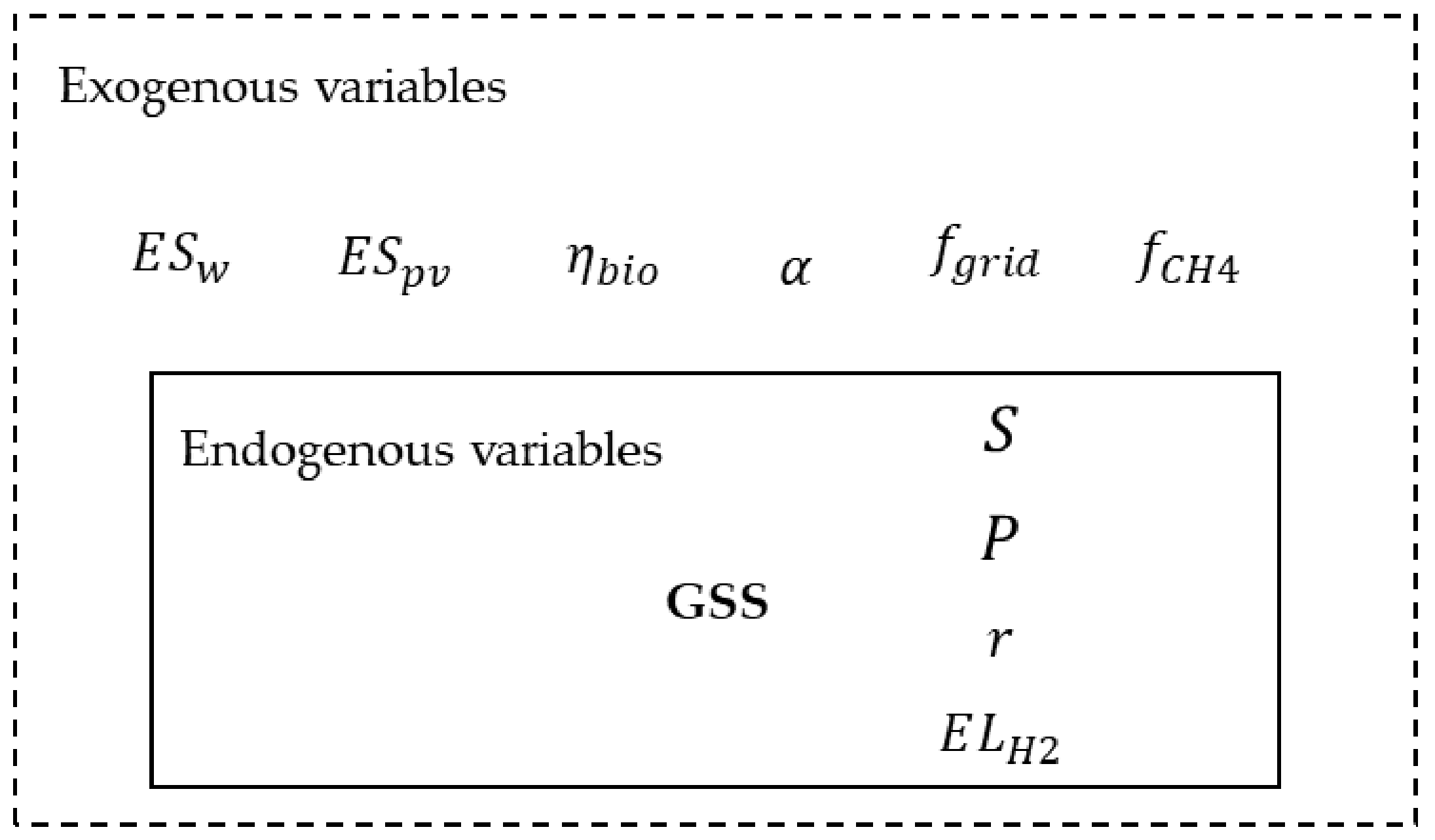
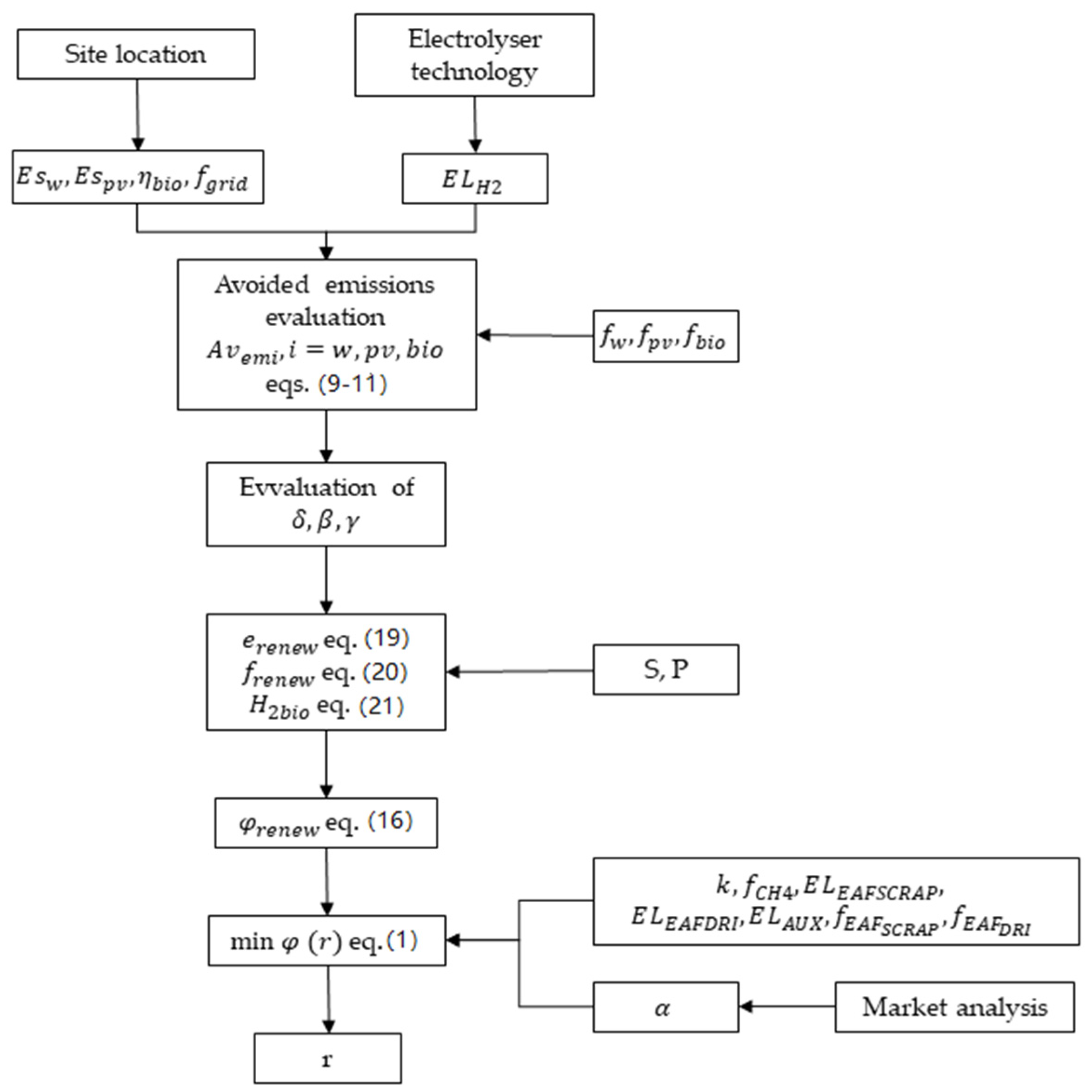
2.2. The Analytical Model for the GESS Minimum Emissions Configuration
- Exogenous variables: they are variables that cannot be influenced by the decision-maker because of the characteristics of the site where the GESS is expected to be located and the dynamic of the raw materials market. In the context of the present work, the energy and hydrogen producibility per unit area, the share of scrap employed to produce LS in EAF, the national grid emission factor, and the natural gas supply chain emission factor are considered in this category. The amount of energy and hydrogen that can be produced per unit area by renewable energy conversion systems (i.e., wind turbines, solar panels, and gasification plants) depends on the characteristics of the installation site, such as windiness and global solar radiation, and the availability of steel scrap on the market cannot be influenced by the needs of a single plant and, finally, emissions from the electricity grid depend on the national energy mix.
- Endogenous variables: they are variables set by the decision-maker during the plant design phase. In the context of the present work, the total area of the energy system, the volumetric share of hydrogen in the reducing gas of the DRI production process, the electrolyzer technology to be adopted, and the expected yearly production volume of liquid steel are considered in this category since they are characteristic choices of a plant design.
- : direct emissions generated by the green steel plant. “direct GHG emissions” are defined as GHG emissions generated in owned or controlled process equipment [34]. In the green steel plant, direct emissions are from DRI plant with an emission factor, , and from the EAF. Different EAF emission factors in case of scraps or DRI feeding it are considered ( and , respectively).
- : emissions generated by the production of electricity and the supply of natural gas to power the DRI process. As established by the GHG protocol [34], “electricity indirect emissions and other GHG emissions” are defined as emissions deriving from the production of electricity consumed by the plant and from activities that can be considered a consequence of the plant’s activity, e.g., the extraction and transport of raw materials. The characteristic of indirect emissions is that, although they do not physically occur at the plant site, they have a significant influence on the total account of the emissions generated. In the case of the analyzed system, lifecycle emissions related to renewable energy conversion systems to produce electricity and hydrogen, emissions related to the production of electricity fed into the national grid (), and emissions generated by the natural gas supply chain to power the DRI production process () have been considered in this category. As far as emissions related to renewable energy and biomass conversion systems are considered, lifecycle emissions have been taken into reference as, on the one hand, all stages of the lifecycle of wind turbines and photovoltaic panels, from production to decommissioning, have been considered (), while, on the other hand, consideration of the carbon sink associated with the growth of biomass has been included (). In this paper, emissions due to iron ore extraction and scrap transport have not been included in the model as they represent invariant variables in the optimization process.
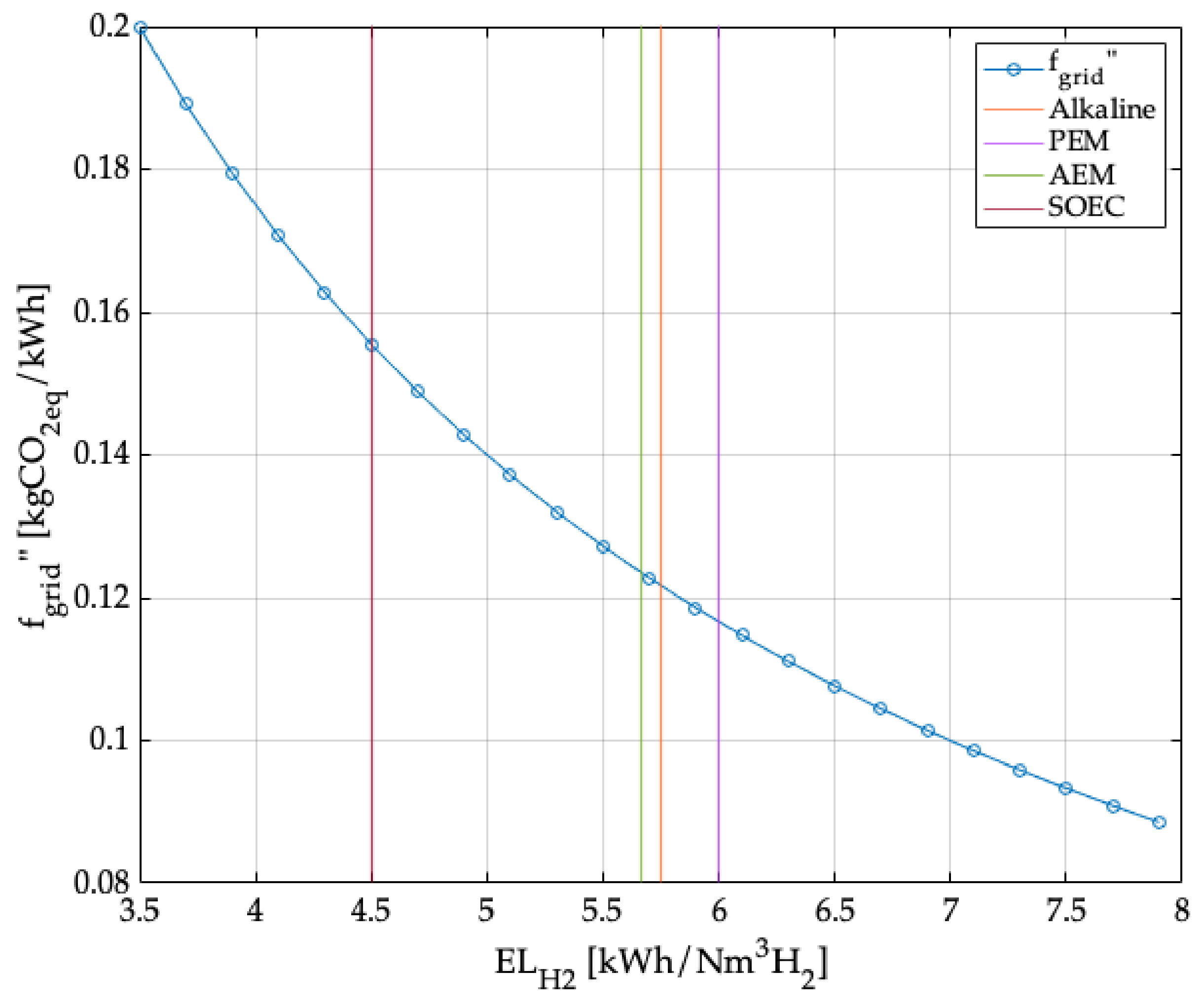
2.3. Avoided Grid Emissions by the Renewable Energy System
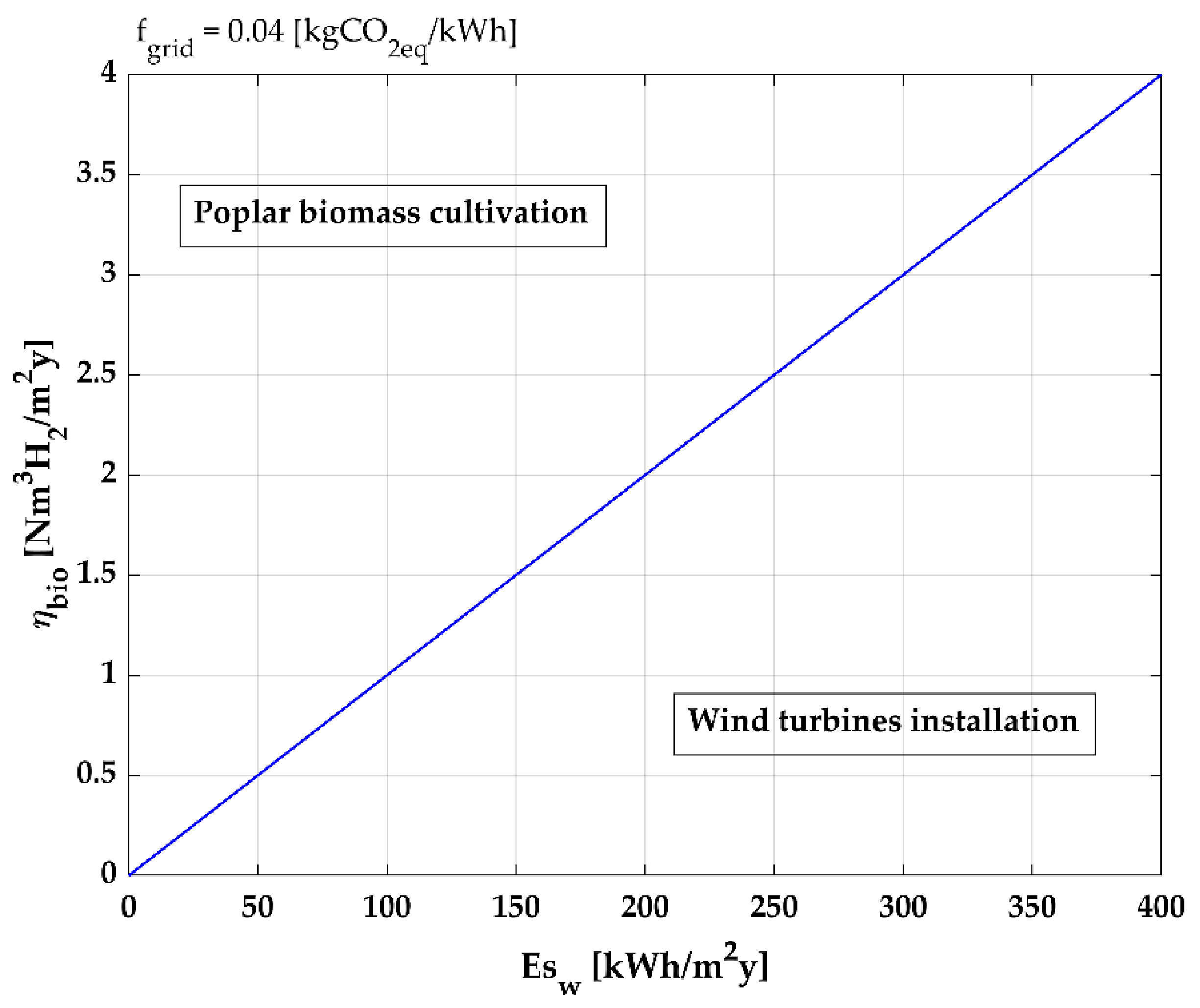
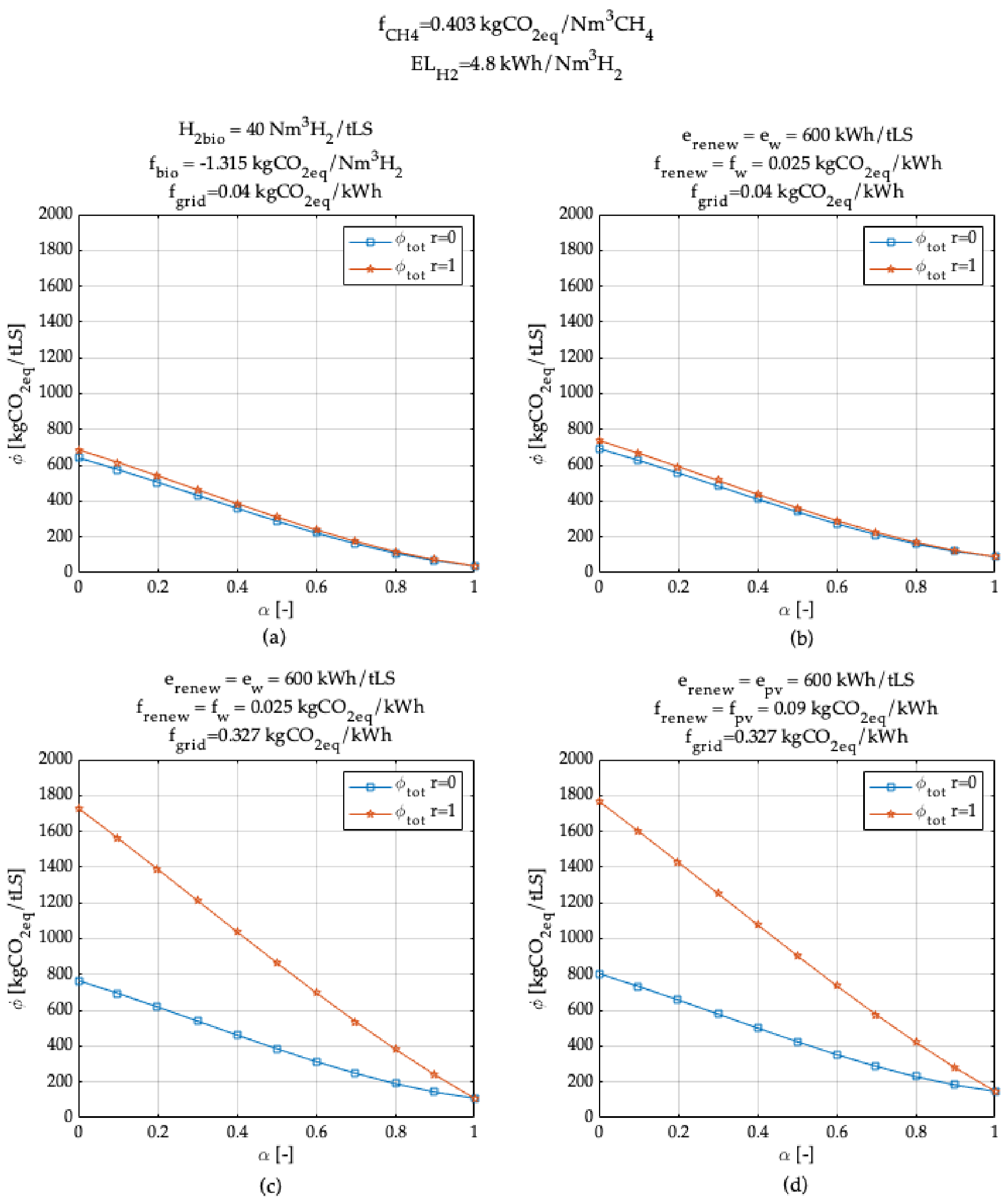
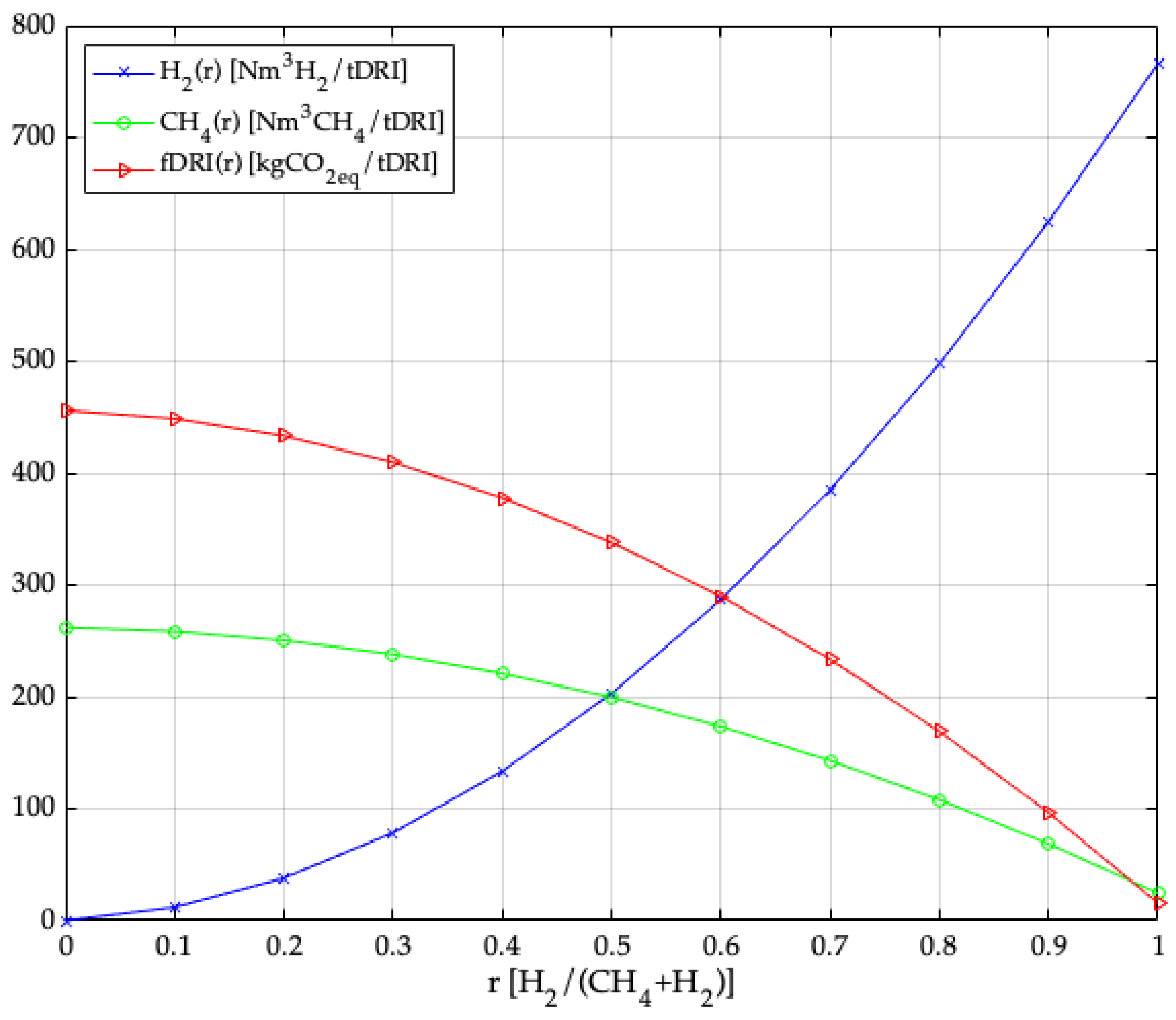
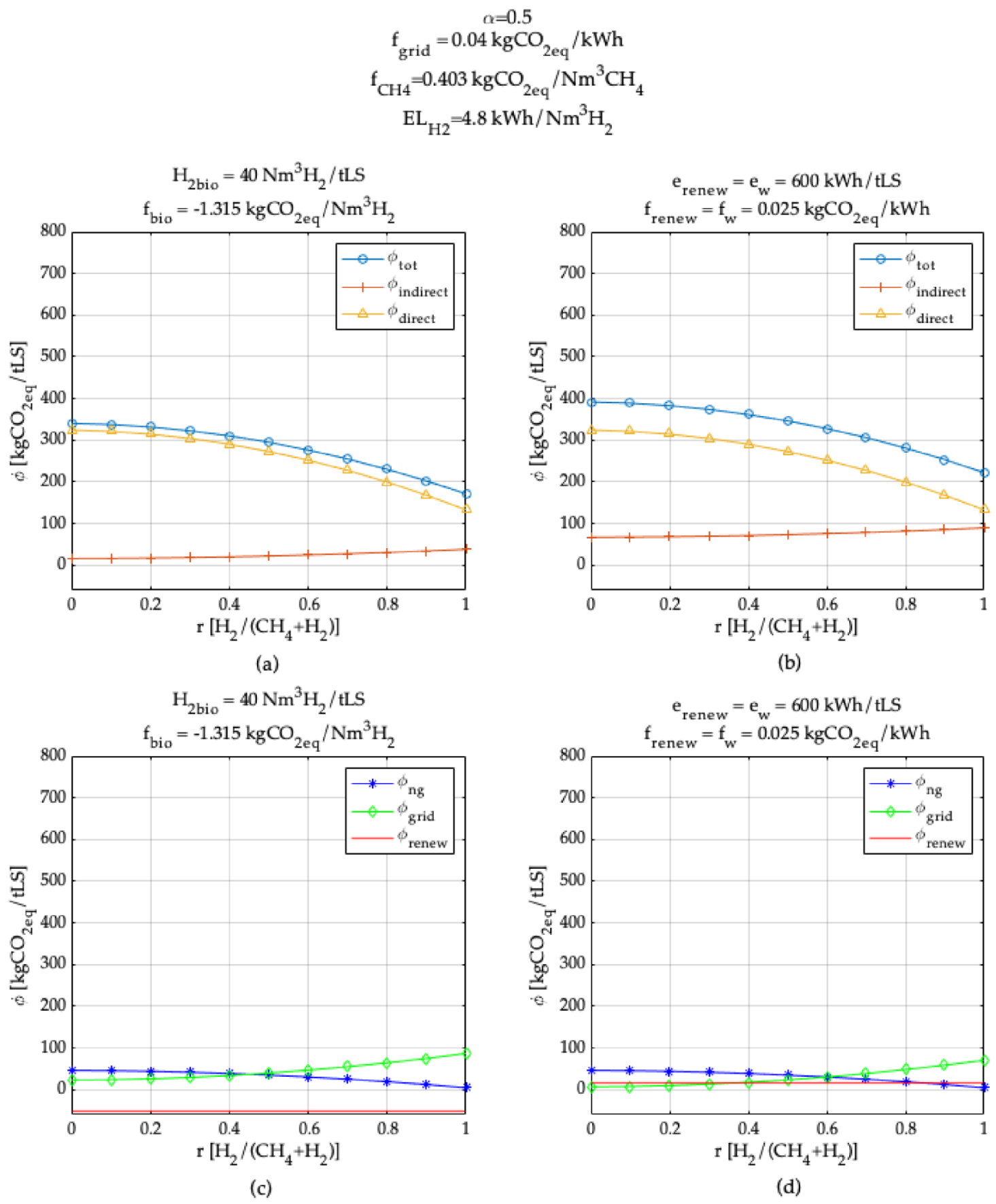
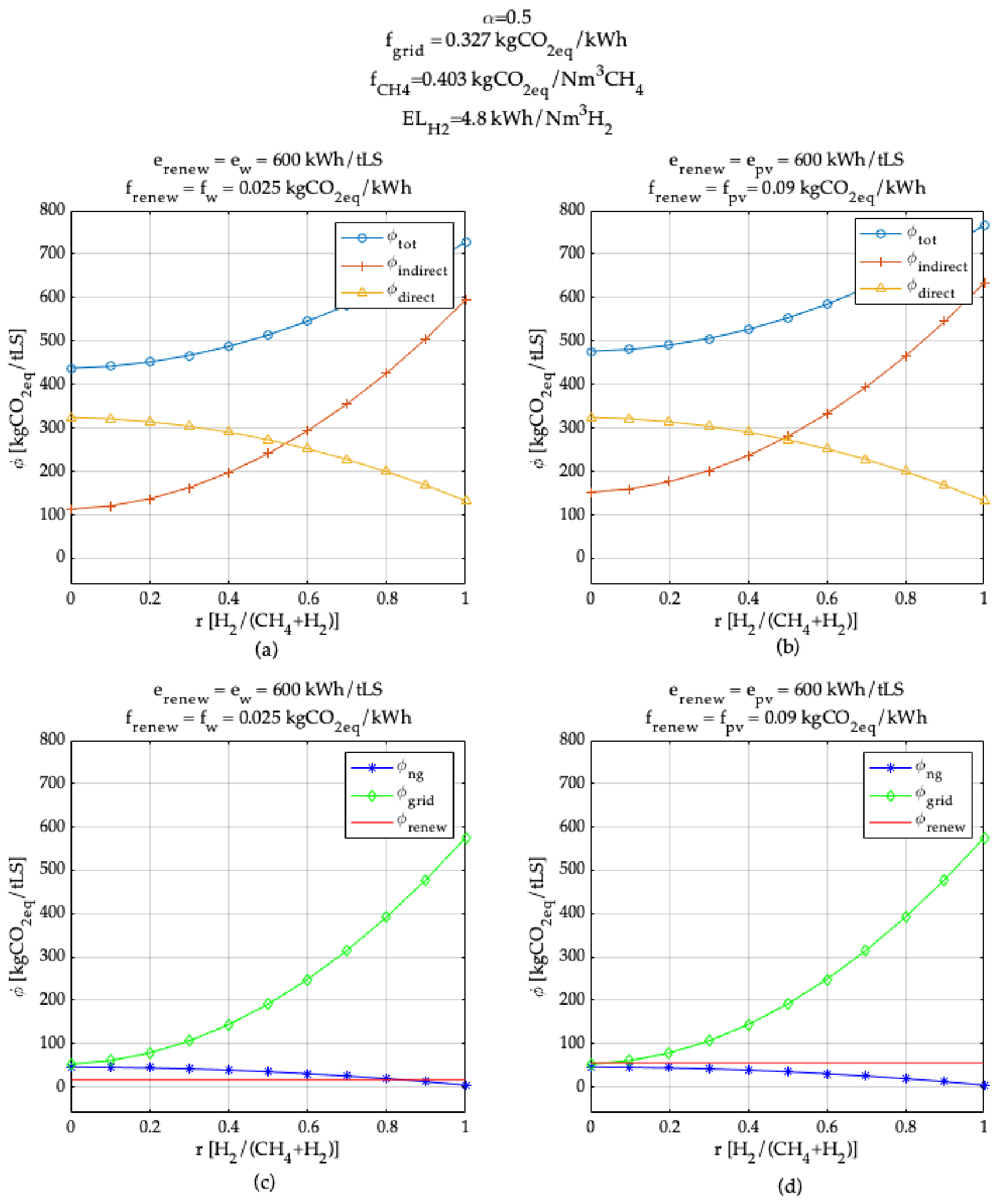
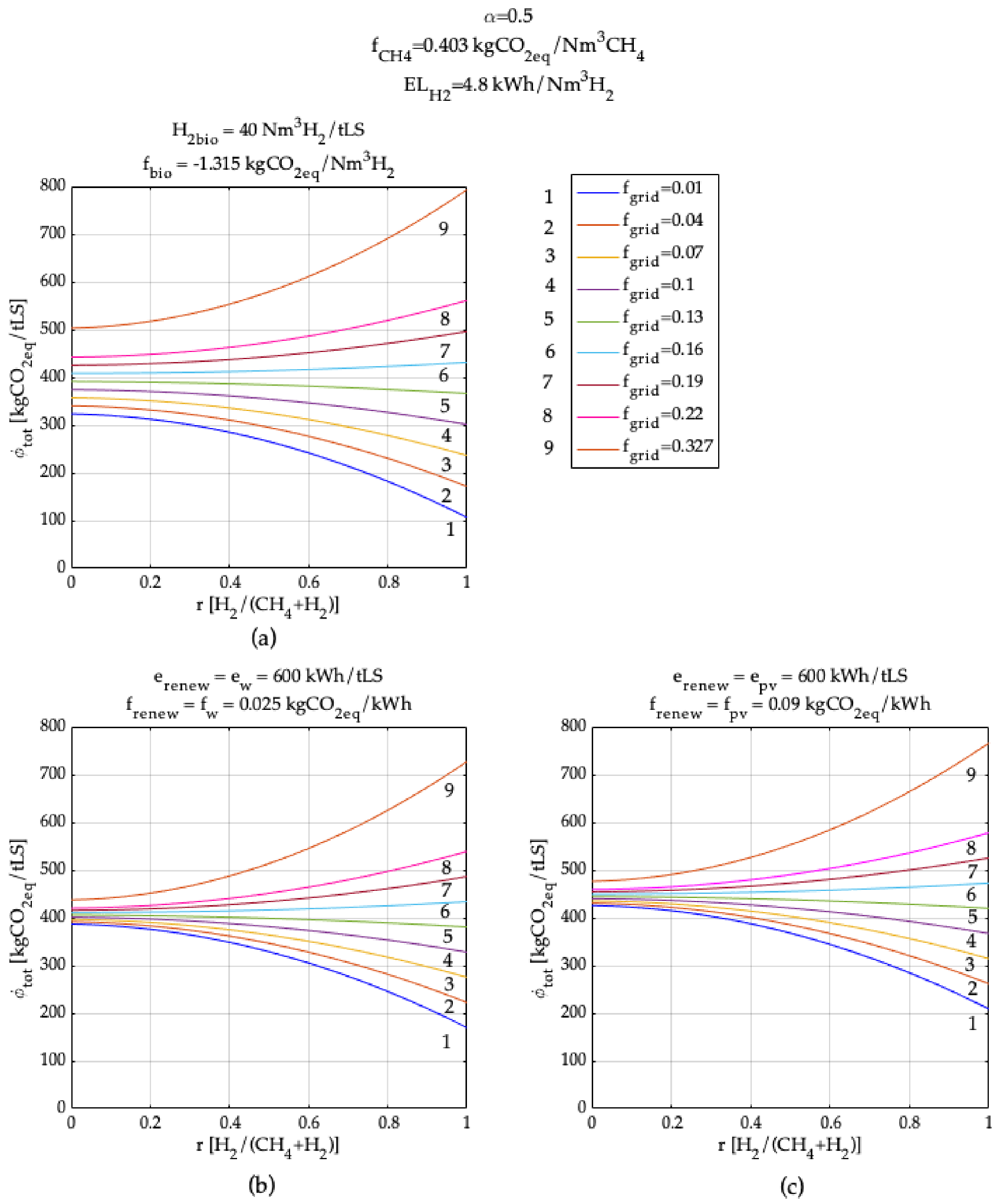
3. Model Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regulation (EU) 2021/1119. Available online: https://eur-lex.europa.eu (accessed on 2 February 2022).
- International Energy Agency. Driving Energy Efficiency in Heavy Industries. Available online: https://www.iea.org/articles/driving-energy-efficiency-in-heavy-industries (accessed on 2 February 2022).
- Ryan, N.A.; Miller, S.A.; Skerlos, S.J.; Cooper, D.R. Reducing CO2 Emissions from U.S. Steel Consumption by 70% by 2050. Environ. Sci. Technol. 2020, 54, 14598–14608. [Google Scholar] [CrossRef] [PubMed]
- World Steel Association. Steel’s Contribution to a Low Carbon Future and Climate Resilient Societies; World Steel Association: Brussels, Belgium, 2020; pp. 1–6. [Google Scholar]
- Pardo, N.; Moya Rivera, J.A.; Vatopoulos, K. Prospective Scenarios on Energy Efficiency and CO2 Emissions in the EU Iron & Steel Industry; Publications Office of the European Union: Luxembourg, 2012; ISBN1 9789279541919. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC74811 (accessed on 2 February 2022)ISBN2 9789279541919.
- World Steel Association. World Steel in Figures Report; World Steel Association: Brussels, Belgium, 2021. [Google Scholar]
- Our World in Data Emissions by Sector. Available online: https://ourworldindata.org/emissions-by-sector (accessed on 2 February 2022).
- World Steel Association. Press Release—Worldsteel Short Range Outlook; World Steel Association: Brussels, Belgium, 2021; p. 7. [Google Scholar]
- United Nations. COP26 The Glasgow Climate Pact. In Proceedings of the United Nations Climate Change Conference, Glasgow, UK, 31 October–13 November 2021; Available online: https://ukcop26.org (accessed on 2 February 2022).
- Chisalita, D.-A.; Petrescu, L.; Cobden, P.; van Dijk, H.A.J.; Cormos, A.-M.; Cormos, C.-C. Assessing the Environmental Impact of an Integrated Steel Mill with Post-Combustion CO2 Capture and Storage Using the LCA Methodology. J. Clean. Prod. 2019, 211, 1015–1025. [Google Scholar] [CrossRef]
- Gul, E.; Riva, L.; Nielsen, H.K.; Yang, H.; Zhou, H.; Yang, Q.; Skreiberg, Ø.; Wang, L.; Barbanera, M.; Zampilli, M.; et al. Substitution of Coke with Pelletized Biocarbon in the European and Chinese Steel Industries: An LCA Analysis. Appl. Energy 2021, 304, 117644. [Google Scholar] [CrossRef]
- Nwachukwu, C.M.; Wang, C.; Wetterlund, E. Exploring the Role of Forest Biomass in Abating Fossil CO2 Emissions in the Iron and Steel Industry—The Case of Sweden. Appl. Energy 2021, 288, 116558. [Google Scholar] [CrossRef]
- Rechberger, K.; Spanlang, A.; Sasiain Conde, A.; Wolfmeir, H.; Harris, C. Green Hydrogen-Based Direct Reduction for Low-Carbon Steelmaking. Steel Res. Int. 2020, 91, 2000110. [Google Scholar] [CrossRef]
- European Parliamentary Research Service. Carbon-Free Steel Production; European Parilament: Brussels, Belgium, 2021; ISBN 9789284678914. [Google Scholar]
- Béchara, R.; Hamadeh, H.; Mirgaux, O.; Patisson, F. Optimization of the Iron Ore Direct Reduction Process through Multiscale Process Modeling. Materials 2018, 11, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Béchara, R.; Hamadeh, H.; Mirgaux, O.; Patisson, F. Carbon Impact Mitigation of the Iron Ore Direct Reduction Process through Computer-Aided Optimization and Design Changes. Metals 2020, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- Alhumaizi, K.; Ajbar, A.; Soliman, M. Modelling the Complex Interactions between Reformer and Reduction Furnace in a Midrex-Based Iron Plant. Can. J. Chem. Eng. 2012, 90, 1120–1141. [Google Scholar] [CrossRef]
- Ajbar, A.; Alhumaizi, K.; Soliman, M.A.; Ali, E. Model-Based Energy Analysis of an Integrated Midrex-Based Iron/Steel Plant. Chem. Eng. Commun. 2014, 201, 1686–1704. [Google Scholar] [CrossRef]
- Sarkar, S.; Bhattacharya, R.; Roy, G.G.; Sen, P.K. Modeling MIDREX Based Process Configurations for Energy and Emission Analysis. Steel Res. Int. 2018, 89, 1700248. [Google Scholar] [CrossRef]
- Shams, A.; Moazeni, F. Modeling and Simulation of the MIDREX Shaft Furnace: Reduction, Transition and Cooling Zones. JOM 2015, 67, 2681–2689. [Google Scholar] [CrossRef]
- Hamadeh, H.; Mirgaux, O.; Patisson, F. Detailed Modeling of the Direct Reduction of Iron Ore in a Shaft Furnace. Materials 2018, 11, 1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Chu, M.; Tang, J.; Liu, Z.; Guo, J.; Yan, R.; Liu, P. Thermodynamic Performance Analysis and Environmental Impact Assessment of an Integrated System for Hydrogen Generation and Steelmaking. Energy 2022, 241, 122922. [Google Scholar] [CrossRef]
- IRENA. Green Hydrogen Cost Reduction; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020; ISBN 9789292602956. [Google Scholar]
- Vogl, V.; Åhman, M.; Nilsson, L.J. Assessment of Hydrogen Direct Reduction for Fossil-Free Steelmaking. J. Clean. Prod. 2018, 203, 736–745. [Google Scholar] [CrossRef]
- Bhaskar, A.; Assadi, M.; Somehsaraei, H.N. Decarbonization of the Iron and Steel Industry with Direct Reduction of Iron Ore with Green Hydrogen. Energies 2020, 13, 758. [Google Scholar] [CrossRef] [Green Version]
- Pimm, A.J.; Cockerill, T.T.; Gale, W.F. Energy System Requirements of Fossil-Free Steelmaking Using Hydrogen Direct Reduction. J. Clean. Prod. 2021, 312, 127665. [Google Scholar] [CrossRef]
- Global Wind Atlas. Available online: https://globalwindatlas.info (accessed on 2 February 2022).
- Global Solar Atlas. Available online: https://globalsolaratlas.info/ (accessed on 2 February 2022).
- Testa, R.; Di Trapani, A.M.; Foderà, M.; Sgroi, F.; Tudisca, S. Economic Evaluation of Introduction of Poplar as Biomass Crop in Italy. Renew. Sustain. Energy Rev. 2014, 38, 775–780. [Google Scholar] [CrossRef]
- Li, K.; Bian, H.; Liu, C.; Zhang, D.; Yang, Y. Comparison of Geothermal with Solar and Wind Power Generation Systems. Renew. Sustain. Energy Rev. 2015, 42, 1464–1474. [Google Scholar] [CrossRef]
- Susmozas, A.; Iribarren, D.; Zapp, P.; Linβen, J.; Dufour, J. Life-Cycle Performance of Hydrogen Production via Indirect Biomass Gasification with CO2 Capture. Int. J. Hydrogen Energy 2016, 41, 19484–19491. [Google Scholar] [CrossRef]
- Balcombe, P.; Anderson, K.; Speirs, J.; Brandon, N.; Hawkes, A. The Natural Gas Supply Chain: The Importance of Methane and Carbon Dioxide Emissions. ACS Sustain. Chem. Eng. 2017, 5, 3–20. [Google Scholar] [CrossRef]
- Country Specific Electricity Grid Greenhouse Gas Emission Factor. Available online: https://www.carbonfootprint.com (accessed on 2 February 2022).
- World Resources Institute and World Business Council for Sustainable Development. The Greenhouse Gas Protocol. Available online: https://ghgprotocol.org (accessed on 2 February 2022).

| Notation | Unit Measure | Description | Value/Range |
|---|---|---|---|
| Total available area for the installation of renewable energy conversion systems or biomass cultivation. | - | ||
| Expected yearly production volume of liquid steel. | - | ||
| Producibility of electricity per unit installation area from wind turbines. | [27] | ||
| [-] | Installation area of wind turbines (share of S). | ||
| Producibility of electricity per unit installation area from photovoltaic panels. | [28] | ||
| [-] | Installation of photovoltaic panels (share of S). | ||
| Yield per unit area of biomass culture in volume of hydrogen produced by indirect gasification. | [29] | ||
| Lifecycle emissions of wind turbines per unit of electricity produced. | 0.025 [30] | ||
| Lifecycle emissions of photovoltaic panels per unit of electricity produced. | 0.090 [30] | ||
| Emissions from hydrogen production by indirect biomass gasification. | −1.315 [31] | ||
| [-] | Biomass cultivation area (share of S). | ||
| [-] | Volumetric share of hydrogen in the reducing gas mixture to produce 1 t of DRI. | ||
| [-] | Ratio of 1 t LS to 1t DRI. | 1.150 [24] | |
| [-] | Share of scrap employed in EAF to produce 1 t of LS. | ||
| Methane requirement in the reducing gas mixture to produce 1 t of DRI. | [13] | ||
| Emissions generated by supplying 1 Nm3 of methane from natural gas supply chain. | 0.404 [32] | ||
| Electrical consumption of DRI production process auxiliaries for producing 1 t LS. | 100 [13] | ||
| EAF electricity consumption for producing 1 t LS from DRI. | 753 [24] | ||
| EAF electricity consumption for producing 1 t of LS from scrap. | 667 [24] | ||
| Emissions from the national grid for the supply of 1 kWh of electricity. | [33] | ||
| Hydrogen requirement in the reducing gas mixture to produce 1 t DRI. | [13] | ||
| Electricity demand of the electrolyzer to produce 1 Nm3 of H2. | 4.8 [13] | ||
| Direct emissions from DRI production process. | [13] | ||
| Direct emissions from EAF producing 1 t LS from scrap. | 72 [13] | ||
| Direct emissions from EAF producing 1 t LS from DRI. | 180 [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Digiesi, S.; Mummolo, G.; Vitti, M. Minimum Emissions Configuration of a Green Energy–Steel System: An Analytical Model. Energies 2022, 15, 3324. https://doi.org/10.3390/en15093324
Digiesi S, Mummolo G, Vitti M. Minimum Emissions Configuration of a Green Energy–Steel System: An Analytical Model. Energies. 2022; 15(9):3324. https://doi.org/10.3390/en15093324
Chicago/Turabian StyleDigiesi, Salvatore, Giovanni Mummolo, and Micaela Vitti. 2022. "Minimum Emissions Configuration of a Green Energy–Steel System: An Analytical Model" Energies 15, no. 9: 3324. https://doi.org/10.3390/en15093324
APA StyleDigiesi, S., Mummolo, G., & Vitti, M. (2022). Minimum Emissions Configuration of a Green Energy–Steel System: An Analytical Model. Energies, 15(9), 3324. https://doi.org/10.3390/en15093324








