Raman Spectroscopy Characterization of Mineral Oil and Palm Oil with Added Multi-Walled Carbon Nanotube for Application in Oil-Filled Transformers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanofluids
2.3. Raman Spectroscopy Analysis
3. Results
3.1. Raman Optical Images
3.2. Qualitative Detection of CNT Nanofluids
3.3. Multiple Peak Fitting Analysis
3.4. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katim, N.I.A.; Ishak, M.T.; Amin, N.A.M.; Hamid, M.H.A.; Ahmad, K.A.; Azis, N. Lightning Breakdown Voltage Evaluation of Palm Oil and Coconut Oil as Transformer Oil under Quasi-Uniform Field Conditions. Energies 2018, 11, 2676. [Google Scholar] [CrossRef] [Green Version]
- Makmud, M.Z.H.; Illias, H.A.; Chee, C.Y. Partial Discharge Behaviour within Palm Oil-Based Fe2O3 Nanofluids under AC Voltage. IOP Conf. Ser. Mater. Sci. Eng. 2017, 210, 012034. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, A.A.; Muhamad, N.A.; Bashir, N.; Murad, N.S.; Arief, Y.Z.; Phung, B.T. Effect of Moisture on Breakdown Voltage and Structure of Palm Based Insulation Oils. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2119–2126. [Google Scholar] [CrossRef]
- Suhaimi, N.S.; Md Din, M.F.; Ishak, M.T.; Rahman, A.R.A. Statistical Analysis on AC Breakdown Voltage of CNT Nanofluid with Mineral Oil and Palm Oil. In Proceedings of the 2018 IEEE 7th International Conference on Power and Energy, PECon 2018, Kuala Lumpur, Malaysia, 3–4 December 2018; pp. 111–116. [Google Scholar]
- Dan, S.A.M.; Hashim, F.H.; Raj, T.; Huddin, A.B.; Hussain, A. Classification of Oil Palm Fresh Fruit Bunches (FFB) Using Raman Spectroscopy. Int. J. Eng. Technol. 2018, 7, 184. [Google Scholar] [CrossRef]
- Suhaimi, S.N.; Rahman, A.R.A.; Din, M.F.M.; Hassan, M.Z.; Ishak, M.T.; Jusoh, M.T.B. A Review on Oil-Based Nanofluid as Next-Generation Insulation for Transformer Application. J. Nanomater. 2020, 2020, 2061343. [Google Scholar] [CrossRef]
- Saidur, R.; Leong, K.Y.; Mohammed, H.A. A Review on Applications and Challenges of Nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 1646–1668. [Google Scholar] [CrossRef]
- Mukherjee, S.P.S. Preparation and Stability of Nanofluids—A Review. IOSR J. Mech. Civ. Eng. 2013, 9, 63–69. [Google Scholar] [CrossRef]
- Ram, A.; Ramaian, P.; Iruthayarajan, W.; Siluvairaj, M.; Karthik, R. Performance Studies on Dielectric and Physical Properties of Eco-Friendly Based Natural Ester Oils Using Semi-Conductive Nanocomposites for Power Transformer Application. IET Sci. Meas. Technol. 2018, 12, 323–327. [Google Scholar] [CrossRef]
- Asse, J.-B.; Mengounou, G.M.; Imano, A.M. Impact of FeO3 on the AC Breakdown Voltage and Acidity Index of a Palm Kernel Oil Methyl Ester Based Nanofluid. Energy Rep. 2022, 8, 275–280. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Suhaimi, N.S.; Md Din, M.F.; Ishak, M.T.; Abdul Rahman, A.R.; Mohd Ariffin, M.; Hashim, N.I.; Wang, J. Systematical Study of Multi-Walled Carbon Nanotube Nanofluids Based Disposed Transformer Oil. Sci. Rep. 2020, 10, 20984. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, N.S.; Din, M.F.; Rashid, A.; Rahman, A.; Hayati, M.; Hamid, A.; Aqilah, N.; Amin, M.; Fathul, W.; Wan, H.; et al. Optimum Electrical and Dielectric Performance of Multi-Walled Carbon Nanotubes Doped Disposed Transformer Oil. Energies 2020, 13, 3181. [Google Scholar] [CrossRef]
- Deb, H.; Nunisa, J.; Moon Sun Naiding, M.; Jidung, S.; Sarmah, D. Transformer Oil Quality Diagnostic Using Spectroscopy Techniques-A Review. ADBU J. Electr. Electron. Eng. 2019, 3, 43–50. [Google Scholar]
- Chen, X.; Chen, S.; Yang, D.; Luo, H.; Yang, P.; Cui, W. Quantitative Prediction of Aging State of Oil-Paper Insulation Based on Raman Spectroscopy. AIP Adv. 2021, 11, 035001. [Google Scholar] [CrossRef]
- Song, R.; Chen, W.; Yang, D.; Shi, H.; Zhang, R.; Wang, Z. Aging Assessment of Oil-Paper Insulation Based on Visional Recognition of the Dimensional Expanded Raman Spectra. IEEE Trans. Instrum. Meas. 2021, 70, 6007110. [Google Scholar] [CrossRef]
- Chen, W.; Gu, Z.; Zou, J.; Wan, F.; Xiang, Y. Analysis of Furfural Dissolved in Transformer Oil Based on Confocal Laser Raman Spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 915–921. [Google Scholar] [CrossRef]
- Somekawa, T.; Fujita, M.; Izawa, Y.; Kasaoka, M.; Nagano, Y. Furfural Analysis in Transformer Oils Using Laser Raman Spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 229–231. [Google Scholar] [CrossRef]
- Yadav, N.; Jarial, R.K.; Rao, U.M. Characterization of Mineral Oil Based Fe3O4 Nanofluid for Application in Oil Filled Transformers. Int. J. Electr. Eng. Inform. 2018, 10, 338–349. [Google Scholar] [CrossRef]
- Kim, B.C.; Lee, I.; Kwon, S.J.; Wee, Y.; Kwon, K.Y.; Jeon, C.; An, H.J.; Jung, H.T.; Ha, S.; Dordick, J.S.; et al. Fabrication of Enzyme-Based Coatings on Intact Multi-Walled Carbon Nanotubes as Highly Effective Electrodes in Biofuel Cells. Sci. Rep. 2017, 7, 40202. [Google Scholar] [CrossRef]
- Mehta, M.; Naffa, R.; Maidment, C.; Holmes, G.; Waterland, M. Raman and ATR-FTIR Spectroscopy Towards Classification of Wet Blue Bovine Leather Using Ratiometric and Chemometric Analysis. J. Leather Sci. Eng. 2020, 2, 3. [Google Scholar] [CrossRef]
- Tsuyama, S.; Taketani, A.; Murakami, T.; Sakashita, M.; Miyajima, S.; Ogawa, T.; Wada, S.; Maeda, H.; Hanada, Y. Quantitative Prediction of a Functional Ingredient in Apple Using Raman Spectroscopy and Multivariate Calibration Analysis. Appl. Phys. B Lasers Opt. 2021, 127, 92. [Google Scholar] [CrossRef]
- Johnson, D.W. Application of Raman Spectroscopy to Lubricants, Lubricated Surfaces, and Lubrication Phenomena. Spectroscopy 2011, 26, 46–50. [Google Scholar]
- Frost, R.L.; López, A.; Xi, Y.; Granja, A.; Scholz, R.; Lima, R.M.F. Vibrational Spectroscopy of the Phosphate Mineral Kovdorskite—Mg 2PO4(OH)·3H2O. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2013, 114, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaskova, H.; Buckova, M. Multivariate Study of Raman Spectral Data of Edible Oils. WSEAS Trans. Environ. Dev. 2018, 14, 02022. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, W.; Pan, J.; Ye, Q.; Lin, S.; Feng, S.; Xie, S.; Zeng, H.; Chen, R. Rapid Detection of Nasopharyngeal Cancer Using Raman Spectroscopy and Multivariate Statistical Analysis. Mol. Clin. Oncol. 2015, 3, 375–380. [Google Scholar] [CrossRef]
- Jin, H.; Li, H.; Yin, Z.; Zhu, Y.; Lu, A.; Zhao, D.; Li, C. Application of Raman Spectroscopy in the Rapid Detection of Waste Cooking Oil. Food Chem. 2021, 362, 130191. [Google Scholar] [CrossRef]
- Orange, D.; Knitile, E.; Farber, D.; Williams, Q. Raman Spectroscopy of Crude Oils and Hydrocarbon Fluid Inclusions: A Feasibility Study. Miner. Spectrosc. 1996, 5, 65–81. [Google Scholar]
- Batakliev, T.; Petrova-Doycheva, I.; Angelov, V.; Georgiev, V.; Ivanov, E.; Kotsilkova, R.; Casa, M.; Cirillo, C.; Adami, R.; Sarno, M.; et al. Effects of Graphene Nanoplatelets and Multiwall Carbon Nanotubes on the Structure and Mechanical Properties of Poly(Lactic Acid) Composites: A Comparative Study. Appl. Sci. 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Ujihara, T.; Munetoh, S.; Kusunoki, K.; Kamei, K.; Usami, N.; Fujiwara, K.; Sazaki, G.; Nakajima, K. Crystal Quality of a 6H-SiC Layer Grown over Macrodefects by Liquid-Phase Epitaxy: A Raman Spectroscopic Study. Thin Solid Film. 2005, 476, 206–209. [Google Scholar] [CrossRef]
- Zhang, N.; Tian, Z.J.; Leng, Y.Y.; Wang, H.T.; Song, F.Q.; Meng, J.H. Raman Characteristics of Hydrocarbon and Hydrocarbon Inclusions. Sci. China Ser. D Earth Sci. 2007, 50, 1171–1178. [Google Scholar] [CrossRef]
- Phillips, L.; Abbas, N.; Mojica, E.-R.E. Raman Spectroscopic Characterization of An Ionic Liquid (1-Butyl-3- Methylimidazolium Thiocyanate). J. Undergrad. Chem. Res. 2021, 2021, 1–4. [Google Scholar]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C—J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Skrzypek, E. First- and Second-Order Raman Spectra of Carbonaceous Material through Successive Contact and Regional Metamorphic Events (Ryoke Belt, SW Japan). Lithos 2021, 388–389, 106029. [Google Scholar] [CrossRef]
- Antunes, E.F.; Lobo, A.O.; Corat, E.J.; Trava-Airoldi, V.J.; Martin, A.A.; Veríssimo, C. Comparative Study of First- and Second-Order Raman Spectra of MWCNT at Visible and Infrared Laser Excitation. Carbon 2006, 44, 2202–2211. [Google Scholar] [CrossRef]
- Barros, I.H.A.S.; Paixão, L.S.; Nascimento, M.H.C.; Lacerda, V.; Filgueiras, P.R.; Romão, W. Use of Portable Raman Spectroscopy in the Quality Control of Extra Virgin Olive Oil and Adulterated Compound Oils. Vib. Spectrosc. 2021, 116, 103299. [Google Scholar] [CrossRef]







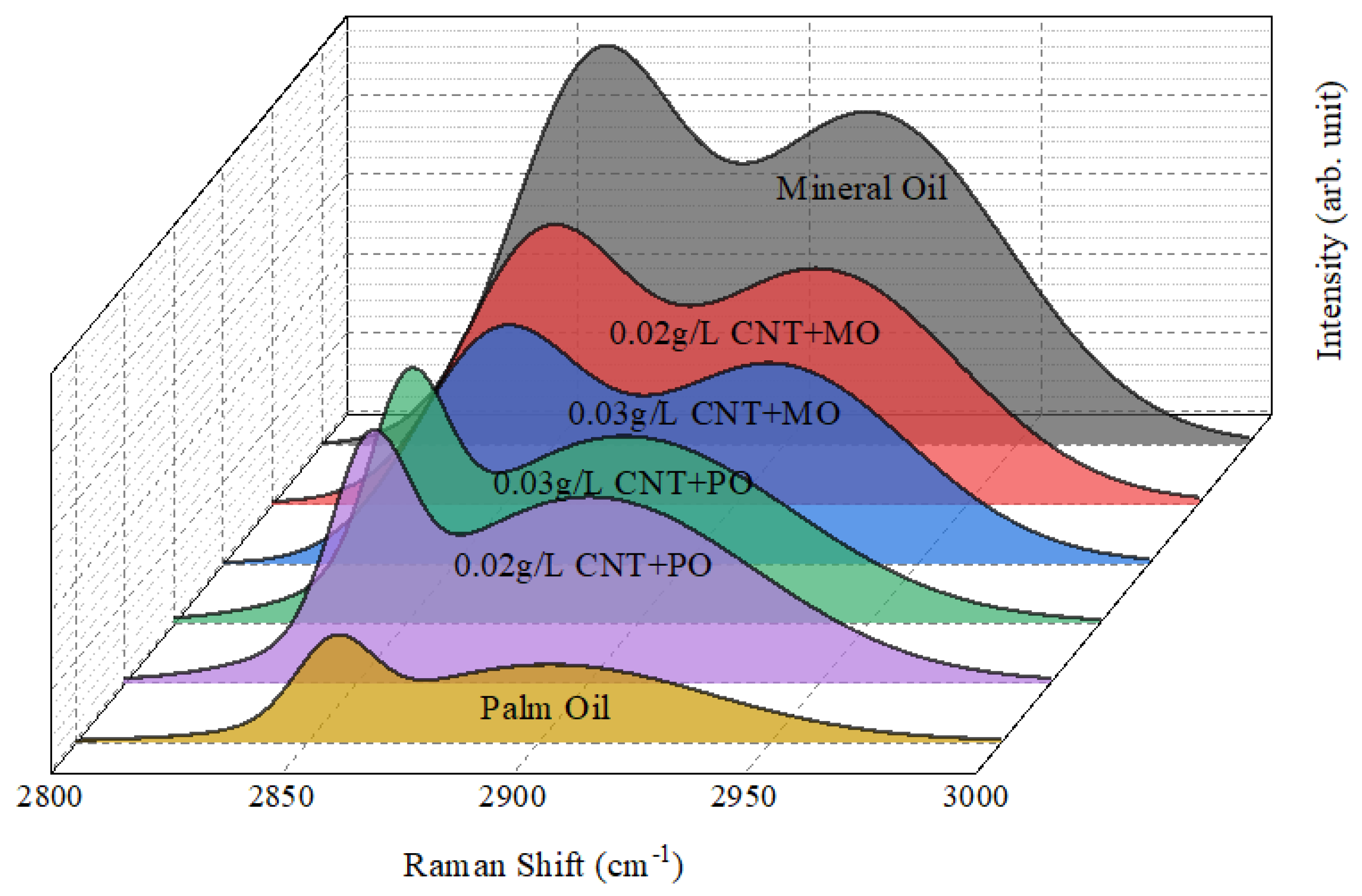


| Oil Samples | Peak 1 | FWHM | Area | Peak 2 | FWHM | Area | IG′/IG″ |
|---|---|---|---|---|---|---|---|
| Mineral Oil | 2858.43 | 41.6049 | 649,083 | 2918.19 | 69.5214 | 1,022,056 | 0.9795 |
| 0.02 g/L CNT + MO | 2857.81 | 41.3858 | 447,516 | 2917.83 | 70.4247 | 729,016 | 0.9794 |
| 0.03 g/L CNT + MO | 2858.66 | 41.4006 | 385,985 | 2918.40 | 69.2314 | 612,169 | 0.9795 |
| Palm Oil | 2855.80 | 19.3357 | 63,697 | 2902.48 | 82.2877 | 279,042 | 0.9839 |
| 0.02 g/L CNT + PO | 2852.80 | 19.4551 | 149,480 | 2900.26 | 84.4675 | 693,816 | 0.9836 |
| 0.03 g/L CNT + PO | 2850.46 | 19.3156 | 148,536 | 2897.26 | 84.5314 | 698,162 | 0.9838 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhaimi, N.S.; Ishak, M.T.; Md Din, M.F.; Hashim, F.R.; Abdul Rahman, A.R. Raman Spectroscopy Characterization of Mineral Oil and Palm Oil with Added Multi-Walled Carbon Nanotube for Application in Oil-Filled Transformers. Energies 2022, 15, 1534. https://doi.org/10.3390/en15041534
Suhaimi NS, Ishak MT, Md Din MF, Hashim FR, Abdul Rahman AR. Raman Spectroscopy Characterization of Mineral Oil and Palm Oil with Added Multi-Walled Carbon Nanotube for Application in Oil-Filled Transformers. Energies. 2022; 15(4):1534. https://doi.org/10.3390/en15041534
Chicago/Turabian StyleSuhaimi, Nur Sabrina, Mohd Taufiq Ishak, Muhamad Faiz Md Din, Fakhroul Ridzuan Hashim, and Abdul Rashid Abdul Rahman. 2022. "Raman Spectroscopy Characterization of Mineral Oil and Palm Oil with Added Multi-Walled Carbon Nanotube for Application in Oil-Filled Transformers" Energies 15, no. 4: 1534. https://doi.org/10.3390/en15041534
APA StyleSuhaimi, N. S., Ishak, M. T., Md Din, M. F., Hashim, F. R., & Abdul Rahman, A. R. (2022). Raman Spectroscopy Characterization of Mineral Oil and Palm Oil with Added Multi-Walled Carbon Nanotube for Application in Oil-Filled Transformers. Energies, 15(4), 1534. https://doi.org/10.3390/en15041534







