A Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle
Abstract
1. Introduction
2. Hydrogen Production through Thermochemical Water Splitting
2.1. Thermodynamics of Water Splitting Cycles Using Metal Oxides
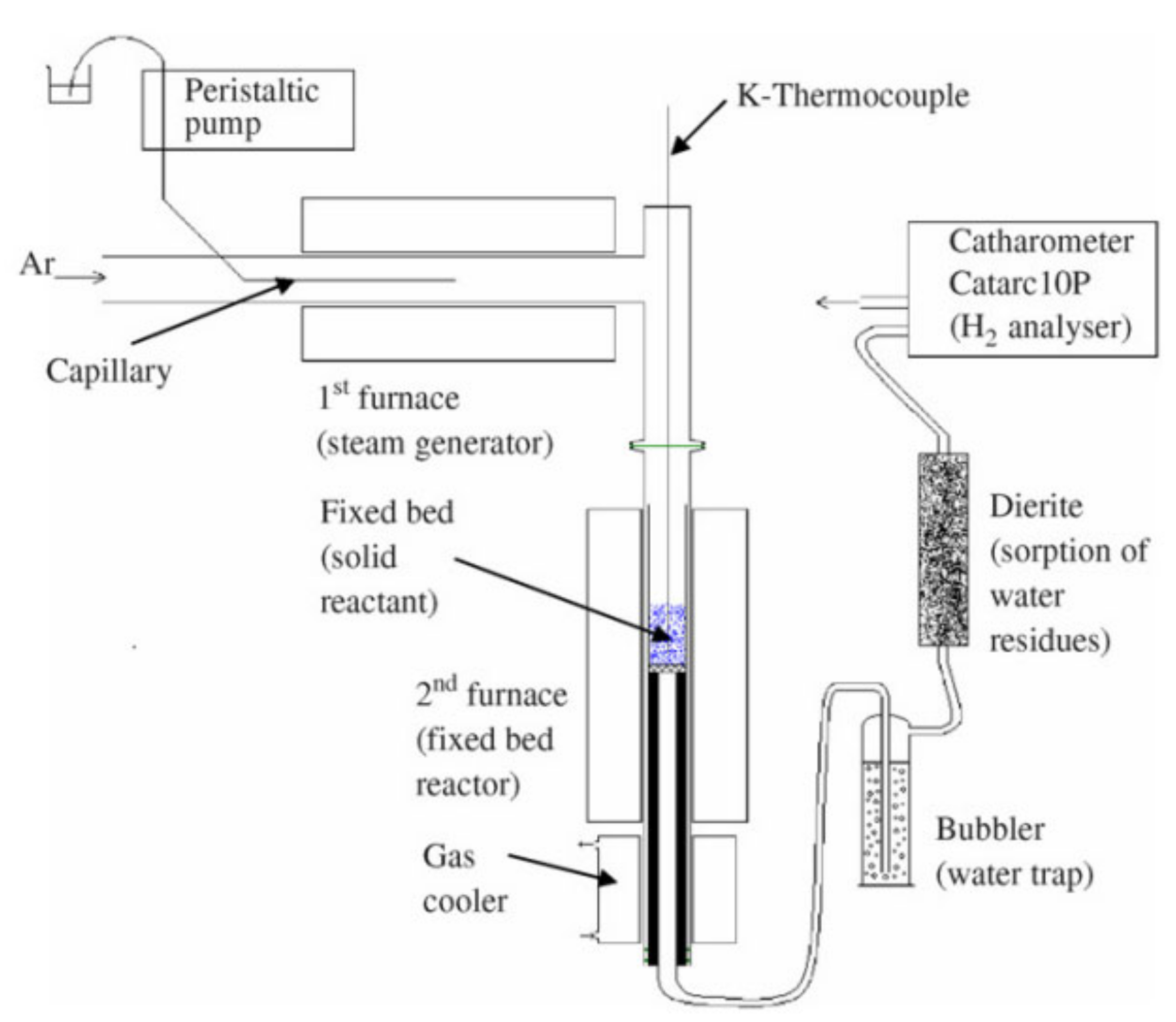
2.2. Reactor Systems and Methodologies
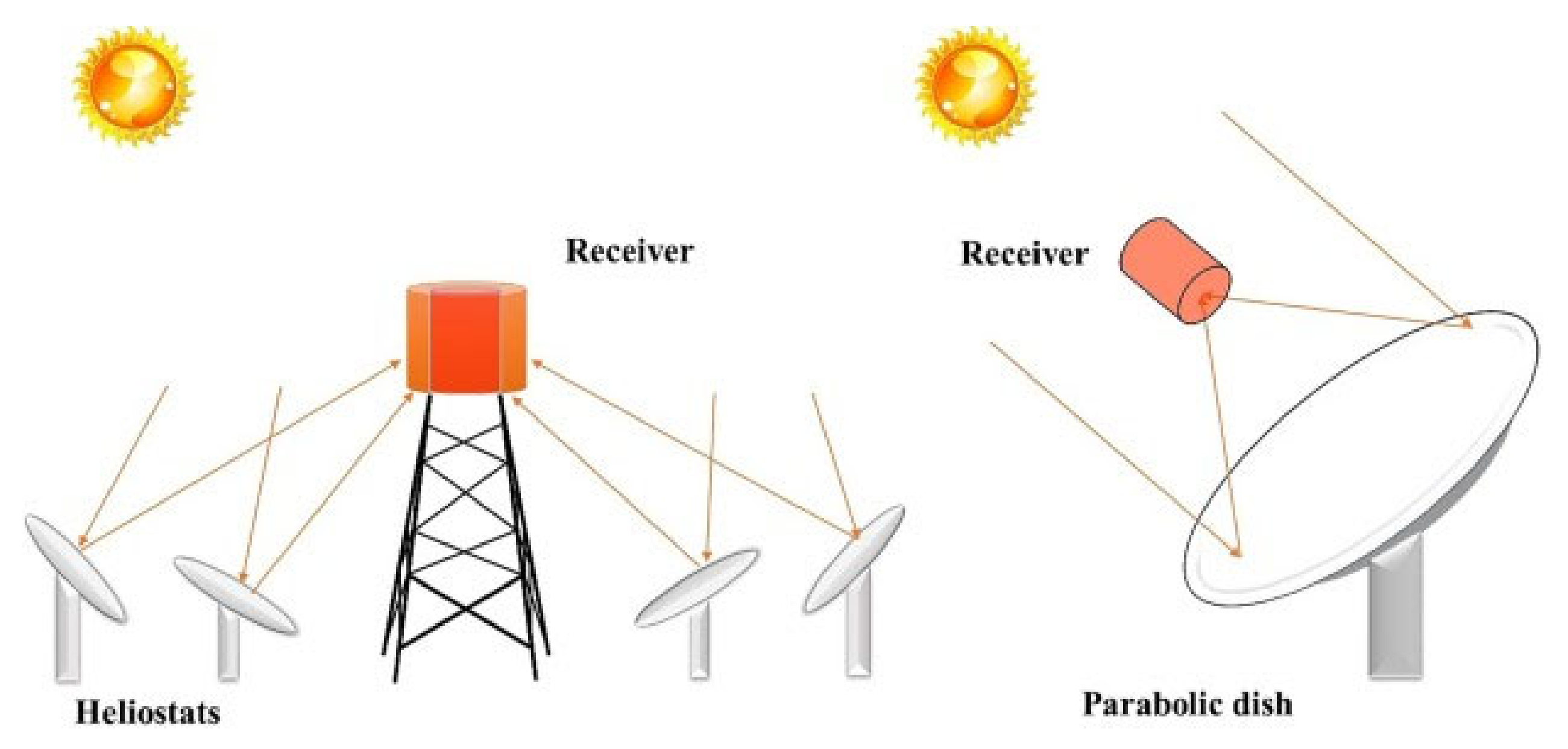
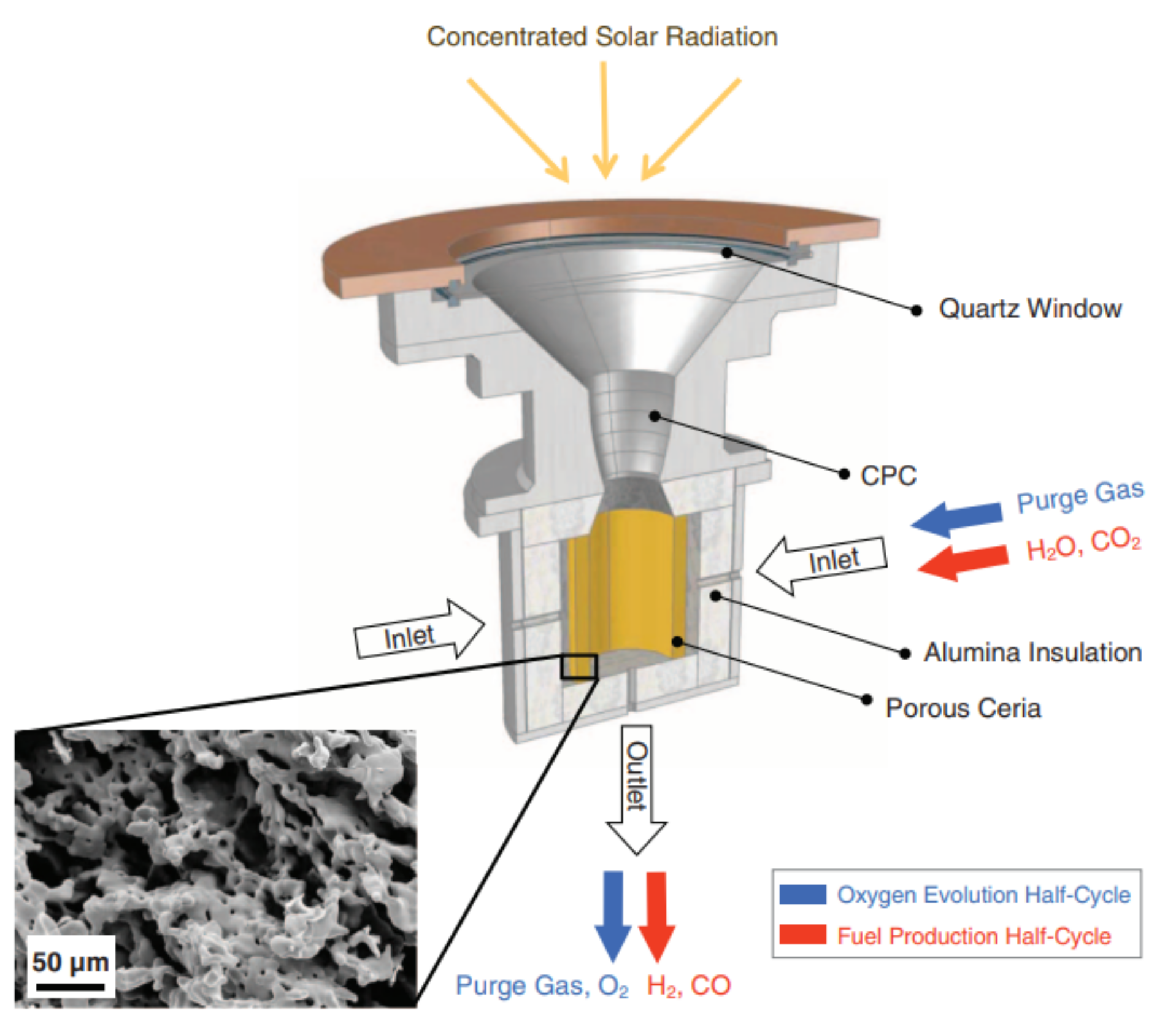
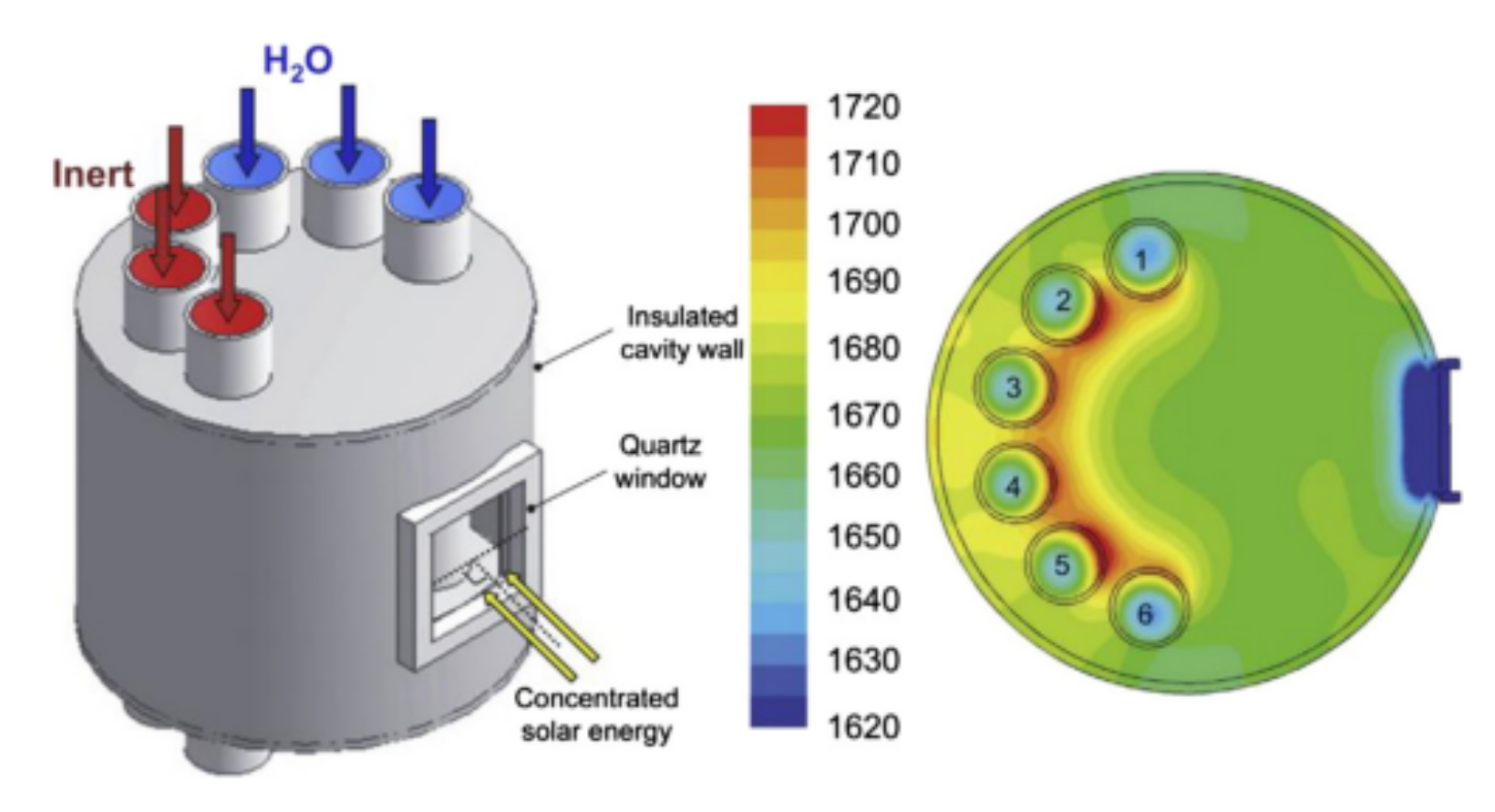
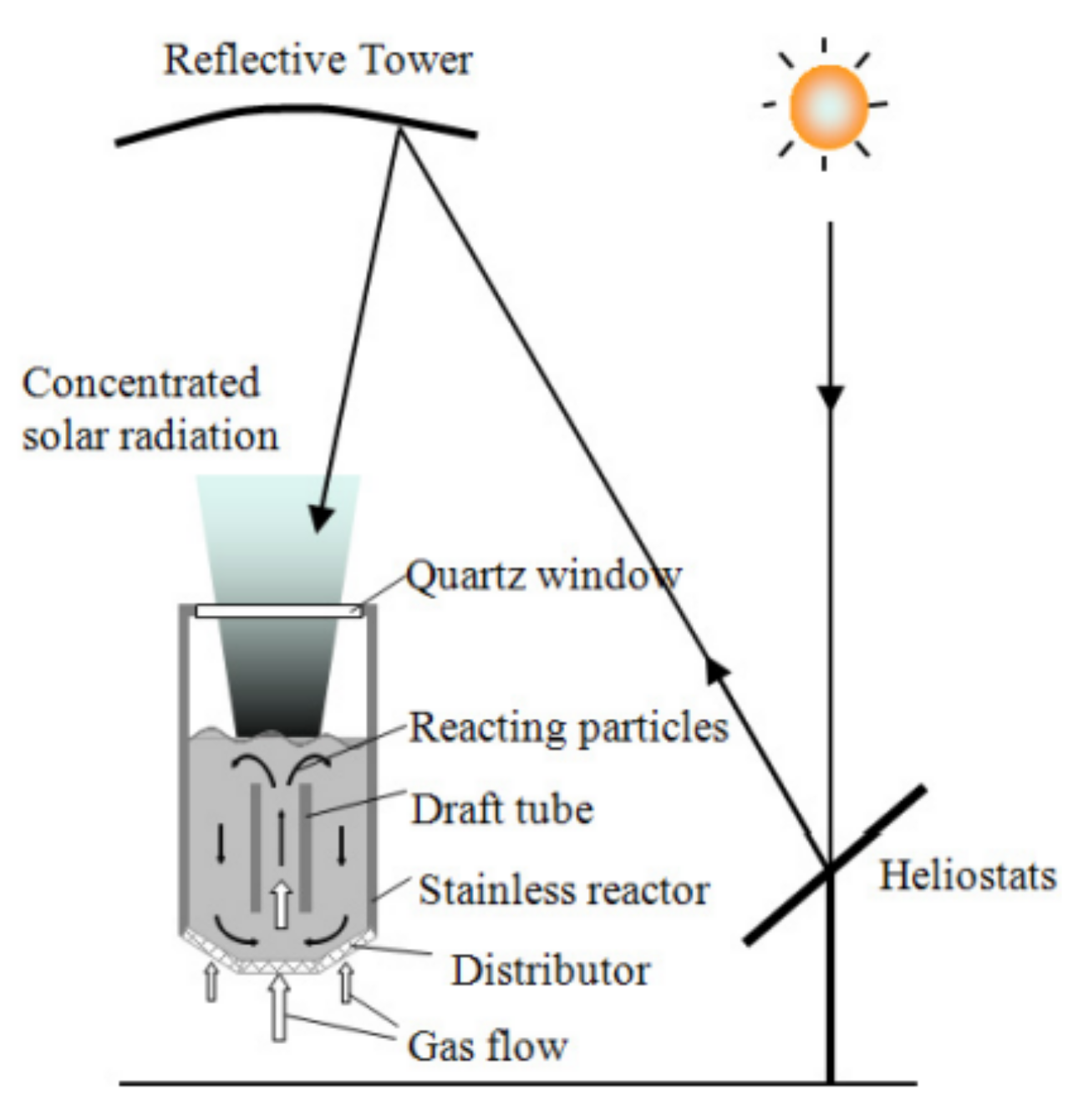
2.2.1. Solar Reactors
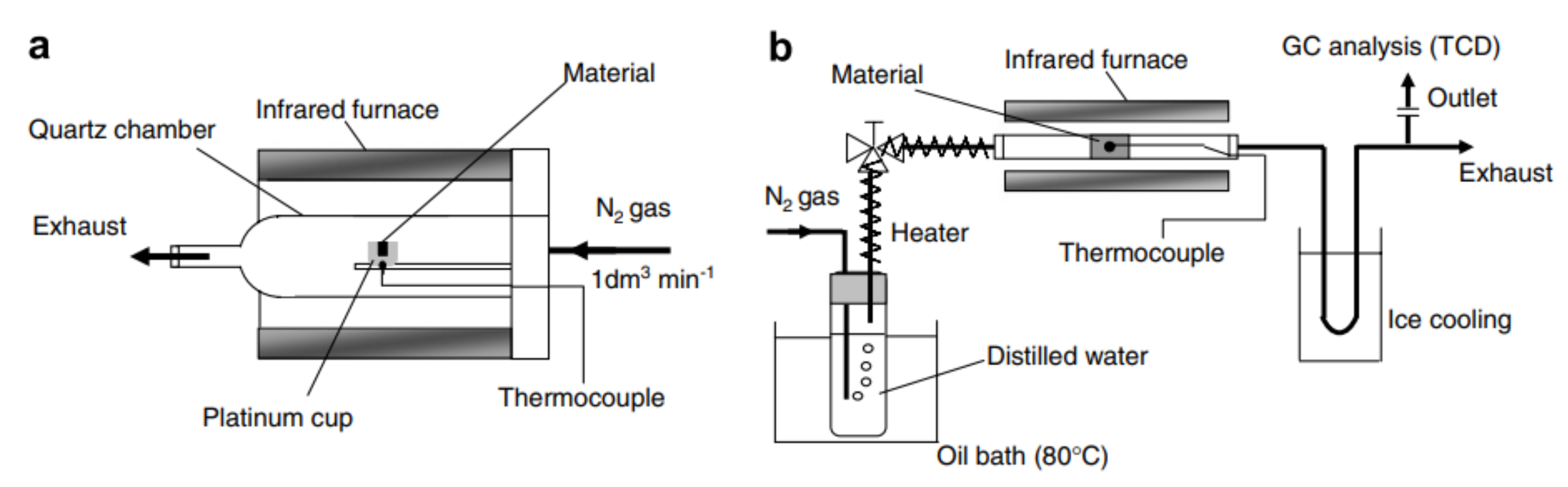
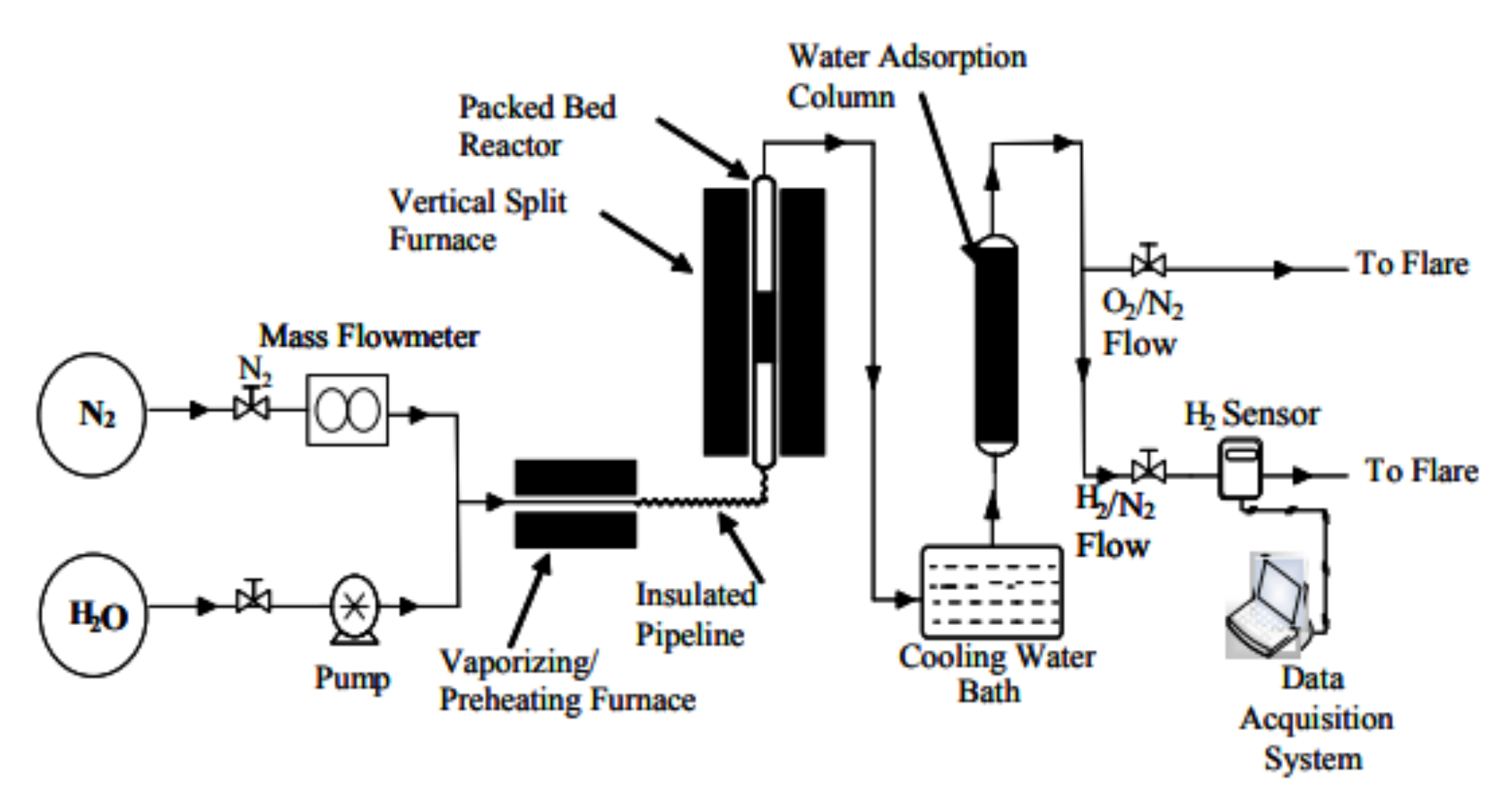
2.2.2. Infrared Reactors
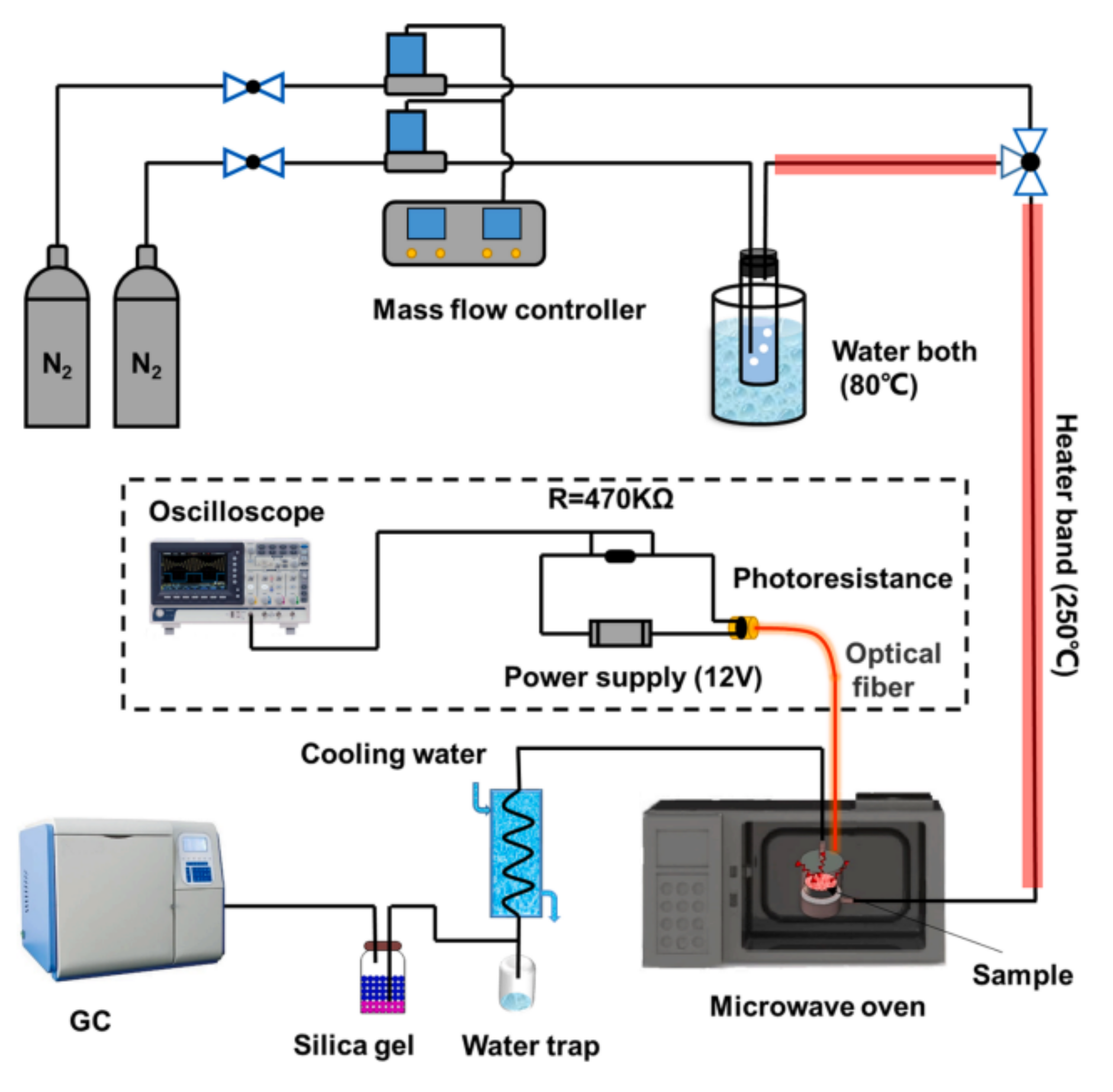
2.2.3. Microwave Oven
2.3. Temperature Swing vs. Pressure Swing Cycling
2.4. Materials for Two-Step Thermochemical Water Splitting
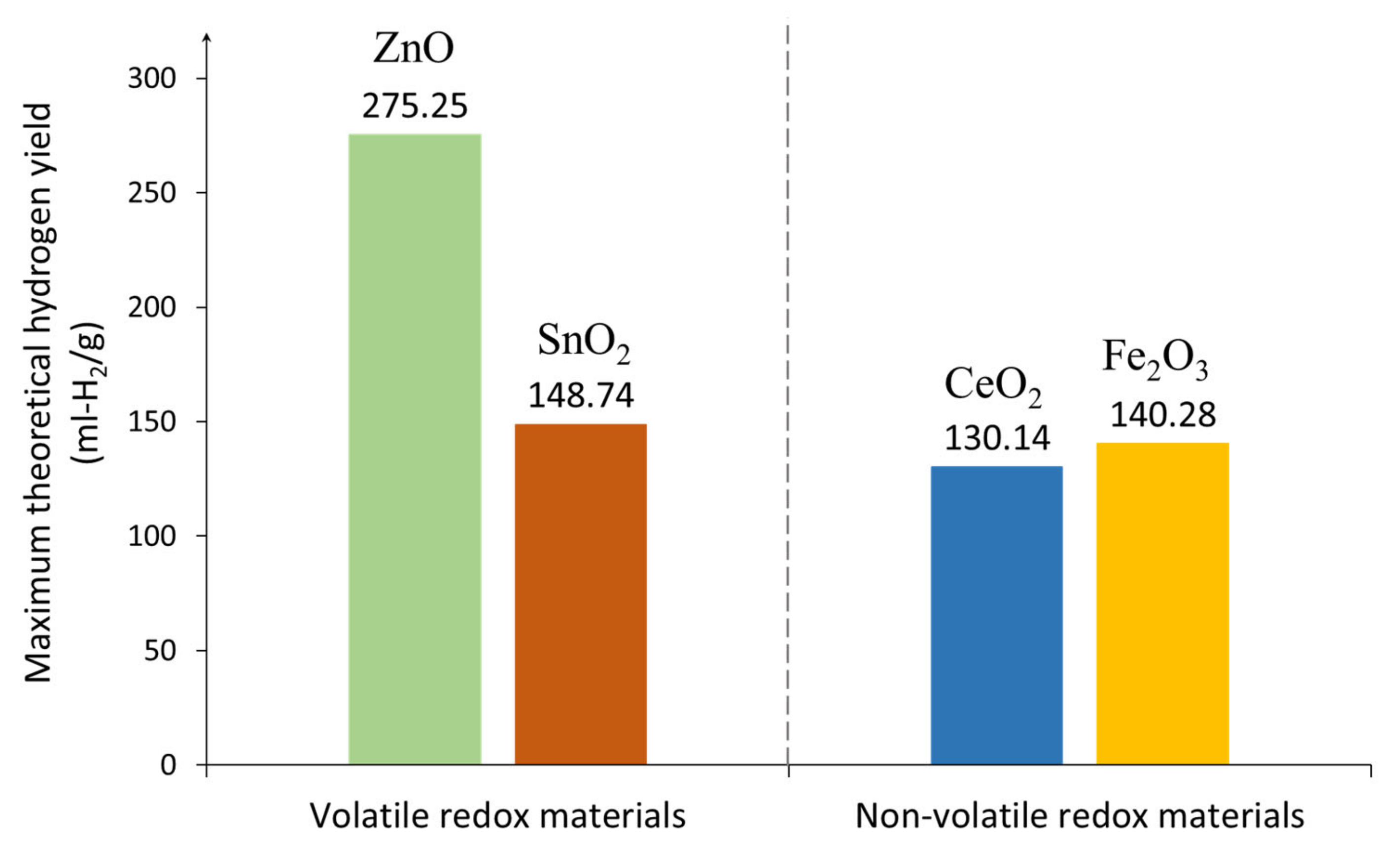
2.4.1. Volatile and Non-Volatile Redox Materials
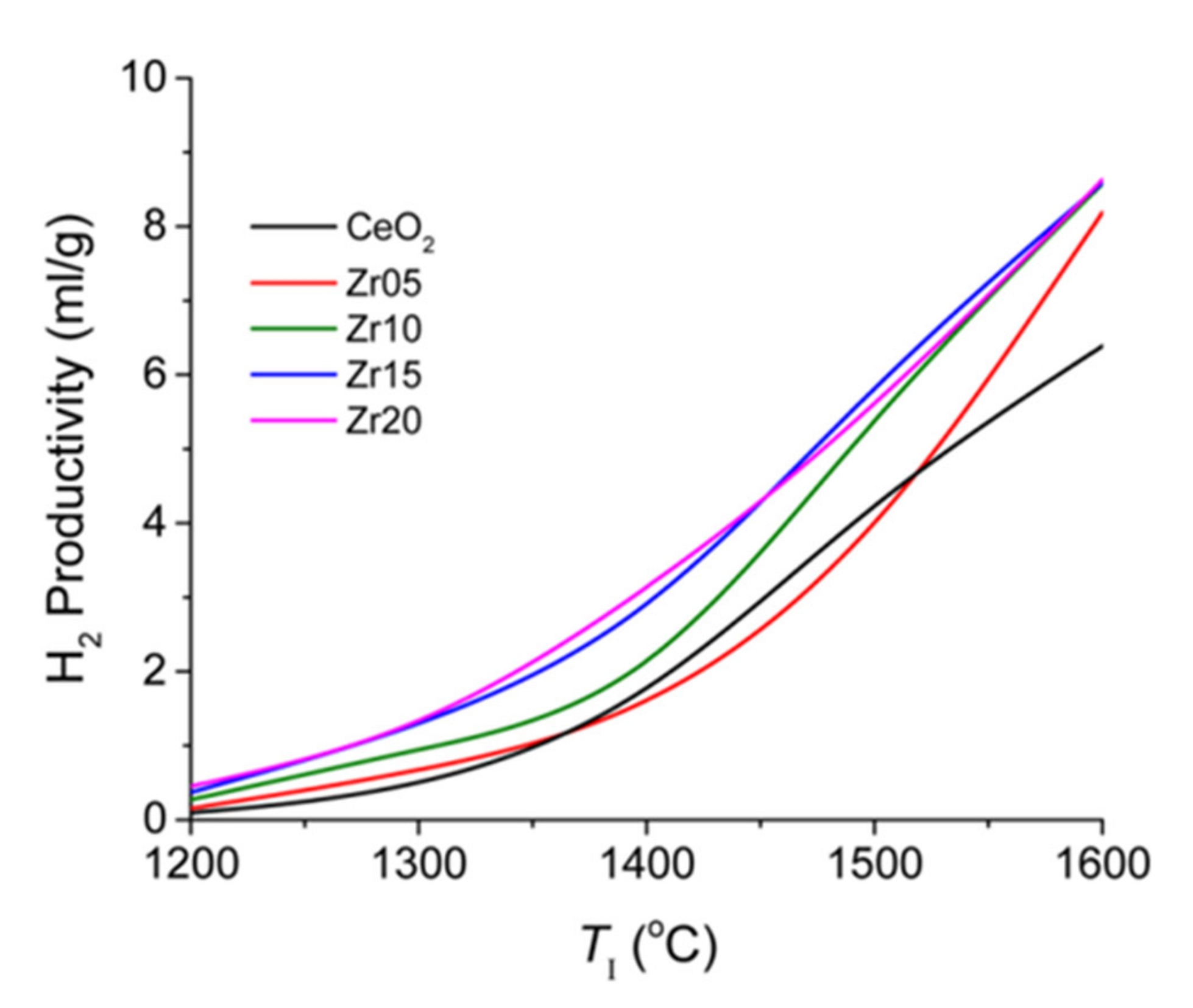
2.4.2. Improvements of the Performance of Non-Volatile Redox Materials
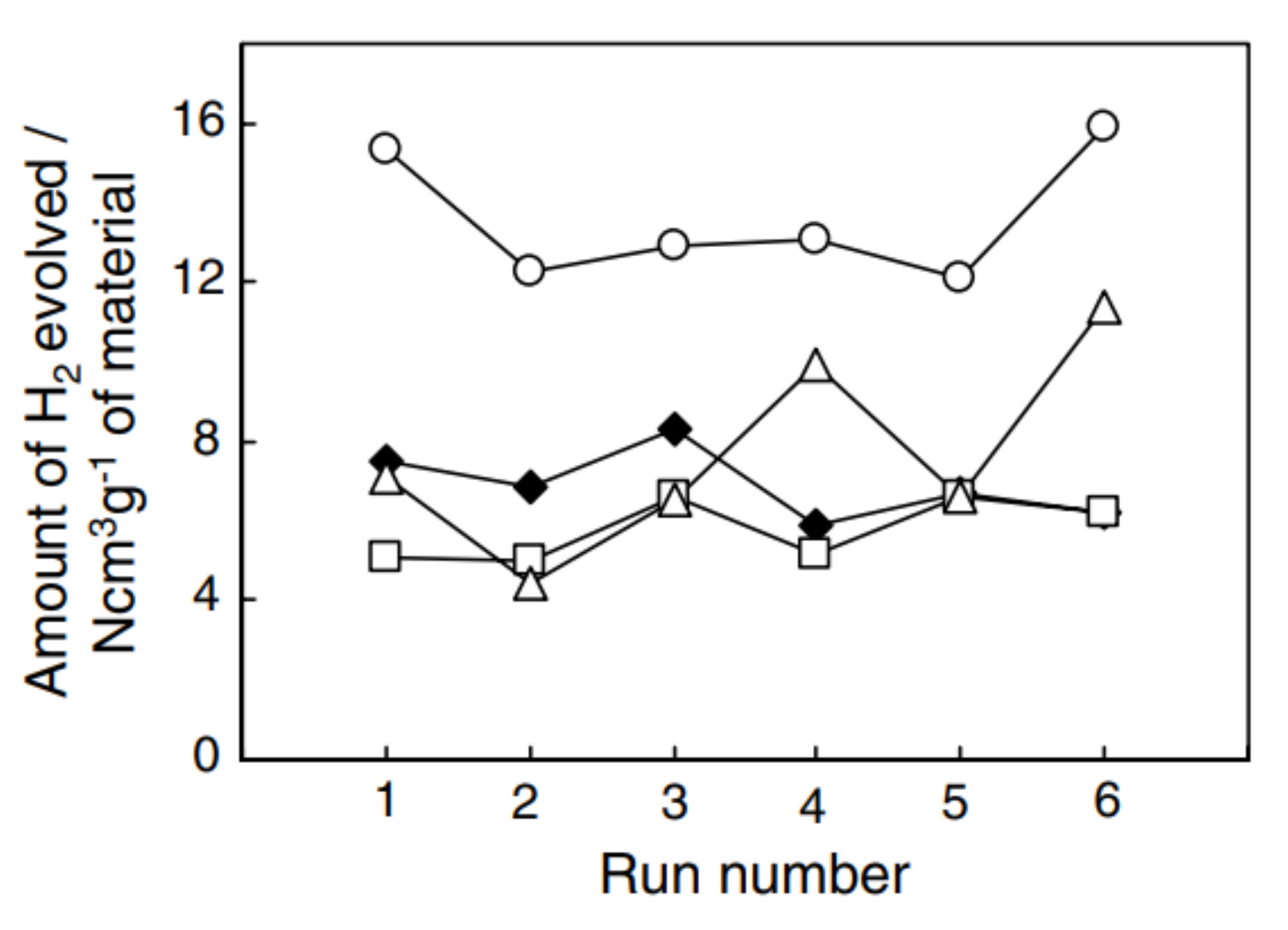
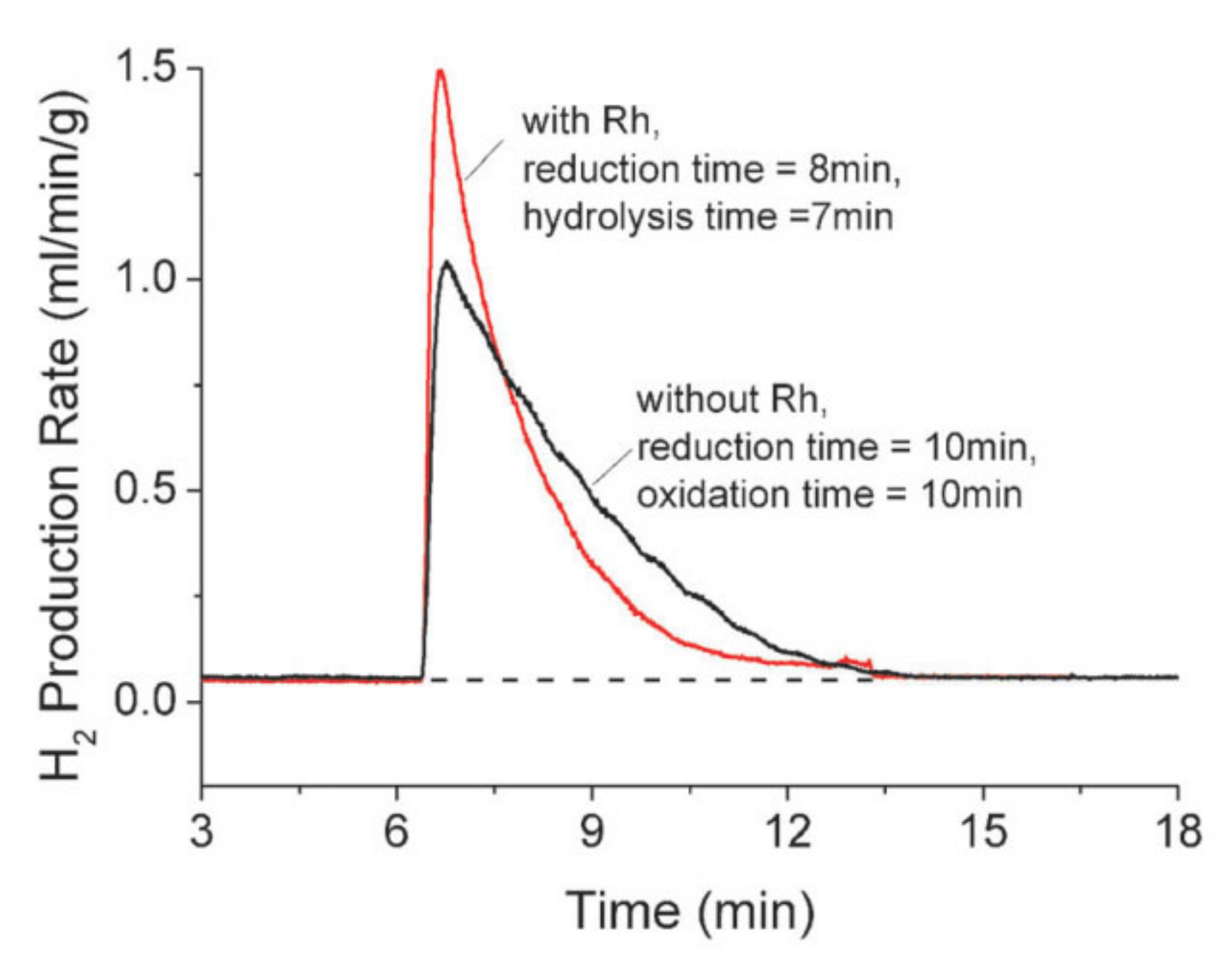
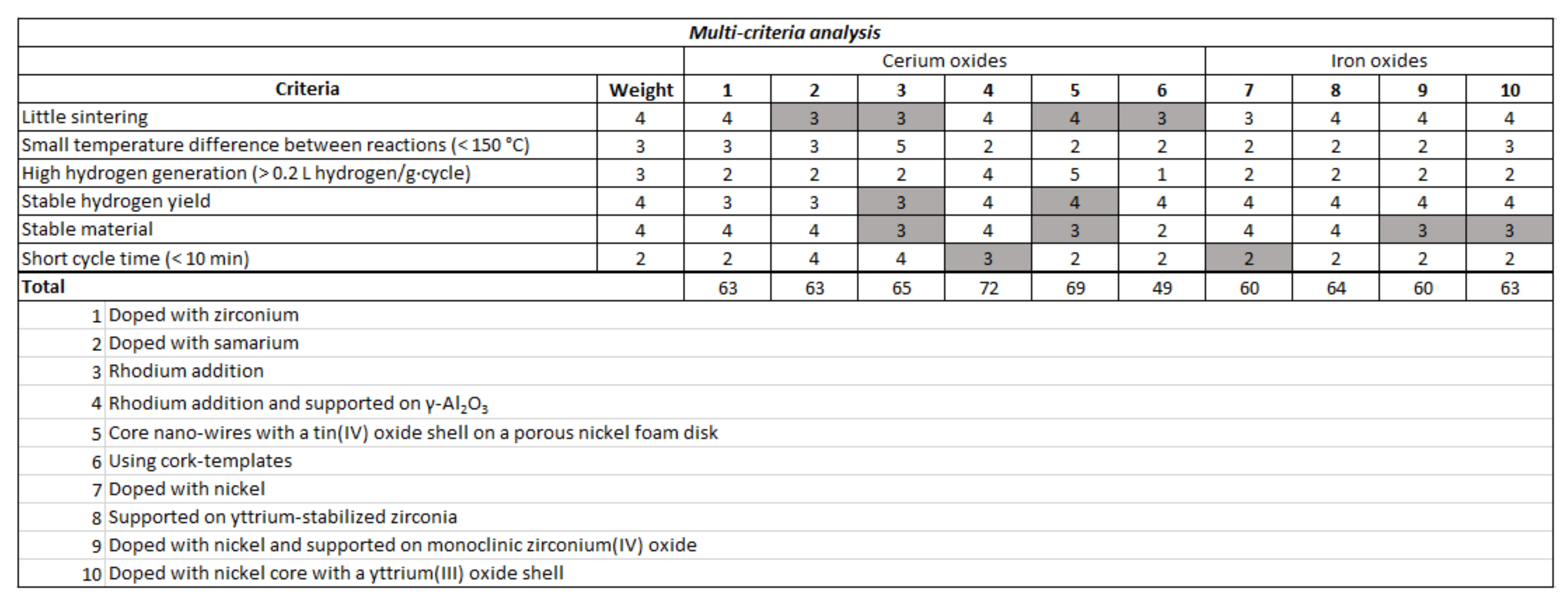
2.4.3. Multi-Criteria Analysis of Non-Volatile Redox Materials
3. Conclusions, Current Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goelzer, H.; Nowicki, S.; Payne, A.; Larour, E.; Seroussi, H.; Lipscomb, W.H.; Gregory, J.; Abe-Ouchi, A.; Shepherd, A.; Simon, E.; et al. The future sea-level contribution of the Greenland ice sheet: A multi-model ensemble study of ISMIP6. Cryosphere 2020, 14, 3071–3096. [Google Scholar] [CrossRef]
- Seo, K.; Lim, T.; Mills, E.M.; Kim, S.; Ju, S. Hydrogen generation enhanced by nano-forest structures. RSC Adv. 2016, 6, 12953–12958. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Mukthar Ali, M.; Dhua, S.; Sundararajan, T.; Ranga Rao, G. Thermochemical hydrogen production using Rh/CeO2/γ-Al2O3 catalyst by steam reforming of ethanol and water splitting in a packed bed reactor. Int. J. Hydrogen Energy 2021, 46, 19254–19269. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Dundar-Tekkaya, E.; Yürüm, Y. Mesoporous MCM-41 material for hydrogen storage: A short review. Int. J. Hydrogen Energy 2016, 41, 9789–9795. [Google Scholar] [CrossRef]
- Luo, Q.-H.; Hu, J.-B.; Sun, B.-G.; Liu, F.-S.; Wang, X.; Li, C.; Bao, L.-Z. Experimental investigation of combustion characteristics and NOx emission of a turbocharged hydrogen internal combustion engine. Int. J. Hydrogen Energy 2019, 44, 5573–5584. [Google Scholar] [CrossRef]
- Ciniviz, M.; Köse, H. Hydrogen Use in Internal Combustion Engine: A Review. Int. J. Automot. Eng. Technol. 2012, 1, 1–15. [Google Scholar]
- White, C.M.; Steeper, R.R.; Lutz, A.E. The hydrogen-fueled internal combustion engine: A technical review. Int. J. Hydrogen Energy 2006, 31, 1292–1305. [Google Scholar] [CrossRef]
- Kulkarni, T.; Slaughter, G. Enzymatic Glucose Biofuel Cell and its Application. J. Biochips Tissue Chips 2015, 5, 1000111. [Google Scholar]
- Tuan, K.N.; Karpukhin, K.E.; Terenchenko, A.S.; Kolbasov, A.F. World trends in the development of vehicles with alternative energy sources. ARPN J. Eng. Appl. Sci. 2018, 13, 2535–2542. [Google Scholar]
- Acar, C.; Dincer, I. 3.1 Hydrogen Production. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Oxford, UK, 2018; pp. 1–40. [Google Scholar]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jäger-Waldau, A. Green hydrogen in Europe—A regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2021, 228, 113649. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Detchusananard, T.; Im-orb, K.; Ponpesh, P.; Arpornwichanop, A. Biomass gasification integrated with CO2 capture processes for high-purity hydrogen production: Process performance and energy analysis. Energy Convers. Manag. 2018, 171, 1560–1572. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, M.; Li, P.; Wang, D.H.; Park, J.H. Water Splitting Progress in Tandem Devices: Moving Photolysis beyond Electrolysis. Adv. Energy Mater. 2016, 6, 1600602. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Ayers, K.; Anderson, E.; Capuano, C.; Carter, B.; Dalton, L.; Hanlon, G.; Manco, J.; Niedzwiecki, M. Research Advances Towards Low Cost, High Efficiency PEM Electrolysis. ECS Trans. 2010, 33, 3. [Google Scholar] [CrossRef]
- Lee, J.E.; Shafiq, I.; Hussain, M.; Lam, S.S.; Rhee, G.H.; Park, Y.-K. A review on integrated thermochemical hydrogen production from water. Int. J. Hydrogen Energy 2022, 47, 4346–4356. [Google Scholar] [CrossRef]
- Funk, J.E.; Conger, W.L.; Carty, R.H. Evaluation of Multi-Step Thermochemical Processes for the Production of Hydrogen from Water. In Hydrogen Energy; Springer: Boston, MA, USA, 1975. [Google Scholar]
- Jansen, G.; Dehouche, Z.; Corrigan, H. Cost-effective sizing of a hybrid Regenerative Hydrogen Fuel Cell energy storage system for remote & off-grid telecom towers. Int. J. Hydrogen Energy 2021, 46, 18153–18166. [Google Scholar]
- Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, S.-Y.; Li, Y.-R. Advances in solar hydrogen production via two-step water-splitting thermochemical cycles based on metal redox reactions. Renew. Energy 2012, 41, 1–12. [Google Scholar] [CrossRef]
- Ohta, T. Chapter 4—Direct Thermal Decomposition of Water. In Solar-Hydrogen Energy Systems; Pergamon: Oxford, UK, 1979; pp. 59–79. [Google Scholar]
- Lorentzou, S.; Pagkoura, C.; Zygogianni, A.; Karagiannakis, G.; Konstandopoulos, A.G. Thermochemical cycles over redox structured reactors. Int. J. Hydrogen Energy 2017, 42, 19664–19682. [Google Scholar] [CrossRef]
- Zhai, S.; Rojas, J.; Ahlborg, N.; Lim, K.; Toney, M.F.; Jin, H.; Chueh, W.C.; Majumdar, A. The use of poly-cation oxides to lower the temperature of two-step thermochemical water splitting. Energy Environ. Sci. 2018, 11, 2172–2178. [Google Scholar] [CrossRef]
- Kodama, T.; Nakamuro, Y.; Mizuno, T. A Two-Step Thermochemical Water Splitting by Iron-Oxide on Stabilized Zirconia. J. Sol. Energy Eng. 2004, 128, 3–7. [Google Scholar] [CrossRef]
- Muhich, C.; Evanko, B.; Weston, K.; Lichty, P.; Liang, X.; Martinek, J.; Musgrave, C.; Weimer, A. Efficient Generation of H-2 by Splitting Water with an Isothermal Redox Cycle. Science 2013, 341, 540–542. [Google Scholar] [CrossRef]
- Le Gal, A.; Abanades, S. Dopant Incorporation in Ceria for Enhanced Water-Splitting Activity during Solar Thermochemical Hydrogen Generation. J. Phys. Chem. C 2012, 116, 13516–13523. [Google Scholar] [CrossRef]
- Han, S.B.; Kang, T.B.; Joo, O.S.; Jung, K.D. Water splitting for hydrogen production with ferrites. Sol. Energy 2007, 81, 623–628. [Google Scholar] [CrossRef]
- Bhosale, R.R. Solar hydrogen production via thermochemical magnesium oxide—Magnesium sulfate water splitting cycle. Fuel 2020, 275, 117892. [Google Scholar] [CrossRef]
- Abanades, S.; Flamant, G. Thermochemical hydrogen production from a two-step solar-driven water-splitting cycle based on cerium oxides. Sol. Energy 2006, 80, 1611–1623. [Google Scholar] [CrossRef]
- Le Gal, A.; Abanades, S.; Bion, N.; Le Mercier, T.; Harlé, V. Reactivity of Doped Ceria-Based Mixed Oxides for Solar Thermochemical Hydrogen Generation via Two-Step Water-Splitting Cycles. Energy Fuels 2013, 27, 6068–6078. [Google Scholar] [CrossRef]
- Tamaura, Y.; Steinfeld, A.; Kuhn, P.; Ehrensberger, K. Production of solar hydrogen by a novel, 2-step, water-splitting thermochemical cycle. Energy 1995, 20, 325–330. [Google Scholar] [CrossRef]
- Mao, Y.; Gao, Y.; Dong, W.; Wu, H.; Song, Z.; Zhao, X.; Sun, J.; Wang, W. Hydrogen production via a two-step water splitting thermochemical cycle based on metal oxide—A review. Appl. Energy 2020, 267, 114860. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar thermochemical production of hydrogen––A review. Sol. Energy 2005, 78, 603–615. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Non-Stoichiometric Redox Active Perovskite Materials for Solar Thermochemical Fuel Production: A Review. Catalysts 2018, 8, 611. [Google Scholar] [CrossRef]
- Gorensek, M.B.; Corgnale, C.; Staser, J.A.; Weidner, J.W. Chapter 3—Thermochemical Hydrogen Processes. In Electrochemical Power Sources: Fundamentals, Systems, and Applications; Smolinka, T., Garche, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 63–82. [Google Scholar]
- Costa Oliveira, F.A.; Barreiros, M.A.; Haeussler, A.; Caetano, A.P.F.; Mouquinho, A.I.; Oliveira e Silva, P.M.; Novais, R.M.; Pullar, R.C.; Abanades, S. High performance cork-templated ceria for solar thermochemical hydrogen production via two-step water-splitting cycles. Sustain. Energy Fuels 2020, 4, 3077–3089. [Google Scholar] [CrossRef]
- Arribas, L.; Gonzalez-Aguilar, J.; Romero, M. Solar-Driven Thermochemical Water-Splitting by Cerium Oxide: Determination of Operational Conditions in a Directly Irradiated Fixed Bed Reactor. Energies 2018, 11, 2451. [Google Scholar] [CrossRef]
- Chueh, W.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.; Steinfeld, A. High-Flux Solar-Driven Thermochemical Dissociation of CO2 and H2O Using Nonstoichiometric Ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Flamant, G.; Lemort, F. Two-step water splitting thermochemical cycle based on iron oxide redox pair for solar hydrogen production. Energy 2007, 32, 1124–1133. [Google Scholar] [CrossRef]
- Al-Shankiti, I.; Ehrhart, B.D.; Weimer, A.W. Isothermal redox for H2O and CO2 splitting—A review and perspective. Sol. Energy 2017, 156, 21–29. [Google Scholar] [CrossRef]
- Martinek, J.; Viger, R.; Weimer, A.W. Transient simulation of a tubular packed bed solar receiver for hydrogen generation via metal oxide thermochemical cycles. Sol. Energy 2014, 105, 613–631. [Google Scholar] [CrossRef]
- Kodama, T.; Gokon, N.; Matsubara, K.; Yoshida, K.; Koikari, S.; Nagase, Y.; Nakamura, K. Flux Measurement of a New Beam-down Solar Concentrating System in Miyazaki for Demonstration of Thermochemical Water Splitting Reactors. Energy Procedia 2014, 49, 1990–1998. [Google Scholar] [CrossRef]
- Gokon, N.; Takahashi, S.; Yamamoto, H.; Kodama, T. Thermochemical two-step water-splitting reactor with internally circulating fluidized bed for thermal reduction of ferrite particles. Int. J. Hydrogen Energy 2008, 33, 2189–2199. [Google Scholar] [CrossRef]
- Kodama, T.; Gokon, N.; Yamamoto, R. Thermochemical two-step water splitting by ZrO2-supported NixFe3−xO4 for solar hydrogen production. Sol. Energy 2008, 82, 73–79. [Google Scholar] [CrossRef]
- Bhosale, R.; Shende, R.; Puszynski, J. H2 Generation from Thermochemical Water-Splitting Using Sol-Gel Derived Ni-Ferrite. J. Energy Power Eng. 2010, 4, 27–38. [Google Scholar]
- Bhosale, R.; Shende, R.; Puszynski, J. H2 generation from thermochemical water-splitting using sol-gel synthesized ferrites (MxFeyOz, M = Ni, Zn, Sn, Co, Mn, Ce). In Proceedings of the AIChE Annual Meeting, Salt Lake City, UT, USA, 7–12 November 2010. [Google Scholar]
- Bhosale, R.; Khadka, R.; Puszynski, J.; Shende, R. H-2 generation from two-step thermochemical water-splitting reaction using sol-gel derived SnxFeyOz. J. Renew. Sustain. Energy 2011, 3, 063104. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Shende, R.V.; Puszynski, J.A. Thermochemical water-splitting for H2 generation using sol-gel derived Mn-ferrite in a packed bed reactor. Int. J. Hydrogen Energy 2012, 37, 2924–2934. [Google Scholar] [CrossRef]
- Gao, Y.; Mao, Y.; Song, Z.; Zhao, X.; Sun, J.; Wang, W.; Chen, G.; Chen, S. Efficient generation of hydrogen by two-step thermochemical cycles: Successive thermal reduction and water splitting reactions using equal-power microwave irradiation and a high entropy material. Appl. Energy 2020, 279, 115777. [Google Scholar] [CrossRef]
- Gedye, R.; Rank, W.; Westaway, K. The rapid synthesis of organic compounds in microwave ovens. II. Can. J. Chem. 1991, 69, 706–711. [Google Scholar] [CrossRef]
- Hoseinzadeh Hesas, R.; Wan Daud, W.M.A.; Sahu, J.N.; Arami-Niya, A. The effects of a microwave heating method on the production of activated carbon from agricultural waste: A review. J. Anal. Appl. Pyrolysis 2013, 100, 1–11. [Google Scholar] [CrossRef]
- Roeb, M.; Sattler, C. Isothermal Water Splitting. Science 2013, 341, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Ganzoury, M.A.; Fateen, S.-E.K.; El Sheltawy, S.T.; Radwan, A.M.; Allam, N.K. Thermodynamic and efficiency analysis of solar thermochemical water splitting using Ce–Zr mixtures. Sol. Energy 2016, 135, 154–162. [Google Scholar] [CrossRef]
- Felinks, J.; Brendelberger, S.; Roeb, M.; Sattler, C.; Pitz-Paal, R. Heat recovery concept for thermochemical processes using a solid heat transfer medium. Appl. Therm. Eng. 2014, 73, 1006–1013. [Google Scholar] [CrossRef]
- Warren, K.J.; Tran, J.T.; Weimer, A.W. A thermochemical study of iron aluminate-based materials: A preferred class for isothermal water splitting. Energy Environ. Sci. 2022, 15, 806–821. [Google Scholar] [CrossRef]
- Ermanoski, I.; Miller, J.E.; Allendorf, M.D. Efficiency maximization in solar-thermochemical fuel production: Challenging the concept of isothermal water splitting. Phys. Chem. Chem. Phys. 2014, 16, 8418–8427. [Google Scholar] [CrossRef]
- Muhich, C.; Ehrhart, B.; Alshankiti, I.; Ward, B.; Musgrave, C.; Weimer, A. A review and perspective of efficient hydrogen generation via solar thermal water splitting: A review and perspective of efficient hydrogen generation. WIREs Energy Environ. 2015, 5, 261–287. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, C.-K.; Haile, S.M. High-temperature isothermal chemical cycling for solar-driven fuel production. Phys. Chem. Chem. Phys. 2013, 15, 17084–17092. [Google Scholar] [CrossRef]
- Kong, H.; Hao, Y.; Jin, H. Isothermal versus two-temperature solar thermochemical fuel synthesis: A comparative study. Appl. Energy 2018, 228, 301–308. [Google Scholar] [CrossRef]
- Brendelberger, S.; Felinks, J.; Roeb, M.; Sattler, C. Solid Phase Heat Recovery and Multi Chamber Reduction for Redox Cycles. In Proceedings of the ASME 2014 8th International Conference on Energy Sustainability Collocated with the ASME 2014 12th International Conference on Fuel Cell Science, Engineering and Technology, Boston, MA, USA, 30 June–2 July 2014. [Google Scholar]
- Demont, A.; Abanades, S.; Beche, E. Investigation of Perovskite Structures as Oxygen-Exchange Redox Materials for Hydrogen Production from Thermochemical Two-Step Water-Splitting Cycles. J. Phys. Chem. C 2014, 118, 12682–12692. [Google Scholar] [CrossRef]
- Yang, C.-K.; Yamazaki, Y.; Aydin, A.; Haile, S.M. Thermodynamic and kinetic assessments of strontium-doped lanthanum manganite perovskites for two-step thermochemical water splitting. J. Mater. Chem. A 2014, 2, 13612–13623. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Dey, S. Solar thermochemical splitting of water to generate hydrogen. Proc. Natl. Acad. Sci. USA 2017, 114, 13385–13393. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, D.R.; Sanders, M.D.; Tong, J.; McDaniel, A.H.; O’Hayre, R.P. BaCe0.25Mn0.75O3−δ—A promising perovskite-type oxide for solar thermochemical hydrogen production. Energy Environ. Sci. 2018, 11, 3256–3265. [Google Scholar] [CrossRef]
- Budama, V.K.; Johnson, N.G.; Ermanoski, I.; Stechel, E.B. Techno-economic analysis of thermochemical water-splitting system for Co-production of hydrogen and electricity. Int. J. Hydrogen Energy 2021, 46, 1656–1670. [Google Scholar] [CrossRef]
- Dimitrakis, D.; Tsongidis, N.; Konstandopoulos, A. Reduction enthalpy and charge distribution of substituted ferrites and doped ceria for thermochemical water and carbon dioxide splitting with DFT+U. Phys. Chem. Chem. Phys. 2016, 18, 23587–23595. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Frei, A.; Albisetti, G.; Lunardi, G.; Steinfeld, A. Solar hydrogen production via a two-step thermochemical process based on MgO/Mg redox reactions—Thermodynamic and kinetic analyses. Int. J. Hydrogen Energy 2008, 33, 2880–2890. [Google Scholar] [CrossRef]
- Abanades, S.; Legal, A.; Cordier, A.; Peraudeau, G.; Flamant, G.; Julbe, A. Investigation of reactive cerium-based oxides for H2 production by thermochemical two-step water-splitting. J. Mater. Sci. 2010, 45, 4163–4173. [Google Scholar] [CrossRef]
- Zhao, Z.; Uddi, M.; Tsvetkov, N.; Yildiz, B.; Ghoniem, A.F. Redox Kinetics Study of Fuel Reduced Ceria for Chemical-Looping Water Splitting. J. Phys. Chem. C 2016, 120, 16271–16289. [Google Scholar] [CrossRef]
- Niazi, Z.; Irankhah, A.; Wang, Y.; Arandiyan, H. Cu, Mg and Co effect on nickel-ceria supported catalysts for ethanol steam reforming reaction. Int. J. Hydrogen Energy 2020, 45, 21512–21522. [Google Scholar] [CrossRef]
- Zamar, F.; Trovarelli, A.; de Leitenburg, C.; Dolcetti, G. CeO 2-based solid solutions with the fluorite structure as novel and effective catalysts for methane combustion. J. Chem. Soc. Chem. Commun. 1995, 9, 965–966. [Google Scholar] [CrossRef]
- Aggarwal, N.; Vasishth, A.; Singh, B.; Singh, B. Investigation of room temperature ferromagnetic behaviour in dilute magnetic oxides. Integr. Ferroelectr. 2018, 186, 10–16. [Google Scholar] [CrossRef]
- Rougab, M.; Gueddouh, A. Light doping effects of rare-earth elements: Sc, Y, La and Lu in rockSalt AlN—first-principles study. Appl. Phys. A 2021, 127, 969. [Google Scholar] [CrossRef]
- Orfila, M.; Linares, M.; Molina, R.; Botas, J.Á.; Sanz, R.; Marugán, J. Perovskite materials for hydrogen production by thermochemical water splitting. Int. J. Hydrogen Energy 2016, 41, 19329–19338. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Steinfeld, A. Oxygen exchange materials for solar thermochemical splitting of H2O and CO2: A review. Mater. Today 2014, 17, 341–348. [Google Scholar] [CrossRef]
- Kaneko, H.; Ishihara, H.; Taku, S.; Naganuma, Y.; Hasegawa, N.; Tamaura, Y. Cerium ion redox system in CeO2–xFe2O3 solid solution at high temperatures (1273–1673 K) in the two-step water-splitting reaction for solar H2 generation. J. Mater. Sci. 2008, 43, 3153–3161. [Google Scholar] [CrossRef]
- Orfila, M.; Sanz, D.; Linares, M.; Molina, R.; Sanz, R.; Marugán, J.; Botas, J.Á. H2 production by thermochemical water splitting with reticulated porous structures of ceria-based mixed oxide materials. Int. J. Hydrogen Energy 2021, 46, 17458–17471. [Google Scholar] [CrossRef]
- Kaneko, H.; Miura, T.; Ishihara, H.; Taku, S.; Yokoyama, T.; Nakajima, H.; Tamaura, Y. Reactive ceramics of CeO2–MOx (M=Mn, Fe, Ni, Cu) for H2 generation by two-step water splitting using concentrated solar thermal energy. Energy 2007, 32, 656–663. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, C.-K.; Haile, S.M. Ceria–Zirconia Solid Solutions (Ce1–xZrxO2−δ, x ≤ 0.2) for Solar Thermochemical Water Splitting: A Thermodynamic Study. Chem. Mater. 2014, 26, 6073–6082. [Google Scholar] [CrossRef]
- Chueh, W.C.; Haile, S.M. Ceria as a Thermochemical Reaction Medium for Selectively Generating Syngas or Methane from H2O and CO2. ChemSusChem 2009, 2, 735–739. [Google Scholar] [CrossRef]
- Muhich, C.; Steinfeld, A. Principles of doping ceria for the solar thermochemical redox splitting of H2O and CO2. J. Mater. Chem. A 2017, 5, 15578–15590. [Google Scholar] [CrossRef]
- Kuhn, M.; Bishop, S.R.; Rupp, J.L.M.; Tuller, H.L. Structural characterization and oxygen nonstoichiometry of ceria-zirconia (Ce1−xZrxO2−δ) solid solutions. Acta Mater. 2013, 61, 4277–4288. [Google Scholar] [CrossRef]
- Andersson, D.A.; Simak, S.I.; Skorodumova, N.V.; Abrikosov, I.A.; Johansson, B. Redox properties of CeO2–MO2 (M=Ti, Zr, Hf, or Th) solid solutions from first principles calculations. Appl. Phys. Lett. 2007, 90, 031909. [Google Scholar] [CrossRef]
- Gokon, N.; Hasegawa, T.; Takahashi, S.; Kodama, T. Thermochemical two-step water-splitting for hydrogen production using Fe-YSZ particles and a ceramic foam device. Energy 2008, 33, 1407–1416. [Google Scholar] [CrossRef]
- Weidenkaff, A.; Nüesch, P.; Wokaun, A.; Reller, A. Mechanistic studies of the water-splitting reaction for producing solar hydrogen. Solid State Ion. 1997, 101–103, 915–922. [Google Scholar] [CrossRef]
- Cui, B.; Zhang, J.; Liu, S.; Liu, X.; Zhang, Z.; Sun, J. A low-temperature electro-thermochemical water-splitting cycle for hydrogen production based on LiFeO2/Fe redox pair. Int. J. Hydrogen Energy 2020, 45, 20800–20807. [Google Scholar] [CrossRef]
- Gokon, N.; Murayama, H.; Umeda, J.; Hatamachi, T.; Kodama, T. Monoclinic zirconia-supported Fe3O4 for the two-step water-splitting thermochemical cycle at high thermal reduction temperatures of 1400–1600 °C. Int. J. Hydrogen Energy 2009, 34, 1208–1217. [Google Scholar] [CrossRef]
- Wegner, K.; Ly, H.C.; Weiss, R.J.; Pratsinis, S.E.; Steinfeld, A. In situ formation and hydrolysis of Zn nanoparticles for H2 production by the 2-step ZnO/Zn water-splitting thermochemical cycle. Int. J. Hydrogen Energy 2006, 31, 55–61. [Google Scholar] [CrossRef]
- Gokon, N.; Mataga, T.; Kondo, N.; Kodama, T. Thermochemical two-step water splitting by internally circulating fluidized bed of NiFe2O4 particles: Successive reaction of thermal-reduction and water-decomposition steps. Int. J. Hydrogen Energy 2011, 36, 4757–4767. [Google Scholar] [CrossRef]
- Gokon, N.; Mizuno, T.; Nakamuro, Y.; Kodama, T. Iron-Containing Yttria-Stabilized Zirconia System For Two-Step Thermochemical Water Splitting. J. Sol. Energy Eng. 2007, 130, 011018. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S. Additive manufacturing and two-step redox cycling of ordered porous ceria structures for solar-driven thermochemical fuel production. Chem. Eng. Sci. 2021, 246, 116999. [Google Scholar] [CrossRef]
- Petkovich, N.D.; Rudisill, S.G.; Venstrom, L.J.; Boman, D.B.; Davidson, J.H.; Stein, A. Control of Heterogeneity in Nanostructured Ce1–xZrxO2 Binary Oxides for Enhanced Thermal Stability and Water Splitting Activity. J. Phys. Chem. C 2011, 115, 21022–21033. [Google Scholar] [CrossRef]
- Venstrom, L.J.; Petkovich, N.; Rudisill, S.; Stein, A.; Davidson, J.H. The Effects of Morphology on the Oxidation of Ceria by Water and Carbon Dioxide. J. Sol. Energy Eng. 2011, 134, 011005. [Google Scholar] [CrossRef]
- Umeda, G.A.; Chueh, W.C.; Noailles, L.; Haile, S.M.; Dunn, B.S. Inverse opal ceria–zirconia: Architectural engineering for heterogeneous catalysis. Energy Environ. Sci. 2008, 1, 484–486. [Google Scholar] [CrossRef][Green Version]
- Stein, A.; Li, F.; Denny, N.R. Morphological Control in Colloidal Crystal Templating of Inverse Opals, Hierarchical Structures, and Shaped Particles. Chem. Mater. 2008, 20, 649–666. [Google Scholar] [CrossRef]
- Reitzmann, A.; Bareiss, A.; Kraushaar-Czarnetzki, B. Simulation of a Reactor for the Partial Oxidation of o-Xylene to Phthalic Anhydride Packed with Ceramic Foam Monoliths. Erdoel Erdgas Kohle 2006, 122, 94–98. [Google Scholar]
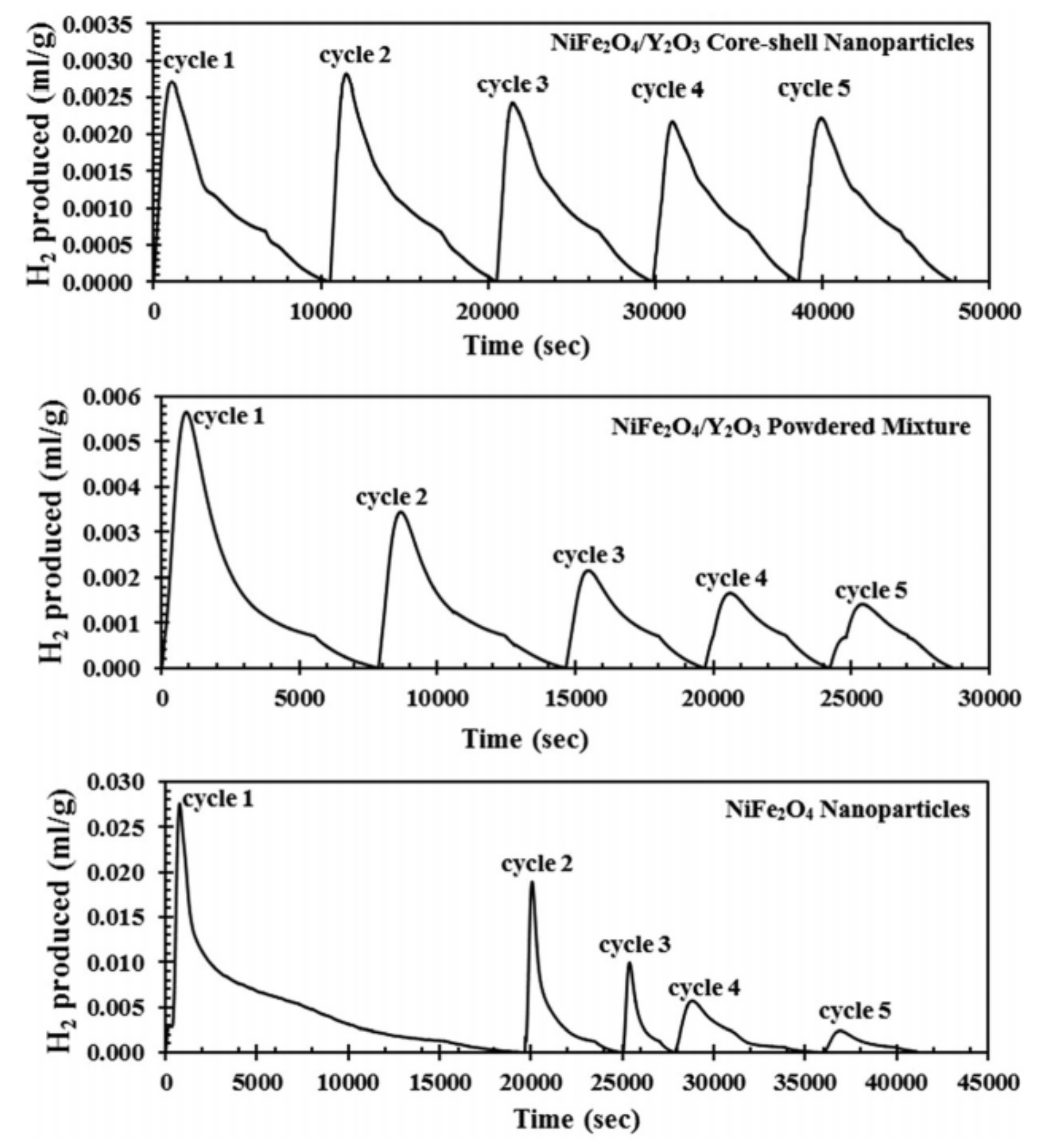
- Amar, V.; Puszynski, J.; Shende, R. H2 generation from thermochemical water-splitting using yttria stabilized NiFe2O4 core-shell nanoparticles. J. Renew. Sustain. Energy 2015, 7, 023113. [Google Scholar] [CrossRef]
- Amar, V.; Shende, R. Core-Shell Nanoscale Ferrite for Thermochemical Hydrogen Generation. In Proceedings of the TechConnect World Innovation Conference & Expo, Anaheim, CA, USA, 13–16 May 2018; 2018; pp. 148–151. [Google Scholar]
- Amar, V.; Houck, J.; Maddipudi, B.; Shende, R. NiFe2O4/ZrO2 Core-Shell Nanoparticles for Hydrogen Generation from Thermochemical Water-Splitting Process. J. Catal. Chem. Eng. Adv. 2020, 7, 105. [Google Scholar] [CrossRef]
- Fresno, F.; Fernández-Saavedra, R.; Belén Gómez-Mancebo, M.; Vidal, A.; Sánchez, M.; Isabel Rucandio, M.; Quejido, A.J.; Romero, M. Solar hydrogen production by two-step thermochemical cycles: Evaluation of the activity of commercial ferrites. Int. J. Hydrogen Energy 2009, 34, 2918–2924. [Google Scholar] [CrossRef]


| Hydrogen Production Method | Feed | Energy Source | Major Advantages | Major Challenges |
|---|---|---|---|---|
| Steam reforming | Fossil fuels | Thermal |
|
|
| Biomass gasification | Biomass | Thermal |
|
|
| Electrolysis | Water | Electricity |
|
|
| Photolysis | Water | Photonic |
|
|
| Photoelectrochemical | Water | Electricity + photonic |
|
|
| Thermochemical | Water | Thermal |
|
|
| Number on the Multi-Criteria Analysis | Redox Material | Reduction Temperature (°C) | Oxidation Temperature (°C) | Maximum H2 Production Rate (mL·g−1·min−1) | H2 Production on a Single Cycle (mL·g−1) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ce0.75Zr0.25O2 | 1450 | 1045 | 0.47 | 5.33 | [38,74] |
| 2 | Ce1−xSmxO2 | 1500 | 900 | 4.1 | 3.8 | [38,86] |
| 3 | Rh/CeO2 | 1500 | 1500 | 1.5 | n.a. | [64] |
| 4 | Rh/CeO2/γ-Al2O3 | 1050 | 1200 | n.a. | 1424.5 | [3] |
| 5 | CeO2/SnO2 core-shell nanowires | Reduced material obtained after CeO2 pulse laser deposition | 800 | 2.14 | 720.4 mL per gram of CeO2 (not referred to total material) | [2] |
| 6 | cork-templated CeO2 | 1450 | 1050 | 1.6 | 3.83 | [42] |
| 7 | NixFe3−xO4 | 1400 | 1000 | n.a. | 11 | [38,50] |
| 1450 | 1000 | 0.314 | 14.11 | [38,106] | ||
| 8 | Fe3O4 on yttrium-stabilized, cubic zirconia (YSZ) | 1400 | 1000 | 0.5 | 9 | [30] |
| 9 | NixFe3−xO4 supported on ZrO2 | 1400 | 1000 | 0.65 | 15 | [50] |
| 10 | NiFe2O4/Y2O3 core-shell nanoparticles | 1100 | 900 | 0.162 | 10.39 | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oudejans, D.; Offidani, M.; Constantinou, A.; Albonetti, S.; Dimitratos, N.; Bansode, A. A Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle. Energies 2022, 15, 3044. https://doi.org/10.3390/en15093044
Oudejans D, Offidani M, Constantinou A, Albonetti S, Dimitratos N, Bansode A. A Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle. Energies. 2022; 15(9):3044. https://doi.org/10.3390/en15093044
Chicago/Turabian StyleOudejans, Daphne, Michele Offidani, Achilleas Constantinou, Stefania Albonetti, Nikolaos Dimitratos, and Atul Bansode. 2022. "A Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle" Energies 15, no. 9: 3044. https://doi.org/10.3390/en15093044
APA StyleOudejans, D., Offidani, M., Constantinou, A., Albonetti, S., Dimitratos, N., & Bansode, A. (2022). A Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle. Energies, 15(9), 3044. https://doi.org/10.3390/en15093044









