Hydrogen-Rich Syngas and Biochar Production by Non-Catalytic Valorization of Date Palm Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Date Seeds
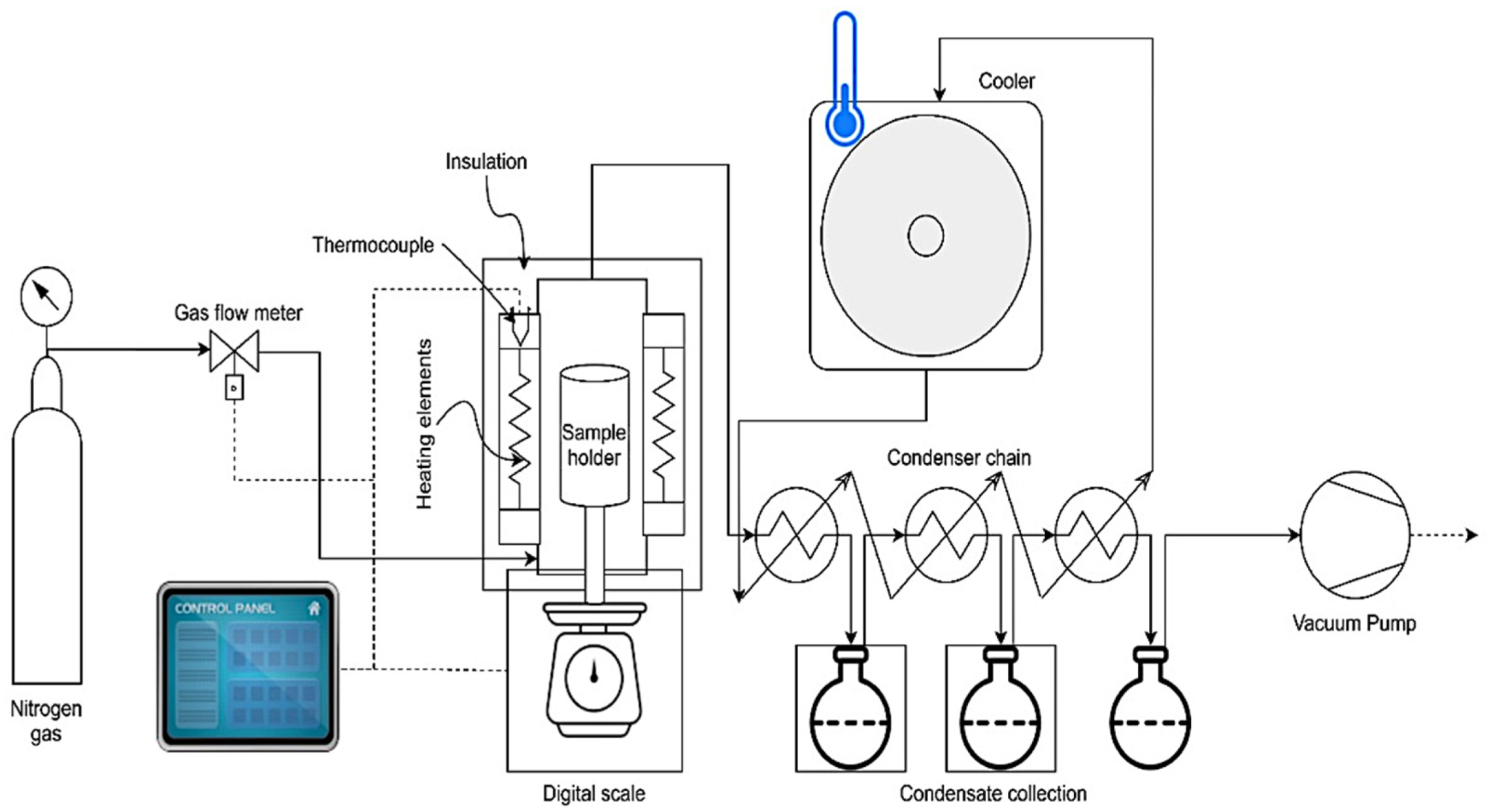
2.2. Fixed-Bed Pyrolyzer Setup
2.3. Kinetics of Pyrolysis
3. Results and Discussion
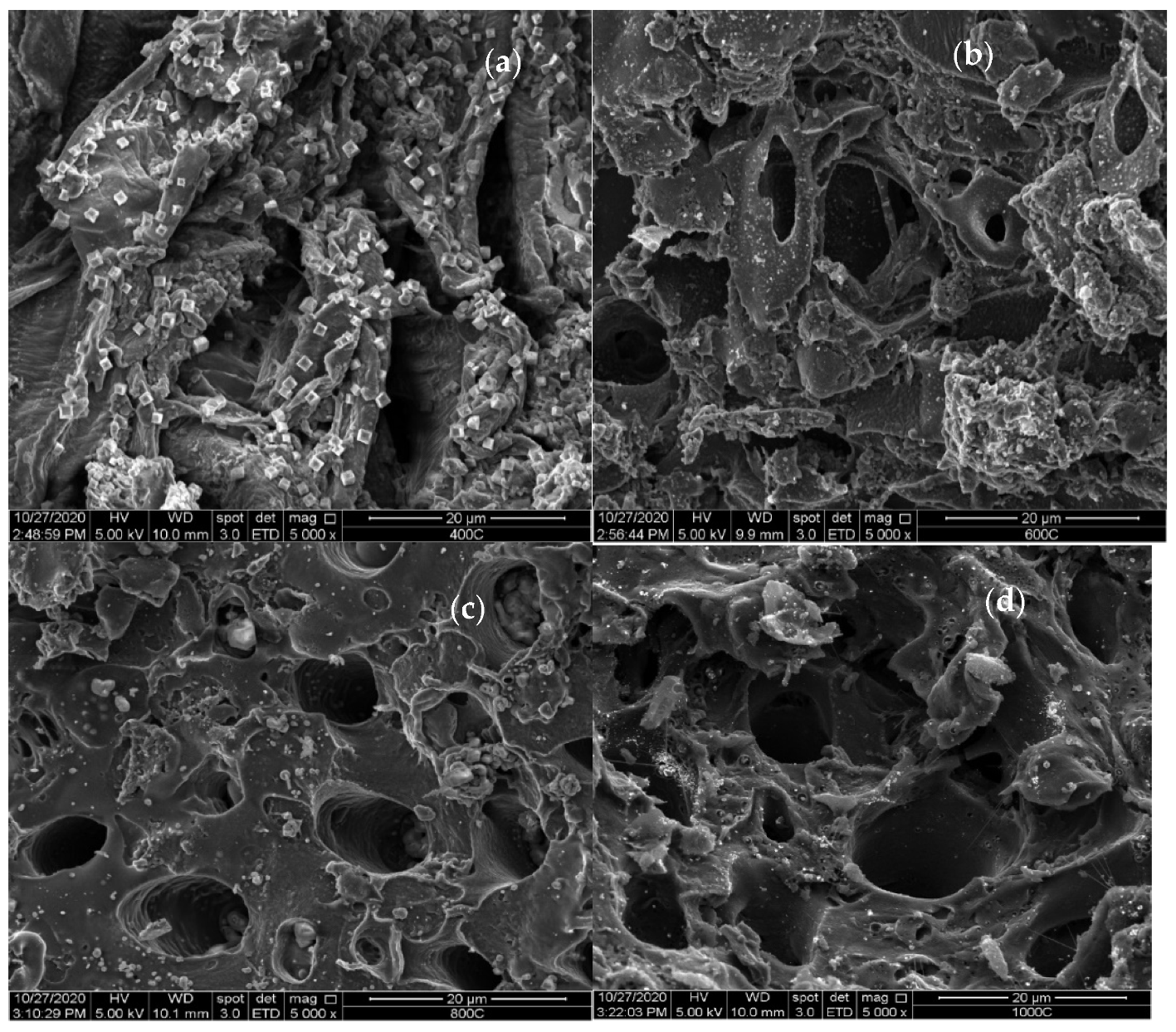
3.1. Characterization of Date Seeds
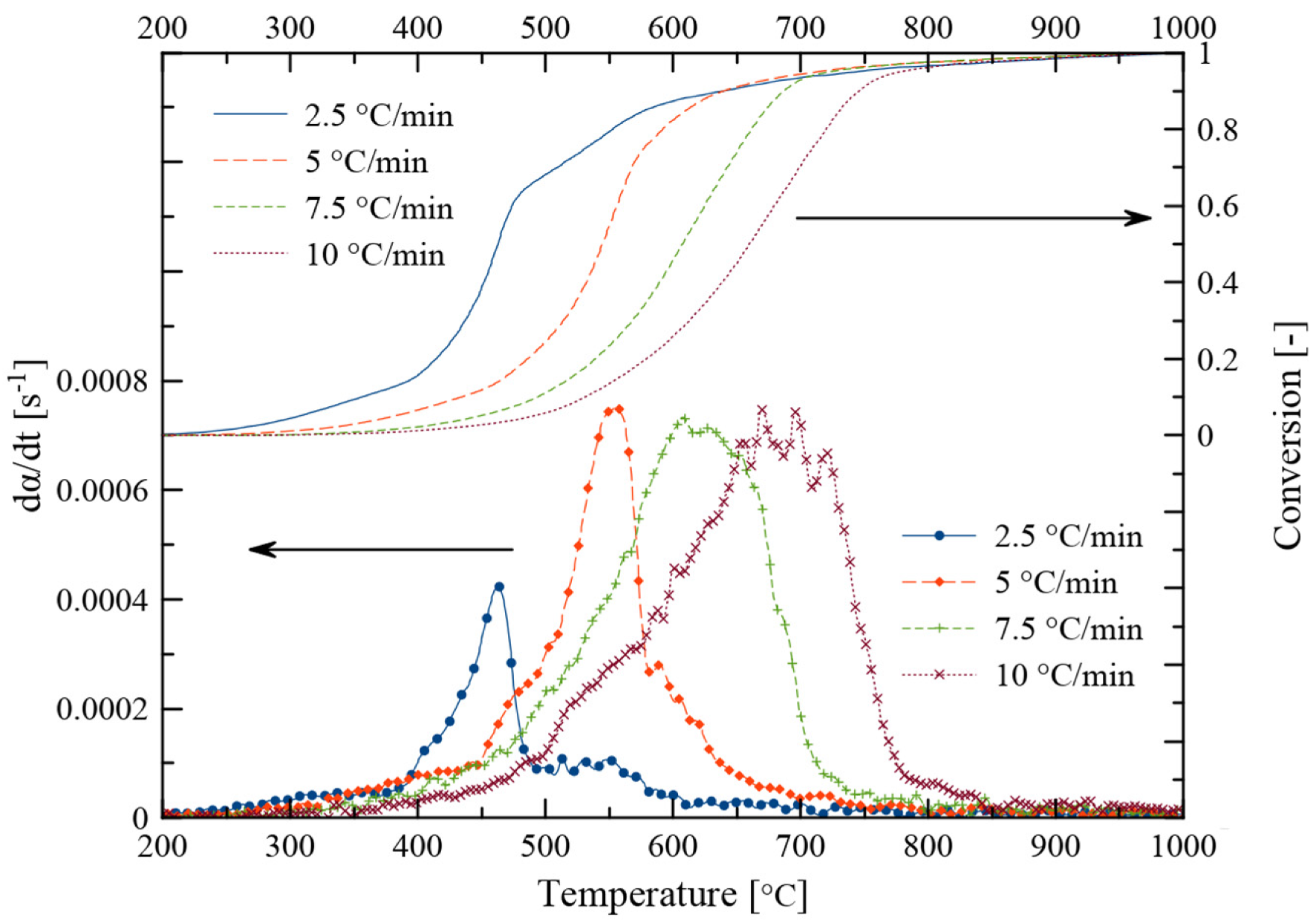
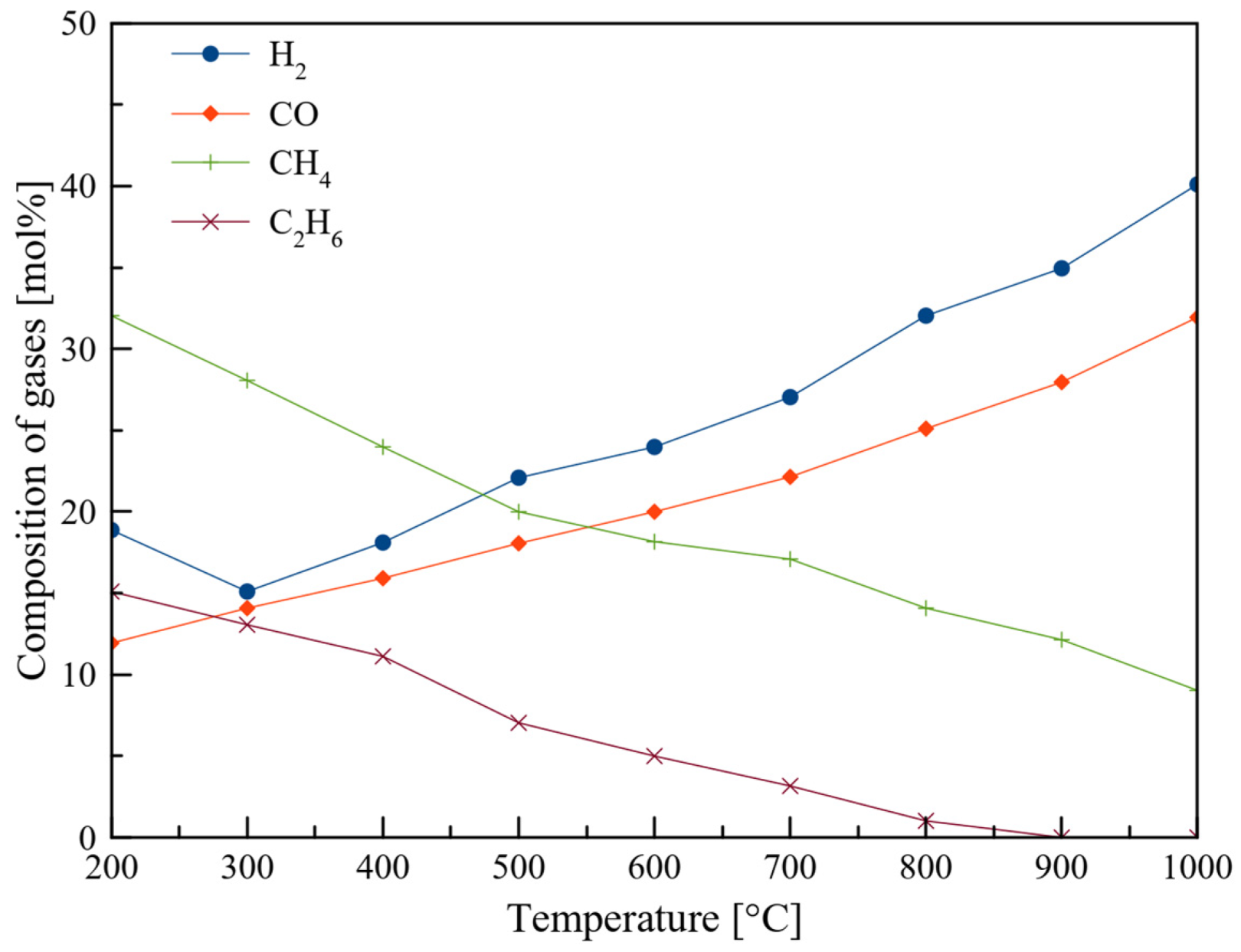
3.2. Fast Pyrolysis Temperature and Heating Rate
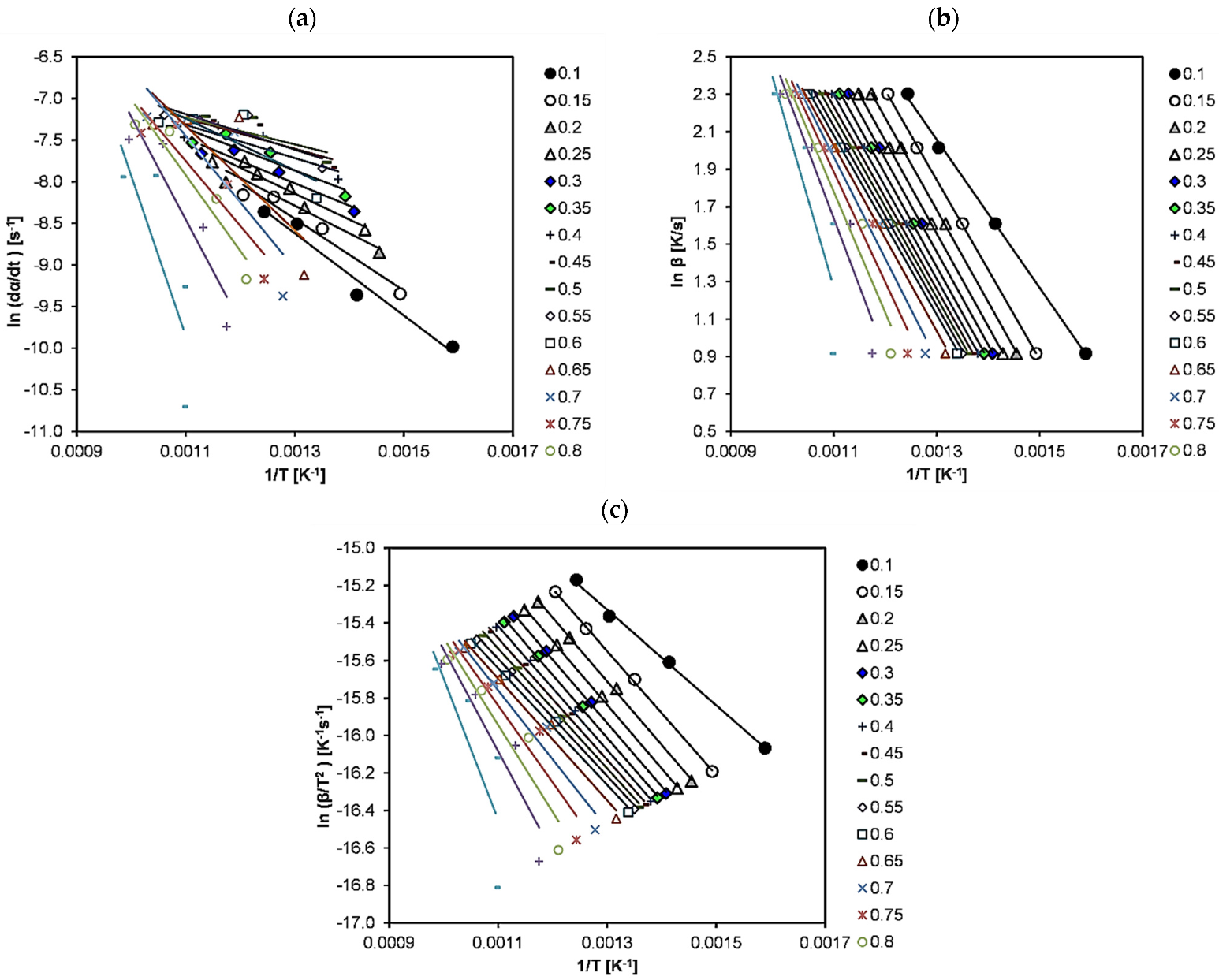
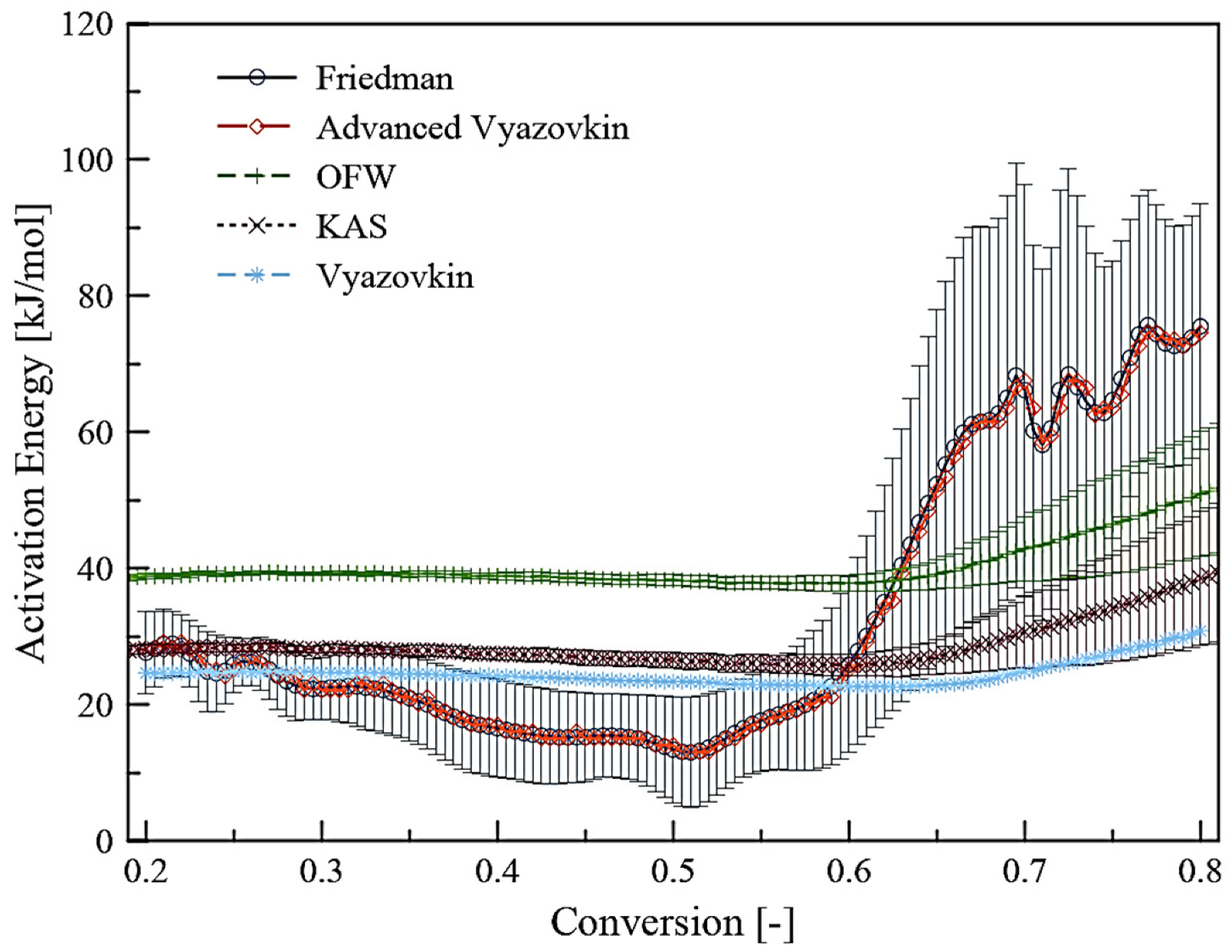
3.3. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sgouridis, S.; Griffiths, S.; Kennedy, S.; Khalid, A.; Zurita, N. A sustainable energy transition strategy for the United Arab Emirates: Evaluation of options using an Integrated Energy Model. Energy Strategy Rev. 2013, 2, 8–18. [Google Scholar] [CrossRef]
- Ray, P. Renewable energy and sustainability. Clean Technol. Environ. Policy 2019, 21, 1517–1533. [Google Scholar] [CrossRef]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials and challenges in lignocellulosic biofuel production technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Habibollahzade, A.; Ahmadi, P.; Rosen, M.A. Biomass gasification using various gasification agents: Optimum feedstock selection, detailed numerical analyses and tri-objective grey wolf optimization. J. Clean. Prod. 2021, 284, 124718. [Google Scholar] [CrossRef]
- Zheng, Y.; Jenkins, B.M.; Kornbluth, K.; Kendall, A.; Træholt, C. Optimization of a biomass-integrated renewable energy microgrid with demand side management under uncertainty. Appl. Energy 2018, 230, 836–844. [Google Scholar] [CrossRef]
- Perea-Moreno, M.-A.; Samerón-Manzano, E.; Perea-Moreno, A.-J. Biomass as Renewable Energy: Worldwide Research Trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef] [Green Version]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Amran, Y.H.A.; Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H. Renewable and sustainable energy production in Saudi Arabia according to Saudi Vision 2030; Current status and future prospects. J. Clean. Prod. 2020, 247, 119602. [Google Scholar] [CrossRef]
- Niphadkar, S.; Bagade, P.; Ahmed, S. Bioethanol production: Insight into past, present and future perspectives. Biofuels 2018, 9, 229–238. [Google Scholar] [CrossRef]
- Hannula, I. Hydrogen enhancement potential of synthetic biofuels manufacture in the European context: A techno-economic assessment. Energy 2016, 104, 199–212. [Google Scholar] [CrossRef]
- Silva, V.; Rouboa, A. Optimizing the gasification operating conditions of forest residues by coupling a two-stage equilibrium model with a response surface methodology. Fuel Process. Technol. 2014, 122, 163–169. [Google Scholar] [CrossRef]
- Salam, M.A.; Ahmed, K.; Akter, N.; Hossain, T.; Abdullah, B. A review of hydrogen production via biomass gasification and its prospect in Bangladesh. Int. J. Hydrogen Energy 2018, 43, 14944–14973. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Chen, S.; Li, Q.; Zhang, Y. A novel method for kinetics analysis of pyrolysis of hemicellulose, cellulose, and lignin in TGA and macro-TGA. RSC Adv. 2015, 5, 26509–26516. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.K. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Ayodele, B.V.; Mustapa, S.I.; Tuan Abdullah, T.A.R.; Bin Salleh, S.F. A Mini-Review on Hydrogen-Rich Syngas Production by Thermo-Catalytic and Bioconversion of Biomass and Its Environmental Implications. Front. Energy Res. 2019, 7, 118. [Google Scholar] [CrossRef]
- Sait, H.H.; Hussain, A.; Salema, A.A.; Ani, F.N. Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour. Technol. 2012, 118, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Salama, K.F.; Randhawa, M.A.; Mulla, A.A.; Al Labib, O.A. Heavy metals in some date palm fruit cultivars in Saudi Arabia and their health risk assessment. Int. J. Food Prop. 2019, 22, 1684–1692. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Farooq, A.; Bassyouni, M.I.; Sait, H.H.; El-Wafa, M.A.; Hasan, S.W.; Ani, F.N. Pyrolysis Of Saudi Arabian Date Palm Waste: A Viable Option For Converting Waste Into Wealth. Life Sci. J. 2014, 11, 667–671. [Google Scholar]
- Mujica, R. 10 World ’s Biggest Countries in Date Production of 2019; Azhan Co.: Shah Alam, Malaysia, 2020. [Google Scholar]
- Rambabu, K.; Show, P.-L.; Bharath, G.; Banat, F.; Naushad, M.; Chang, J.-S. Enhanced biohydrogen production from date seeds by Clostridium thermocellum ATCC 27405. Int. J. Hydrogen Energy 2020, 45, 22271–22280. [Google Scholar] [CrossRef]
- Mrabet, A.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Sindic, M. Date Seeds: A Promising Source of Oil with Functional Properties. Foods 2020, 9, 787. [Google Scholar] [CrossRef]
- Kabir, G.; Mohd Din, A.T.; Hameed, B.H. Pyrolysis of oil palm mesocarp fiber catalyzed with steel slag-derived zeolite for bio-oil production. Bioresour. Technol. 2018, 249, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Sharma, A.; Pareek, V.; Wu, H.; Yu, Y. A review on biomass pyrolysis models: Kinetic, network and mechanistic models. Biomass Bioenergy 2019, 123, 104–122. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Santos, V.O.; Queiroz, L.S.; Araujo, R.O.; Ribeiro, F.C.P.; Guimarães, M.N.; da Costa, C.E.F.; Chaar, J.S.; de Souza, L.K.C. Pyrolysis of acai seed biomass: Kinetics and thermodynamic parameters using thermogravimetric analysis. Bioresour. Technol. Rep. 2020, 12, 100553. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Insights into pyrolysis and catalytic co-pyrolysis upgrading of biomass and waste rubber seed oil to promote the formation of aromatics hydrocarbon. Int. J. Hydrogen Energy 2018, 43, 16479–16496. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Thermal and catalytic pyrolysis of pine sawdust (Pinus ponderosa) and Gulmohar seed (Delonix regia) towards production of fuel and chemicals. Mater. Sci. Energy Technol. 2019, 2, 139–149. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, V.; Mohanty, K. Pyrolysis kinetics behaviour and thermal pyrolysis of Samanea saman seeds towards the production of renewable fuel. J. Energy Inst. 2020, 93, 1148–1162. [Google Scholar] [CrossRef]
- Aboulkas, A.; Hammani, H.; El Achaby, M.; Bilal, E.; Barakat, A. Valorization of algal waste via pyrolysis in a fixed-bed reactor: Production and characterization of bio-oil and bio-char. Bioresour. Technol. 2017, 1, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.S.; Abdelmottaleb, M.; Cheira, M.F.; Abdel-Aziz, G.; Gomaa, H.; Hassanein, T.F. Date seed as an efficient, eco-friendly, and cost-effective bio-adsorbent for removal of thorium ions from acidic solutions. Aswan Univ. J. Environ. Stud. 2020, 1, 106–124. [Google Scholar] [CrossRef]
- Al Haddabi, M.; Ahmed, M.; Al Jebri, Z.; Vuthaluru, H.; Znad, H.; Al Kindi, M. Boron removal from seawater using date palm (Phoenix dactylifera) seed ash. Desalination Water Treat. 2016, 57, 5130–5137. [Google Scholar] [CrossRef]
- Mahdi, Z.; El Hanandeh, A.; Yu, Q.J. Preparation, characterization and application of surface modified biochar from date seed for improved lead, copper, and nickel removal from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103379. [Google Scholar] [CrossRef]
- Khiari, B.; Jeguirim, M. Pyrolysis of Grape Marc from Tunisian Wine Industry: Feedstock Characterization, Thermal Degradation and Kinetic Analysis. Energies 2018, 11, 730. [Google Scholar] [CrossRef] [Green Version]
- Ondro, T.; Vitázek, I.; Húlan, T.; Lawson, M.K.; Csáki, Š. Non-isothermal kinetic analysis of the thermal decomposition of spruce wood in air atmosphere. Res. Agric. Eng. 2018, 64, 41–46. [Google Scholar]
- Gao, W.; Chen, K.; Zeng, J.; Xu, J.; Wang, B. Thermal pyrolysis characteristics of macroalgae Cladophora glomerata. Bioresour. Technol. 2017, 243, 212–217. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, S.; Su, W.; Wu, W.; Tang, J.; Qi, W. Kinetic studies on the pyrolysis of plastic waste using a combination of model-fitting and model-free methods. Waste Manag. Res. 2020, 38, 77–85. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, S.; Su, W.; Wu, W.; Tang, J.; Qi, W. Comparative study on the pyrolysis kinetics of polyurethane foam from waste refrigerators. Waste Manag. Res. 2020, 38, 271–278. [Google Scholar] [CrossRef]

| This Study | [8] | [9] | [11] | [12] | ||
|---|---|---|---|---|---|---|
| Proximate (wt%) | Moisture | 4.1 | 4–4.5 | |||
| Volatile matter | 78.5 | 78–80.1 | 83.33 | |||
| Fixed carbon | 9.2 | 5.4–9.9 | 14.94 | |||
| Ash | 8.2 | 7.7–11.5 | 1.4 | |||
| Ultimate (wt%) | C | 47.15 | 47.14 | |||
| H | 7.52 | 6.63 | ||||
| N | 0.82 | 0.9 | ||||
| O | 44.51 | 45.3 | ||||
| Lignocellulose (wt%) | Cellulose | 33.1 | 33.77 | 26.6–33.92 | 23.9 | |
| Hemicellulose | 24.3 | 30.20 | 31.97–42.3 | 26.8 | ||
| Lignin | 22.6 | 37.03 | 21.2–24.06 | 216 | ||
| Thermal (MJ/kg) | HHV | 18.7 | 18.226–18.548 |
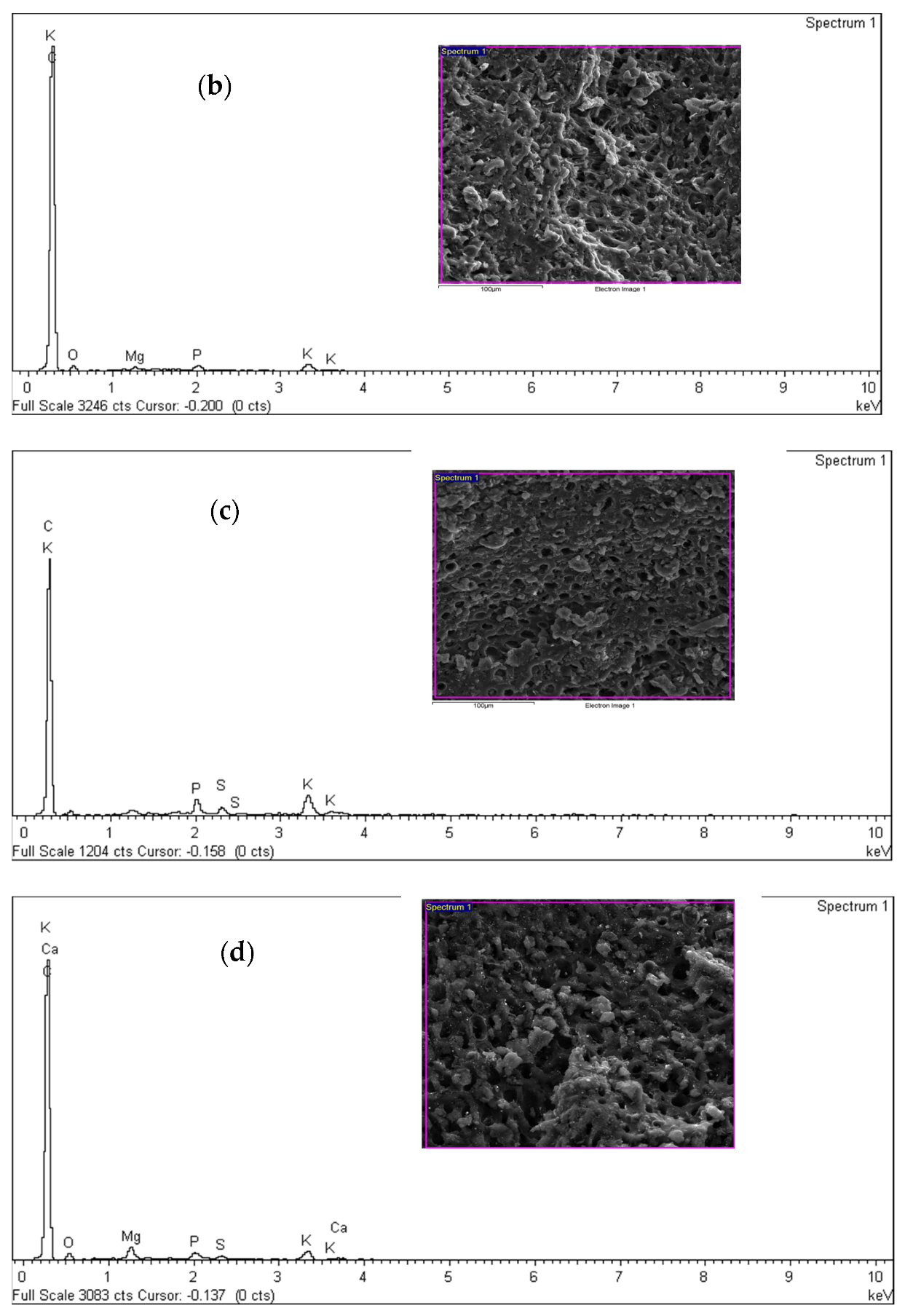
| Element | Atomic Weight (%) at 400 °C | Atomic Weight (%) at 600 °C | Atomic Weight (%) at 800 °C | Atomic Weight (%) at 1000 °C |
|---|---|---|---|---|
| Carbon (C) | 88.96 0.21 | 94.69 0.20 | 97.99 0.14 | 93.88 0.16 |
| Oxygen (O) | 10.28 0.11 | 4.66 0.10 | 0.70 0.20 | 4.75 0.14 |
| Magnesium (Mg) | 0.28 0.02 | 0.13 0.03 | 0.32 0.02 | 0.52 0.10 |
| Phosphorus (P) | 0.21 0.02 | 0.99 0.11 | 0.24 0.03 | |
| Sulfur (S) | 0.14 0.01 | 0.13 0.01 | ||
| Potassium (K) | 0.48 0.01 | 0.31 0.10 | 0.40 0.01 | |
| Calcium (Ca) | 0.08 0.12 |
| α | Vyazovkin | Advanced Vyazovkin | Friedman | KAS | FWO | |||
|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | Ea (kJ/mol) | Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | Ea (kJ/mol) | R2 | |
| 0.20 | 24.66 | 29.19 | 27.62 | 0.91 | 28.19 | 1.00 | 38.85 | 1.00 |
| 0.25 | 24.85 | 25.15 | 25.85 | 0.95 | 28.29 | 1.00 | 39.18 | 1.00 |
| 0.30 | 24.67 | 22.12 | 22.34 | 0.92 | 28.15 | 1.00 | 39.24 | 1.00 |
| 0.35 | 24.50 | 21.11 | 20.95 | 0.85 | 27.90 | 1.00 | 39.17 | 1.00 |
| 0.40 | 24.18 | 16.06 | 16.53 | 0.73 | 27.44 | 1.00 | 38.87 | 1.00 |
| 0.45 | 23.78 | 15.05 | 15.24 | 0.73 | 26.94 | 1.00 | 38.53 | 1.00 |
| 0.50 | 23.40 | 16.06 | 13.40 | 0.60 | 26.57 | 1.00 | 38.30 | 1.00 |
| 0.55 | 22.92 | 16.06 | 17.69 | 0.74 | 26.01 | 0.99 | 37.87 | 1.00 |
| 0.60 | 22.69 | 24.14 | 25.91 | 0.67 | 25.85 | 0.99 | 37.84 | 1.00 |
| 0.65 | 22.88 | 50.40 | 52.39 | 0.68 | 27.03 | 0.98 | 39.16 | 0.99 |
| 0.70 | 24.89 | 66.56 | 66.08 | 0.71 | 30.66 | 0.94 | 42.90 | 0.98 |
| 0.75 | 27.56 | 63.53 | 64.72 | 0.84 | 34.13 | 0.91 | 46.48 | 0.95 |
| 0.80 | 30.83 | 74.64 | 75.49 | 0.90 | 38.56 | 0.88 | 50.97 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sait, H.H.; Hussain, A.; Bassyouni, M.; Ali, I.; Kanthasamy, R.; Ayodele, B.V.; Elhenawy, Y. Hydrogen-Rich Syngas and Biochar Production by Non-Catalytic Valorization of Date Palm Seeds. Energies 2022, 15, 2727. https://doi.org/10.3390/en15082727
Sait HH, Hussain A, Bassyouni M, Ali I, Kanthasamy R, Ayodele BV, Elhenawy Y. Hydrogen-Rich Syngas and Biochar Production by Non-Catalytic Valorization of Date Palm Seeds. Energies. 2022; 15(8):2727. https://doi.org/10.3390/en15082727
Chicago/Turabian StyleSait, Hani Hussain, Ahmed Hussain, Mohamed Bassyouni, Imtiaz Ali, Ramesh Kanthasamy, Bamidele Victor Ayodele, and Yasser Elhenawy. 2022. "Hydrogen-Rich Syngas and Biochar Production by Non-Catalytic Valorization of Date Palm Seeds" Energies 15, no. 8: 2727. https://doi.org/10.3390/en15082727
APA StyleSait, H. H., Hussain, A., Bassyouni, M., Ali, I., Kanthasamy, R., Ayodele, B. V., & Elhenawy, Y. (2022). Hydrogen-Rich Syngas and Biochar Production by Non-Catalytic Valorization of Date Palm Seeds. Energies, 15(8), 2727. https://doi.org/10.3390/en15082727








