Thermal Stress Simulation and Structure Failure Analyses of Nitrogen–Oxygen Sensors under a Gradual Temperature Field
Abstract
:1. Introduction
2. Experimental
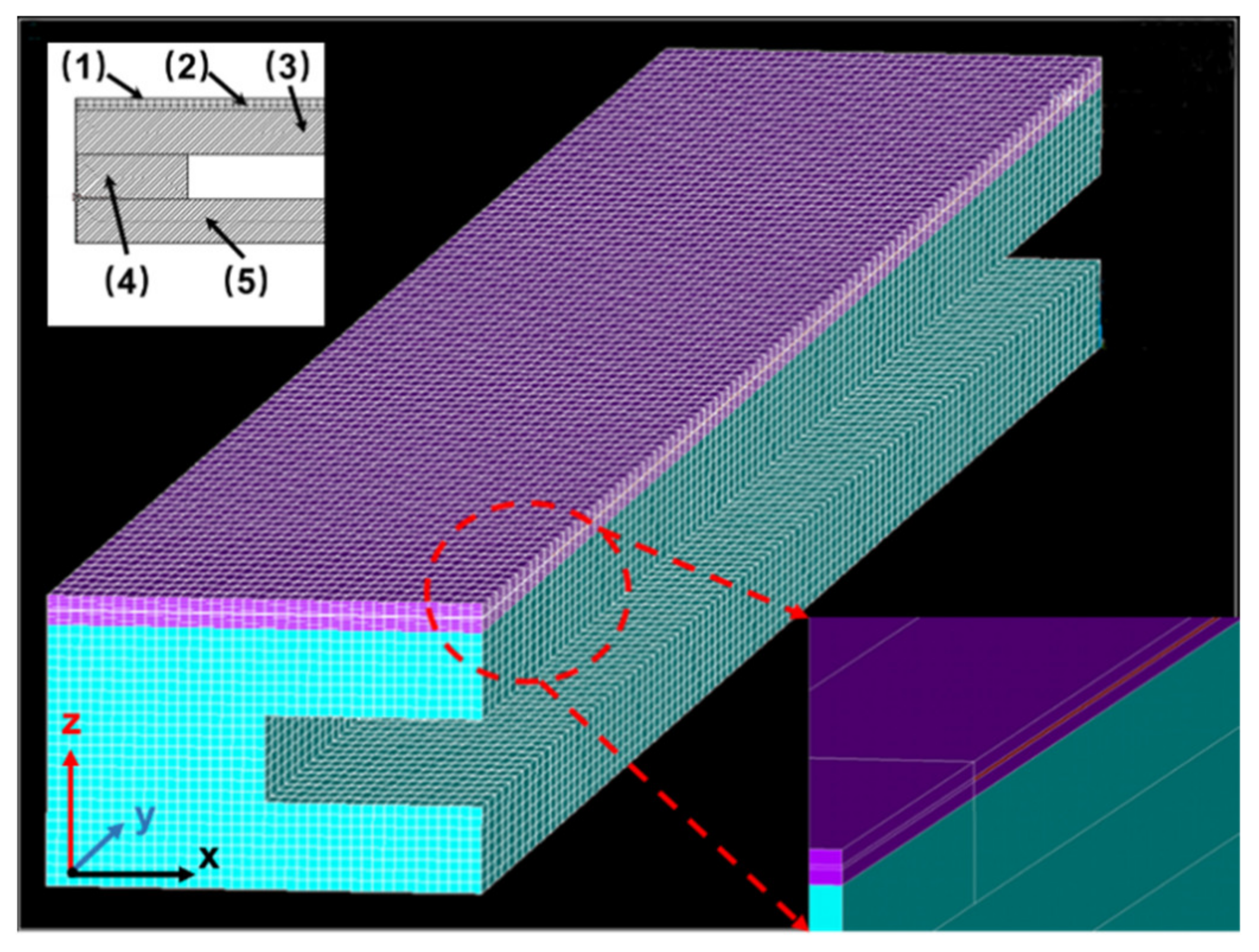
2.1. Structure of the NOx Sensor
2.2. Modeling and Mesh Generation
2.3. Boundary Conditions and Parameters
2.4. Micromorphology Observation
3. Results and Discussions
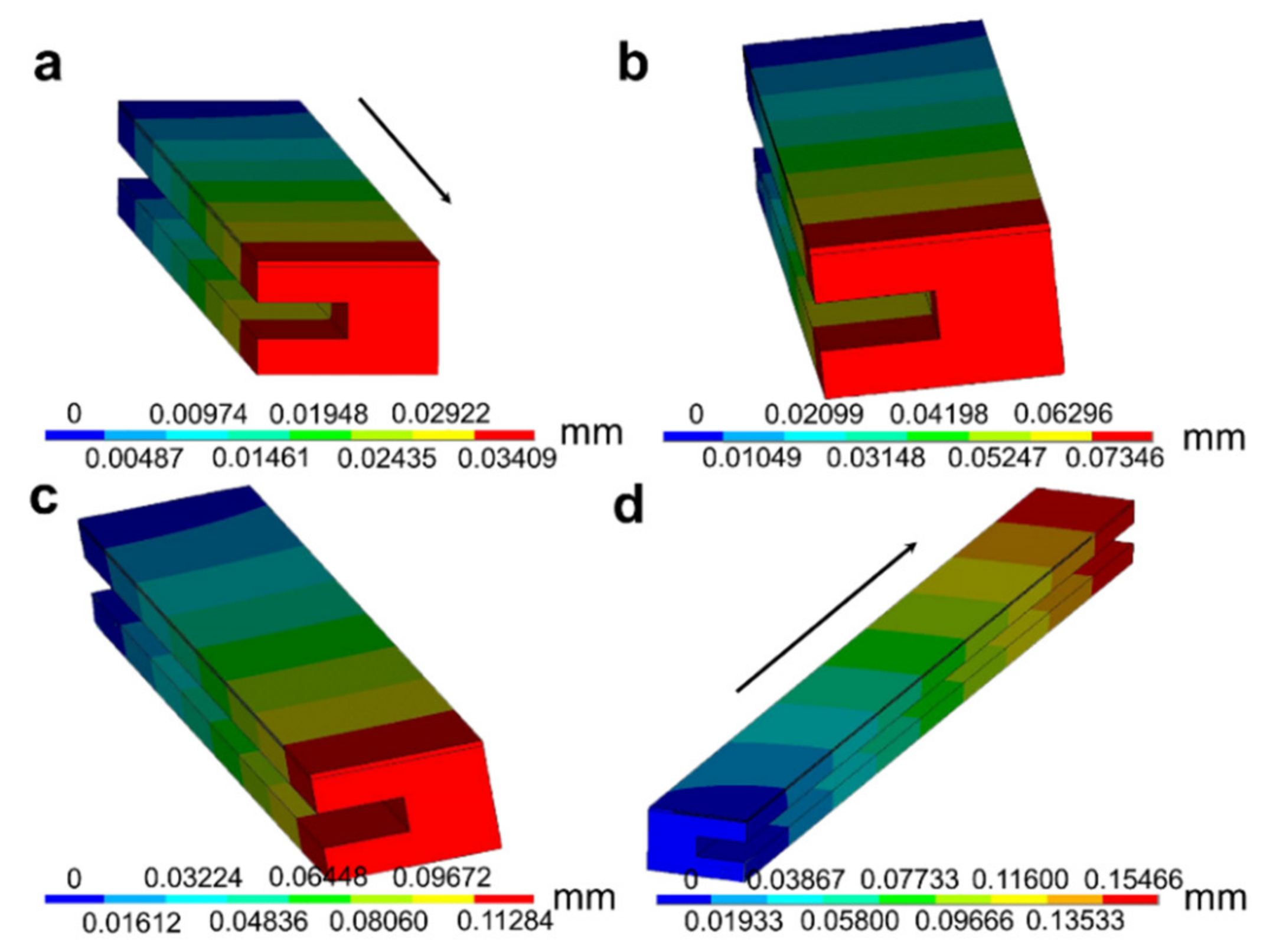
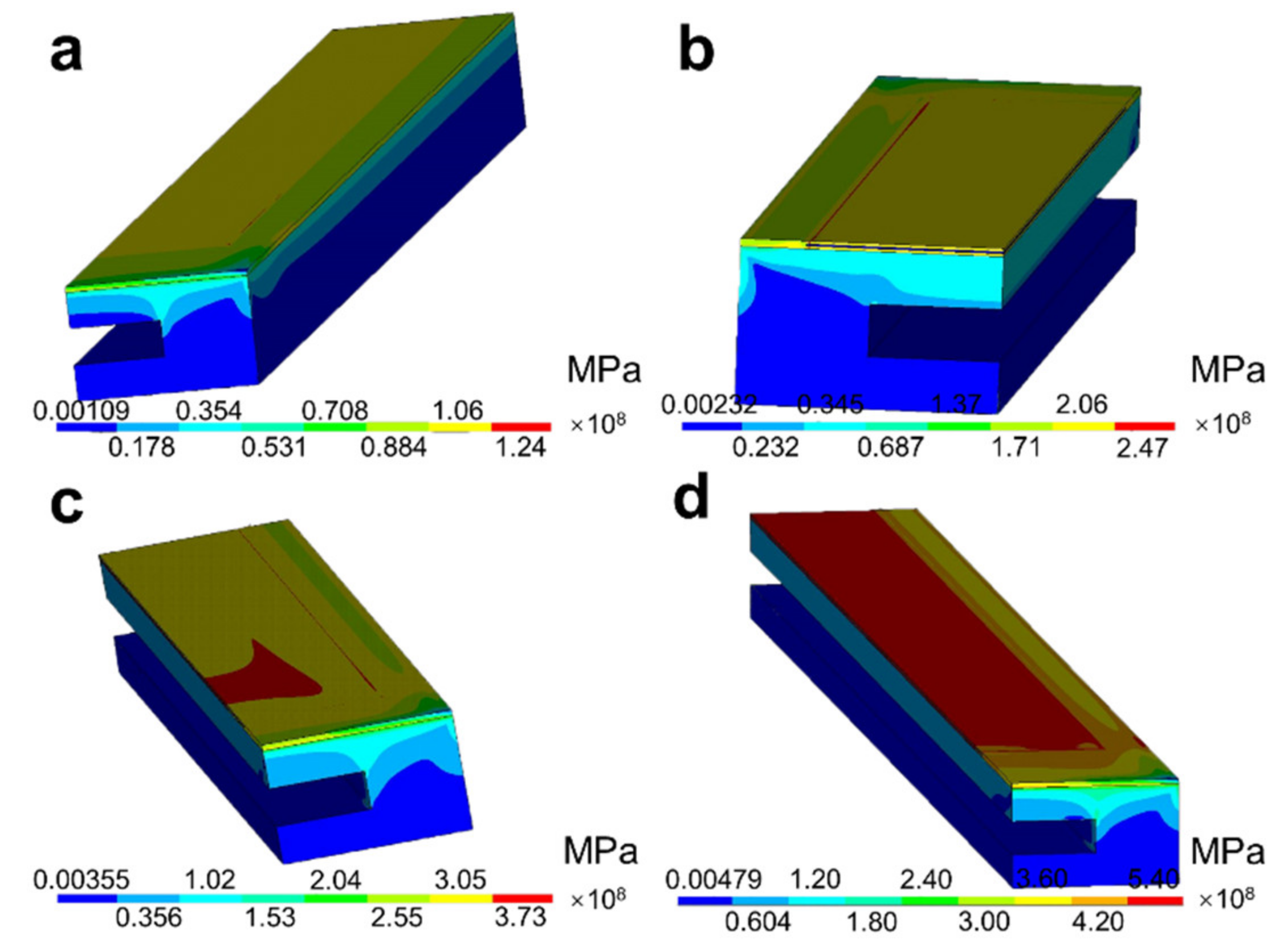
3.1. Temperature and Thermal Stress Distribution Based on Numerical Simulation
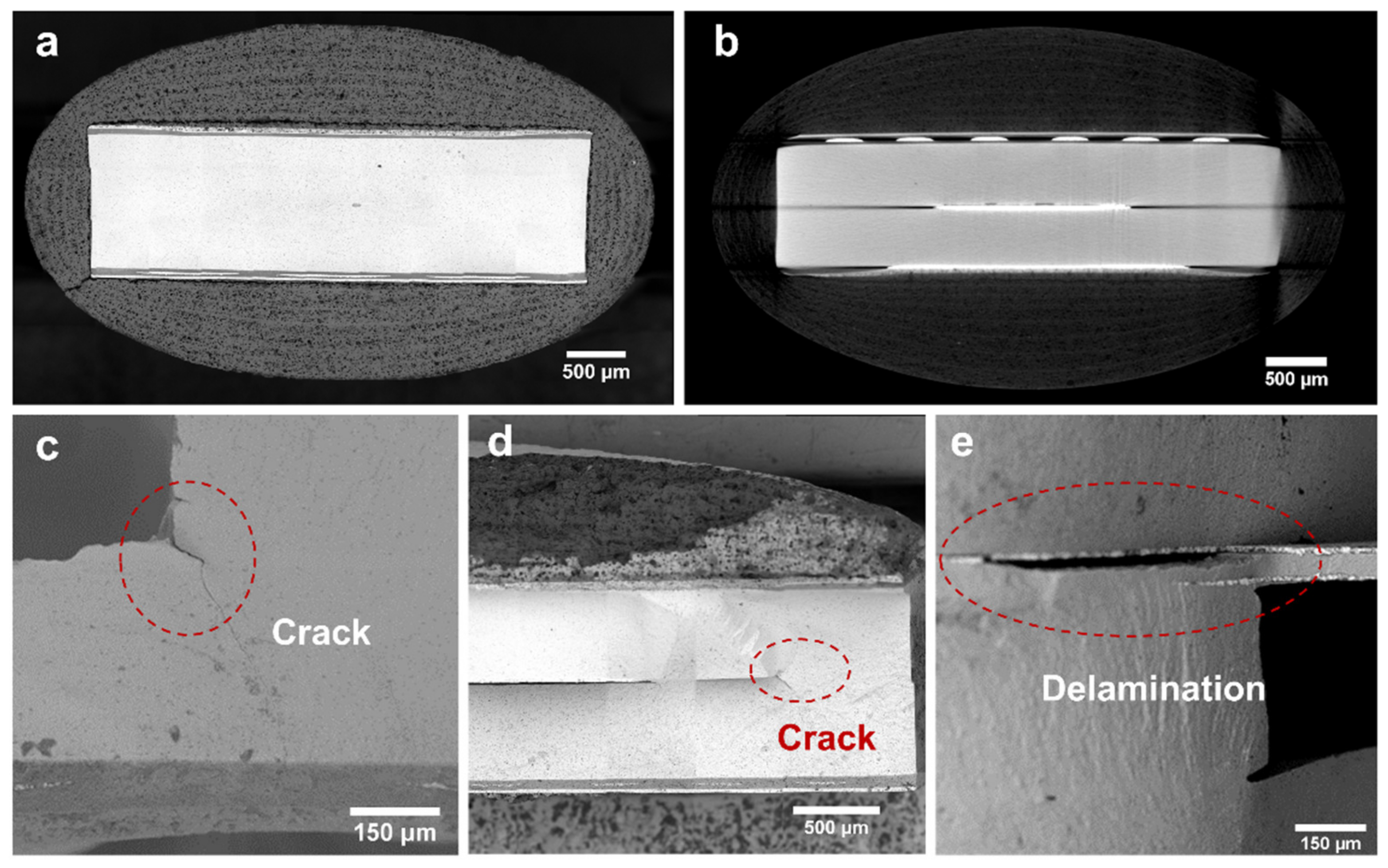
3.2. Thermal Stress Failure Analysis of NOx Sensor
4. Conclusions
- (1)
- In the testing of NOx sensors, the temperature distribution near the Pt heating circuit was relatively uniform, and there was an obvious temperature gradient along the length direction that increased with the increase in heating temperature. In contrast, the temperature gradient along the interlayer was smaller owing to the higher heat transfer efficiency of the Pt heating circuit;
- (2)
- The thermal deformation of the NOx sensor presented a gradient distribution along the length direction. The maximum thermal deformation location observed was consistent to that of the highest temperature gradient, which indicates that the temperature gradient had a significantly impact on the thermal deformation of the sensor;
- (3)
- The overall thermal stress increased when the temperature increased. The maximum thermal stress was observed at the region of the Pt heating circuit and two Al2O3 layers below 600 °C, and the region changed to the interfaces between the Al2O3 layer and the ZrO2 layer when the temperature reached 800 °C;
- (4)
- Based on the simulations and microstructural observations, most of the defects were found around the air chamber in the form of cracks and delamination, which may cause decay in the mechanical strength and localized overheating of the sensors. It can be further confirmed that the cracks mainly occurred at the right-angle position of the air chamber, while the delamination occurred between the Al2O3 and the ZrO2 layers.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, R.; Pin, Y.; Meng, X.; Wang, L.; Liu, C.; Niu, Y. Associations between ambient nitrogen dioxide and daily cause-specific mortality. Epidemiology 2018, 29, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, S.; Sun, Y.; Liu, Y.; Beazley, R.; Hou, X. Assessing NO2-related health effects by non-linear and linear methods on a national level. Sci. Total Environ. 2020, 744, 140909. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, S.; Cirach, M.; Pereira-Barboza, E.; Mueller, N.; Barrera-Gómez, J.; Rojas-Rueda, D. Premature mortality due to air pollution in European cities: A health impact assessment. Lancet. Planet Health 2021, 5, e121–e134. [Google Scholar] [CrossRef]
- Rood, S.; Eslava, S.; Manigrasso, A.; Bannister, C. Recent advances in gasoline three-way catalyst formulation: A review. Proc. Inst. Mech. Eng. Part. D J. Automob. Eng. 2020, 234, 936–949. [Google Scholar] [CrossRef]
- Deng, B.; Li, Q.; Chen, Y.; Li, M.; Liu, A.; Ran, J. The effect of air/fuel ratio on the CO and NOx emissions for a twin-spark motorcycle gasoline engine under wide range of operating conditions. Energy 2019, 169, 1202–1213. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakano, H.; Takase, S.; Song, J.H. Solid electrolyte impedancemetric NOx sensor attached with zeolite receptor. Sens. Actuators B Chem. 2018, 264, 177–183. [Google Scholar] [CrossRef]
- Zaporozhets, A. Analysis of control system of fuel combustion in boilers with oxygen sensor. Period. Polytech. Mech. Eng. 2019, 64, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Cirera, A.; López-Gándara, C.; Ramos, F.M. YSZ-based oxygen sensors and the use of nanomaterials: A review from classical models to current trends. J. Sens. 2009, 2009, 258489. [Google Scholar] [CrossRef]
- Miura, N.; Jin, H.; Wama, R.; Nakakubo, S.; Elumalai, P.; Plashnitsa, V. Novel solid-state manganese oxide-based reference electrode for YSZ-based oxygen sensors. Sens. Actuators B Chem. 2011, 152, 261–266. [Google Scholar] [CrossRef]
- Yoo, Y.-S.; Bhardwaj, A.; Hong, J.-W.; Im, H.-N.; Song, S.-J. Sensing Performance of a YSZ-Based Electrochemical NO2 Sensor Using Nanocomposite Electrodes. J. Electrochem. Soc. 2019, 166, B799–B804. [Google Scholar] [CrossRef]
- Song, S.-W.; Martin, L.P.; Glass, R.S.; Murray, E.P.; Visser, J.H.; Soltis, R.E. Aging Studies of Sr-Doped LaCrO3/YSZ/Pt Cells for an Electrochemical NOx Sensor. J. Electrochem. Soc. 2006, 153, H171. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; Chadwick, A.V.; Greaves, G.N.; Moroney, L.M. EXAFS Study of Yttria-Stabilized Zirconia. J. Am. Ceram. Soc. 1986, 69, 272–277. [Google Scholar] [CrossRef]
- Kurumada, M.; Hara, H.; Iguchi, E. Oxygen vacancies contributing to intragranular electrical conduction of yttria-stabilized zirconia (YSZ) ceramics. Acta Mater. 2005, 53, 4839–4846. [Google Scholar] [CrossRef]
- Kukk, F.; Möller, P.; Kanarbik, R.; Nurk, G. Study of Long-Term Stability of Ni-Zr0.92Y0.08O2−δ|Zr0.92Y0.08O2−δ|Ce0.9Gd0.1O2−δ |Pr0.6Sr0.4CoO3−δ at SOFC and SOEC Mode. Energies 2021, 14, 824. [Google Scholar] [CrossRef]
- Fang, X.; Zhu, J.; Lin, Z. Effects of electrode composition and thickness on the mechanical performance of a solid oxide fuel cell. Energies 2018, 11, 1735. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Tang, X.; Yin, H.; Peng, S. Fabrication, microstructure and properties of a YSZ electrolyte for SOFCs. J. Power Sources 2007, 165, 757–763. [Google Scholar] [CrossRef]
- Ikeda, H.; Iio, A.; Anggraini, S.A.; Miura, N. Impedancemetric YSZ-based oxygen sensor using BaFeO3 sensing-electrode. Sens. Actuators B Chem. 2017, 243, 279–282. [Google Scholar] [CrossRef]
- Wang, J.; Yan, D.; Pu, J.; Chi, B.; Jian, L. Fabrication and performance evaluation of planar solid oxide fuel cell with large active reaction area. Int. J. Hydrogen Energy 2011, 36, 7234–7239. [Google Scholar] [CrossRef]
- Ahamer, C.; Opitz, A.K.; Rupp, G.M.; Fleig, J. Revisiting the Temperature Dependent Ionic Conductivity of Yttria Stabilized Zirconia (YSZ). J. Electrochem. Soc. 2017, 164, F790–F803. [Google Scholar] [CrossRef]
- Pan, J.; Yang, J.; Yan, D.; Pu, J.; Chi, B.; Li, J. Effect of thermal cycling on durability of a solid oxide fuel cell stack with external manifold structure. Int. J. Hydrogen Energy 2020, 45, 17927–17934. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Bae, H.; Namgung, Y.; Lim, J.; Song, S.J. Influence of sintering temperature on the physical, electrochemical and sensing properties of α-Fe2O3-SnO2 nanocomposite sensing electrode for a mixed-potential type NOx sensor. Ceram. Int. 2019, 45, 2309–2318. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wu, J.X.; Chen, H.I. High-selectivity NOx sensors based on an Au/InGaP Schottky diode functionalized with self-assembled monolayer of alkanedithiols. Sens. Actuators B Chem. 2020, 305, 127269. [Google Scholar] [CrossRef]
- Inaba, T.; Saji, K. Low temperature operation of thin-film limiting-current type oxygen sensor using graded-composition layer electrodes. Sens. Actuators B Chem. 2008, 129, 874–880. [Google Scholar] [CrossRef]
- Huang, D.T. An integrated computer model for simulating electrothermo-mechanical interactions of an exhaust oxygen sensor. Finite Elem. Anal. Des. 2001, 37, 657–672. [Google Scholar] [CrossRef]
- Elsheikh, A.H.; Guo, J.; Lee, K.-M. Thermal deflection and thermal stresses in a thin circular plate under an axisymmetry heat source. J. Therm. Stresses 2019, 42, 361–373. [Google Scholar] [CrossRef]
- Ahmadein, M.; Elsheikh, A.H.; Alsaleh, N.A. Modeling of cooling and heat conduction in permanent mold casting process. Alex. Eng. J. 2021, 61, 1757–1768. [Google Scholar] [CrossRef]



| Parameters | YSZ | Al2O3 | Metallic Pt |
|---|---|---|---|
| Elastic modulus (GPa) | 210 | 350 | 169 |
| Bending strength (MPa) | 1100 | 2500 | 922 |
| Density (g/cm3) | 6.00 | 3.85 | 21.45 |
| Coefficient of thermal expansion (K−1) | 10 × 10−6 | 8.2 × 10−6 | 9 × 10−6 |
| Thermal conductivity (W/(m·K)) | 22.0 | 25.0 | 71.4 |
| Poisson ratio | 0.30 | 0.23 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Geng, J.; She, H.; Zhang, T.; Chi, B.; Pu, J. Thermal Stress Simulation and Structure Failure Analyses of Nitrogen–Oxygen Sensors under a Gradual Temperature Field. Energies 2022, 15, 2799. https://doi.org/10.3390/en15082799
Feng J, Geng J, She H, Zhang T, Chi B, Pu J. Thermal Stress Simulation and Structure Failure Analyses of Nitrogen–Oxygen Sensors under a Gradual Temperature Field. Energies. 2022; 15(8):2799. https://doi.org/10.3390/en15082799
Chicago/Turabian StyleFeng, Jiangtao, Jiaqi Geng, Hangyu She, Tao Zhang, Bo Chi, and Jian Pu. 2022. "Thermal Stress Simulation and Structure Failure Analyses of Nitrogen–Oxygen Sensors under a Gradual Temperature Field" Energies 15, no. 8: 2799. https://doi.org/10.3390/en15082799
APA StyleFeng, J., Geng, J., She, H., Zhang, T., Chi, B., & Pu, J. (2022). Thermal Stress Simulation and Structure Failure Analyses of Nitrogen–Oxygen Sensors under a Gradual Temperature Field. Energies, 15(8), 2799. https://doi.org/10.3390/en15082799





