Pitfalls of Using Biomarker Maturity Parameters for Organic Matter Maturity Assessment Suggested by Coal Hydrous Pyrolysis
Abstract
:1. Introduction
2. Samples and Analytical Methods
2.1. Petrographic Analysis
2.2. Hydrous Pyrolysis
2.3. Bulk Geochemical Composition Analysis
3. Results
3.1. Original Bitumen Characterization
3.2. Variations in Bulk Compositions and Biomarker Distributions with Heating Temperature
4. Discussion
4.1. Hopane and Sterane Distribution in the OB–275 °C tSOM (0.49 %Ro–0.73 %Rm) Stage
4.2. Hopane and Sterane Distribution in the 275–340 °C (0.73–1.43 %Rm) Stage
5. Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grantham, P.J. Sterane isomerisation and moretane/hopane ratios in crude oils derived from Tertiary source rocks. Org. Geochem. 1986, 9, 293–304. [Google Scholar] [CrossRef]
- Mackenzie, A.S. Applications of Biological makes in petroleum geochemistry. In Advances in Petroleum Geochemistry; Brooks, J., Welte, D., Eds.; Academic Press: New York, NY, USA, 1984; Volume 1, pp. 115–2104. [Google Scholar]
- Seifert, W.K.; Moldowan, J.M. Applications of steranes, terpanes and monoaromatics to the maturation, migration and source of crude oils. Geochim. Cosmochim. Acta 1978, 42, 77–95. [Google Scholar] [CrossRef]
- Seifert, W.K.; Moldowan, J.M. The effect of thermal stress on source-rock quality as measured by hopane stereochemistry. Phys. Chem. Earth 1980, 12, 229–237. [Google Scholar] [CrossRef]
- van Graas, G.W. Biomarker maturity parameters for high maturities: Calibration of the working range up to the oil/condensate threshold. Org. Geochem. 1990, 16, 1025–1032. [Google Scholar] [CrossRef]
- Waples, D.W.; Machihara, T. Biomarkers as maturity indicators. In Biomarkers for Geologists: A Practical Guide to the Application of Steranes and Triterpanes in Petroleum Exploration; AAPG Methods in Exploration Series, 9; The American Association of Petroleum Geologists: Tulsa, OK, USA, 1991; pp. 19–40. [Google Scholar]
- Huang, H.; di Primio, R.; Pedersen, J.H.; Silva, R.; Algeer, R.; Ma, J.; Larter, S. On the determination of oil charge history and the practical application of molecular maturity markers. Mar. Pet. Geol. 2022, 139, 105586. [Google Scholar] [CrossRef]
- Dahl, J.; Moldowan, J.M.; Suandararaman, P. Relationship of biomarker distribution to depositional environment: Phosphoria Formation, Montana, USA. Org. Geochem. 1993, 20, 1001–1017. [Google Scholar] [CrossRef]
- Rullkötter, J.; Marzi, R. Natural and artificial maturation of biological markers in a Toarcian shale from northern Germany. Org. Geochem. 1988, 13, 639–645. [Google Scholar] [CrossRef]
- Bechtel, A.; Oberauer, K.; Kostić, A.; Gratzer, R.; Milisavljević, V.; Aleksić, N.; Stojanović, K.; Groß, D.; Sachsenhofer, R.F. Depositional environment and hydrocarbon source potential of the Lower Miocene oil shale deposit in the Aleksinac Basin (Serbia). Org. Geochem. 2018, 115, 93–112. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Wang, C.; Duan, Y.; Li, Y.; Hu, B. The organic geochemistry of the Eocene–Oligocene black shales from the Lunpola Basin, central Tibet. J. Asian Earth Sci. 2014, 79, 468–476. [Google Scholar] [CrossRef]
- Yin, M.; Huang, H.; Cheng, L. Molecular fingerprints in shales from the Sanhu biogenic gas fields in eastern Qaidam Basin, NW China: Evidence of biodegradation of shale organic matter. Mar. Pet. Geol. 2021, 133, 105289. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, T.; Sun, Y.; Milliken, K.L.; Sun, D. Geochemical evidence of organic matter source input and depositional environments in the lower and upper Eagle Ford Formation, south Texas. Org. Geochem. 2016, 98, 66–81. [Google Scholar] [CrossRef]
- Hackley, P.C.; Ryder, R.T.; Trippi, M.H.; Alimi, H. Thermal maturity of northern Appalachian Basin Devonian shales: Insights from sterane and terpane biomarkers. Fuel 2013, 106, 455–462. [Google Scholar] [CrossRef]
- Stojanović, K.; Šajnović, A.; Sabo, T.J.; Golovko, A.; Jovančićević, B. Pyrolysis and catalyzed pyrolysis in the investigation of a neogene shale potential from Valjevo-Mionica Basin, Serbia. Energy Fuels 2010, 24, 4357–4368. [Google Scholar] [CrossRef]
- Vuković, N.; Životić, D.; Mendonça Filho, J.G.; Kravić-Stevović, T.; Hámor-Vidó, M.; de Oliveira Mendonça, J.; Stojanović, K. The assessment of maturation changes of humic coal organic matter—Insights from closed-system pyrolysis experiments. Int. J. Coal Geol. 2016, 154–155, 213–239. [Google Scholar] [CrossRef]
- Mackenzie, A.S.; McKenzie, D. Isomerization and aromatization of hydrocarbons in sedimentary basins formed by extension. Geol. Mag. 1983, 120, 417–470. [Google Scholar] [CrossRef]
- Mackenzie, A.S.; Patience, R.L.; Maxwell, J.R.; Vandenbroucke, M.; Durand, B. Molecular parameters of maturation in the Toarcian shales, Paris Basin, France—I. Changes in the configurations of acyclic isoprenoid alkanes, steranes and triterpanes. Geochim. Cosmochim. Acta 1980, 44, 1709–1721. [Google Scholar] [CrossRef]
- Dzou, L.I.P.; Noble, R.A.; Senftle, J.T. Maturation effects on absolute biomarker concentration in a suite of coals and associated vitrinite concentrates. Org. Geochem. 1995, 23, 681–697. [Google Scholar] [CrossRef]
- Raymond, A.C.; Murchison, D.G. Effect of igneous activity on molecular-maturation indices in different types of organic matter. Org. Geochem. 1992, 18, 725–735. [Google Scholar] [CrossRef]
- Abbott, G.D.; Wang, G.Y.; Eglinton, T.I.; Home, A.K.; Petch, G.S. The kinetics of sterane biological marker release and degradation processes during the hydrous pyrolysis of vitrinite kerogen. Geochim. Cosmochim. Acta 1990, 54, 2451–2461. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Sinninghe Damsté, J.S.; Koopmans, M.P.; van de Graaf, B.; De Leeuw, J.W. A kinetic calculation method of homohopanoid maturation: Applications in the reconstruction of burial histories of sedimentary basins. Geochim. Cosmochim. Acta 1997, 61, 2409–2429. [Google Scholar] [CrossRef]
- Murray, I.P.; Love, G.D.; Snape, C.E.; Bailey, N.J.L. Comparison of covalently-bound aliphatic biomarkers released via hydropyrolysis with their solvent-extractable counterparts for a suite of Kimmeridge clays. Org. Geochem. 1998, 29, 1487–1505. [Google Scholar] [CrossRef]
- Peters, K.E.; Moldowan, J.M.; Sundararaman, P. Effects of hydrous pyrolysis on biomarker thermal maturity parameters: Monterey Phosphatic and Siliceous members. Org. Geochem. 1990, 15, 249–265. [Google Scholar] [CrossRef]
- Li, M.; Wang, P.; Johns, R.B. Changes to unbound biomarkers in low-rank coals during simulated coalification. Energy Fuels 1991, 5, 885–895. [Google Scholar] [CrossRef]
- Eglinton, T.I.; Douglas, A.G. Quantitative study of biomarker hydrocarbons released from kerogens during hydrous pyrolysis. Energy Fuels 1988, 2, 81–88. [Google Scholar] [CrossRef]
- Farrimond, P.; Bevan, J.C.; Bishop, A.N. Hopanoid hydrocarbon maturation by an igneous intrusion. Org. Geochem. 1996, 25, 149–164. [Google Scholar] [CrossRef]
- Chen, Z.; Simoneit, B.R.; Wang, T.G.; Yang, Y.; Ni, Z.; Cheng, B.; Luo, B.; Yang, C.; Chen, T. Biomarker signatures of Sinian bitumens in the Moxi–Gaoshiti Bulge of Sichuan Basin, China: Geological significance for paleo-oil reservoirs. Precambrian Res. 2017, 296, 1–19. [Google Scholar] [CrossRef]
- Huang, H.; Li, J. Biomarker signatures of Sinian bitumens in the Moxi–Gaoshiti bulge of Sichuan Basin, China: Geological significance for paleo-oil reservoirs: Discussion. Precambrian Res. 2018, 314, 487–491. [Google Scholar] [CrossRef]
- Ritter, U.; Aareskjold, K.; Schou, L. Distributed activation energy models of isomerisation reactions from hydrous pyrolysis. Org. Geochem. 1993, 20, 511–520. [Google Scholar] [CrossRef]
- Landais, P.; Michels, R.; Elie, M. Are time and temperature the only constraints to the simulation of organic matter maturation? Org. Geochem. 1994, 22, 617–630. [Google Scholar] [CrossRef]
- Tannenbaum, E.; Ruth, E.; Kaplan, I.R. Steranes and triterpanes generated from kerogen pyrolysis in the absence and presence of minerals. Geochim. Cosmochim. Acta 1986, 50, 805–812. [Google Scholar] [CrossRef]
- Bishop, A.N.; Love, G.D.; McAulay, A.D.; Snape, C.E.; Farrimond, P. Release of kerogen-bound hopanoids by hydropyrolysis. Org. Geochem. 1998, 29, 989–1001. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.; Zhang, S. The effect of biodegradation on bound biomarkers released from intermediate-temperature gold-tube pyrolysis of severely biodegraded Athabasca bitumen. Fuel 2020, 263, 116669. [Google Scholar] [CrossRef]
- Lockhart, R.S.; Meredith, W.; Love, G.D.; Snape, C.E. Release of bound aliphatic biomarkers via hydropyrolysis from Type II kerogen at high maturity. Org. Geochem. 2008, 39, 1119–1124. [Google Scholar] [CrossRef]
- Love, G.D.; McAulay, A.; Snape, C.E.; Bishop, A.N. Effect of process variables in catalytic hydropyrolysis on the release of covalently bound aliphatic hydrocarbons from sedimentary organic matter. Energy Fuels 1997, 11, 522–531. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, T.; Wang, Z.; Tuo, J.; Zhang, M.; Wu, C.; Tian, C. Organic geochemical characteristics and generating potential of source rocks from the Lower-Middle Jurassic coal-bearing strata in the East Junggar Basin, NW China. Mar. Pet. Geol. 2018, 93, 113–126. [Google Scholar] [CrossRef]
- Zeng, Q.; Pu, Y.; Cao, Z. Kinetics of oxidation and spontaneous combustion of major super-thick coal seam in Eastern Junggar Coalfield, Xinjiang, China. J. Loss Prev. Process Ind. 2018, 56, 128–136. [Google Scholar] [CrossRef]
- Luo, Q.; Zhong, N.; Dai, N.; Zhang, W. Graptolite-derived organic matter in the Wufeng-Longmaxi Formations (Upper Ordovician-Lower Silurian) of southeastern Chongqing, China: Implications for gas shale evaluation. Int. J. Coal Geol. 2016, 153, 87–98. [Google Scholar] [CrossRef]
- Luo, Q.; Qu, Y.; Chen, Q.; Xiong, Z. Organic geochemistry and petrology of mudrocks from the upper Carboniferous Batamayineishan Formation, Wulungu Area, Junggar Basin, China: Implications for petroleum exploration. Energy Fuels 2017, 31, 10628–10638. [Google Scholar] [CrossRef]
- Barker, C.E.; Lewan, M.D.; Pawlewicz, M.J. The influence of extractable organic matter on vitrinite reflectance suppression: A survey of kerogen and coal types. Int. J. Coal Geol. 2007, 70, 67–78. [Google Scholar] [CrossRef]
- Guan, D.; Xu, X.; Li, Z.; Zheng, L.; Tan, C.; Yao, Y. Theory and practice of hydrocarbon generation within space-limited source rocks. In Springer Geology; Springer: Singapore, 2017; pp. 1–189. [Google Scholar]
- Lewan, M.D.; Winters, J.C.; McDonald, J.H. Generation of oil-like pyrolyzates from organic-rich shales. Science 1979, 203, 897–899. [Google Scholar] [CrossRef]
- Eseme, E.; Littke, R.; Krooss, B.M.; Schwarzbauer, J. Experimental investigation of the compositional variation of petroleum during primary migration. Org. Geochem. 2007, 38, 1373–1397. [Google Scholar] [CrossRef]
- Fan, Z.; Philp, R.P. Laboratory biomarker fractionations and implications for migration studies. Org. Geochem. 1987, 11, 169–175. [Google Scholar]
- Jiang, Z.; Philp, R.P.; Lewis, C.A. Fractionation of biological markers in crude oils during migration and the effects on correlation and maturation parameters. Org. Geochem. 1988, 13, 561–571. [Google Scholar]
- Shi, Q.; Hou, D.; Chung, K.H.; Xu, C.; Zhao, S.; Zhang, Y. Characterization of heteroatom compounds in a crude oil and its saturates, aromatics, resins, and asphaltenes (SARA) and non-basic nitrogen fractions analyzed by negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2010, 24, 2545–2553. [Google Scholar]
- Shao, X.; Pang, X.; Li, M.; Li, Z.; Zhao, Y. Hydeocarbon generation from lacustrine shales with retained oil during thermal maturation. Pet. Sci. 2020, 17, 1478–1490. [Google Scholar] [CrossRef]
- Spigolon, A.L.D.; Lewan, M.D.; de Barros Penteado, H.L.; Coutinho, L.F.C.; Mendonça Filho, J.G. Evaluation of the petroleum composition and quality with increasing thermal maturity as simulated by hydrous pyrolysis: A case study using a Brazilian source rock with Type I kerogen. Org. Geochem. 2015, 83–84, 27–53. [Google Scholar] [CrossRef]
- Garcia, R.; Moinelo, S.R.; Lafferty, C.J.; Snape, C.E. Pyrolytic desulfurization of some high-sulfur coals. Energy Fuels 1991, 5, 582–586. [Google Scholar] [CrossRef]
- Vu, T.T.A.; Zink, K.G.G.; Mangelsdorf, K.; Sykes, R.; Wilkes, H.; Horsfield, B. Changes in bulk properties and molecular compositions within New Zealand Coal Band solvent extracts from early diagenetic to catagenetic maturity levels. Org. Geochem. 2009, 40, 963–977. [Google Scholar] [CrossRef]
- Elie, M.; Mazurek, M. Biomarker transformations as constraints for the depositional environment and for maximum temperatures during burial of Opalinus Clay and Posidonia Shale in northern Switzerland. Appl. Geochem. 2008, 23, 3337–33354. [Google Scholar] [CrossRef]
- Jenisch, A.; Richnow, H.H.; Michaelis, W. Chemical structural units of macromolecular coal components. Org. Geochem. 1990, 16, 917–929. [Google Scholar] [CrossRef]
- Koopmans, M.P.; De Leeuw, J.W.; Lewan, M.D.; Damsté, J.S.S. Impact of dia- and catagenesis on sulphur and oxygen sequestration of biomarkers as revealed by artificial maturation of an immature sedimentary rock. Org. Geochem. 1996, 25, 391–426. [Google Scholar] [CrossRef]
- Farrimond, P.; Love, G.D.; Bishop, A.N.; Innes, H.E.; Watson, D.F.; Snape, C.E. Evidence for the rapid incorporation of hopanoids into kerogen. Geochim. Cosmochim. Acta 2003, 67, 1383–1394. [Google Scholar] [CrossRef]
- Lee, C.; Love, G.D.; Jahnke, L.L.; Kubo, M.D.; Des Marais, D.J. Early diagenetic sequestration of microbial mat lipid biomarkers through covalent binding into insoluble macromolecular organic matter (IMOM) as revealed by sequential chemolysis and catalytic hydropyrolysis. Org. Geochem. 2019, 132, 11–22. [Google Scholar] [CrossRef]
- Bowden, S.A.; Farrimond, P.; Snape, C.E.; Love, G.D. Compositional differences in biomarker constituents of the hydrocarbon, resin, asphaltene and kerogen fractions: An example from the Jet Rock (Yorkshire, UK). Org. Geochem. 2006, 37, 369–383. [Google Scholar] [CrossRef]
- Sinninghe Damsté, J.S.; Van Duin, A.C.T.; Hollander, D.; Kohnen, M.E.L.; De Leeuw, J.W. Early diagenesis of bacteriohopanepolyol derivatives: Formation of fossil homohopanoids. Geochim. Cosmochim. Acta 1995, 59, 5141–5157. [Google Scholar] [CrossRef]
- Farrimond, P.; Taylor, A.; TelnÆs, N. Biomarker maturity parameters: The role of generation and thermal degradation. Org. Geochem. 1998, 29, 1181–1197. [Google Scholar] [CrossRef]
- Norgate, C.M.; Boreham, C.J.; Wilkins, A.J. Changes in hydrocarbon maturity indices with coal rank and type, Buller Coalfield, New Zealand. Org. Geochem. 1999, 30, 985–1010. [Google Scholar] [CrossRef]
- Farrimond, P.; Bevan, J.C.; Bishop, A.N. Tricyclic terpane maturity parameters: Response to heating by an igneous intrusion. Org. Geochem. 1999, 30, 1011–1019. [Google Scholar] [CrossRef]
- Farrimond, P.; Telnæs, N. Three series of rearranged hopanes in Toarcian sediments (northern Italy). Org. Geochem. 1996, 25, 165–177. [Google Scholar] [CrossRef]
- Tannenbaum, E.; Aizenshtat, Z. Formation of immature asphalt from organic-rich carbonate rocks—II. Correlation of maturation indicators. Org. Geochem. 1984, 6, 503–511. [Google Scholar] [CrossRef]
- Ensminger, A.; van Dorsselaer, A.; Spyckerelle, C.; Albrecht, P.; Ourisson, G. Pentacyclic triterpenes of the hopane type as ubiquitous geochemical markers: Origin and significance. In Advances in Organic Geochemistry; Tissot, B., Bienner, F., Eds.; Editions Technip: Paris, France, 1973; pp. 245–260. [Google Scholar]
- Rosa-Putra, S.; Nalin, R.; Domenach, A.M.; Rohmer, M. Novel hopanoids from Frankia spp. and related soil bacteria. Eur. J. Biochem. 2001, 268, 4300–4306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.; Qiu, X.; Huang, X.; Xie, S. Occurrence of highly abundant bacterial hopanoids in Dajiuhu peatland, central China. Front. Earth Sci. China 2009, 3, 320–326. [Google Scholar] [CrossRef]
- Sinninghe Damsté, J.S.; Schouten, S.; Volkman, J.K. C27–C30 neohop-13(18)-enes and their saturated and aromatic derivatives in sediments: Indicators for diagenesis and water column stratification. Geochim. Cosmochim. Acta 2014, 133, 402–421. [Google Scholar] [CrossRef] [Green Version]
- ten Haven, H.L.; De Leeuw, J.W.; Peakman, T.M.; Maxwell, J.R. Anomalies in steroid and hopanoid maturity indices. Geochim. Cosmochim. Acta 1986, 50, 853–855. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Wu, Y.; Wang, Z.; Shen, Q. Features of liquid hydrocarbon and biomarker maturity ratios during HTHP semi-open system pyrolysis of type II and III source rocks. Pet. Sci. Technol. 2017, 35, 1063–1069. [Google Scholar] [CrossRef]
- Lu, S.T.; Ruth, E.; Kaplan, I.R. Pyrolysis of kerogens in the absence and presence of montmorillonite-I. The generation, degradation and isomerization of steranes and triterpanes at 200 and 300 °C. Org. Geochem. 1989, 14, 491–499. [Google Scholar] [CrossRef]
- Love, G.D.; Snape, C.E.; Carr, A.D.; Houghton, R.C. Release of covalently-bound alkane biomarkers in high yields from kerogen via catalytic hydropyrolysis. Org. Geochem. 1995, 23, 981–986. [Google Scholar] [CrossRef]
- Fang, R.; Littke, R.; Zieger, L.; Baniasad, A.; Li, M.; Schwarzbauer, J. Changes of composition and content of tricyclic terpane, hopane, sterane, and aromatic biomarkers throughout the oil window: A detailed study on maturity parameters of Lower Toarcian Posidonia Shale of the Hils Syncline, NW Germany. Org. Geochem. 2019, 138, 103928. [Google Scholar] [CrossRef]
- Koopmans, M.P.; Schaeffer-Reiss, C.; De Leeuw, J.W.; Lewan, M.D.; Maxwell, J.R.; Schaeffer, P.; Damsté, J.S.S. Sulphur and oxygen sequestration of n-C37 and n-C38 unsaturated ketones in an immature kerogen and the release of their carbon skeletons during early stages of thermal maturation. Geochim. Cosmochim. Acta 1997, 61, 2397–2408. [Google Scholar] [CrossRef]
- Seifert, W.K. Steranes and terpanes in kerogen pyrolysis for correlation of oils and source rocks. Geochim. Cosmochim. Acta 1978, 42, 473–484. [Google Scholar] [CrossRef]
- Alexander, R.; Kagi, R.I.; Noble, R.; Volkman, J.K. Identification of some bicyclic alkanes in petroleum. Org. Geochem. 1984, 6, 63–72. [Google Scholar] [CrossRef]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1984; p. 699. [Google Scholar]
- Moldowan, J.M.; Dahl, J.; Zinniker, D.; Barbanti, S.M. Underutilized advanced geochemical technologies for oil and gas exploration and production-1. The diamondoids. J. Pet. Sci. Eng. 2015, 126, 87–96. [Google Scholar] [CrossRef]
- van Graas, G.W. Biomarker distributions in asphaltenes and kerogens analysed by flash pyrolysis-gas chromatography-mass spectrometry. Org. Geochem. 1986, 10, 1127–1135. [Google Scholar] [CrossRef]
- Pan, C.; Geng, A.; Zhong, N.; Liu, J. Kerogen pyrolysis in the presence and absence of water and minerals: Steranes and triterpenoids. Fuel 2010, 89, 336–345. [Google Scholar] [CrossRef]
- Chen, Z.; Simoneit, B.R.; Wang, T.G.; Huang, W.; Yan, D.; Ni, Z.; Liu, K. Effects of high temperatures on biomarker ratios during oil-to-gas cracking experiments at two pressures. Org. Geochem. 2016, 101, 108–131. [Google Scholar] [CrossRef]
- Peters, K.E.; Moldowan, J.M. Effects of source, thermal maturity, and biodegradation on the distribution and isomerization of homohopanes in petroleum. Org. Geochem. 1991, 17, 47–61. [Google Scholar] [CrossRef]
- Strachan, M.G.; Alexander, R.; Subroto, E.A.; Kagi, R.I. Constraints upon the use of 24-ethylcholestane diastereomer ratios as indicators of the maturity of petroleum. Org. Geochem. 1989, 14, 423–432. [Google Scholar] [CrossRef]
- Bishop, A.N.; Abbott, G.D. The interrelationship of biological marker maturity parameters and molecular yields during contact metamorphism. Geochim. Cosmochim. Acta 1993, 57, 3661–3668. [Google Scholar] [CrossRef]
- Mißbach, H.; Duda, J.P.; Lünsdorf, N.K.; Schmidt, B.C.; Thiel, V. Testing the preservation of biomarkers during experimental maturation of an immature kerogen. Int. J. Astrobiol. 2016, 15, 165–175. [Google Scholar] [CrossRef]
- Requejo, A.G. Maturation of petroleum source rocks—II. Quantitative changes in extractable hydrocarbon content and composition associated with hydrocarbon generation. Org. Geochem. 1994, 21, 91–105. [Google Scholar] [CrossRef]
- Pan, C.; Peng, D.; Zhang, M.; Yu, L.; Sheng, G.; Fu, J. Distribution and isomerization of C31–C35 homohopanes and C29 steranes in oligocene saline lacustrine sediments from Qaidam Basin, Northwest China. Org. Geochem. 2008, 39, 646–657. [Google Scholar] [CrossRef]
- Köster, J.; Van Kaam-Peters, H.M.E.; Koopmans, M.P.; De Leeuw, J.W.; Sinninghe Damsté, J.S. Sulphurisation of homohopanoids: Effects on carbon number distribution, speciation, and 22S/22R epimer ratios. Geochim. Cosmochim. Acta 1997, 61, 2431–2452. [Google Scholar] [CrossRef]
- Peakman, T.M.; Maxwell, J.R. Early diagenetic pathways of steroid alkenes. Org. Geochem. 1988, 13, 583–592. [Google Scholar] [CrossRef]
- Han, Z.; Yang, Q.; Pang, Z. Artificial maturation study of a humic coal and a torbanite. Int. J. Coal Geol. 2001, 46, 133–143. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Zheng, J.; Li, X.; Wang, X.; Gao, Z.; Luo, H.; Li, Z.; Qian, Y. Hydrous pyrolysis of different kerogen types of source rock at high temperature-bulk results and biomarkers. J. Pet. Sci. Eng. 2015, 125, 209–217. [Google Scholar] [CrossRef]
- Strachan, M.G.; Alexander, R.; Van Bronswijk, W.; Kagi, R.I. Source and heating rate effects upon maturity parameters based on ratios of 24-ethylcholestane diastereomers. J. Geochem. Explor. 1989, 31, 285–294. [Google Scholar] [CrossRef]
- Lewan, M.D.; Bjorøy, M.; Dolcater, D.L. Effects of thermal maturation on steroid hydrocarbons as determined by hydrous pyrolysis of Phosphoria Retort Shale. Geochim. Cosmochim. Acta 1986, 50, 1977–1987. [Google Scholar] [CrossRef]

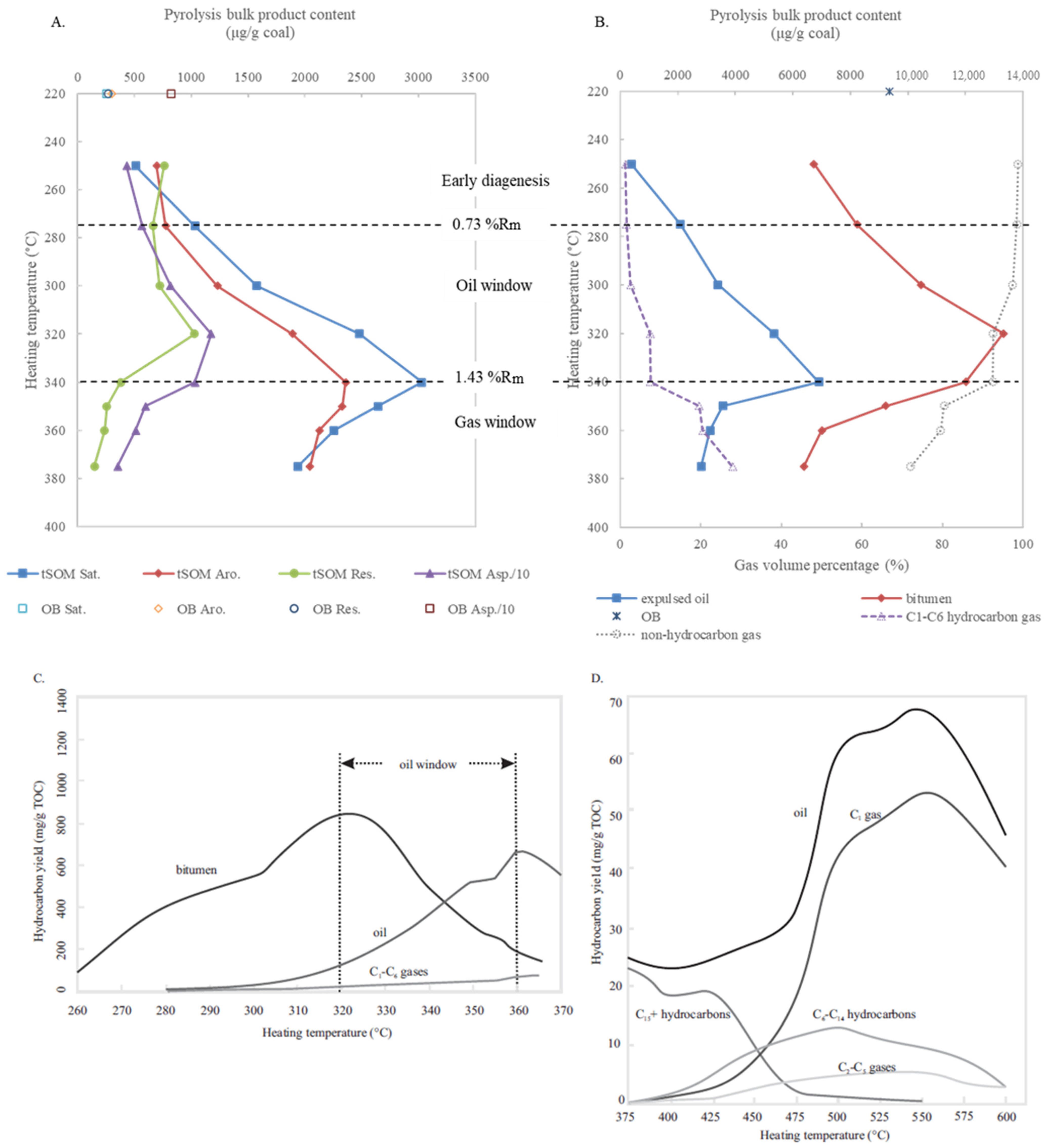

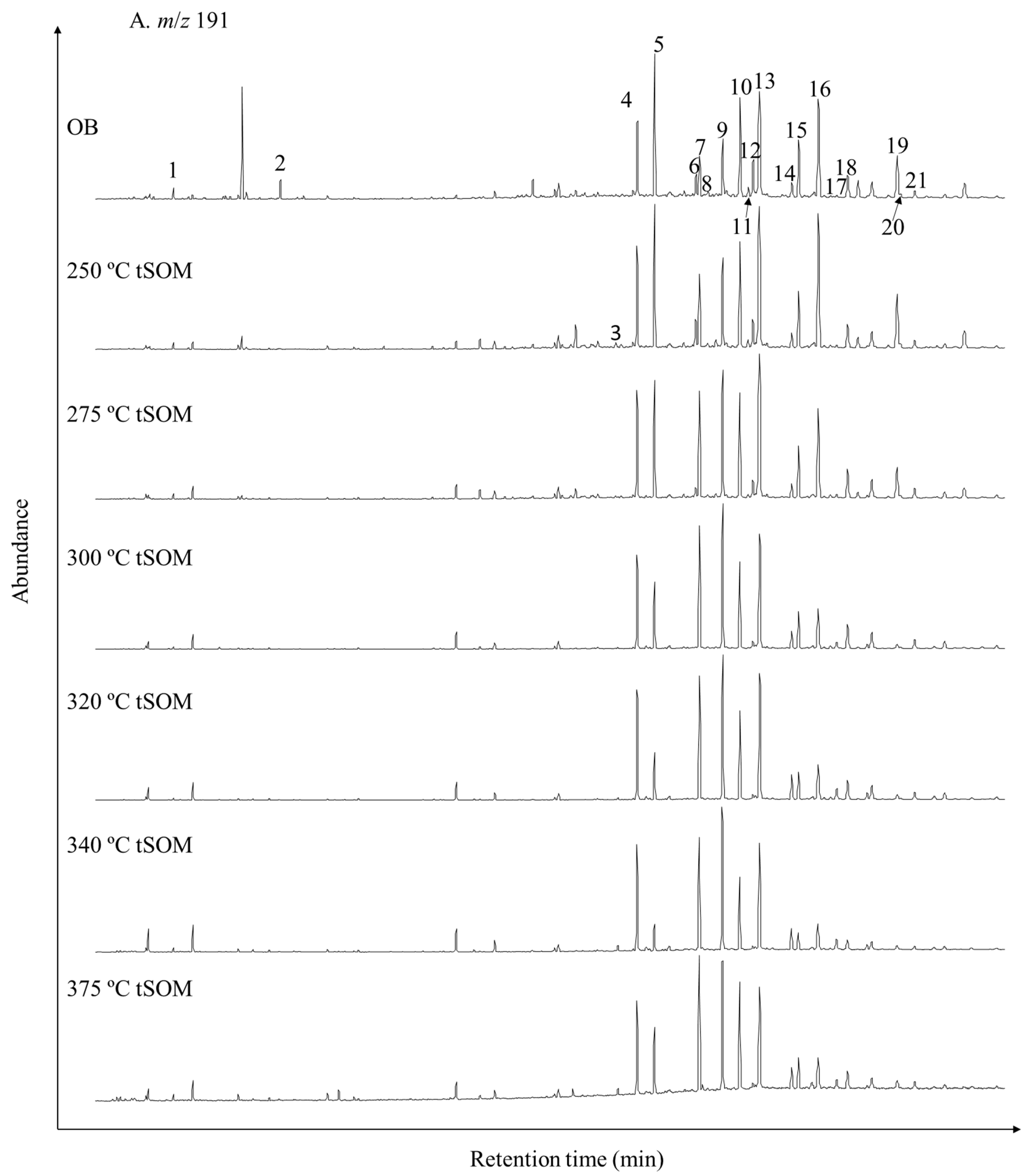
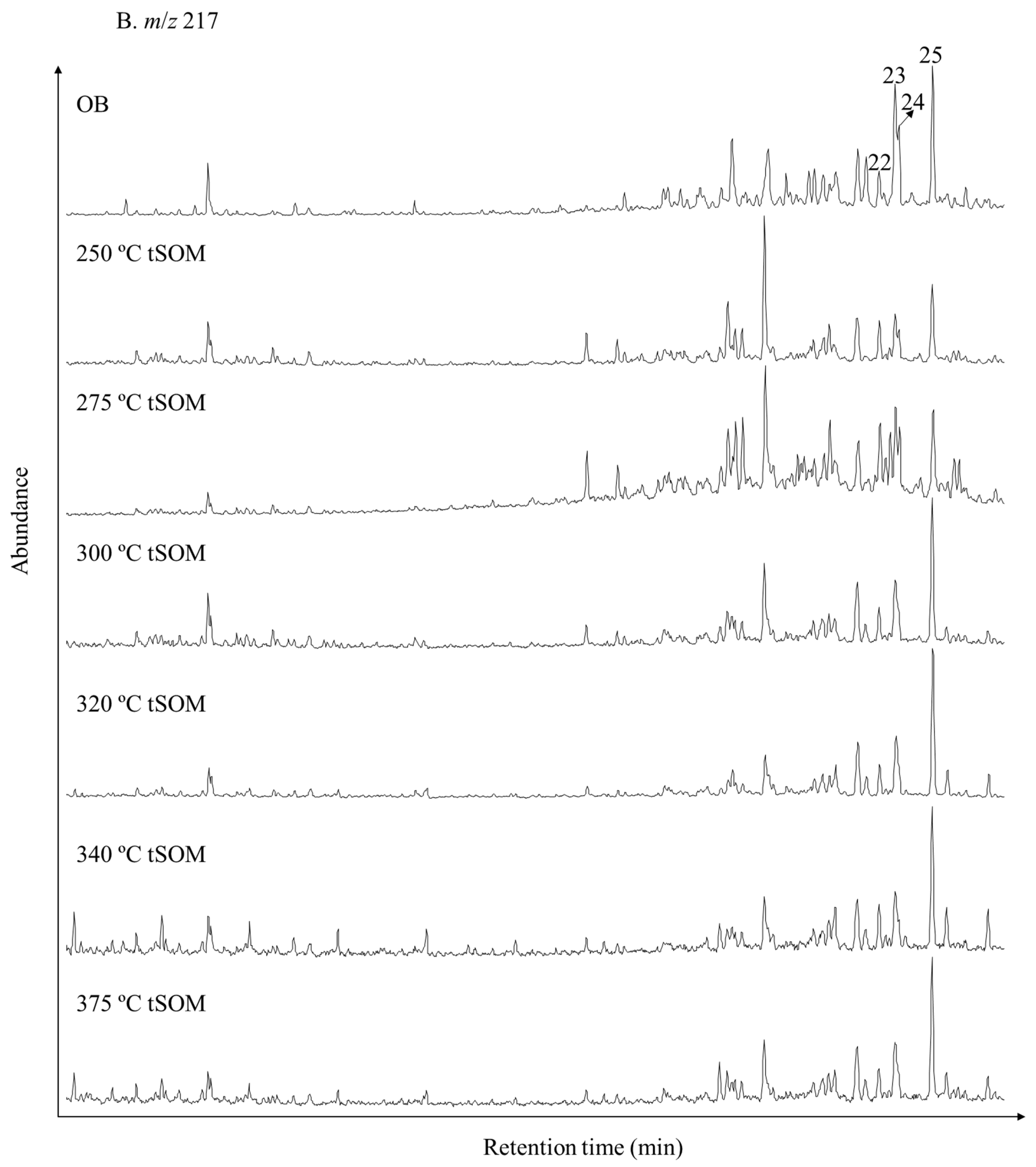
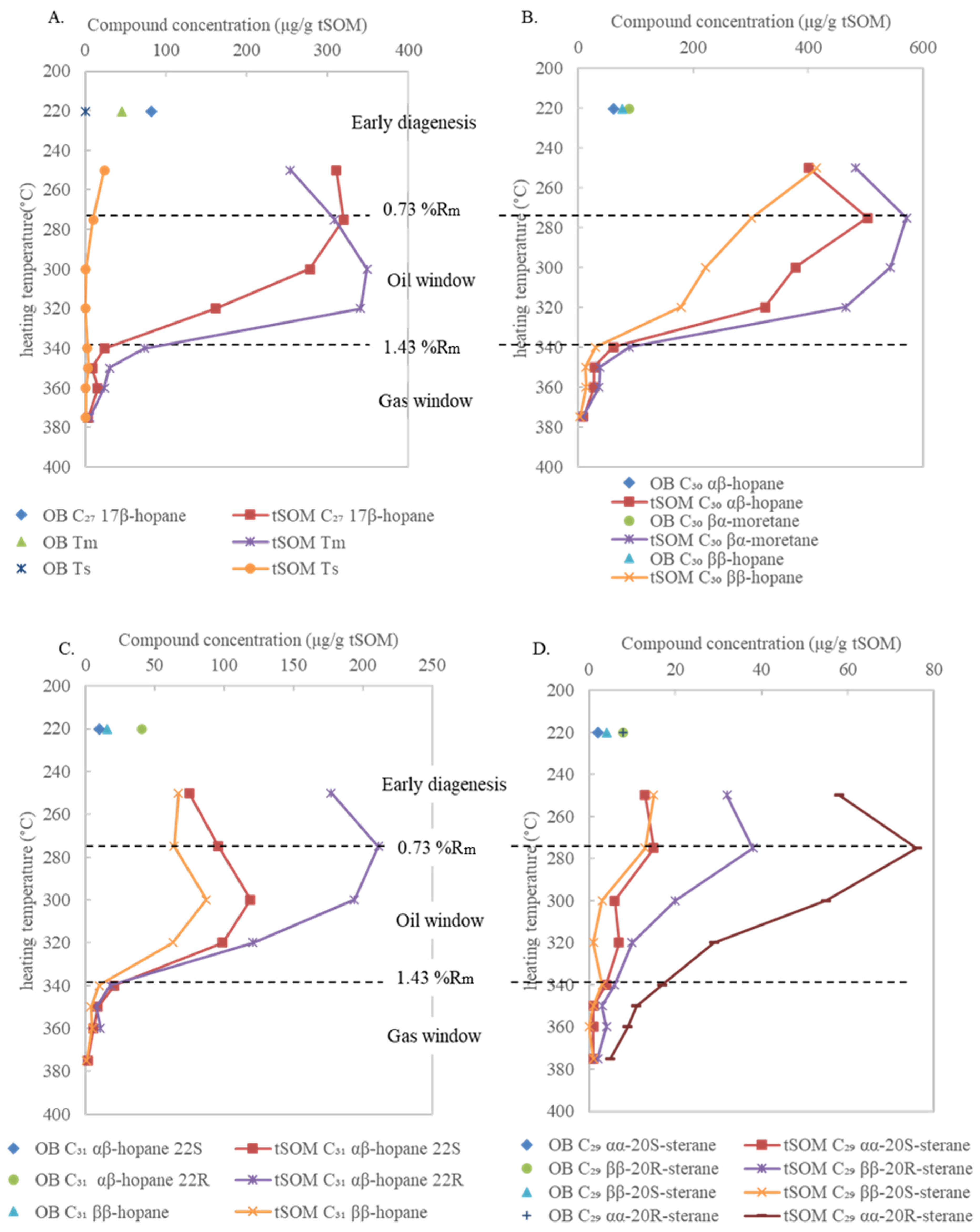

| Peak No. in Figure 4 | Sample Type | OB | tSOM | tSOM | tSOM | tSOM | tSOM | tSOM | tSOM | tSOM |
|---|---|---|---|---|---|---|---|---|---|---|
| heating target temperature (°C) | \ | 250 | 275 | 300 | 320 | 340 | 350 | 360 | 375 | |
| Ro (%) | 0.49 | 0.71 | 0.73 | 0.9 | 1.19 | 1.43 | 1.5 | 1.74 | 1.91 | |
| 3 | Ts | 0 | 24 | 10 | 1 | 0 | 3 | 4 | 1 | 1 |
| 4 | Tm | 46 | 254 | 309 | 349 | 341 | 73 | 30 | 24 | 6 |
| 5 | C27 17β-hopane | 82 | 311 | 320 | 278 | 161 | 24 | 9 | 16 | 4 |
| 6 | C29 neohop13(18)ene | 13 | 56 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | C29 αβ-hopane | 25 | 237 | 510 | 474 | 424 | 86 | 40 | 34 | 9 |
| 8 | C29Ts | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | C29 βα-moretane | 34 | 270 | 561 | 574 | 515 | 104 | 47 | 40 | 8 |
| 10 | C30 αβ-hopane | 61 | 401 | 503 | 378 | 325 | 61 | 28 | 27 | 9 |
| 11 | C30 neohop13(18)ene | 4 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | C29 ββ-hopane | 25 | 82 | 73 | 6 | 0 | 0 | 0 | 0 | 0 |
| 13 | C30 βα-moretane | 88 | 483 | 571 | 543 | 465 | 89 | 38 | 36 | 8 |
| 14 | C31 αβ-hopane 22S | 10 | 75 | 96 | 119 | 99 | 21 | 9 | 6 | 2 |
| 15 | C31 αβ-hopane 22R | 41 | 177 | 211 | 194 | 121 | 18 | 8 | 11 | 3 |
| 16 | C30 ββ-hopane | 76 | 415 | 302 | 222 | 179 | 30 | 13 | 14 | 3 |
| 19 | C31 ββ-hopane | 16 | 67 | 64 | 87 | 63 | 10 | 4 | 5 | 1 |
| 22 | C29 αα-20S-sterane | 2 | 13 | 15 | 6 | 7 | 4 | 1 | 1 | 1 |
| 23 | C29 ββ-20R-sterane | 8 | 32 | 38 | 20 | 10 | 6 | 3 | 4 | 2 |
| 24 | C29 ββ-20S-sterane | 4 | 15 | 13 | 3 | 1 | 3 | 1 | 0 | 1 |
| 25 | C29 αα-20R-sterane | 8 | 58 | 76 | 55 | 29 | 17 | 11 | 9 | 5 |
| C29 βα-moretane/C29 αβ-hopane | 1.36 | 1.14 | 1.10 | 1.21 | 1.21 | 1.21 | 1.18 | 1.18 | 0.89 | |
| C30 βα-moretane/C30 αβ-hopane | 1.44 | 1.20 | 1.14 | 1.44 | 1.43 | 1.46 | 1.36 | 1.33 | 0.89 | |
| Ts/(Ts + Tm) | 0 | 0.09 | 0.03 | 0 | 0 | 0.04 | 0.12 | 0.04 | 0.14 | |
| C29Ts/(C29Ts + C29 αβ-hopane) | 0.17 | 0.01 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 22S/(22S + 22R) | 0.20 | 0.30 | 0.31 | 0.38 | 0.45 | 0.54 | 0.53 | 0.35 | 0.40 | |
| 20S/(20R + 20S) | 0.27 | 0.24 | 0.20 | 0.11 | 0.17 | 0.23 | 0.13 | 0.07 | 0.22 | |
| ββ/(αα + ββ) | 0.55 | 0.40 | 0.36 | 0.27 | 0.23 | 0.30 | 0.25 | 0.29 | 0.33 | |
| n-alkanes | 1504 | 20,341 | 20,003 | 26,630 | 39,717 | 24,922 | 27,895 | 18,111 | 6085 | |
| Pr + Ph | 338 | 1855 | 2716 | 3365 | 2765 | 1030 | 704 | 721 | 149 | |
| alkyl-cyclohexanes | 77 | 1442 | 1580 | 729 | 1072 | 705 | 738 | 456 | 164 | |
| bicyclic sesquiterpanes | 9 | 77 | 105 | 319 | 422 | 83 | 150 | 33 | 3 | |
| tricyclic terpanes | 21 | 251 | 239 | 114 | 115 | 35 | 31 | 16 | 8 | |
| pentacyclic terpanes | 380 | 2562 | 3154 | 2500 | 2200 | 425 | 193 | 175 | 46 | |
| steranes | 42 | 313 | 343 | 134 | 80 | 14 | 9 | 12 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, M.; Huang, H. Pitfalls of Using Biomarker Maturity Parameters for Organic Matter Maturity Assessment Suggested by Coal Hydrous Pyrolysis. Energies 2022, 15, 2595. https://doi.org/10.3390/en15072595
Yin M, Huang H. Pitfalls of Using Biomarker Maturity Parameters for Organic Matter Maturity Assessment Suggested by Coal Hydrous Pyrolysis. Energies. 2022; 15(7):2595. https://doi.org/10.3390/en15072595
Chicago/Turabian StyleYin, Mengsha, and Haiping Huang. 2022. "Pitfalls of Using Biomarker Maturity Parameters for Organic Matter Maturity Assessment Suggested by Coal Hydrous Pyrolysis" Energies 15, no. 7: 2595. https://doi.org/10.3390/en15072595
APA StyleYin, M., & Huang, H. (2022). Pitfalls of Using Biomarker Maturity Parameters for Organic Matter Maturity Assessment Suggested by Coal Hydrous Pyrolysis. Energies, 15(7), 2595. https://doi.org/10.3390/en15072595






