1. Introduction
From its very beginning, corrosion has posed a serious and complex problem for the petroleum industry and as such, requires a detailed analysis and a systematic management approach. On one hand, most of the pipelines and equipment in the petroleum industry are made of carbon steel, which, under certain conditions and in specific (corrosive) environments, is subject to corrosion. On the other hand, the petroleum industry deals with fluids that are susceptible to the formation of a corrosive environment. During hydrocarbon production, depending on the type of reservoir, there is always a certain amount of brine produced, with the quantities increasing as the production field matures. Along with brine (containing a significant amount of salt), the production fluid also contains some other impurities such as carbon dioxide (CO2) and hydrogen sulfide (H2S), which create highly corrosive environments.
The fluid flow has a great influence on the corrosion rate and surface processes. When a protective ferrous-carbonate (FeCO
3) layer is formed or an inhibitor film covers the steel surface, the effect of flow becomes meaningless because in that case, the main corrosion resistance is that layer/inhibitor film. On the other hand, turbulent flow could interfere with the forming of the mentioned protective ferrous-carbonate (FeCO
3) layer/inhibitor film, or it could mechanically damage it, which can lead to greater risk of localized corrosion attack [
1].
Corrosion damages equipment, reduces infrastructure lifetime, has potentially harmful effects on the environment, and consequently causes high costs. The total costs of corrosion in the oil and gas production industry have been estimated to be 1.372 × 10
9 US dollars per year, of which the highest share is attributed to the costs of the corrosion of surface facilities (pipelines and surface processing equipment) (43%) and downhole equipment (tubing) (34%) [
2]. According to the latest report of the National Association of Corrosion Engineers (NACE), the
International Measures and Prevention,
Application and Economics of Corrosion Technologies (the IMPACT report), the global corrosion costs have been estimated to 2.5 × 10
18 US dollars, which, in 2016 when the IMPACT report was issued, was equivalent to 3.4% of the global Gross Domestic Product (GDP) [
3]. Although the corrosion problem in the petroleum industry cannot be fully avoided, this problem can be mitigated, among others, by the application of corrosion inhibitors. Since commonly used, conventional corrosion inhibitors (usually organic inhibitors with polar functional groups, such as pyridines, imidazolines or amides [
4,
5] containing sulphur (S), phosphorus (P), oxygen (O) and nitrogen (N) atoms) are considered to be harmful to the environment, there is a strong initiative aimed at developing equally effective, less toxic and biodegradable so-called green corrosion inhibitors. There are several groups of compounds that are considered to be green corrosion inhibitors, such as medicaments, amino acids, biopolymers, Rare Earth elements, and plant extracts [
6,
7,
8]. Out of the stated green corrosion inhibitors, due to their non-toxic and biodegradable nature and simple and economical extraction process, nowadays, most research has been performed using plant extracts. The extracts of many plants have been considered to be green corrosion inhibitors [
9].
Most of the plants’ extracts considered to be green corrosion inhibitors have been tested in acidic environments (HCl, H
2SO
4, H
2CO
3) [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19] with fewer being tested in neutral media [
20,
21,
22]. As mentioned before, in the oil and gas production system, inner corrosion mostly occurs because of the formation of a salty-acid environment due to sour gas dissolution in brine, so, in order to test green corrosion inhibitors’ efficiency for such systems, the initial tests should at least be performed in brine saturated with CO
2.
In the selection phase, ten plants were tested [
23]. The results of the preliminary tests pointed to dandelion-root extract as a potential corrosion inhibitor in brine solution saturated with CO
2. In this paper, the results of the inhibitor efficiency of dandelion-root extract in static and flow conditions, determined through electrochemical methods (Tafel polarization and electrochemical-impedance spectroscopy (EIS)), are presented. Additionally, the results of scanning electron microscopy (SEM) and Fourier-transform infrared spectroscopy (FTIR), which were conducted to determine how the inhibitor is adsorbed on a steel surface and through which active groups, are shown. Lastly, the biodegradability and toxicity of the extract were determined.
2. Materials and Methods
In this study, dandelion-root extract was tested as a green corrosion inhibitor for carbon steel in brine saturated with CO
2. The inhibitor efficiency was tested in static and flow conditions by using electrochemical methods (Tafel polarization and EIS). The chemical composition of the carbon-steel sample is shown in
Table 1. The composition was determined by optical emission spectrometry on spectrophotometer GDS 850 A, LECO. Before each measurement, the carbon-steel sample was polished with 300, 600 and 1200 grit abrasive paper, then washed with distilled water and finally degreased with ethanol (96%). The carbon-steel-sample area exposed to the solution was 1 cm
2.
The solution that served as a corrosive media was a simulated brine solution with or without the added dandelion-root extract. The chemical composition of the simulated brine solution is shown in
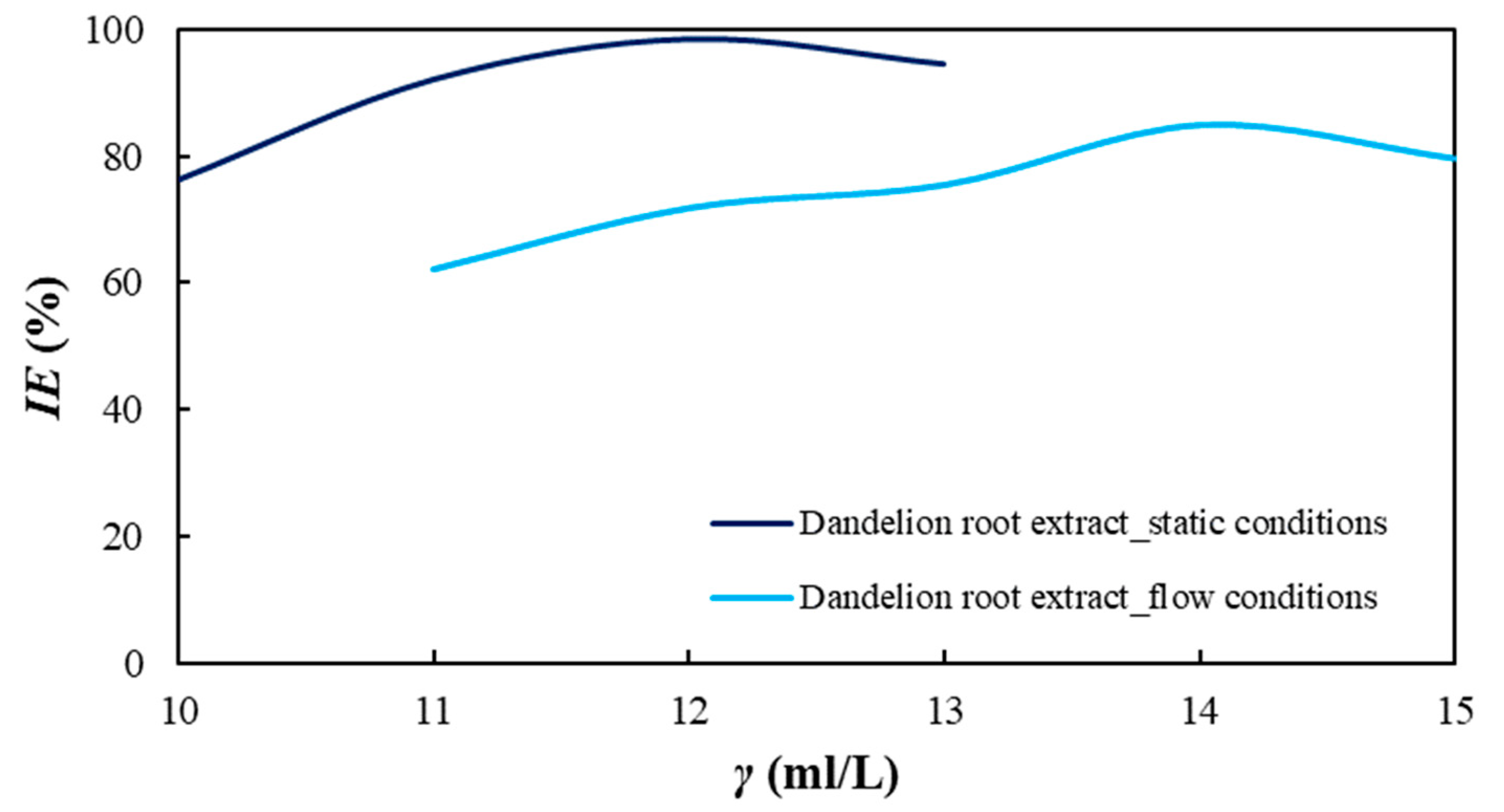
Table 2. Prior to the tests, the solution was firstly saturated with carbon dioxide for 45 min, followed by continuous saturation during the measurement. Commercially available liquid dandelion-root extract (with 80% flavonoids), Soria Natural, Spain, was used for these measurements. Dandelion-root extract was added at concentrations ranging from 10 mL/L to 13 mL/L with a concentration increase of 1 mL/L per measurement for static conditions. For flow conditions, the tested concentration range of dandelion-root extract was from 11 mL/L to 15 mL/L with an increase of 1 mL/L per measurement. The concentration range was selected based on the available literature on the previously conducted research on dandelion-root extract and the results of the self-performed preliminary research [
23].
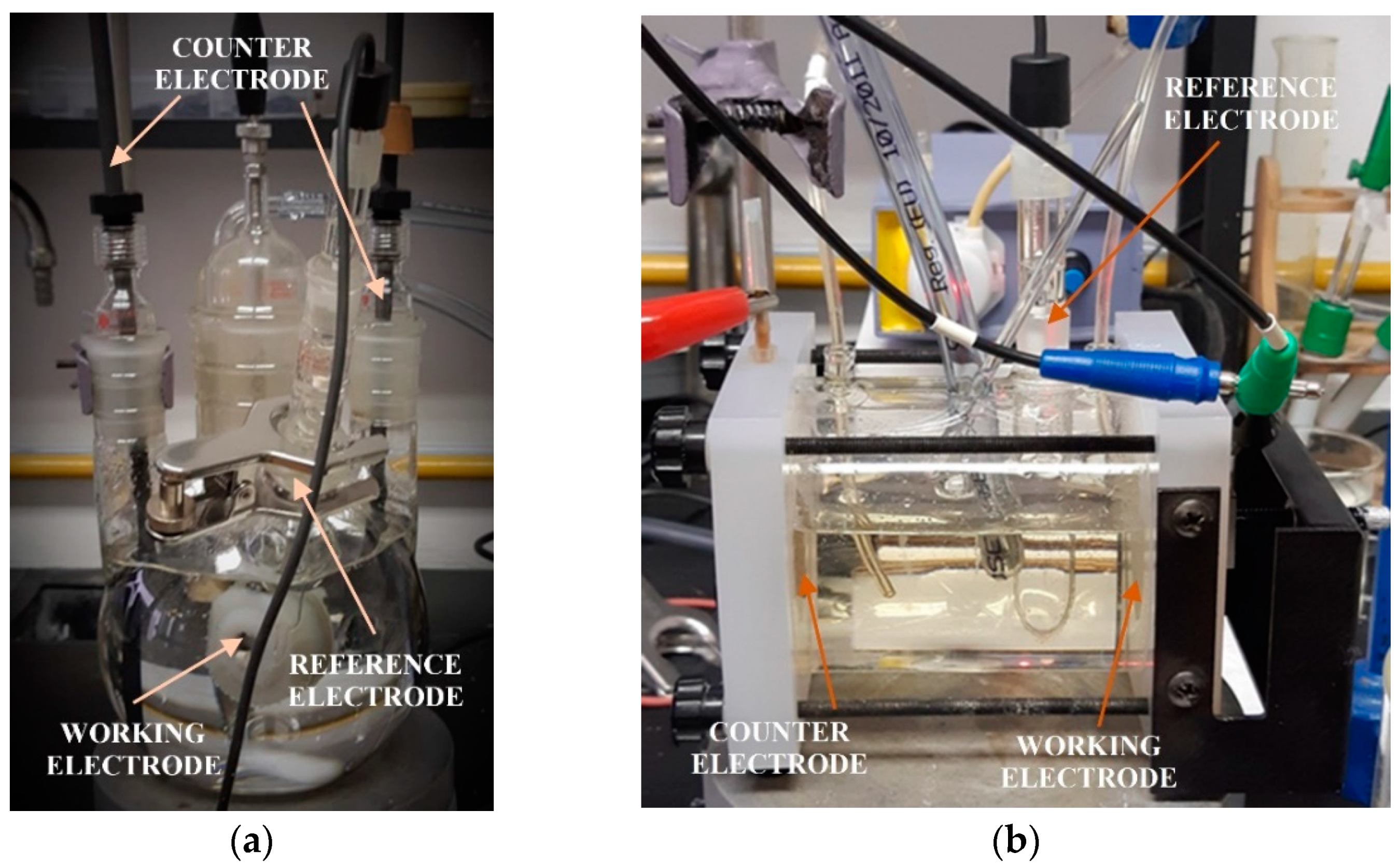
The measurements were conducted by using a three-electrode corrosion cell, as shown in
Figure 1. After immersing a carbon-steel sample into the solution, the electrode was stabilized for 1 h before performing electrochemical measurements. All measurements were performed at ambient temperature. As a counter electrode, for measurement in static conditions, two graphite rods were used, and for measurement in flow conditions, a platinum electrode was used. As a reference electrode for measurements in both conditions, a saturated calomel electrode (SCE) was used. In flow conditions, measurements were conducted under a maximum flow of 50 cm
3/min, which is equivalent to a maximum flow rate of 400 cm/min for this flow cell. This flow rate is achieved in real field conditions in flowlines in the case of a flow of approximately 20 m
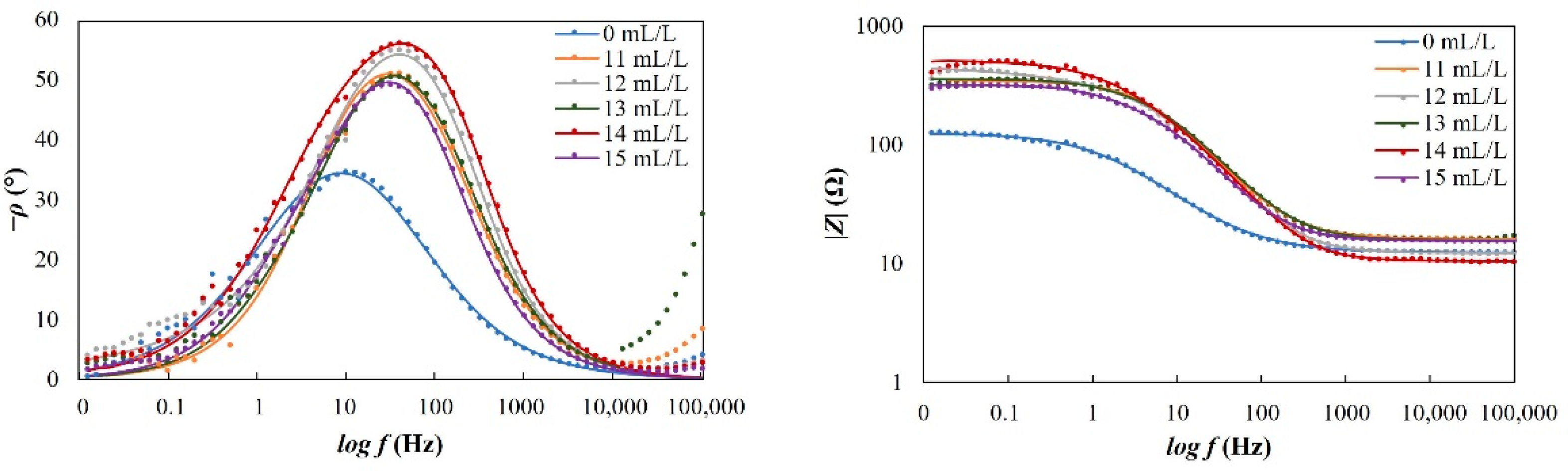
3/d. Electrochemical measurements were performed using SP1 Potentiostat and SmartManager software with IVMAN and ZMAN programs used to analyze data. All measurements were performed three times for better data reproducibility. Very good reproducibility was achieved with less than 5% of relative standard deviation.
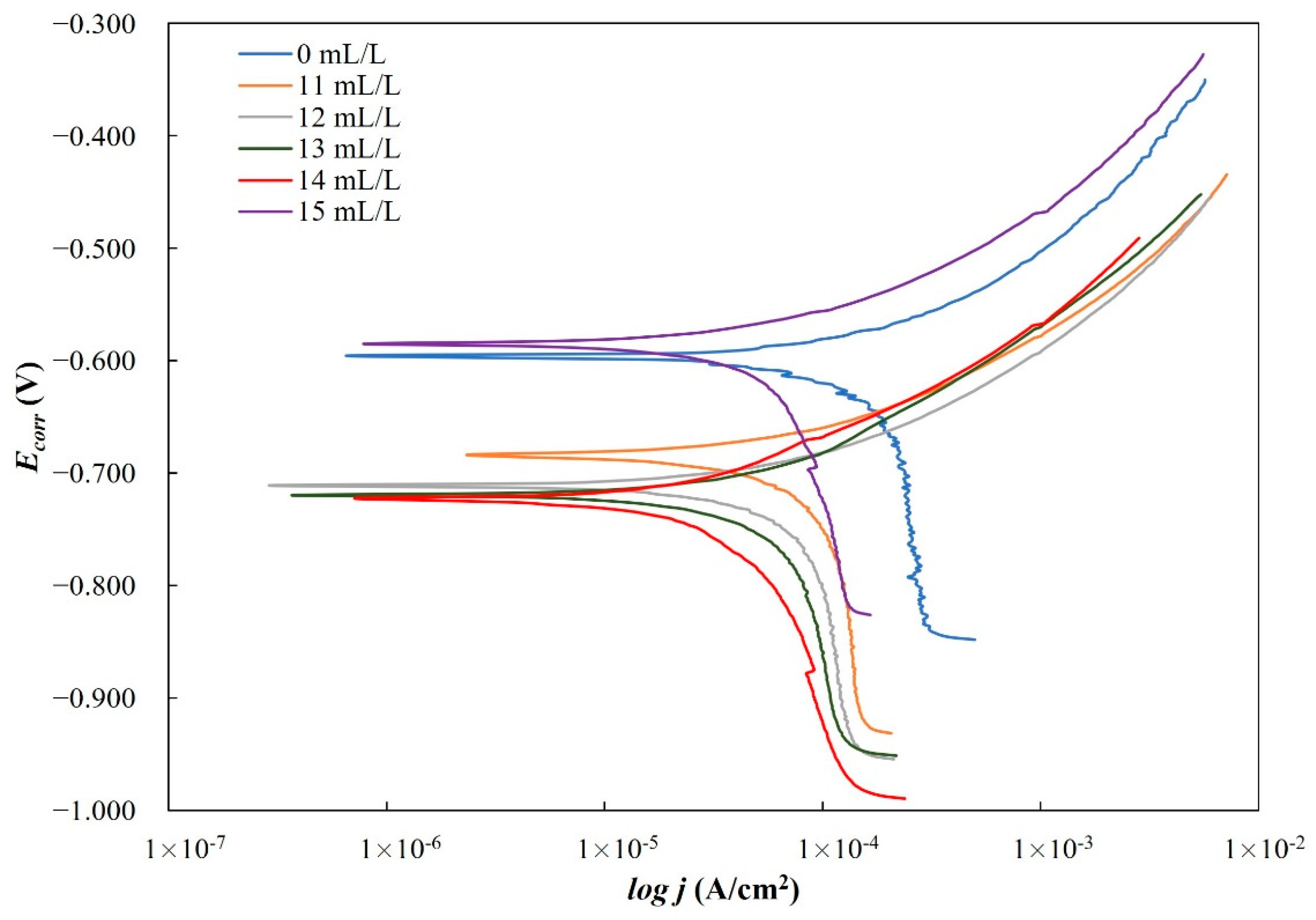
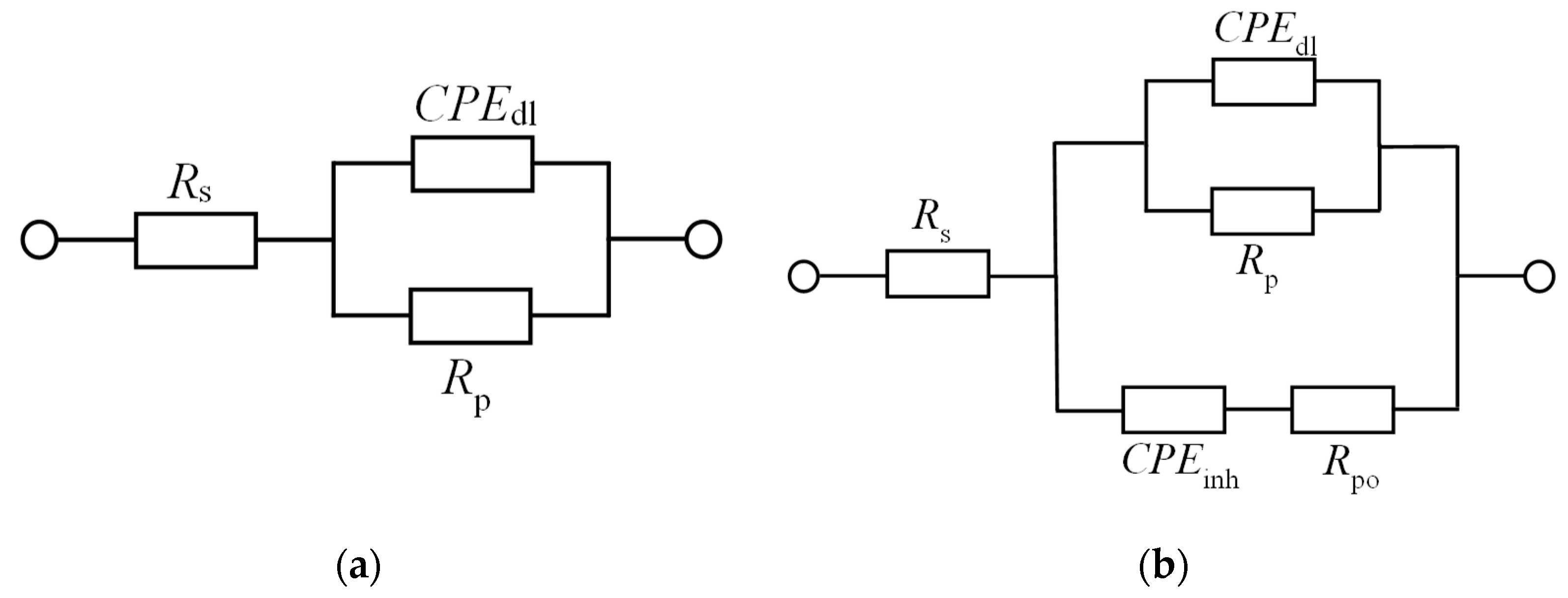
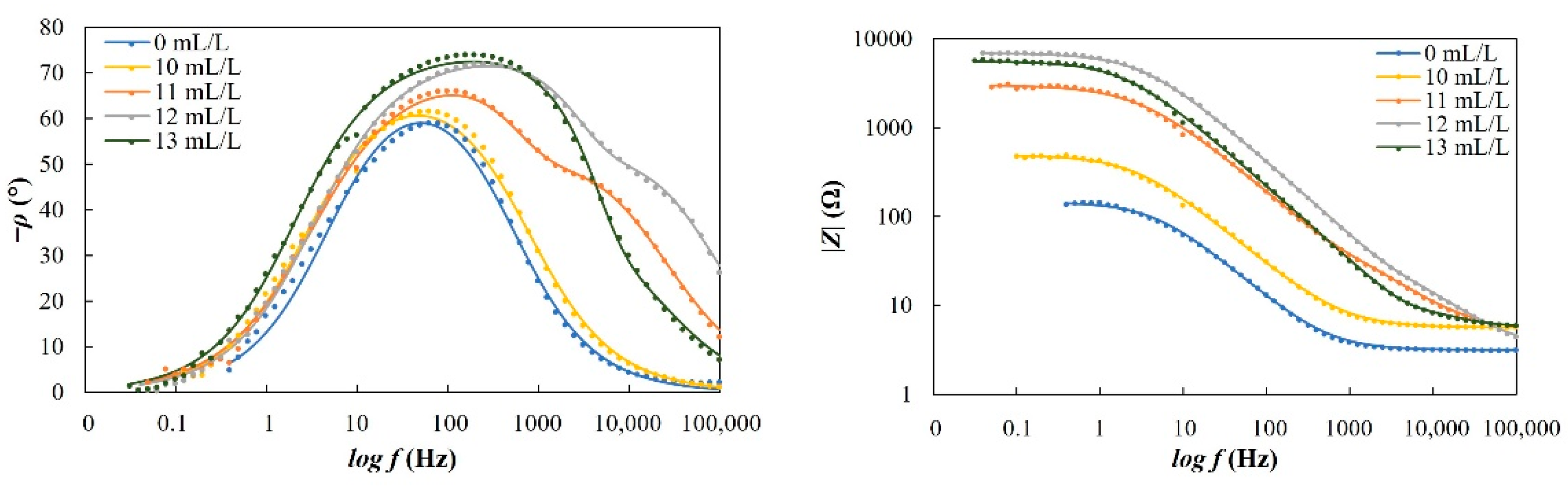
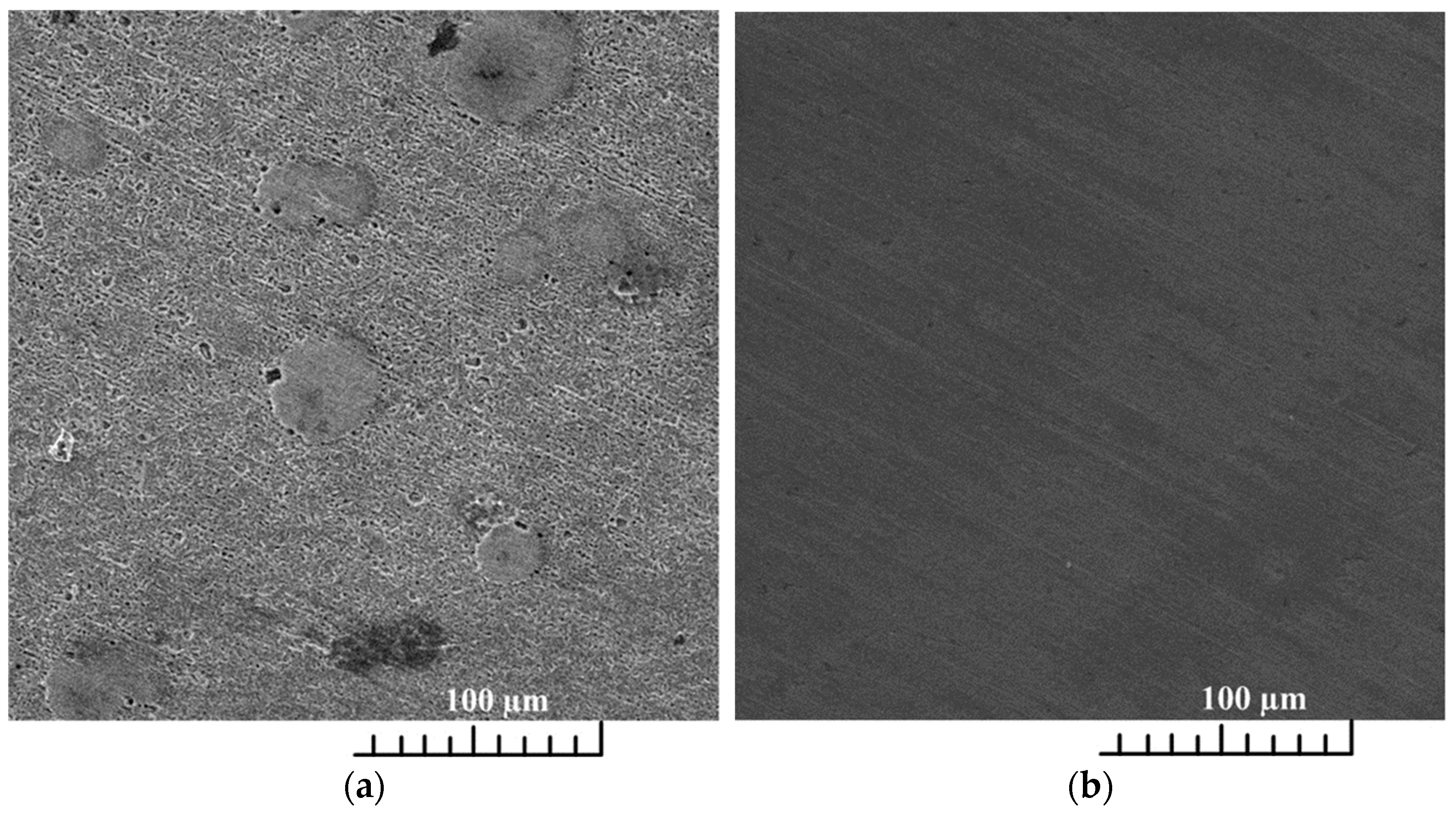
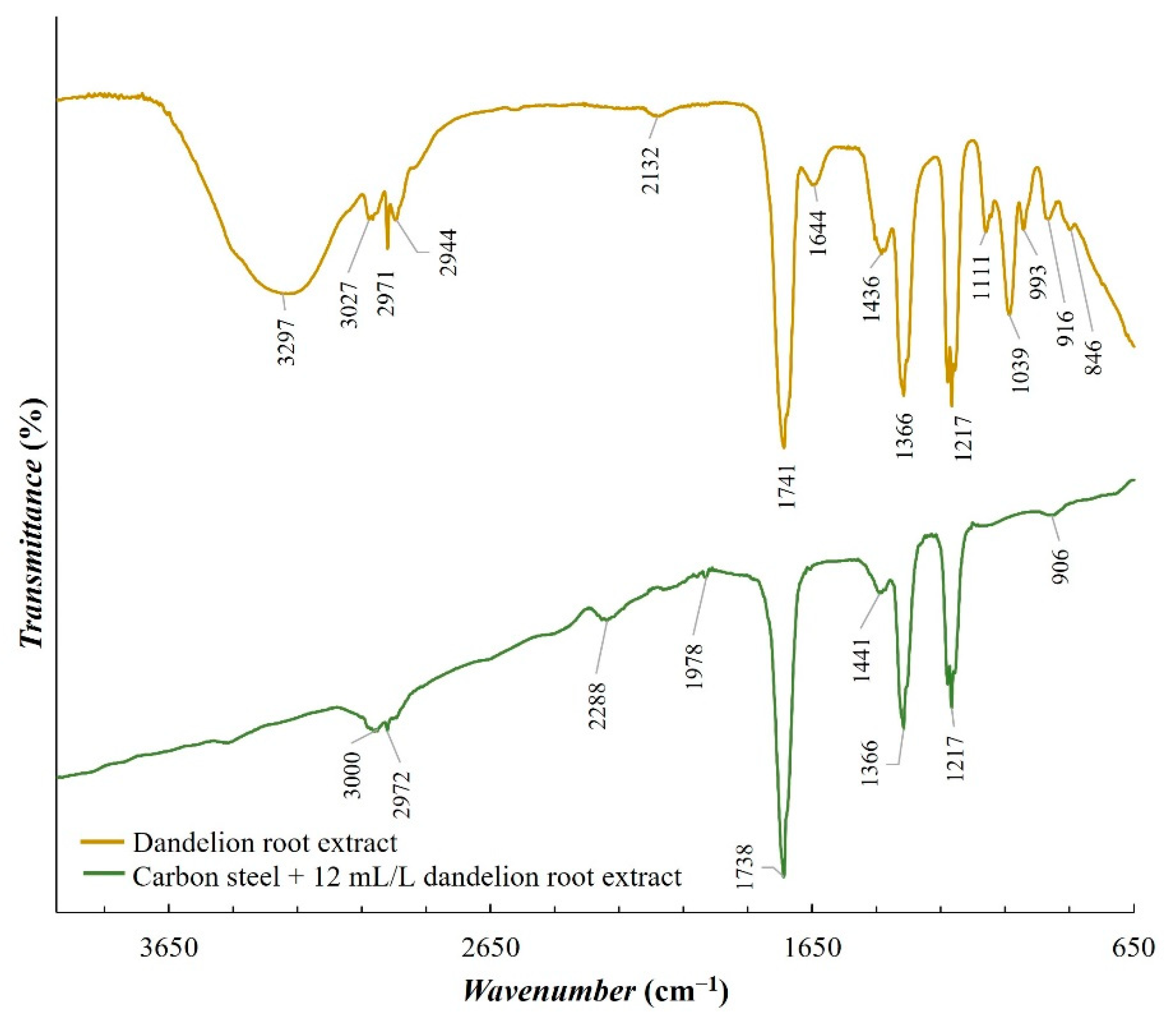
For potentiodynamic polarization with Tafel extrapolation, the corrosion potential was set to ±250 mV versus open circuit potential after stabilizing the electrode for 1 h, with a scan rate of 0.166 mV/s. Using this method, the corrosion rate and inhibition efficiency were determined. Electrochemical-impedance spectroscopy was performed in a frequency range from 100 kHz to 1 mHz. Some of the EIS measurements were stopped before reaching the frequency of 1 mHz, which in the case of our measurements did not affect the results nor the selected equivalent electrical circuits. Characterization of a steel surface with or without an inhibitor was conducted through the nondestructive EIS method. Besides electrochemical measurements, the surface-examination methods of SEM and FTIR were used to determine the mechanism of the inhibitor adsorption on the metal surface. The morphology of the steel surface was observed using a TESCAN VEGA TS5136LS scanning electron microscope and FTIR spectra were recorded in Fourier-transform infrared Spectrum One, Perkin Elmer spectrophotometer (Great Britain), which extended from 650 cm−1 to 4000 cm−1. Both surface-examination methods were performed with carbon-steel samples that had been immersed for a period of four hours in the brine solution to which the inhibitor (dandelion-root extract) was added at the most effective concentration and compared with the pure liquid extract. To determine the environmental impact of the inhibitor, the biodegradability and toxicity of dandelion-root extract was examined. The value of chemical oxygen demand (COD) was spectrophotometrically determined at 670 nm (Hach model DR/2400), while biological oxygen demand (BOD) was determined by Winkler’s method. For the determination of toxicity, bioluminescent bacteria Vibrio fischeri and luminometer LUMIStox 300 (Dr Lange GmbH, Germany) were used.
4. Conclusions
Dandelion-root extract was analyzed in static and flow conditions in a simulated brine solution saturated with CO2 by potentiodynamic polarization and electrochemical-impedance spectroscopy. The extract proved to be an effective corrosion inhibitor in both conditions. In static conditions, the highest inhibitor efficiency of 98.37% was achieved at a concentration of 12 mL/L of the inhibitor, but in flow conditions, the efficiency was 82.8% at a concentration of 14 mL/L. The extract behaved as a mixed-type inhibitor. An SEM scan of the carbon-steel-sample surface showed a uniform surface compared to the steel sample surface from the uninhibited solution. This indicates the adsorption of the inhibitor onto the surface of the examined steel sample. Regarding the FTIR scans, dandelion-root extract was adsorbed to the steel sample surface by C-N (1217 cm−1), C-C (1366 cm−1) and C=O (1738 cm−1) functional groups. The conducted biodegradability and toxicity analyses showed that dandelion-root extract is almost completely biodegradable (0.96) and with a value of toxicity of 2.38, from which it can be concluded that this extract is environmentally acceptable. Future research should be aimed at testing the inhibition efficiency of dandelion-root extract in systems in which, in addition to brine and dissolved CO2, there are also hydrocarbon components. Additionally, in order to simulate as realistic conditions as possible, future research should be conducted under conditions of different high temperatures and pressures.