Extraction of Rice Bran Oil Using CO2-Expanded Hexane in the Two-Phase Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
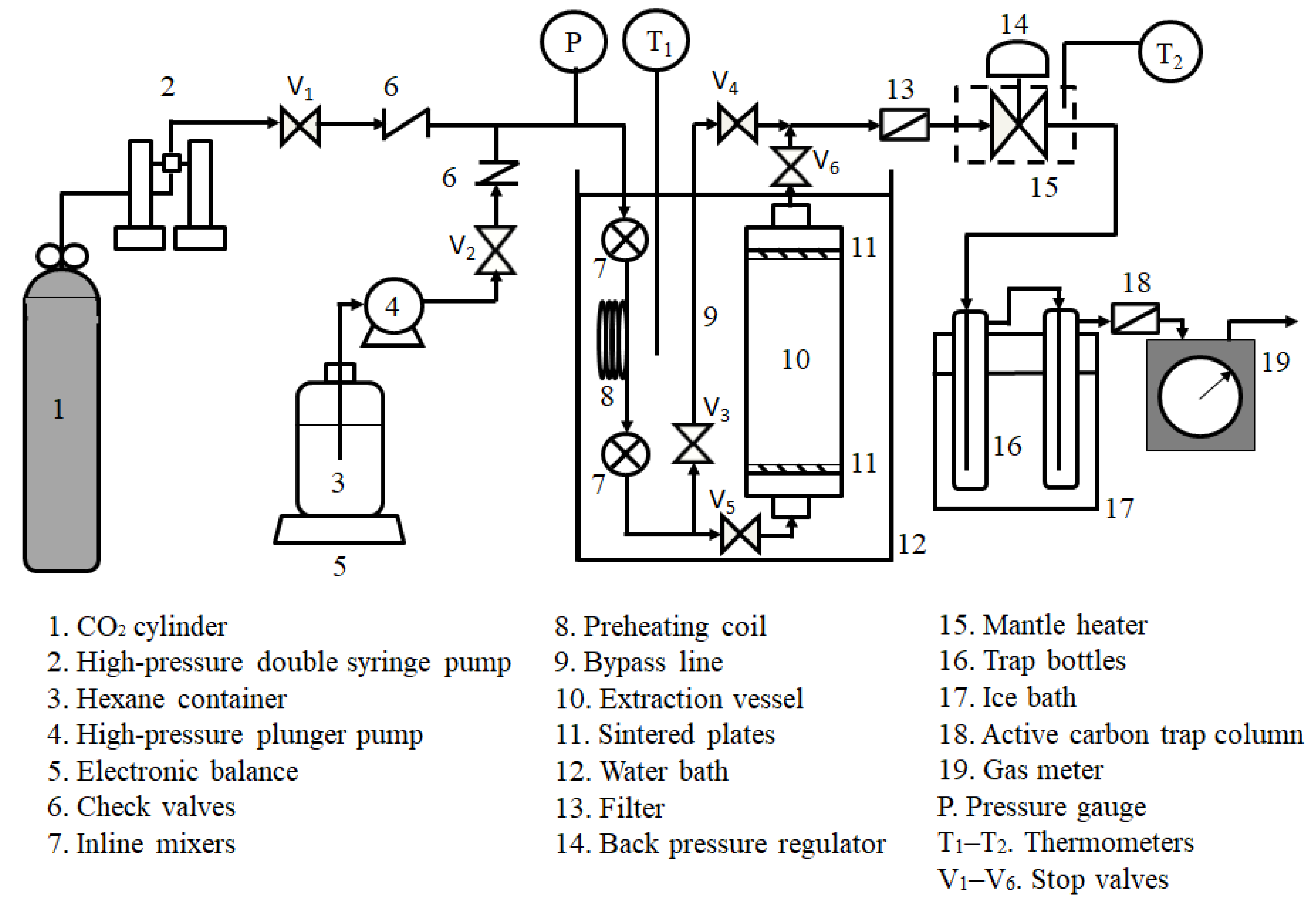
2.2. Experimental Setup and Procedure
3. Results and Discussion
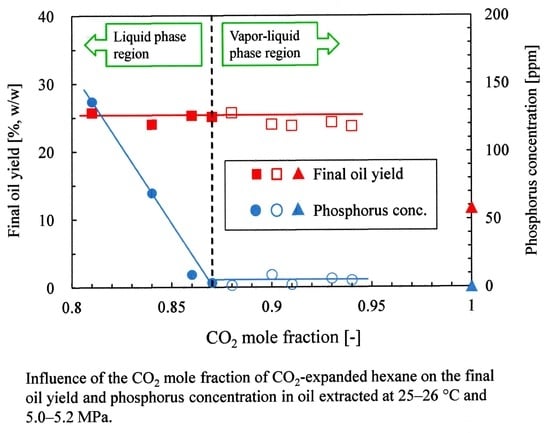
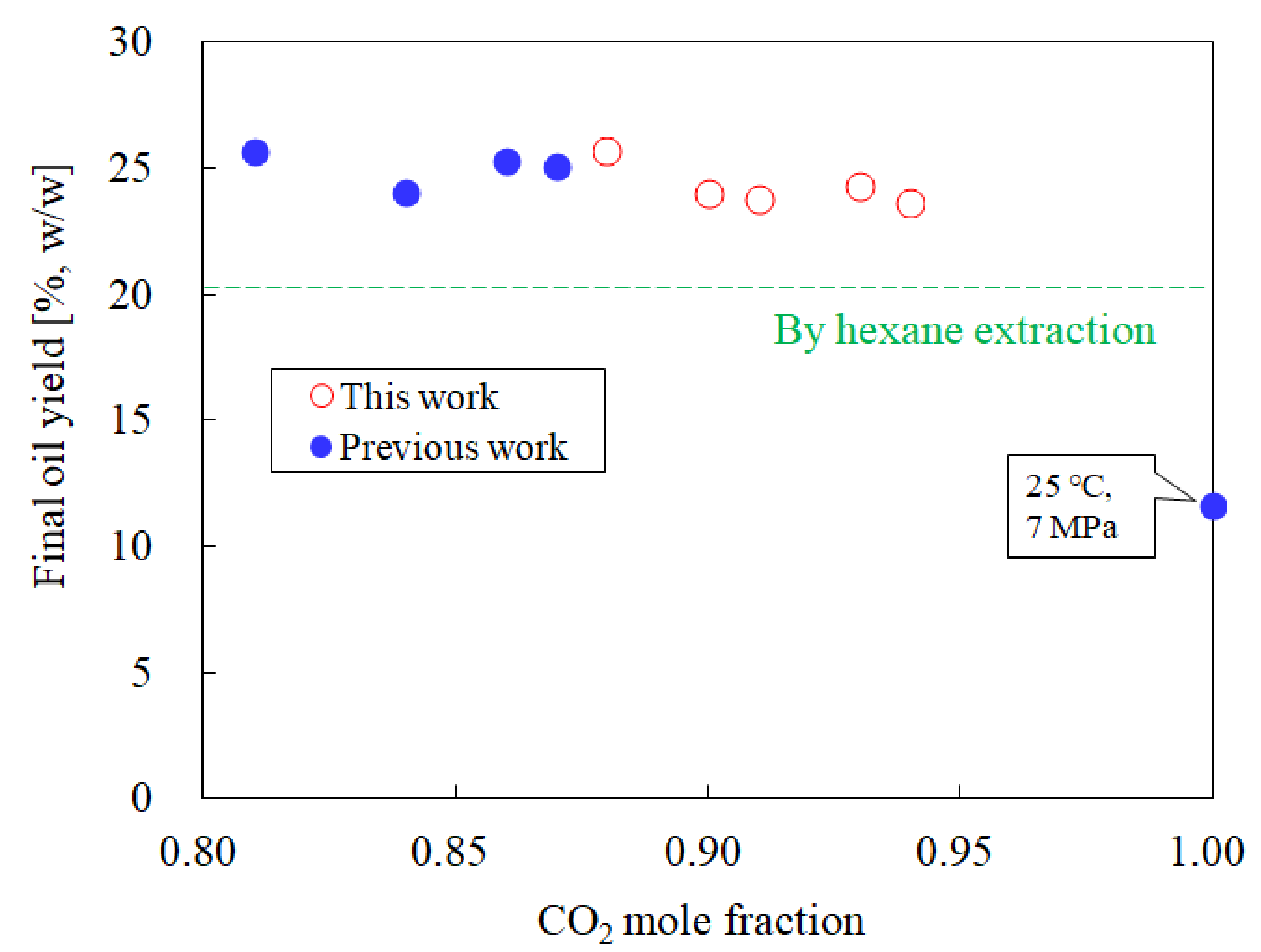
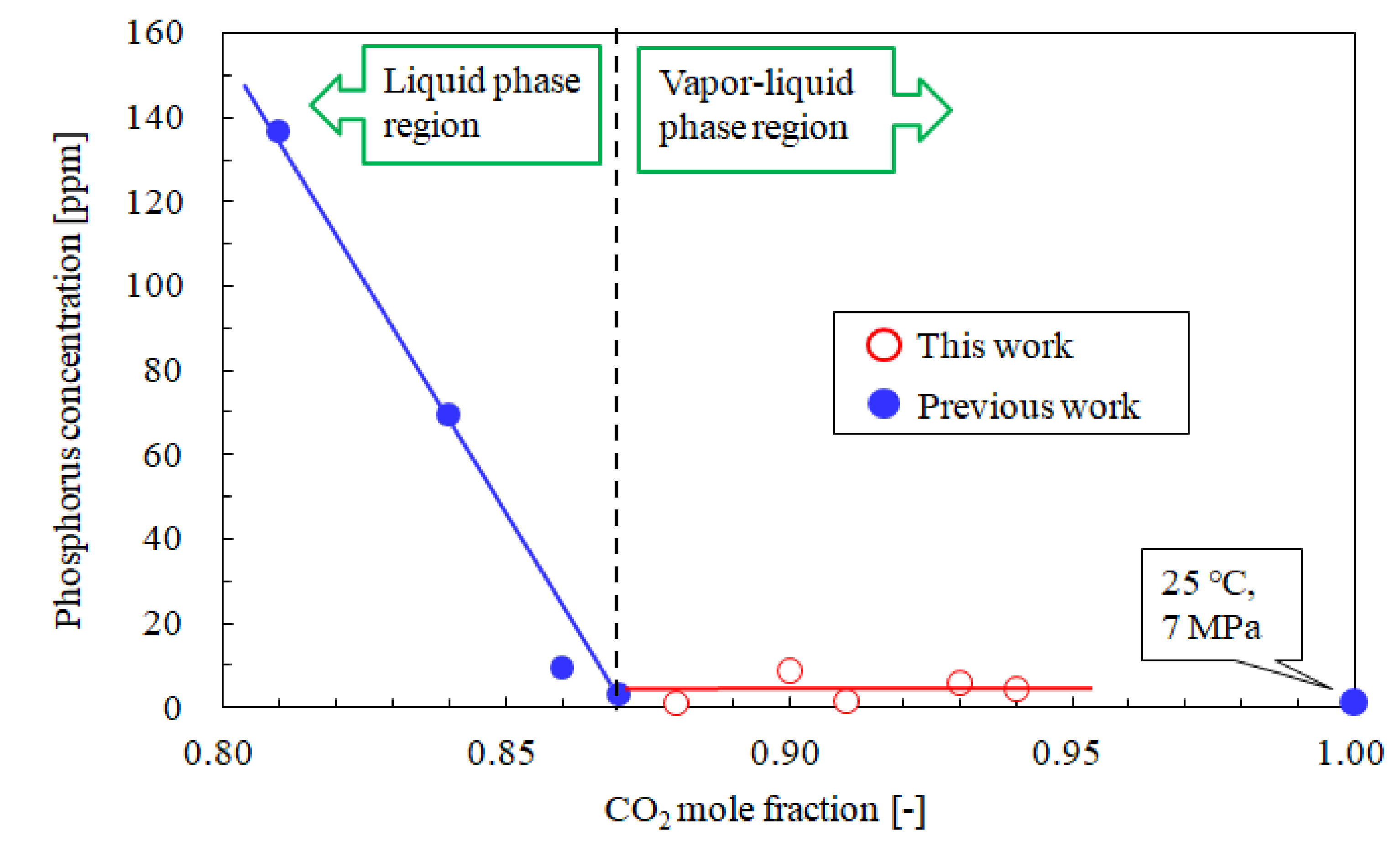
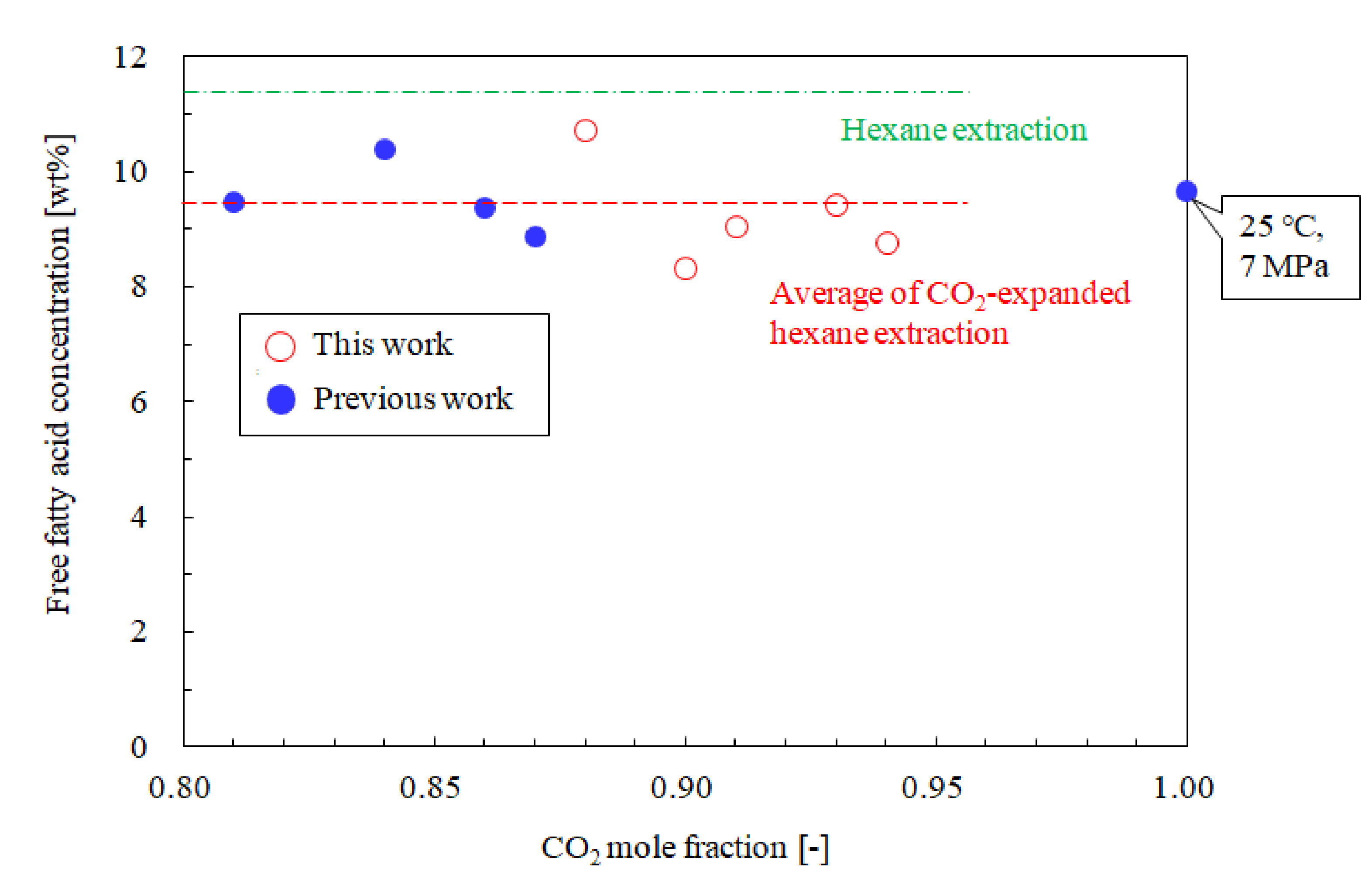
| No. | T [°C] | P [MPa] | CO2 Mole Fraction [-] | Oil Yield [%] | Oil Solubility [g/mol] | Phosphorus conc. [ppm] | Free Fatty Acid conc. [wt%] (3) | Literature |
|---|---|---|---|---|---|---|---|---|
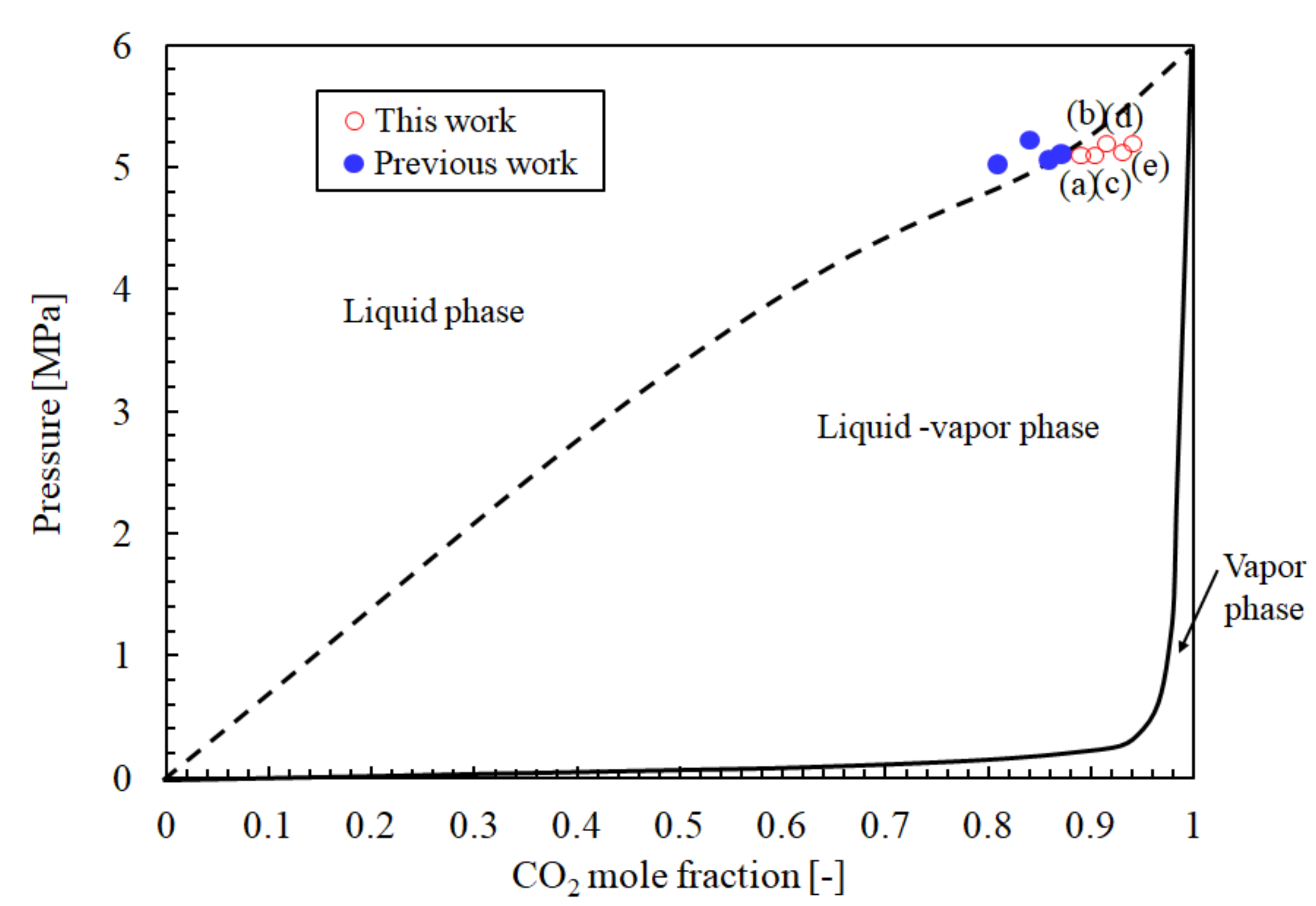
| (a) | 25 | 5.1 | 0.88 | 25.7 | 2.382 | 1.3 | 10.8 | This work |
| (b) | 25 | 5.1 | 0.90 | 24.0 | 1.894 | 9.1 | 8.4 | |
| (c) | 25 | 5.2 | 0.91 | 23.8 | 1.160 | 1.9 | 9.1 | |
| (d) | 25 | 5.1 | 0.93 | 24.3 | 0.987 | 6.3 | 9.5 | |
| (e) | 25 | 5.2 | 0.94 | 23.7 | 0.593 | 5.0 | 8.8 | |
| (f) | 25 | 7.0 | 1 | 11.6 (1) | 0.151 | 0.2 | 9.7 | [21] |
| (g) (2) | ‒ | 0.1 | 0 | 20.3 | ‒ | 277.9 | 11.4 |
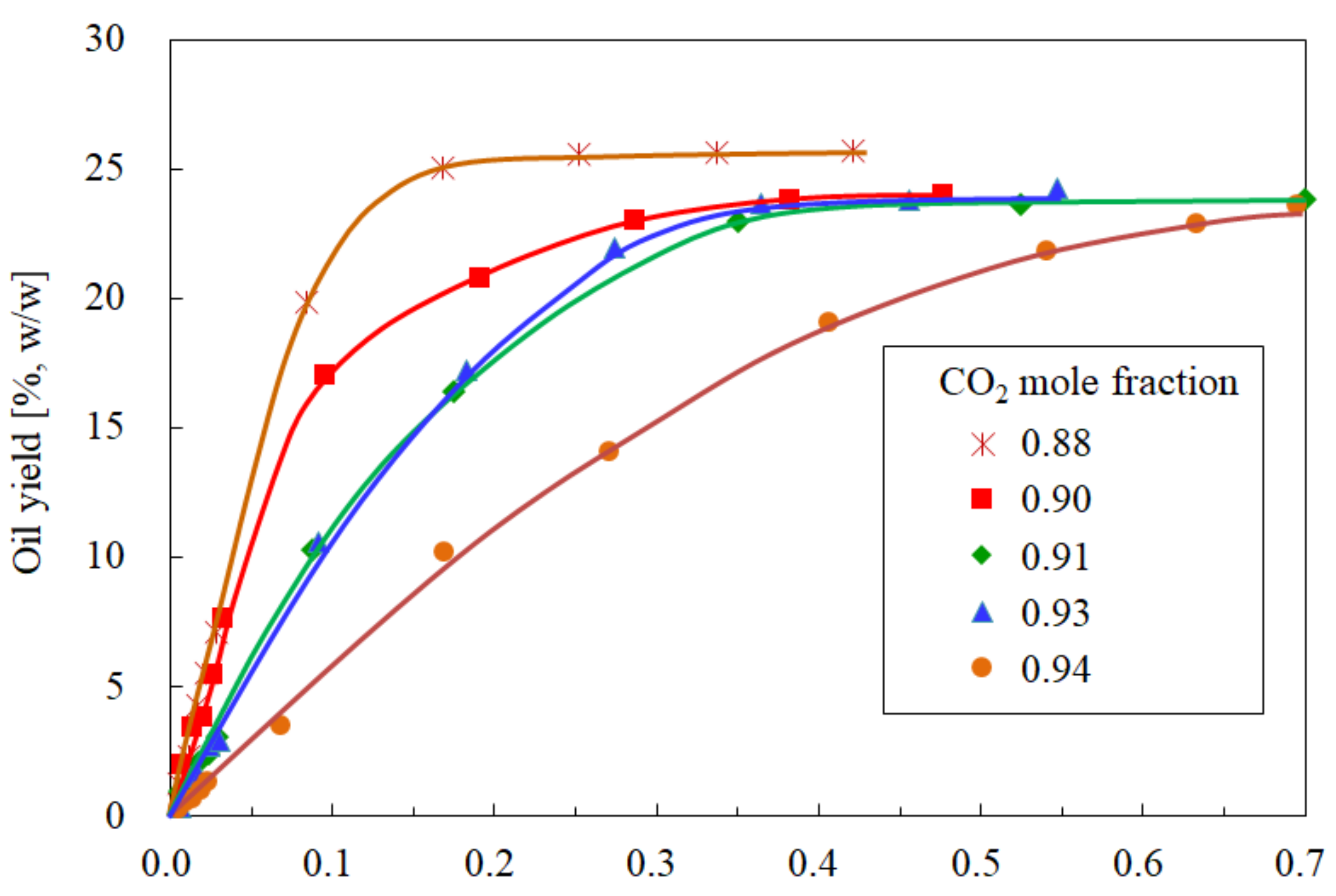
3.1. Influence of the CO2 Mole Fraction on the Oil Yield
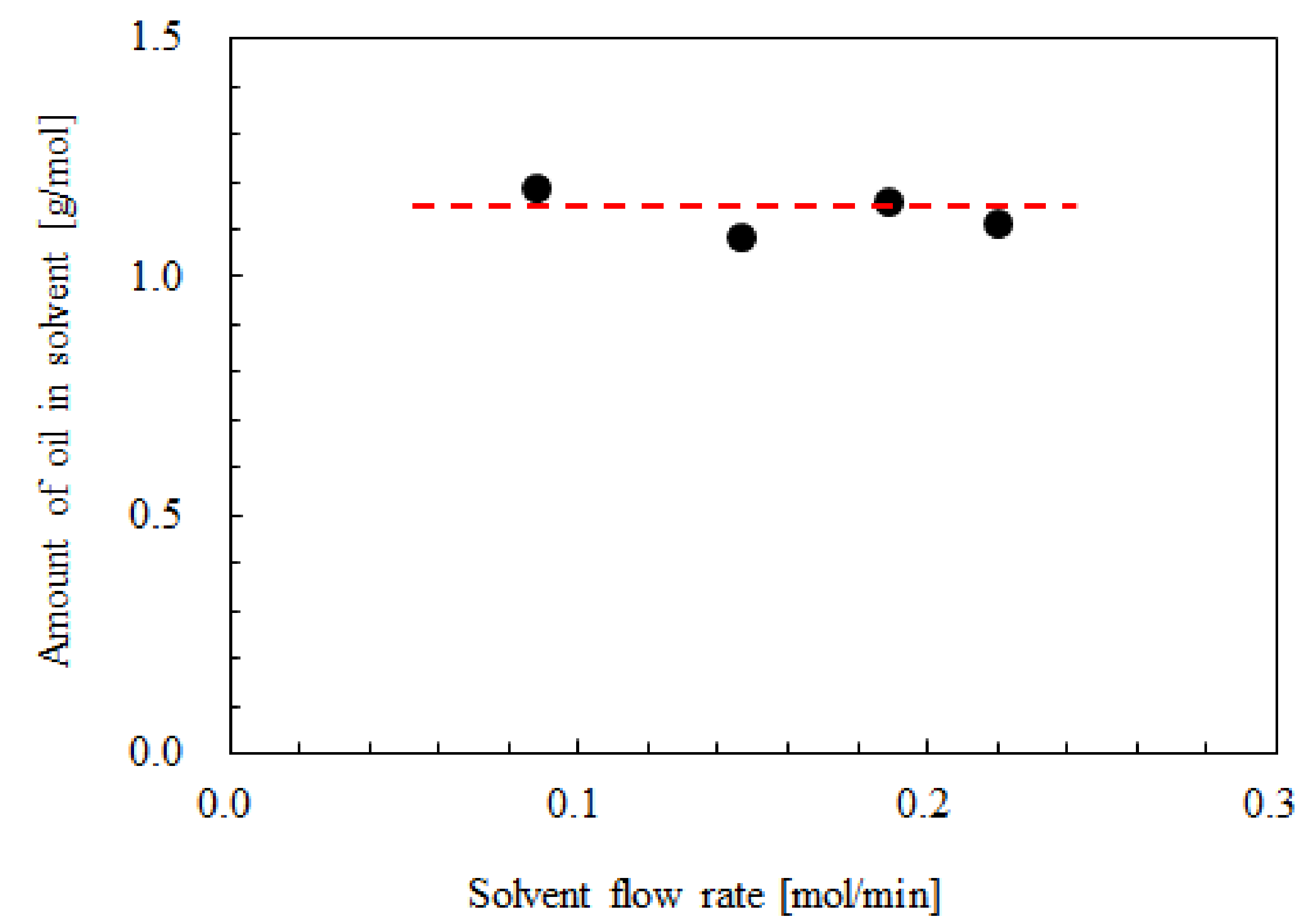
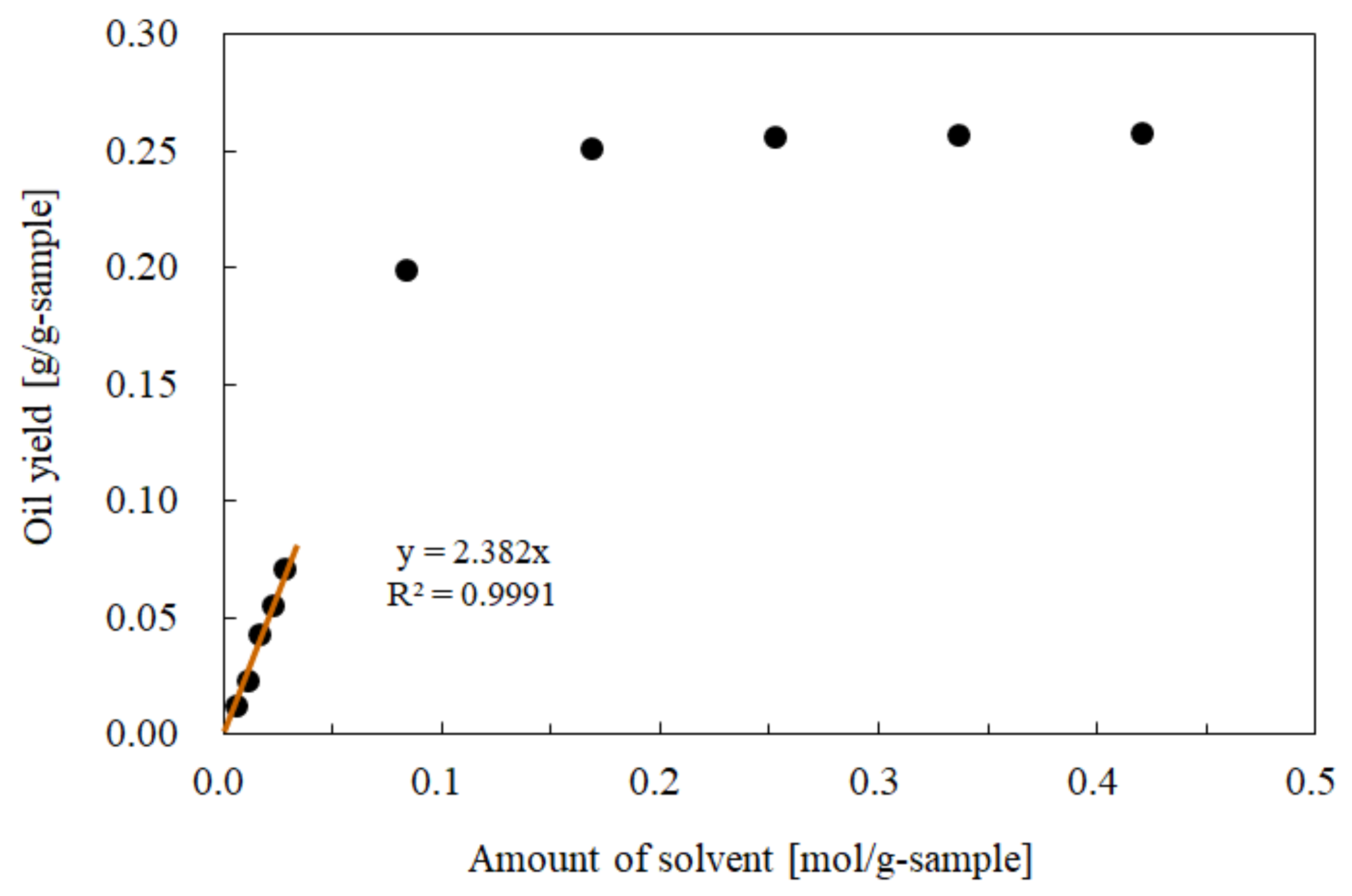
3.2. Influence of the CO2 Mole Fraction on the Oil Solubility
3.3. Influence of the CO2 Mole Fraction on the Phosphorus and Free Fatty Acid Concentrations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture, Forestry and Fisheries, Overseas Food Supply and Demand Report. Available online: https://www.maff-go.jp/j/zyukyu/jki/j-rep/monthly/attach/pdf/r2index-35.pdf (accessed on 20 November 2021).
- Pandey, R.; Shrivastava, S.L. Comparative evaluation of rice bran oil obtained with two-step microwave assisted extraction and conventional solvent extraction. J. Food Eng. 2018, 218, 106–114. [Google Scholar] [CrossRef]
- Japan Oilseed Processors Association, Basic Knowledge about Vegetable Oils. Available online: https://www.oil.or.jp/en/ (accessed on 20 November 2021).
- Danielski, L.; Zetzl, C.; Hense, H.; Brunner, G. A process line for the production of raffinated rice oil from rice bran. J. Supercrit. Fluids. 2005, 34, 133–141. [Google Scholar] [CrossRef]
- Wang, C.H.; Chen, C.R.; Wu, J.J.; Wang, L.Y.; Chang, C.M.; Ho, W.J. Designing supercritical carbon dioxide extraction of rice bran oil that contain oryzanols using response surface methodology. J. Sep. Sci. 2008, 31, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Machmudah, S.; Wahyudiono, N.; Fukuzato, R.; Kanda, H.; Quitain, A.T.; Sasaki, M.; Goto, M. Extraction of rice bran oil by supercritical carbon dioxide and solubility consideration. Sep. Purif. Technol. 2014, 125, 319–325. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M.; Siroha, A.K.; Purewal, S.S. Rice bran oil: Emerging trends in extraction, health benefit, and its industrial application. Rice Sci. 2021, 28, 217–232. [Google Scholar] [CrossRef]
- Go, A.W.; Pham, T.Y.N.; Truong, C.T.; Quijote, K.L.; Angkawijaya, A.E.; Agapay, R.C.; Gunarto, C.; Ju, Y.-H.; Santoso, S.P. Improved solvent economy and rate of rice bran lipid extraction using hydrolyzed rice bran with hexane as solvent. Biomass Bioenerg. 2020, 142, 105773. [Google Scholar] [CrossRef]
- Jessop, P.G.; Subramaniam, B. Gas-expanded liquids. Chem. Rev. 2007, 107, 2666–2694. [Google Scholar] [CrossRef]
- Wyatt, V.T.; Bush, D.; Lu, J.; Hallett, J.P.; Liotta, C.L.; Eckert, C.A. Determination of solvatochromic solvent parameters for the characterization of gas-expanded liquids. J. Supercrit. Fluids. 2005, 36, 16–22. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Mendiola, J.A.; Rezaei, K.; Ibáñez, E. Expanded ethanol with CO2 and pressurized ethyl lactate to obtain fractions enriched in γ-linolenic acid from Arthrospira platensis (Spirulina). J. Supercrit. Fluids. 2012, 62, 109–115. [Google Scholar] [CrossRef]
- Li, P.-L.; Li, H.-N.; Jing, K.-J.; David, A.; Lin, J.-R.; Deng, G. Evaluation of lipid extraction from microalgae based on different phase regions of CO2-expanded ethanol. Chem. Eng. Process. 2019, 138, 1–6. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Mendiola, J.A.; Quirantes-Piné, R.; Ibáñez, E.; Segura-Carretero, A. Green downstream processing using supercritical carbon dioxide, CO2-expanded ethanol and pressurized hot water extractions for recovering bioactive compounds from Moringa oleifera leaves. J. Supercrit. Fluids. 2016, 116, 90–100. [Google Scholar] [CrossRef]
- Otsu, M.; Suzuki, Y.; Koesoema, A.A.; Hoang, H.N.; Tamura, M.; Matsuda, T. CO2-expanded liquids as solvents to enhance activity of Pseudozyma antarctica lipase B towards ortho-substituted 1-phenylethanols. Tetrahedron Lett. 2020, 61, 152424. [Google Scholar] [CrossRef]
- Ming, J.; Wu, C.; Cheng, H.; Yu, Y.; Zhao, F. Reaction of hydrous inorganic metal salts in CO2 expanded ethanol: Fabrication of nanostructured materials via supercritical technology. J. Supercrit. Fluids. 2011, 57, 137–142. [Google Scholar] [CrossRef]
- Pu, D.W.; Devitt, M.P.; Thickett, S.C.; Lucien, F.P.; Zetterlund, P.B. Dispersion polymerization of styrene in CO2-expanded ethanol. Polymer 2013, 54, 6689–6694. [Google Scholar] [CrossRef]
- Li, H.; Fu, X.; Deng, G.; David, A.; Huang, L. Extraction of oil from grape seeds (Vitis vinifera L.) using recyclable CO2-expanded ethanol. Chem. Eng. Process. 2020, 157, 108147. [Google Scholar] [CrossRef]
- Chhouk, K.; Uemori, C.; Wahyudiono, H.K.; Kanda, H.; Goto, M. Extraction of phenolic compounds and antioxidant activity from garlic husk using carbon dioxide expanded ethanol. Chem. Eng. Process. 2017, 117, 113–119. [Google Scholar] [CrossRef]
- Duereh, A.; Inomata, H. Prediction of solvatochromic parameters of electronic transition energy for characterizing dipolarity/polarizability and hydrogen bonding donor interactions in binary solvent systems of liquid nonpolar-polar mixtures, CO2-expanded liquids and supercritical carbon dioxide with cosolvent. J. Mol. Liq. 2020, 320, 114394. [Google Scholar]
- Ye, K.; Freund, H.; Xie, Z.; Subramaniam, B.; Sundmacher, K. Prediction of multicomponent phase behavior of CO2-expanded liquids using CEoS/GE models and comparison with experimental data. J. Supercrit. Fluid. 2012, 67, 41–52. [Google Scholar] [CrossRef]
- Okajima, I.; Ly, L.T.T.; Kong, C.Y.; Sako, T. Phosphorus-free oil extraction from rice bran using CO2-expanded hexane. Chem. Eng. Process. 2021, 166, 108502. [Google Scholar] [CrossRef]
- Knapp, H.; Döring, R.; Oellrich, L.; Plöcker, U.; Prausnitz, J.M. Vapor–Liquid Equilibria for Mixtures of Low Boiling Substances; Dechema: Frankfurt, Germany, 1982; pp. 625–626. [Google Scholar]
- Wang, H.C.; Klinthong, W.; Yang, Y.H.; Tan, C.S. Continuous extraction of lipids from Schizochytrium sp. by CO2-expanded ethanol. Bioresour. Technol. 2015, 189, 162–168. [Google Scholar] [CrossRef]
- Yang, Y.H.; Klinthong, W.; Tan, C.S. Optimization of continuous lipid extraction from Chlorella vulgaris by CO2-expanded methanol for biodiesel production. Bioresour. Technol. 2015, 198, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Jessop, M.J.; Stubbins, S.H.; Champagne, P.; Jessop, P.G. Extraction of lipids from microalgae using CO2-expanded methanol and liquid CO2. Bioresour. Technol. 2015, 184, 286–290. [Google Scholar] [CrossRef] [PubMed]
- DIN EN 14214-European Standards. Available online: https://www.en-standard.eu/din-en-14214-liquid-petroleum-products-fatty-acid-methyl-esters-fame-for-use-in-diesel-engines-and-heating-applications-requirements-and-test-methods-includes-amendment-2019/ (accessed on 17 February 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okajima, I.; Ito, K.; Aoki, Y.; Kong, C.Y.; Sako, T. Extraction of Rice Bran Oil Using CO2-Expanded Hexane in the Two-Phase Region. Energies 2022, 15, 2594. https://doi.org/10.3390/en15072594
Okajima I, Ito K, Aoki Y, Kong CY, Sako T. Extraction of Rice Bran Oil Using CO2-Expanded Hexane in the Two-Phase Region. Energies. 2022; 15(7):2594. https://doi.org/10.3390/en15072594
Chicago/Turabian StyleOkajima, Idzumi, Kaichi Ito, Yusuke Aoki, Chang Yi Kong, and Takeshi Sako. 2022. "Extraction of Rice Bran Oil Using CO2-Expanded Hexane in the Two-Phase Region" Energies 15, no. 7: 2594. https://doi.org/10.3390/en15072594
APA StyleOkajima, I., Ito, K., Aoki, Y., Kong, C. Y., & Sako, T. (2022). Extraction of Rice Bran Oil Using CO2-Expanded Hexane in the Two-Phase Region. Energies, 15(7), 2594. https://doi.org/10.3390/en15072594