CO2—A Crisis or Novel Functionalization Opportunity?
Abstract
:1. Introduction
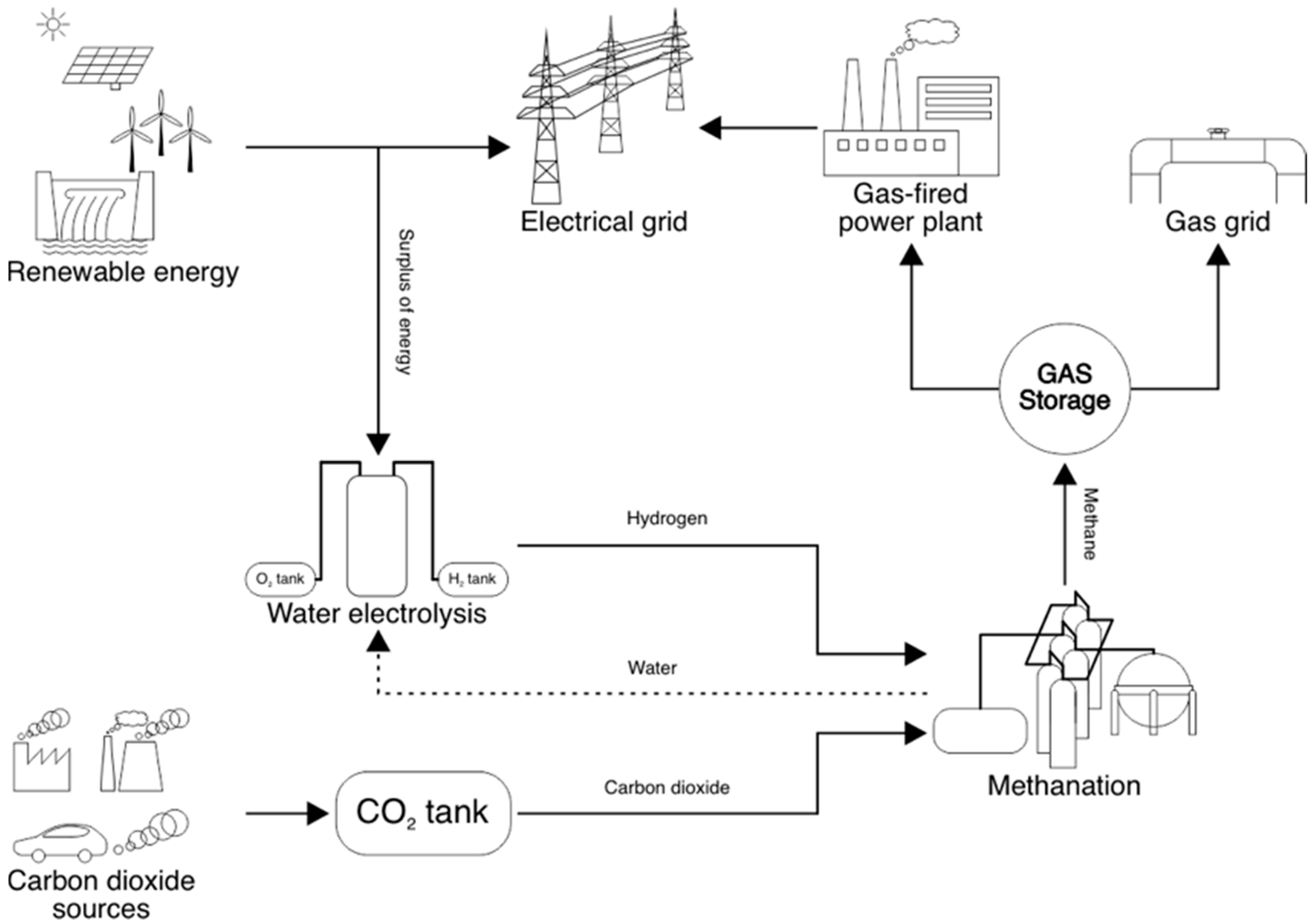
2. Carbon Dioxide Employment
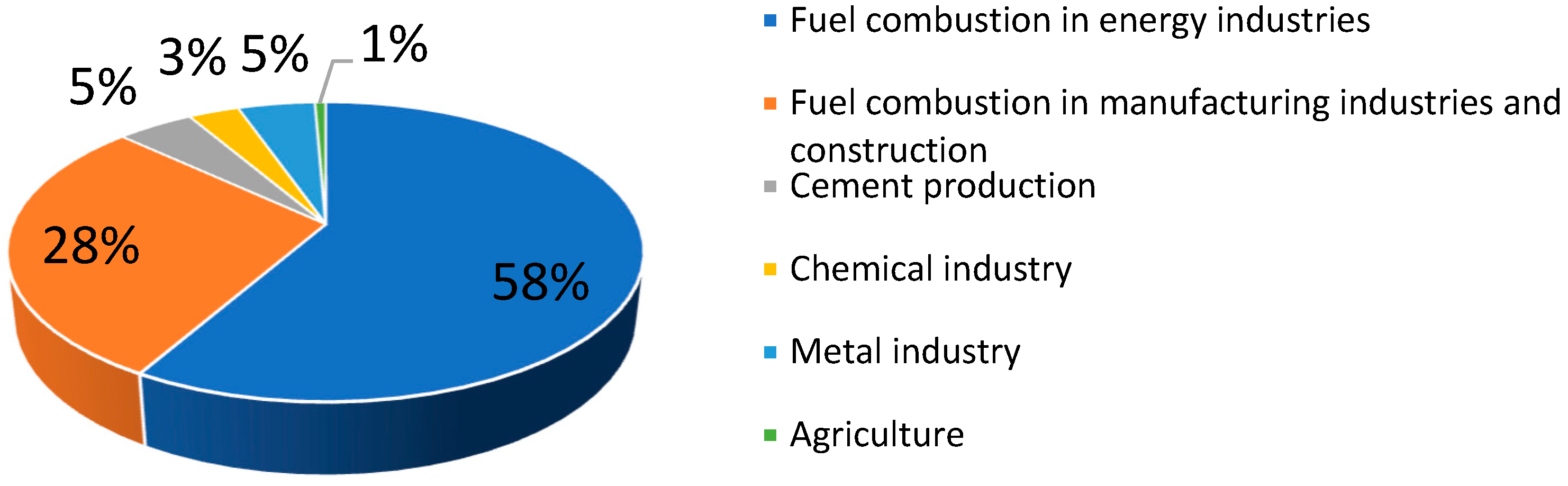
2.1. CO2 Management—Obligation and Opportunity
2.2. CO2 Processing—Examples
- in impregnating the compound in pre-formed carrier particles, e.g., of the active compound on the drug carrier [48],
3. Carbon Dioxide Methanation and Nanocatalysis—The Focal Point in CO2 Conversion
3.1. CO2 Methanation
3.2. Catalyst in Methanation
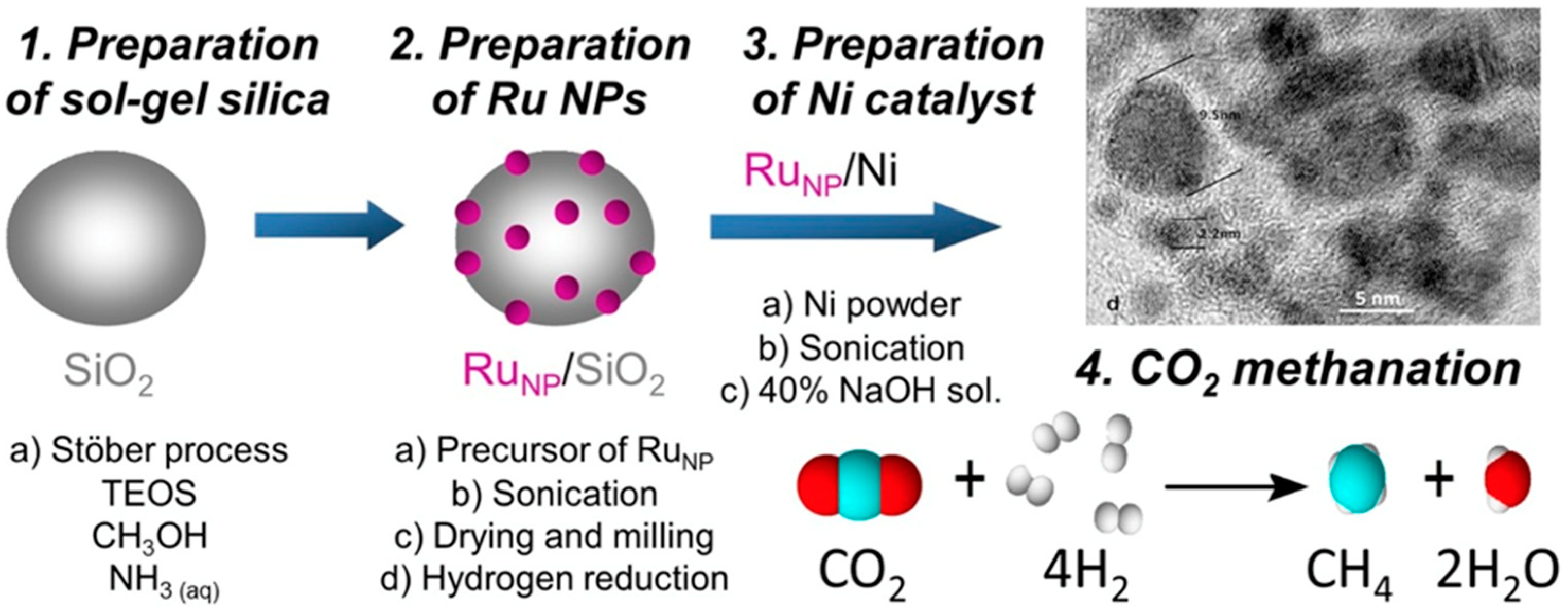
4. Modeling of the Methanation Catalysis—The Determination of Research Clues
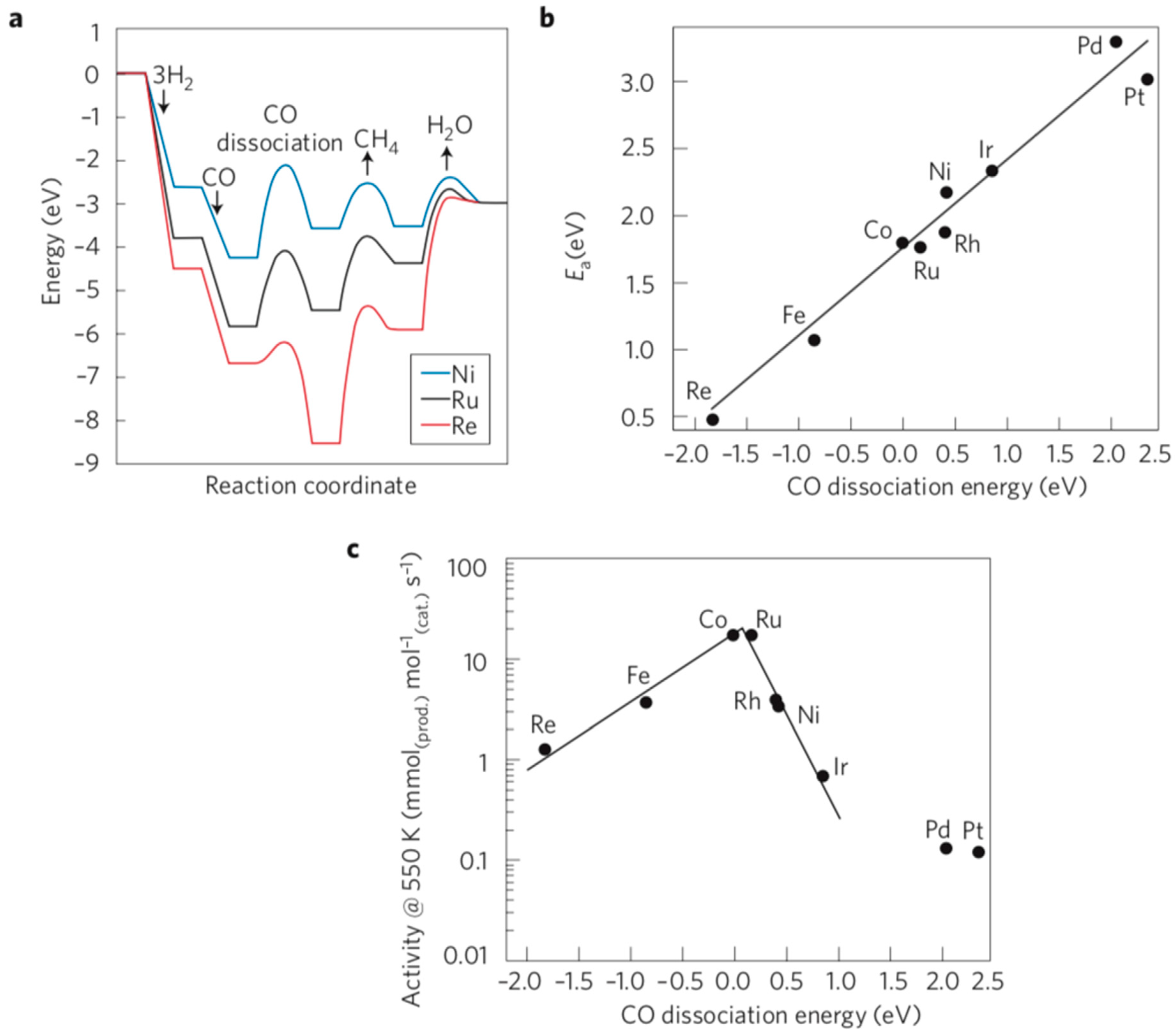
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CO2 Annual Mean Data—NOAA Data. Available online: Ftp://aftp.cmdl.noaa.gov/products/trends/co2/co2_annmean_mlo.txt (accessed on 20 August 2021).
- Brodziński, Z.; Kramarz, M.; Sławomirski, M.R. Energia Odnawialna Wizytówką Nowoczesnej Gospodarki; Wydawnictwo Adam Marszałek: Toruń, Poland, 2016; ISBN 978-83-8019-509-7. [Google Scholar]
- Smith, M.R.; Myers, S.S. Impact of Anthropogenic CO2 Emissions on Global Human Nutrition. Nat. Clim. Chang. 2018, 8, 834–839. [Google Scholar] [CrossRef]
- Fletcher, S.E.M. Ocean Circulation Drove Increase in CO2 Uptake. Nature 2017, 542, 169–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeVries, T.; Holzer, M.; Primeau, F. Recent Increase in Oceanic Carbon Uptake Driven by Weaker Upper-Ocean Overturning. Nature 2017, 542, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Fernández-Martínez, M.; Vallicrosa, H.; Maspons, J.; Zuccarini, P.; Carnicer, J.; Sanders, T.G.M.; Krüger, I.; Obersteiner, M.; Janssens, I.A.; et al. Increasing Atmospheric CO2 Concentrations Correlate with Declining Nutritional Status of European Forests. Commun. Biol. 2020, 3, 125. [Google Scholar] [CrossRef]
- European Union Emissions Trading System, EU ETS. Available online: https://ec.europa.eu/clima/policies/ets_en (accessed on 20 August 2021).
- Pre-2020 Ambition and Implementation-CO2 Reduction-Kyoto Protocol. Available online: https://unfccc.int/topics/pre-2020 (accessed on 20 August 2021).
- Kyoto Protocol—Reference Manual. Available online: https://unfccc.int/sites/default/files/08_unfccc_kp_ref_manual.pdf (accessed on 20 August 2020).
- De Falco, M.D.; Iaquaniello, G.; Centi, G. (Eds.) CO2: A Valuable Source of Carbon, 1st ed.; Green Energy and Technology; Springer: London, UK, 2013; ISBN 978-1-4471-5119-7. [Google Scholar]
- 2030 Climate & Energy Framework. Available online: https://ec.europa.eu/clima/policies/strategies/2030_en (accessed on 20 August 2021).
- Crippa, M.; Oreggioni, G.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Lo Vullo, E.; Solazzo, E.; Monforti-Ferrario, F.; Olivier, J.G.J.; Vignati, E. Fossil CO2 and GHG Emissions of All World Countries: 2019 Report; Publications Office of the European Union: Luxemburg, 2019; ISBN 978-92-76-11100-9. [Google Scholar]
- Infografika: Emisje Gazów Cieplarnianych w Unii Europejskiej. Available online: https://www.europarl.europa.eu/news/pl/headlines/society/20180301STO98928/infografika-emisje-gazow-cieplarnianych-w-unii-europejskiej (accessed on 20 August 2021).
- Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 20 August 2021).
- Rozporządzenie Parlamentu Europejskiego i Rady (UE) w Sprawie Wiążących Rocznych Redukcji Emisji Gazów Cieplarnianych. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/?qid=1582275556293&uri=CELEX:32018R0842 (accessed on 20 August 2021).
- Quadrelli, E.A.; Centi, G.; Duplan, J.-L.; Perathoner, S. Carbon Dioxide Recycling: Emerging Large-Scale Technologies with Industrial Potential. ChemSusChem 2011, 4, 1194–1215. [Google Scholar] [CrossRef]
- Aresta, M. (Ed.) Carbon Dioxide as Chemical Feedstock, 1st ed.; Wiley: Weinheim, Germany, 2010; ISBN 978-3-527-32475-0. [Google Scholar]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The Teraton Challenge. A Review of Fixation and Transformation of Carbon Dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Peters, M.; Köhler, B.; Kuckshinrichs, W.; Leitner, W.; Markewitz, P.; Müller, T.E. Chemical Technologies for Exploiting and Recycling Carbon Dioxide into the Value Chain. ChemSusChem 2011, 4, 1216–1240. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and Prospects in the Chemical Recycling of Carbon Dioxide to Fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Willauer, H.D. Heterogeneous Catalytic CO2 Conversion to Value-Added Hydrocarbons. Energy Environ. Sci. 2010, 3, 884. [Google Scholar] [CrossRef]
- International Energy Agency; United Nations Industrial Development Organization. Carbon Capture and Storage in Industrial Applications; IEA Technology Roadmaps; OECD: Paris, France, 2012; ISBN 978-92-64-13066-1. [Google Scholar]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 Methanation over Heterogeneous Catalysts: Recent Progress and Future Prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Carbon Dioxide Emission by Source Sector (Source: EEA). Available online: https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (accessed on 20 August 2021).
- Triantafyllidis, K.S.; Lappas, A.A.; Stöcker, M. (Eds.) The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals, 1st ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2013; ISBN 978-0-444-56330-9. [Google Scholar]
- Energy Efficiency and Renewable Energy. Energy-Intensive Processes Portfolio: Addressing Key Energy Challenges Across U.S. Industry; Industrial Technologies Program; U.S. Department of Energy: Washington, DC, USA, 2011.
- Metz, B.; Intergovernmental Panel on Climate Change (Eds.) IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press, for the Intergovernmental Panel on Climate Change: Cambridge, UK, 2005; ISBN 978-0-521-86643-9. [Google Scholar]
- Wulf, C.; Linßen, J.; Zapp, P. Review of Power-to-Gas Projects in Europe. Energy Procedia 2018, 155, 367–378. [Google Scholar] [CrossRef]
- Sterner, M. Bioenergy and Renewable Power Methane in Integrated 100% Renewable Energy Systems: Limiting Global Warming by Transforming Energy Systems; Erneuerbare Energien und Energieeffizienz/Renewable Energies and Energy Efficiency; Kassel University Press: Kassel, Germany, 2010; ISBN 978-3-89958-798-2. [Google Scholar]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-64463-6. [Google Scholar]
- Grignard, B.; Gennen, S.; Jérôme, C.; Kleij, A.W.; Detrembleur, C. Advances in the Use of CO2 as a Renewable Feedstock for the Synthesis of Polymers. Chem. Soc. Rev. 2019, 48, 4466–4514. [Google Scholar] [CrossRef]
- Pescarmona, P.P. Cyclic Carbonates Synthesised from CO2: Applications, Challenges and Recent Research Trends. Curr. Opin. Green Sustain. Chem. 2021, 29, 100457. [Google Scholar] [CrossRef]
- Kelly, M.J.; Barthel, A.; Maheu, C.; Sodpiban, O.; Dega, F.-B.; Vummaleti, S.V.C.; Abou-Hamad, E.; Pelletier, J.D.A.; Cavallo, L.; D’Elia, V.; et al. Conversion of Actual Flue Gas CO2 via Cycloaddition to Propylene Oxide Catalyzed by a Single-Site, Recyclable Zirconium Catalyst. J. CO2 Util. 2017, 20, 243–252. [Google Scholar] [CrossRef]
- Sodpiban, O.; Phungpanya, C.; Del Gobbo, S.; Arayachukiat, S.; Piromchart, T.; D’Elia, V. Rational Engineering of Single-Component Heterogeneous Catalysts Based on Abundant Metal Centers for the Mild Conversion of Pure and Impure CO2 to Cyclic Carbonates. Chem. Eng. J. 2021, 422, 129930. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, R.; Xu, Q.; Jiang, J.; Zhou, X.; Ji, H. Charged Metalloporphyrin Polymers for Cooperative Synthesis of Cyclic Carbonates from CO2 under Ambient Conditions. ChemSusChem 2017, 10, 2534–2541. [Google Scholar] [CrossRef]
- Fukuoka, S.; Fukawa, I.; Tojo, M.; Oonishi, K.; Hachiya, H.; Aminaka, M.; Hasegawa, K.; Komiya, K. A Novel Non-Phosgene Process for Polycarbonate Production from CO2: Green and Sustainable Chemistry in Practice. Catal. Surv. Asia 2010, 14, 146–163. [Google Scholar] [CrossRef]
- Demirbas, A.; Demirbas, M.F. Algae Energy: Algae as a New Source of Biodiesel; Green Energy and Technology; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2010; ISBN 978-1-84996-050-2. [Google Scholar]
- Chiappe, C.; Mezzetta, A.; Pomelli, C.S.; Iaquaniello, G.; Gentile, A.; Masciocchi, B. Development of Cost-Effective Biodiesel from Microalgae Using Protic Ionic Liquids. Green Chem. 2016, 18, 4982–4989. [Google Scholar] [CrossRef]
- Antonovsky, N.; Gleizer, S.; Noor, E.; Zohar, Y.; Herz, E.; Barenholz, U.; Zelcbuch, L.; Amram, S.; Wides, A.; Tepper, N.; et al. Sugar Synthesis from CO2 in Escherichia Coli. Cell 2016, 166, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Callaway, E.E. Coli Bacteria Engineered to Eat Carbon Dioxide. Nature 2019, 576, 19–20. [Google Scholar] [CrossRef]
- Sharma, T.; Sharma, S.; Kamyab, H.; Kumar, A. Energizing the CO2 Utilization by Chemo-Enzymatic Approaches and Potentiality of Carbonic Anhydrases: A Review. J. Clean. Prod. 2020, 247, 119138. [Google Scholar] [CrossRef]
- Eckert, C.A.; Knutson, B.L.; Debenedetti, P.G. Supercritical Fluids as Solvents for Chemical and Materials Processing. Nature 1996, 383, 313–318. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Solubility Measurement and Preparation of Nanoparticles of an Anticancer Drug (Letrozole) Using Rapid Expansion of Supercritical Solutions with Solid Cosolvent (RESS-SC). J. Supercrit. Fluids 2018, 133, 239–252. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Saadati Ardestani, N.; Razmimanesh, F. Production of Loratadine Drug Nanoparticles Using Ultrasonic-Assisted Rapid Expansion of Supercritical Solution into Aqueous Solution (US-RESSAS). J. Supercrit. Fluids 2019, 147, 241–253. [Google Scholar] [CrossRef]
- Sodeifian, G.; Garlapati, C.; Razmimanesh, F.; Ghanaat-Ghamsari, M. Measurement and Modeling of Clemastine Fumarate (Antihistamine Drug) Solubility in Supercritical Carbon Dioxide. Sci. Rep. 2021, 11, 24344. [Google Scholar] [CrossRef]
- Sodeifian, G.; Surya Alwi, R.; Razmimanesh, F.; Abadian, M. Solubility of Dasatinib Monohydrate (Anticancer Drug) in Supercritical CO2: Experimental and Thermodynamic Modeling. J. Mol. Liq. 2022, 346, 117899. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Derakhsheshpour, R. CO2 Utilization as a Supercritical Solvent and Supercritical Antisolvent in Production of Sertraline Hydrochloride Nanoparticles. J. CO2 Util. 2022, 55, 101799. [Google Scholar] [CrossRef]
- Ameri, A.; Sodeifian, G.; Sajadian, S.A. Lansoprazole Loading of Polymers by Supercritical Carbon Dioxide Impregnation: Impacts of Process Parameters. J. Supercrit. Fluids 2020, 164, 104892. [Google Scholar] [CrossRef]
- Saadati Ardestani, N.; Sodeifian, G.; Sajadian, S.A. Preparation of Phthalocyanine Green Nano Pigment Using Supercritical CO2 Gas Antisolvent (GAS): Experimental and Modeling. Heliyon 2020, 6, e04947. [Google Scholar] [CrossRef]
- Sodeifian, G.; Saadati Ardestani, N.; Sajadian, S.A.; Soltani Panah, H. Experimental Measurements and Thermodynamic Modeling of Coumarin-7 Solid Solubility in Supercritical Carbon Dioxide: Production of Nanoparticles via RESS Method. Fluid Phase Equilibria 2019, 483, 122–143. [Google Scholar] [CrossRef]
- Sodeifian, G.; Ansari, K. Optimization of Ferulago Angulata Oil Extraction with Supercritical Carbon Dioxide. J. Supercrit. Fluids 2011, 57, 38–43. [Google Scholar] [CrossRef]
- Sodeifian, G.; Saadati Ardestani, N.; Sajadian, S.A.; Ghorbandoost, S. Application of Supercritical Carbon Dioxide to Extract Essential Oil from Cleome Coluteoides Boiss: Experimental, Response Surface and Grey Wolf Optimization Methodology. J. Supercrit. Fluids 2016, 114, 55–63. [Google Scholar] [CrossRef]
- White, M.T.; Bianchi, G.; Chai, L.; Tassou, S.A.; Sayma, A.I. Review of Supercritical CO2 Technologies and Systems for Power Generation. Appl. Therm. Eng. 2021, 185, 116447. [Google Scholar] [CrossRef]
- Climeworks-CO2 Removal. Available online: https://climeworks.com (accessed on 23 August 2021).
- Ghiasi, M.; Zeinali, P.; Gholami, S.; Zahedi, M. Separation of CH4, H2S, N2 and CO2 Gases Using Four Types of Nanoporous Graphene Cluster Model: A Quantum Chemical Investigation. J. Mol. Model. 2021, 27, 201. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Ashraf, M.; AlMayef, T.; Chawla, M.; Poater, A.; Cavallo, L. Amino Acid Ionic Liquids as Potential Candidates for CO2 Capture: Combined Density Functional Theory and Molecular Dynamics Simulations. Chem. Phys. Lett. 2020, 745, 137239. [Google Scholar] [CrossRef] [Green Version]
- Sabatier, P.; Senderens, J.-B. Nouvelles Synthèses Du Méthane. Comptes Rendus l’Académie Sci. 1902, 134, 514–516. [Google Scholar]
- Barbarossa, V.; Vanga, G. Energia, Ambiente e Innovazione; ENEA: Rome, Italy, 2011; pp. 82–85. [Google Scholar]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent Advances in Methanation Catalysts for the Production of Synthetic Natural Gas. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Heterogeneous Catalytic Reactions with CO2: Status and Perspectives. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 153, pp. 1–8. ISBN 978-0-444-51600-8. [Google Scholar]
- Jessop, P.G.; Joó, F.; Tai, C.-C. Recent Advances in the Homogeneous Hydrogenation of Carbon Dioxide. Coord. Chem. Rev. 2004, 248, 2425–2442. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on Methanation—From Fundamentals to Current Projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Choe, S.J.; Kang, H.-J.; Kim, S.-J.; Park, S.-B.; Park, D.H.; Huh, D.S. Adsorbed Carbon Formation and Carbon Hydrogenation for CO2 Methanation on the Ni(111) Surface: ASED-MO Study. Bull. Korean Chem. Soc. 2005, 26, 1682–1688. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Lee, J.; Hong, U.G.; Seo, J.G.; Jung, J.C.; Koh, D.J.; Lim, H.; Byun, C.; Song, I.K. Methane Production from Carbon Monoxide and Hydrogen over Nickel–Alumina Xerogel Catalyst: Effect of Nickel Content. J. Ind. Eng. Chem. 2011, 17, 154–157. [Google Scholar] [CrossRef]
- Tada, S.; Ikeda, S.; Shimoda, N.; Honma, T.; Takahashi, M.; Nariyuki, A.; Satokawa, S. Sponge Ni Catalyst with High Activity in CO2 Methanation. Int. J. Hydrogen Energy 2017, 42, 30126–30134. [Google Scholar] [CrossRef]
- Polanski, J.; Lach, D.; Kapkowski, M.; Bartczak, P.; Siudyga, T.; Smolinski, A. Ru and Ni—Privileged Metal Combination for Environmental Nanocatalysis. Catalysts 2020, 10, 992. [Google Scholar] [CrossRef]
- Bligaard, T.; Nørskov, J.K.; Dahl, S.; Matthiesen, J.; Christensen, C.H.; Sehested, J. The Brønsted–Evans–Polanyi Relation and the Volcano Curve in Heterogeneous Catalysis. J. Catal. 2004, 224, 206–217. [Google Scholar] [CrossRef]
- Kai, T.; Yamasaki, Y.; Takahashi, T.; Masumoto, T.; Kimura, H. Increase in the Thermal Stability during the Methanation of CO2 over a Rh Catalyst Prepared from an Amorphous Alloy. Can. J. Chem. Eng. 1998, 76, 331–335. [Google Scholar] [CrossRef]
- Martin, N.M.; Hemmingsson, F.; Wang, X.; Merte, L.R.; Hejral, U.; Gustafson, J.; Skoglundh, M.; Meira, D.M.; Dippel, A.-C.; Gutowski, O.; et al. Structure–Function Relationship during CO2 Methanation over Rh/Al2O3 and Rh/SiO2 Catalysts under Atmospheric Pressure Conditions. Catal. Sci. Technol. 2018, 8, 2686–2696. [Google Scholar] [CrossRef] [Green Version]
- Benítez, J.J.; Alvero, R.; Capitán, M.J.; Carrizosa, I.; Odriozola, J.A. DRIFTS Study of Adsorbed Formate Species in the Carbon Dioxide and Hydrogen Reaction over Rhodium Catalysts. Appl. Catal. 1991, 71, 219–231. [Google Scholar] [CrossRef]
- Abe, T.; Tanizawa, M.; Watanabe, K.; Taguchi, A. CO2 Methanation Property of Ru Nanoparticle-Loaded TiO2 Prepared by a Polygonal Barrel-Sputtering Method. Energy Environ. Sci. 2009, 2, 315. [Google Scholar] [CrossRef]
- Siudyga, T.; Kapkowski, M.; Janas, D.; Wasiak, T.; Sitko, R.; Zubko, M.; Szade, J.; Balin, K.; Klimontko, J.; Lach, D.; et al. Nano-Ru Supported on Ni Nanowires for Low-Temperature Carbon Dioxide Methanation. Catalysts 2020, 10, 513. [Google Scholar] [CrossRef]
- Snytnikov, P.V.; Zyryanova, M.M.; Sobyanin, V.A. CO-Cleanup of Hydrogen-Rich Stream for LT PEM FC Feeding: Catalysts and Their Performance in Selective CO Methanation. Top. Catal. 2016, 59, 1394–1412. [Google Scholar] [CrossRef]
- Ross, J.R.H. Contemporary Catalysis: Fundamentals and Current Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-444-63474-0. [Google Scholar]
- Martínez, J.; Hernández, E.; Alfaro, S.; López Medina, R.; Valverde Aguilar, G.; Albiter, E.; Valenzuela, M. High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects. Catalysts 2018, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 Methanation over Supported Ni Catalysts. Catal. Today 2017, 293–294, 89–96. [Google Scholar] [CrossRef]
- Fan, M.-T.; Miao, K.-P.; Lin, J.-D.; Zhang, H.-B.; Liao, D.-W. Mg-Al Oxide Supported Ni Catalysts with Enhanced Stability for Efficient Synthetic Natural Gas from Syngas. Appl. Surf. Sci. 2014, 307, 682–688. [Google Scholar] [CrossRef]
- Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Enhanced Investigation of CO Methanation over Ni/Al2O3 Catalysts for Synthetic Natural Gas Production. Ind. Eng. Chem. Res. 2012, 51, 4875–4886. [Google Scholar] [CrossRef]
- Qin, H.; Guo, C.; Wu, Y.; Zhang, J. Effect of La2O3 Promoter on NiO/Al2O3 Catalyst in CO Methanation. Korean J. Chem. Eng. 2014, 31, 1168–1173. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, F.; Lu, X.; Liu, Y.; Li, H.; Zhong, Z.; Xu, G.; Su, F. Enhanced Catalytic Performances of Ni/Al2O3 Catalyst via Addition of V2O3 for CO Methanation. Appl. Catal. A-Gen. 2014, 488, 37–47. [Google Scholar] [CrossRef]
- Liu, H.; Zou, X.; Wang, X.; Lu, X.; Ding, W. Effect of CeO2 Addition on Ni/Al2O3 Catalysts for Methanation of Carbon Dioxide with Hydrogen. J. Nat. Gas Chem. 2012, 21, 703–707. [Google Scholar] [CrossRef]
- Campbell, C.T.; Goodman, D.W. A Surface Science Investigation of the Role of Potassium Promoters in Nickel Catalysts for CO Hydrogenation. Surf. Sci. 1982, 123, 413–426. [Google Scholar] [CrossRef]
- Aldana, P.A.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 Valorization into CH4 on Ni-Based Ceria-Zirconia. Reaction Mechanism by Operando IR Spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Pan, Q.; Peng, J.; Wang, S.; Wang, S. In Situ FTIR Spectroscopic Study of the CO2 Methanation Mechanism on Ni/Ce0.5Zr0.5O2. Catal. Sci. Technol. 2014, 4, 502–509. [Google Scholar] [CrossRef]
- Fujita, S.; Nakamura, M.; Doi, T.; Takezawa, N. Mechanisms of Methanation of Carbon Dioxide and Carbon Monoxide over Nickel/Alumina Catalysts. Appl. Catal. A-Gen. 1993, 104, 87–100. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Improving the Hydrogenation Function of Pd/γ-Al2O3 Catalyst by Rh/γ-Al2O3 Addition in CO2 Methanation at Low Temperature. ACS Catal. 2013, 3, 2799–2812. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Sidik, S.M. Methanation of Carbon Dioxide on Metal-Promoted Mesostructured Silica Nanoparticles. Appl. Catal. A-Gen. 2014, 486, 115–122. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly Active Ni-Promoted Mesostructured Silica Nanoparticles for CO2 Methanation. Appl. Catal. B 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Saad, M.W.A. CO2 Methanation over Ni-Promoted Mesostructured Silica Nanoparticles: Influence of Ni Loading and Water Vapor on Activity and Response Surface Methodology Studies. Chem. Eng. J. 2015, 260, 757–764. [Google Scholar] [CrossRef]
- Kärger, J.; Ruthven, D.M.; Theodorou, D.N. Diffusion in Nanoporous Materials, 1st ed.; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-3-527-31024-1. [Google Scholar]
- Kärger, J.; Goepel, M.; Gläser, R. Diffusion in Nanocatalysis. In Nanotechnology in Catalysis; Van de Voorde, M., Sels, B., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 293–334. ISBN 978-3-527-69982-7. [Google Scholar]
- Tesser, R.; Santacesaria, E. Revisiting the Role of Mass and Heat Transfer in Gas–Solid Catalytic Reactions. Processes 2020, 8, 1599. [Google Scholar] [CrossRef]
- Dai, X.; Sun, Y. Reduction of Carbon Dioxide on Photoexcited Nanoparticles of VIII Group Metals. Nanoscale 2019, 11, 16723–16732. [Google Scholar] [CrossRef]
- Robatjazi, H.; Zhao, H.; Swearer, D.F.; Hogan, N.J.; Zhou, L.; Alabastri, A.; McClain, M.J.; Nordlander, P.; Halas, N.J. Plasmon-Induced Selective Carbon Dioxide Conversion on Earth-Abundant Aluminum-Cuprous Oxide Antenna-Reactor Nanoparticles. Nat. Commun. 2017, 8, 27. [Google Scholar] [CrossRef]
- Kale, M.J.; Avanesian, T.; Xin, H.; Yan, J.; Christopher, P. Controlling Catalytic Selectivity on Metal Nanoparticles by Direct Photoexcitation of Adsorbate–Metal Bonds. Nano Lett. 2014, 14, 5405–5412. [Google Scholar] [CrossRef]
- Kim, Y.; Dumett Torres, D.; Jain, P.K. Activation Energies of Plasmonic Catalysts. Nano Lett. 2016, 16, 3399–3407. [Google Scholar] [CrossRef]
- Pinchuk, A.; von Plessen, G.; Kreibig, U. Influence of Interband Electronic Transitions on the Optical Absorption in Metallic Nanoparticles. J. Phys. D Appl. Phys. 2004, 37, 3133–3139. [Google Scholar] [CrossRef]
- Pinchuk, A.; Kreibig, U.; Hilger, A. Optical Properties of Metallic Nanoparticles: Influence of Interface Effects and Interband Transitions. Surf. Sci. 2004, 557, 269–280. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, T.; Fu, Z.; Zhang, Z.; Zheng, H. Hot Electron and Thermal Effects in Plasmonic Catalysis of Nanocrystal Transformation. Nanoscale 2020, 12, 8768–8774. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hyeon, S.; Lee, J.; Kim, W.D.; Lee, D.C.; Kim, J.; Lee, H. Energy-Efficient CO2 Hydrogenation with Fast Response Using Photoexcitation of CO2 Adsorbed on Metal Catalysts. Nat. Commun. 2018, 9, 3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, J.A.; Contescu, C.; Contescu, A. Methods for Preparation of Catalytic Materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Rao, K.S.; El-Hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A Novel Method for Synthesis of Silica Nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef]
- Polanski, J.; Bartczak, P.; Ambrozkiewicz, W.; Sitko, R.; Siudyga, T.; Mianowski, A.; Szade, J.; Balin, K.; Lelątko, J. Ni-Supported Pd Nanoparticles with Ca Promoter: A New Catalyst for Low-Temperature Ammonia Cracking. PLoS ONE 2015, 10, e0136805. [Google Scholar] [CrossRef]
- Polanski, J.; Siudyga, T.; Bartczak, P.; Kapkowski, M.; Ambrozkiewicz, W.; Nobis, A.; Sitko, R.; Klimontko, J.; Szade, J.; Lelątko, J. Oxide Passivated Ni-Supported Ru Nanoparticles in Silica: A New Catalyst for Low-Temperature Carbon Dioxide Methanation. Appl. Catal. B. 2017, 206, 16–23. [Google Scholar] [CrossRef]
- Siudyga, T.; Kapkowski, M.; Bartczak, P.; Zubko, M.; Szade, J.; Balin, K.; Antoniotti, S.; Polanski, J. Ultra-Low Temperature Carbon (Di)Oxide Hydrogenation Catalyzed by Hybrid Ruthenium–Nickel Nanocatalysts: Towards Sustainable Methane Production. Green Chem. 2020, 22, 5143–5150. [Google Scholar] [CrossRef]
- Kapkowski, M.; Bartczak, P.; Korzec, M.; Sitko, R.; Szade, J.; Balin, K.; Lelątko, J.; Polanski, J. SiO2-, Cu-, and Ni-Supported Au Nanoparticles for Selective Glycerol Oxidation in the Liquid Phase. J. Catal. 2014, 319, 110–118. [Google Scholar] [CrossRef]
- Korzec, M.; Bartczak, P.; Niemczyk, A.; Szade, J.; Kapkowski, M.; Zenderowska, P.; Balin, K.; Lelątko, J.; Polanski, J. Bimetallic Nano-Pd/PdO/Cu System as a Highly Effective Catalyst for the Sonogashira Reaction. J. Catal. 2014, 313, 1–8. [Google Scholar] [CrossRef]
- Rönsch, S.; Köchermann, J.; Schneider, J.; Matthischke, S. Global Reaction Kinetics of CO and CO2 Methanation for Dynamic Process Modeling. Chem. Eng. Technol. 2016, 39, 208–218. [Google Scholar] [CrossRef]
- Hernandez Lalinde, J.A.; Roongruangsree, P.; Ilsemann, J.; Bäumer, M.; Kopyscinski, J. CO2 Methanation and Reverse Water Gas Shift Reaction. Kinetic Study Based on in Situ Spatially-Resolved Measurements. Chem. Eng. J. 2020, 390, 124629. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Methanation in a Fluidized Bed Reactor with High Initial CO Partial Pressure: Part II— Modeling and Sensitivity Study. Chem. Eng. Sci. 2011, 66, 1612–1621. [Google Scholar] [CrossRef]
- Champon, I.; Bengaouer, A.; Chaise, A.; Thomas, S.; Roger, A.-C. Carbon Dioxide Methanation Kinetic Model on a Commercial Ni/Al2O3 Catalyst. J. CO2 Util. 2019, 34, 256–265. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane Steam Reforming, Methanation and Water-Gas Shift: I. Intrinsic Kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the Computational Design of Solid Catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Bahn, S.; Hansen, L.B.; Bollinger, M.; Bengaard, H.; Hammer, B.; Sljivancanin, Z.; Mavrikakis, M.; et al. Universality in Heterogeneous Catalysis. J. Catal. 2002, 209, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Ciobica, I.M.; van Santen, R.A. Carbon Monoxide Dissociation on Planar and Stepped Ru(0001) Surfaces. J. Phys. Chem. B 2003, 107, 3808–3812. [Google Scholar] [CrossRef]
- Michaelides, A.; Liu, Z.-P.; Zhang, C.J.; Alavi, A.; King, D.A.; Hu, P. Identification of General Linear Relationships between Activation Energies and Enthalpy Changes for Dissociation Reactions at Surfaces. J. Am. Chem. Soc. 2003, 125, 3704–3705. [Google Scholar] [CrossRef]
- Andersson, M.; Bligaard, T.; Kustov, A.; Larsen, K.; Greeley, J.; Johannessen, T.; Christensen, C.; Norskov, J. Toward Computational Screening in Heterogeneous Catalysis: Pareto-Optimal Methanation Catalysts. J. Catal. 2006, 239, 501–506. [Google Scholar] [CrossRef]
- Sabatier, P. Hydrogénations et déshydrogénations par catalyse. Ber. Dtsch. Chem. Ges. 1911, 44, 1984–2001. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Nikolla, E.; Linic, S.; Medlin, J.W.; Janik, M.J. Multicomponent Catalysts: Limitations and Prospects. ACS Catal. 2018, 8, 3202–3208. [Google Scholar] [CrossRef]
- Greeley, J. Theoretical Heterogeneous Catalysis: Scaling Relationships and Computational Catalyst Design. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 605–635. [Google Scholar] [CrossRef]
- Goldsmith, B.R.; Esterhuizen, J.; Liu, J.; Bartel, C.J.; Sutton, C. Machine Learning for Heterogeneous Catalyst Design and Discovery. AIChE J. 2018, 64, 2311–2323. [Google Scholar] [CrossRef]
- Suzuki, K.; Toyao, T.; Maeno, Z.; Takakusagi, S.; Shimizu, K.; Takigawa, I. Statistical Analysis and Discovery of Heterogeneous Catalysts Based on Machine Learning from Diverse Published Data. ChemCatChem 2019, 11, 4537–4547. [Google Scholar] [CrossRef]
- Ouyang, R.; Xie, Y.; Jiang, D. Global Minimization of Gold Clusters by Combining Neural Network Potentials and the Basin-Hopping Method. Nanoscale 2015, 7, 14817–14821. [Google Scholar] [CrossRef] [Green Version]
- Lach, D.; Zhdan, U.; Smolinski, A.; Polanski, J. Functional and Material Properties in Nanocatalyst Design: A Data Handling and Sharing Problem. IJMS 2021, 22, 5176. [Google Scholar] [CrossRef]
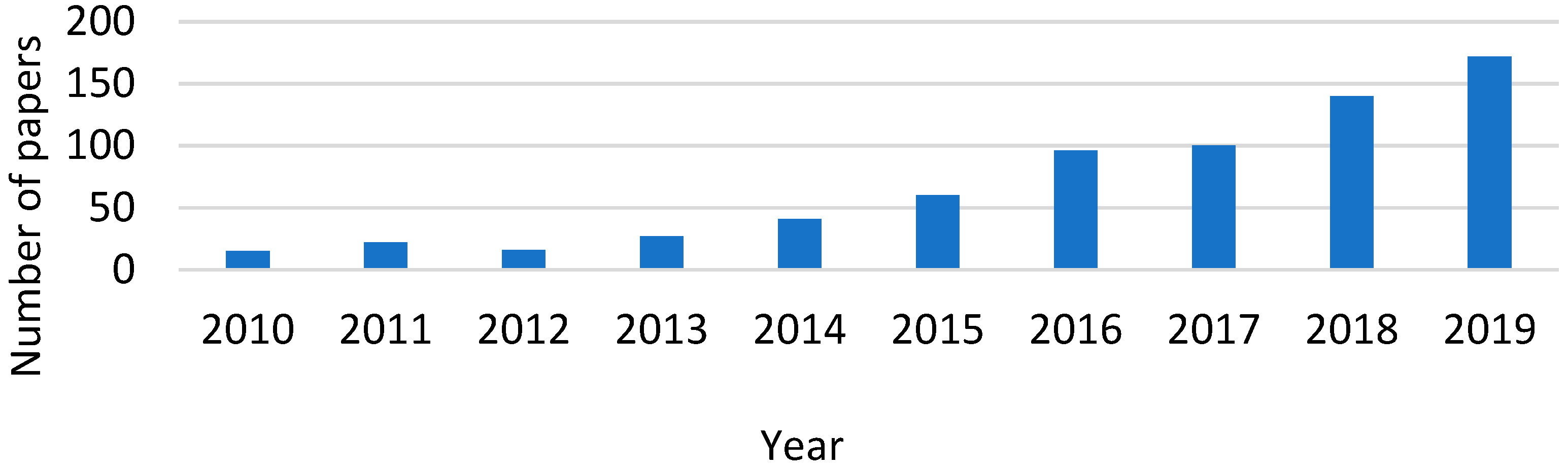
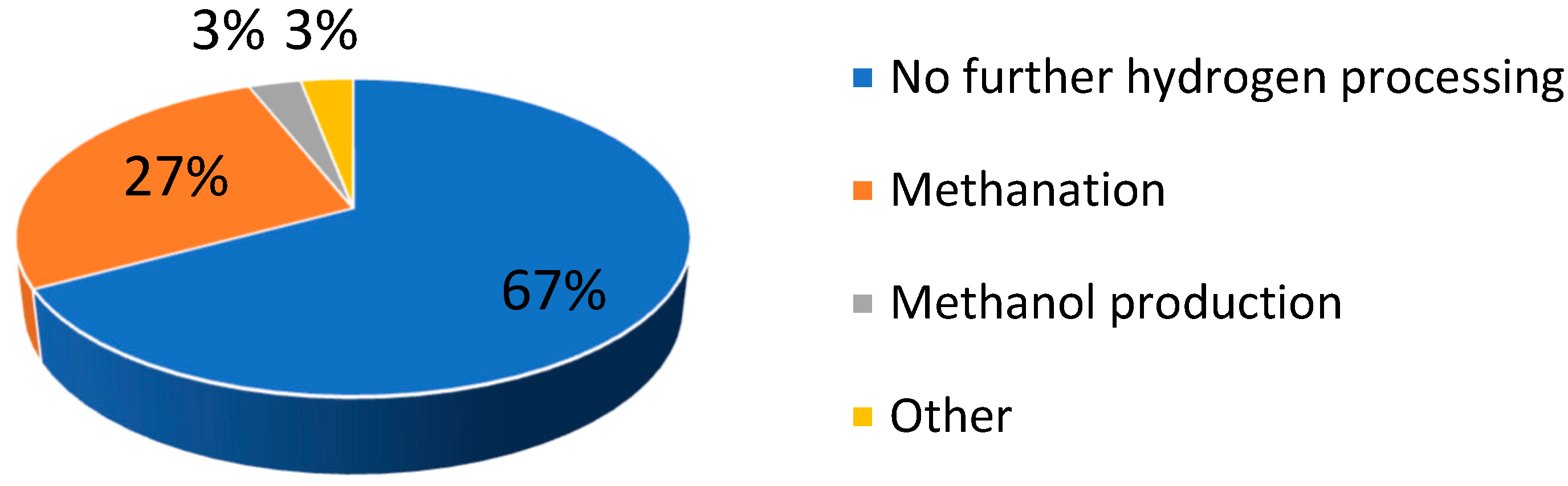


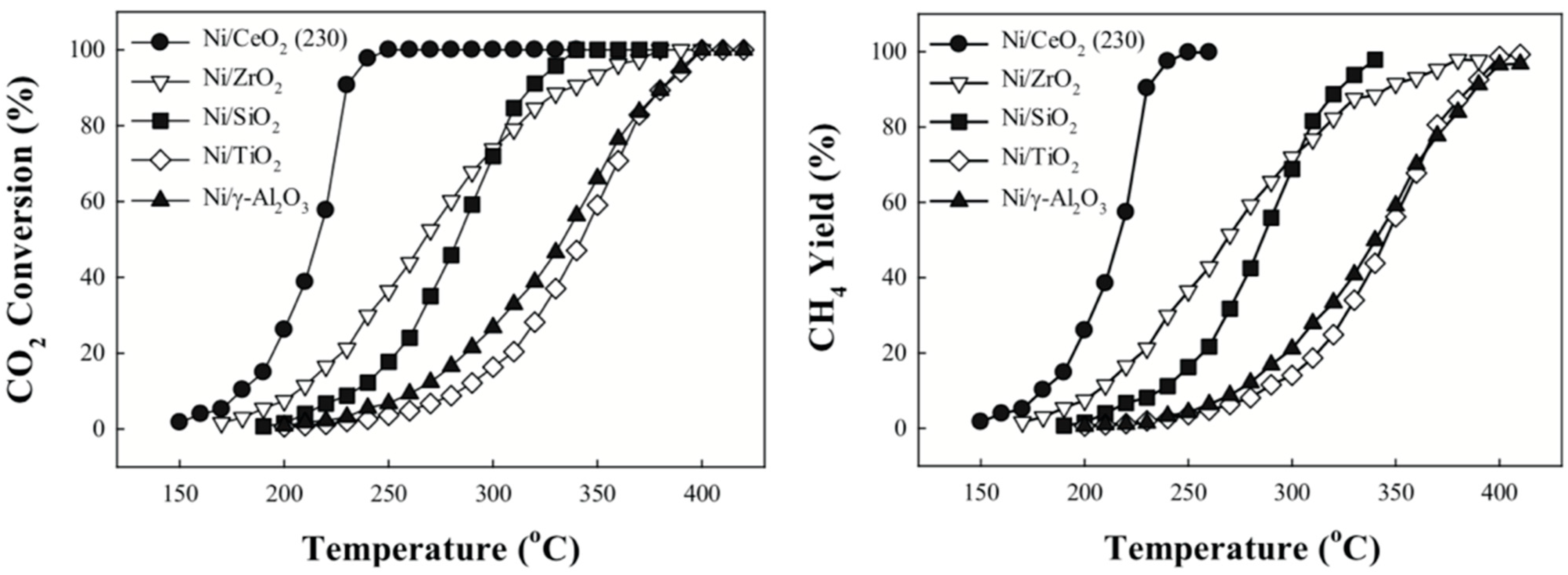
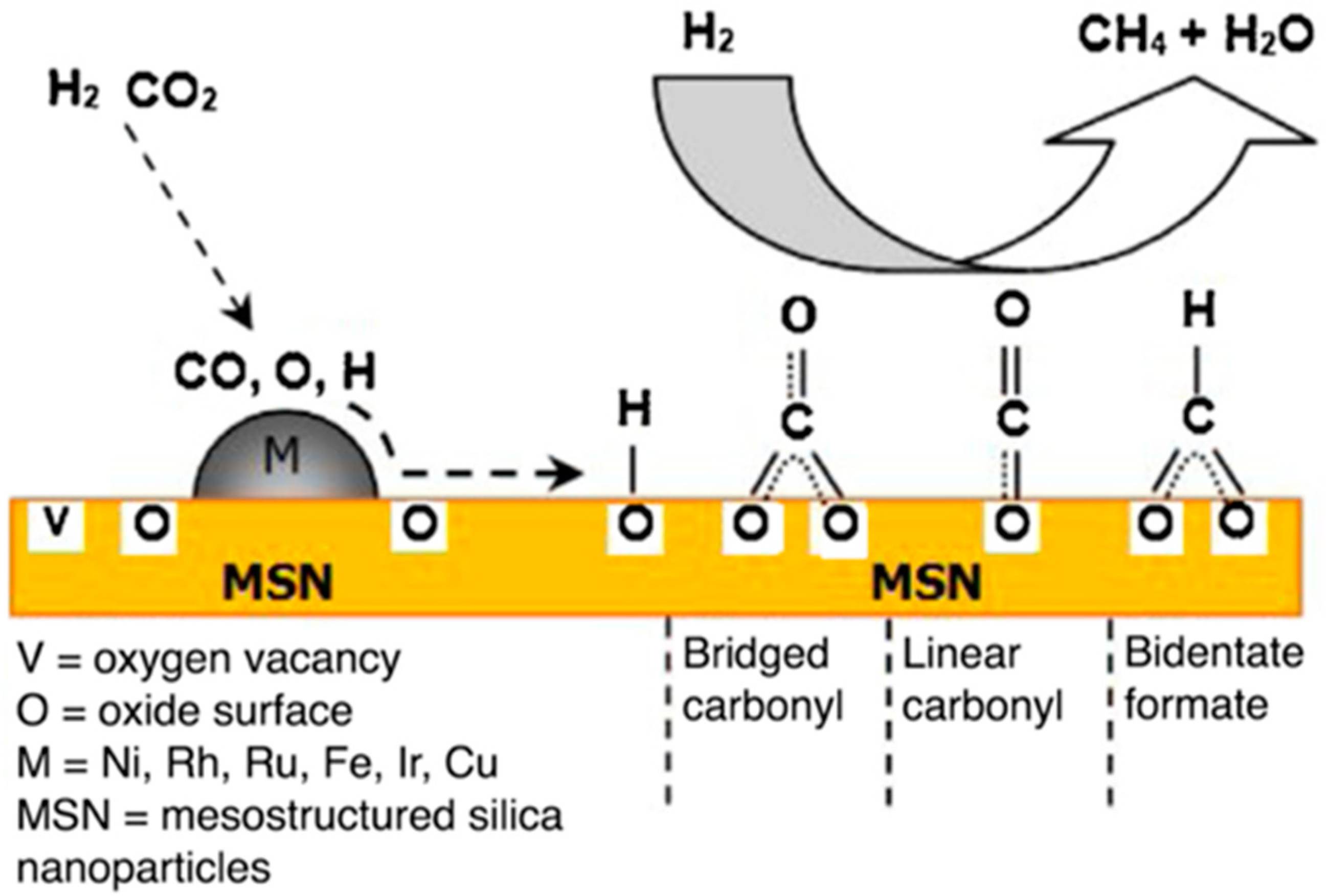
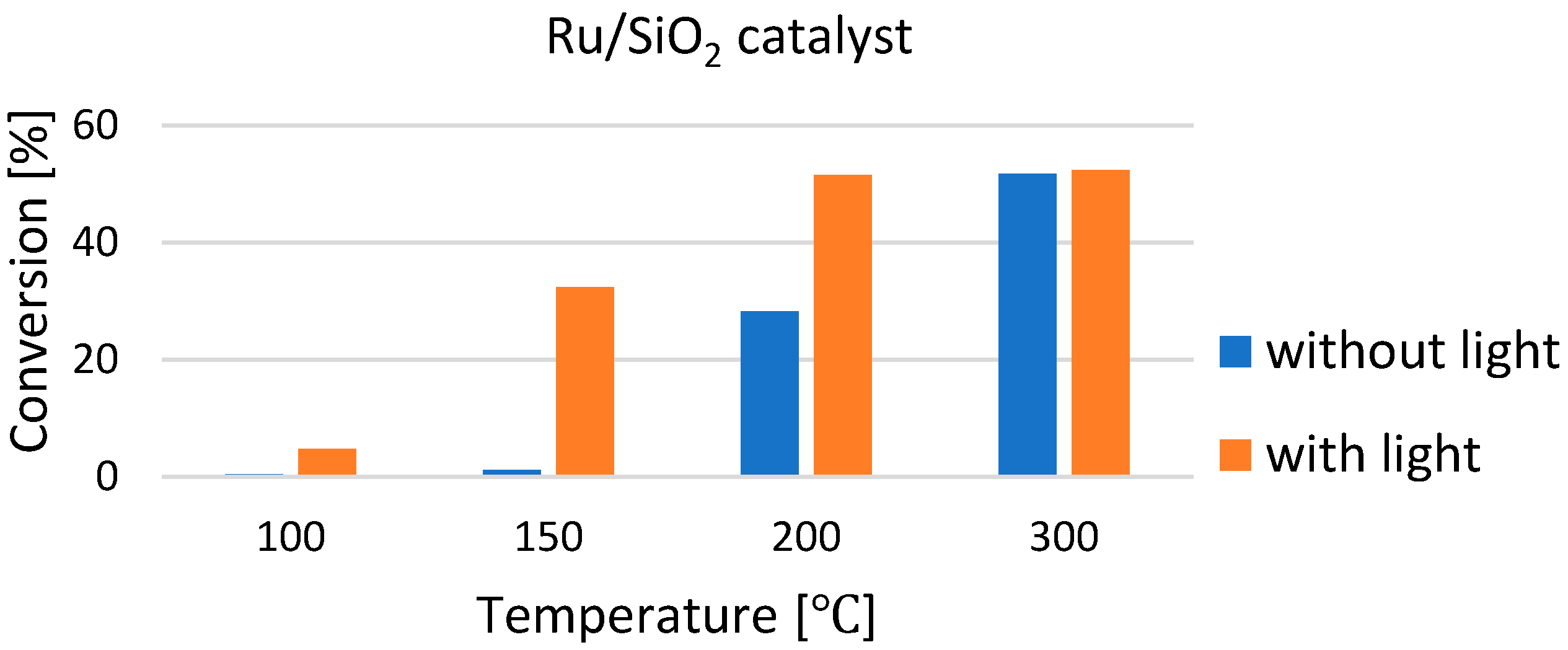
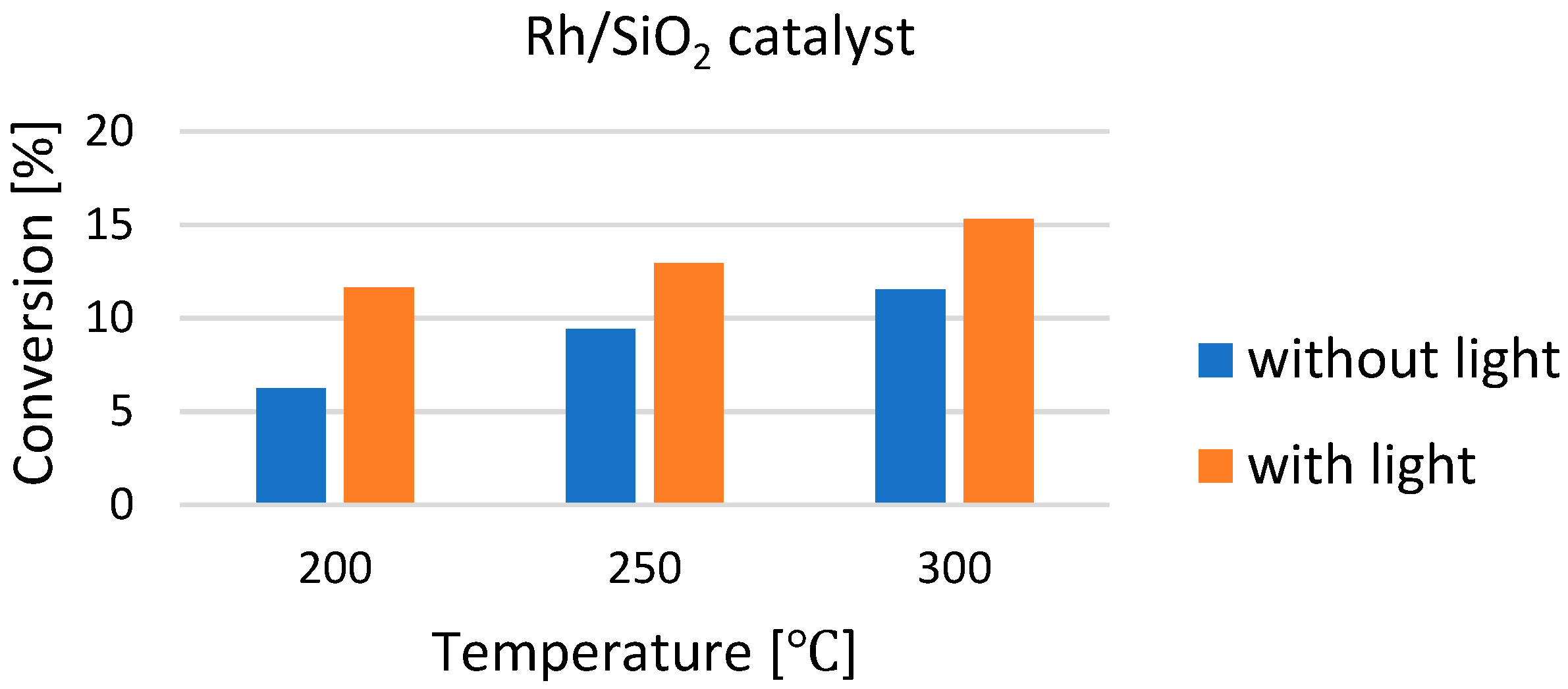
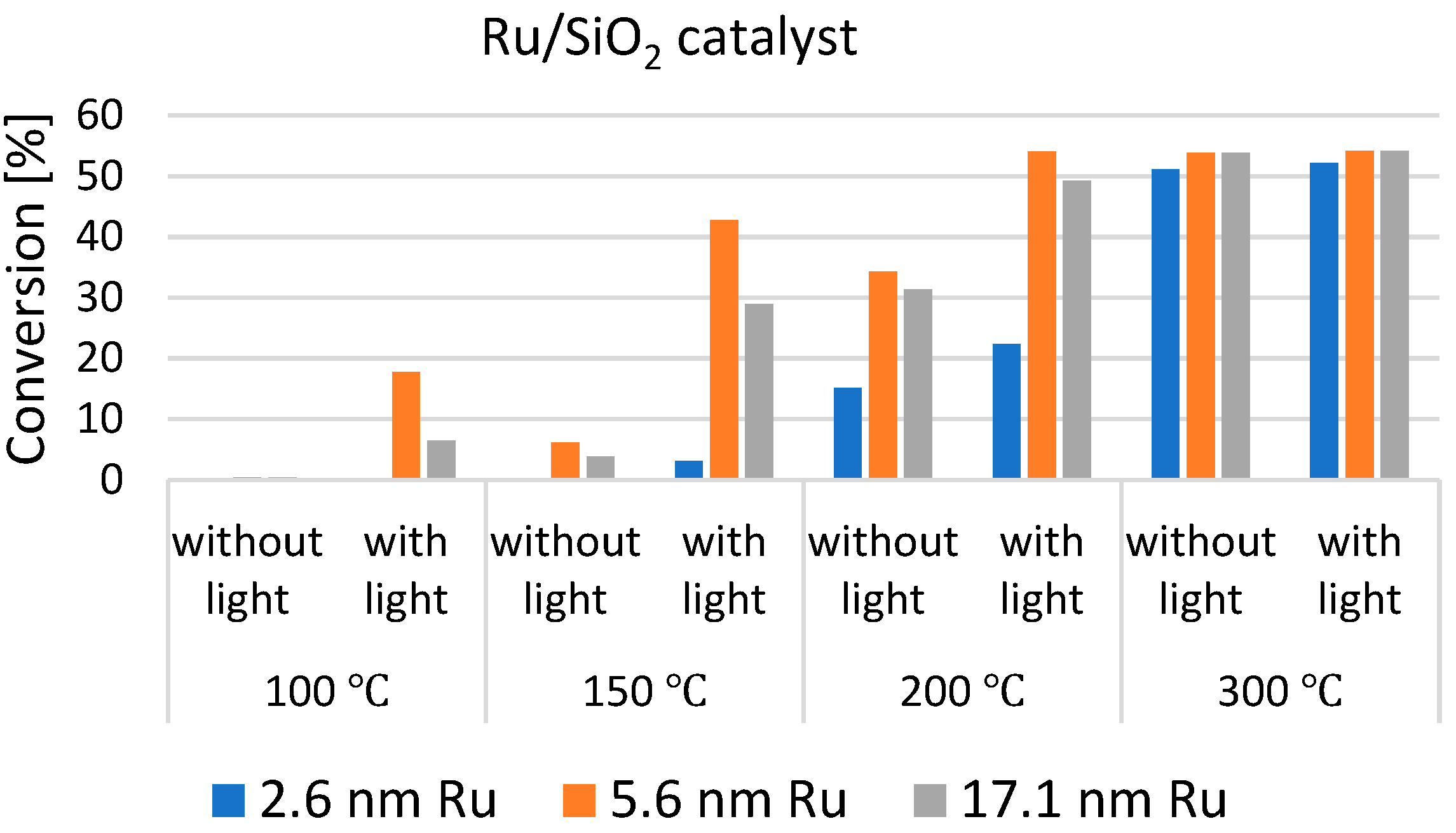
| Selected EU States | 1 GHG Emission Reduction by 2030, % | 2 GHG Emission in 2005, CO2 Equivalent, Mt/yr | GHG Emission Limit in 2030, CO2 Equivalent, Mt/yr | 3 Averaged Value of CO2 Share in GHG, % | CO2 Emission Limit in 2030, Mt/yr |
|---|---|---|---|---|---|
| Belgium | 35 | 147.174 | 95.663 | 73 | 69.834 |
| Luxembourg | 40 | 13.166 | 7.900 | 5.767 | |
| Netherlands | 36 | 225.725 | 144.464 | 105.459 | |
| Germany | 38 | 993.712 | 616.101 | 449.754 | |
| Czech Republic | 14 | 148.874 | 128.032 | 93.463 | |
| Poland | 7 | 412.938 | 384.032 | 280.343 | |
| Slovakia | 12 | 49.748 | 43.778 | 31.958 | |
| Lithuania | 9 | 23.668 | 21.538 | 15.723 | |
| Latvia | 6 | 13.081 | 12.296 | 8.976 |
| Reaction Number | Reaction Equation | ΔH298 K, kJ mol−1 | ΔG298 K, kJ mol−1 |
|---|---|---|---|
| R1 | CO + 3H2 ⟺ CH4 + H2O | −206.1 | −141.8 |
| R2 | CO2 + 4H2 ⟺ CH4 + 2H2O | −165.0 | −113.2 |
| R3 | 2CO + 2H2 ⟺ CH4 + CO2 | −247.3 | −170.4 |
| R4 | 2CO ⟺ C + CO2 | −172.4 | −119.7 |
| R5 | CO + H2O ⟺ CO2 + H2 | −41.2 | −28.6 |
| R6 | 2H2 + C ⟺ CH4 | −74.8 | −50.7 |
| R7 | CO + H2 ⟺ C + H2O | −131.3 | −91.1 |
| R8 | CO2 + 2H2 ⟺ C + 2H2O | −90.1 | −62.5 |
| Catalyst | 1 WHSV, cm3 g−1 h−1 | 2 GHSV, h−1 | Composition of Inlet Gases, % | 3 Reaction Characteristic and Yield | ||||
|---|---|---|---|---|---|---|---|---|
| CO/CO2/H2O/H2 | Tmin, °C | Smin, % | Tmax, °C | Smax, % | mol CO g−1 h−1 | |||
| 10% w/w Ni/CeO2 | 26,000 | 46,000 | 1.5/20/10/60 | 250 | 89 | 320 | 50 | 0.0160 |
| 1/20/10/60 | 240 | 100 | 315 | 45 | 0.0106 | |||
| 0.5/20/10/60 | 230 | 99 | 290 | 31 | 0.0053 | |||
| 6000 | 12,000 | 1/20/10/60 | 210 | 100 | 265 | 50 | 0.0025 | |
| 13,000 | 26,000 | 225 | 100 | 280 | 50 | 0.0053 | ||
| 43,000 | 84,000 | 265 | 94 | 295 | 54 | 0.0176 | ||
| 10% w/w Ni/ZrO2 | ~150,000 | - | 0.5/14.8/0.8/59.2 | 280 | ~90 | 300 | ~70 | 0.0307 |
| 1.6% w/w Ni/ZrO2 | - | 10,000 | 1.14/21.43/1.8/74.8 | 260 | ~60 | 280 | ~60 | - |
| 10% w/w Ni/TiO2 | - | 10,000 | 0.2/16.1/18.4/65.3 | 200 | ~80 | - | - | - |
| 5% w/w Ru/TiO2 | ~150,000 | - | 0.5/14.8/0.8/59.2 | 220 | ~70 | 260 | ~20 | 0.0307 |
| 5% w/w Ru/TiO2 | 27,000 | - | 0.5/18/15/40 | 220 | 60 | 260 | 20 | 0.0055 |
| 5% w/w Ru/ZrO2 | 265 | 80 | 310 | 50 | 0.0055 | |||
| 5% w/w Ru/CeO2 | 250 | 75 | 300 | 30 | 0.0055 | |||
| 3% w/w Ru/Al2O3 | - | 13,500 | 0.9/24.5/5.7/68.9 | 220 | <50 | - | - | - |
| 2% w/w Ru/Al2O3 | - | 10,000 | 0.3/4.8/75/18.8 | 270 | <20 | - | - | - |
| 30% w/w Ru/CNT | 12,000 | - | 1.2/20/0/78.8 | 220 | - | - | - | 0.0059 |
| 30% w/w Ru-ZrO2/CNT | 180 | 100 | 240 | 35 | 0.0059 | |||
| 1% w/w Ru/MA-33Ni | - | 2800 | 0.9/17/15/67.1 | 185 | 100 | 245 | 50 | - |
| 1% w/w Ru/MA-40Ni | - | 185 | 100 | 260 | 50 | - | ||
| 1% w/w Ru/MA-50Ni | - | 195 | 100 | 270 | 50 | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lach, D.; Polanski, J.; Kapkowski, M. CO2—A Crisis or Novel Functionalization Opportunity? Energies 2022, 15, 1617. https://doi.org/10.3390/en15051617
Lach D, Polanski J, Kapkowski M. CO2—A Crisis or Novel Functionalization Opportunity? Energies. 2022; 15(5):1617. https://doi.org/10.3390/en15051617
Chicago/Turabian StyleLach, Daniel, Jaroslaw Polanski, and Maciej Kapkowski. 2022. "CO2—A Crisis or Novel Functionalization Opportunity?" Energies 15, no. 5: 1617. https://doi.org/10.3390/en15051617
APA StyleLach, D., Polanski, J., & Kapkowski, M. (2022). CO2—A Crisis or Novel Functionalization Opportunity? Energies, 15(5), 1617. https://doi.org/10.3390/en15051617








