Properties of Animal-Origin Ash—A Valuable Material for Circular Economy
Abstract
:1. Introduction
2. Materials and Methods
- Cow manure from free-range farming CD1_FR, CD2_FR
- Cow manure from industrial farming CD3_IF, CD4_IF
- Chicken litter from free-range farming CL1_FR
- Chicken litter from industrial farming CL2_IF, CL3_IF, CL4_IF, CL5_IF
2.1. Feedstock and Ash Analysis
2.2. Ash Deposition, Slagging and Fouling Prediction
3. Results and Discussion
3.1. Feedstock and Ash Characteristics
3.2. Ash Morphology
3.3. Ash Deposition Tendencies
3.4. Metals and Metalloids Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szymajda, A.; Łaska, G.; Joka, M. Assessment of Cow Dung Pellets as a Renewable Solid Fuel in Direct Combustion Technologies. Energies 2021, 14, 1192. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Villamil, J.A.; Qaramaleki, S.V.; Coronella, C.J.; Mohedano, A.F.; Rubia, M.A. de la Energy Valorization of Cow Manure by Hydrothermal Carbonization and Anaerobic Digestion. Renew. Energy 2020, 160, 623–632. [Google Scholar] [CrossRef]
- Atimtay, A.; Yurdakul, S. Combustion and Co-Combustion Characteristics of Torrefied Poultry Litter with Lignite. Renew. Energy 2020, 148, 1292–1301. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.-M. Trace Element Supplementation and Enzyme Addition to Enhance Biogas Production by Anaerobic Digestion of Chicken Litter. Energies 2020, 13, 3477. [Google Scholar] [CrossRef]
- Williams, A.G.; Leinonen, I.; Kyriazakis, I. Environmental Benefits of Using Turkey Litter as a Fuel Instead of a Fertiliser. J. Clean. Prod. 2016, 113, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Pan-in, S.; Sukasem, N. Methane Production Potential from Anaerobic Co-Digestions of Different Animal Dungs and Sweet Corn Residuals. Energy Procedia 2017, 138, 943–948. [Google Scholar] [CrossRef]
- Theofanous, E.; Kythreotou, N.; Panayiotou, G.; Florides, G.; Vyrides, I. Energy Production from Piggery Waste Using Anaerobic Digestion: Current Status and Potential in Cyprus. Renew. Energy 2014, 71, 263–270. [Google Scholar] [CrossRef]
- Orlando, M.-Q.; Borja, V.-M. Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies 2020, 13, 3573. [Google Scholar] [CrossRef]
- Nordin, A.; Strandberg, A.; Elbashir, S.; Åmand, L.-E.; Skoglund, N.; Pettersson, A. Co-Combustion of Municipal Sewage Sludge and Biomass in a Grate Fired Boiler for Phosphorus Recovery in Bottom Ash. Energies 2020, 13, 1708. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, J.; Sheng, L.; Liu, X.; Zong, M.; Yao, D. Anaerobic Digestion Technology for Methane Production Using Deer Manure Under Different Experimental Conditions. Energies 2019, 12, 1819. [Google Scholar] [CrossRef] [Green Version]
- Stępień, P.; Świechowski, K.; Hnat, M.; Kugler, S.; Stegenta-Dąbrowska, S.; Koziel, J.A.; Manczarski, P.; Białowiec, A. Waste to Carbon: Biocoal from Elephant Dung as New Cooking Fuel. Energies 2019, 12, 4344. [Google Scholar] [CrossRef] [Green Version]
- Dobrzański, Z. The Relationship between Modern Poultry Production Systems and the Protection of Natural and Productive Environment. First Agricultural Portal. 2002. Available online: http://www.ppr.pl/artykul-ppr-2924.php?_resourcePK=2924 (accessed on 1 May 2021). (In Polish).
- Dalólio, F.S.; da Silva, J.N.; de Oliveira, A.C.C.; Tinôco, I.D.F.F.; Barbosa, R.C.; de Oliveira Resende, M.; Albino, L.F.T.; Coelho, S.T. Poultry Litter as Biomass Energy: A Review and Future Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 941–949. [Google Scholar] [CrossRef]
- The Agency for Restructuring and Modernization of Agriculture (Poland). Available online: https://www.arimr.gov.pl/fileadmin/pliki/kontrole/2018/Zal_06.pdf (accessed on 1 May 2021).
- Vandecasteele, B.; Reubens, B.; Willekens, K.; de Neve, S. Composting for Increasing the Fertilizer Value of Chicken Manure: Effects of Feedstock on P Availability. Waste Biomass Valorization 2014, 5, 491–503. [Google Scholar] [CrossRef]
- Szogi, A.A.; Vanotti, M.B. Prospects for Phosphorus Recovery from Poultry Litter. Bioresour. Technol. 2009, 100, 5461–5465. [Google Scholar] [CrossRef] [PubMed]
- Keesstra, S.; Sannigrahi, S.; López-Vicente, M.; Pulido, M.; Novara, A.; Visser, S.; Kalantari, Z. The Role of Soils in Regulation and Provision of Blue and Green Water. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 1–8. [Google Scholar] [CrossRef]
- Billen, P.; Costa, J.; van der Aa, L.; van Caneghem, J.; Vandecasteele, C. Electricity from Poultry Manure: A Cleaner Alternative to Direct Land Application. J. Clean. Prod. 2015, 96, 467–475. [Google Scholar] [CrossRef]
- Tańczuk, M.; Junga, R.; Kolasa-Więcek, A.; Niemiec, P. Assessment of the Energy Potential of Chicken Manure in Poland. Energies 2019, 12, 1244. [Google Scholar] [CrossRef] [Green Version]
- Olugbade, T.O.; Ojo, O.T. Binderless Briquetting Technology for Lignite Briquettes: A Review. Energy Ecol. Environ. 2021, 6, 69–79. [Google Scholar] [CrossRef]
- Olugbade, T.; Ojo, O.; Mohammed, T. Influence of Binders on Combustion Properties of Biomass Briquettes: A Recent Review. BioEnergy Res. 2019, 12, 241–259. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Ojo, O.T. Biomass Torrefaction for the Production of High-Grade Solid Biofuels: A Review. BioEnergy Res. 2020, 13, 999–1015. [Google Scholar] [CrossRef]
- Dróżdż, D.; Wystalska, K.; Malińska, K.; Grosser, A.; Grobelak, A.; Kacprzak, M. Management of Poultry Manure in Poland—Current State and Future Perspectives. J. Environ. Manag. 2020, 264, 110327. [Google Scholar] [CrossRef]
- Lynch, D.; Henihan, A.M.; Bowen, B.; Lynch, D.; McDonnell, K.; Kwapinski, W.; Leahy, J.J. Utilisation of Poultry Litter as an Energy Feedstock. Biomass Bioenergy 2013, 49, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Keesstra, S.; Mol, G.; de Leeuw, J.; Okx, J.; Molenaar, C.; de Cleen, M.; Visser, S. Soil-Related Sustainable Development Goals: Four Concepts to Make Land Degradation Neutrality and Restoration Work. Land 2018, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Novara, A.; Pulido, M.; Rodrigo-Comino, J.; di Prima, S.; Smith, P.; Gristina, L.; Gimenez-Morera, A.; Terol, E.; Salesa, D.; Keesstra, S. Long-Term Organic Farming on a Citrus Plantation Results in Soil Organic Carbon Recovery. Cuad. Investig. Geográfica 2019, 45, 271. [Google Scholar] [CrossRef] [Green Version]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef] [Green Version]
- Jarosz-Krzemińska, E.; Poluszyńska, J. Repurposing Fly Ash Derived from Biomass Combustion in Fluidized Bed Boilers in Large Energy Power Plants as a Mineral Soil Amendment. Energies 2020, 13, 4805. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in Poultry Litter Disposal Technology—A Review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef]
- Codling, E.E.; Chaney, R.L.; Sherwell, J. Poultry Litter Ash as a Potential Phosphorus Source for Agricultural Crops. J. Environ. Qual. 2002, 31, 954–961. [Google Scholar] [CrossRef]
- Sugiyama, S.; Wakisaka, K.; Imanishi, K.; Kurashina, M.; Shimoda, N.; Katoh, M.; Liu, J.-C. Recovery of Phosphate Rock Equivalents from Incineration Ash of Chicken Manure by Elution-Precipitation Treatment. J. Chem. Eng. Jpn. 2019, 52, 778–782. [Google Scholar] [CrossRef]
- Kaikake, K.; Sekito, T.; Dote, Y. Phosphate Recovery from Phosphorus-Rich Solution Obtained from Chicken Manure Incineration Ash. Waste Manag. 2009, 29, 1084–1088. [Google Scholar] [CrossRef]
- Bolan, N.S.; Szogi, A.A.; Chuasavathi, T.; Seshadri, B.; Rothrock, M.J.; Panneerselvam, P. Uses and Management of Poultry Litter. World’s Poult. Sci. J. 2010, 66, 673–698. [Google Scholar] [CrossRef] [Green Version]
- Faridullah, F.; Irshad, M.; Yamamoto, S.; Honna, T.; Eneji, A.E. Characterization of Trace Elements in Chicken and Duck Litter Ash. Waste Manag. 2009, 29, 265–271. [Google Scholar] [CrossRef]
- Fiameni, L.; Fahimi, A.; Marchesi, C.; Sorrentino, G.P.; Zanoletti, A.; Moreira, K.; Valentim, B.; Predeanu, G.; Depero, L.E.; Bontempi, E. Phosphorous and Silica Recovery from Rice Husk Poultry Litter Ash: A Sustainability Analysis Using a Zero-Waste Approach. Materials 2021, 14, 6297. [Google Scholar] [CrossRef] [PubMed]
- Maj, I.; Kalisz, S.; Szymajda, A.; Łaska, G.; Gołombek, K. The Influence of Cow Dung and Mixed Straw Ashes on Steel Corrosion. Renew. Energy 2021, 177, 1198–1211. [Google Scholar] [CrossRef]
- Vankát, A.; Krepl, V.; Kára, J. Animal Dung as a Source of Energy in Remote Areas of Indian Himalayas. Agricult. Trop. Subtrop. 2009, 43, 140–142. [Google Scholar]
- Tran, Q.T.; Maeda, M.; Oshita, K.; Takaoka, M. Phosphorus Release from Cattle Manure Ash as Soil Amendment in Laboratory-Scale Tests. Soil Sci. Plant Nutr. 2017, 63, 369–376. [Google Scholar] [CrossRef]
- Fahimi, A.; Bilo, F.; Assi, A.; Dalipi, R.; Federici, S.; Guedes, A.; Valentim, B.; Olgun, H.; Ye, G.; Bialecka, B.; et al. Poultry Litter Ash Characterisation and Recovery. Waste Manag. 2020, 111, 10–21. [Google Scholar] [CrossRef]
- Vassilev, S.v.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Díaz-Ramírez, M.; Frandsen, F.J.; Glarborg, P.; Sebastián, F.; Royo, J. Partitioning of K, Cl, S and P during Combustion of Poplar and Brassica Energy Crops. Fuel 2014, 134, 209–219. [Google Scholar] [CrossRef]
- Kofstad, P. High Temperature Corrosion; Elsevier: London, UK, 1988. [Google Scholar]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpio, M.; Perez-Jimenez, J.A. Critical Review of Predictive Coefficients for Biomass Ash Deposition Tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Weber, R.; Poyraz, Y.; Beckmann, A.M.; Brinker, S. Combustion of Biomass in Jet Flames. Proc. Combust. Inst. 2015, 35, 2749–2758. [Google Scholar] [CrossRef]
- Miles, T.R.; Miles, T.R.; Baxter, L.L.; Bryers, R.W.; Jenkins, B.M.; Oden, L.L. Boiler Deposits from Firing Biomass Fuels. Biomass Bioenergy 1996, 10, 125–138. [Google Scholar] [CrossRef]
- Lachman, J.; Baláš, M.; Lisý, M.; Lisá, H.; Milčák, P.; Elbl, P. An Overview of Slagging and Fouling Indicators and Their Applicability to Biomass Fuels. Fuel Processing Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Kalemba-Rec, I.; Nowak, W. The Influence of Potassium-Rich Biomass Ashes on Steel Corrosion above 550 °C. Energy Convers. Manag. 2019, 187, 15–28. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R.; Miles, T.R. Combustion Properties of Biomass. Fuel Processing Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Vamvuka, D.; Zografos, D. Predicting the Behaviour of Ash from Agricultural Wastes during Combustion. Fuel 2004, 83, 2051–2057. [Google Scholar] [CrossRef]
- McLennan, A.R.; Bryant, G.W.; Bailey, C.W.; Stanmore, B.R.; Wall, T.F. Index for Iron-Based Slagging for Pulverized Coal Firing in Oxidizing and Reducing Conditions. Energy Fuels 2000, 14, 349–354. [Google Scholar] [CrossRef]
- Qian, X.; Lee, S.; Soto, A.; Chen, G. Regression Model to Predict the Higher Heating Value of Poultry Waste from Proximate Analysis. Resources 2018, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Xing, P.; Mason, P.E.; Chilton, S.; Lloyd, S.; Jones, J.M.; Williams, A.; Nimmo, W.; Pourkashanian, M. A Comparative Assessment of Biomass Ash Preparation Methods Using X-ray Fluorescence and Wet Chemical Analysis. Fuel 2016, 182, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Nutalapati, D.; Gupta, R.; Moghtaderi, B.; Wall, T.F. Assessing Slagging and Fouling during Biomass Combustion: A Thermodynamic Approach Allowing for Alkali/Ash Reactions. Fuel Processing Technol. 2007, 88, 1044–1052. [Google Scholar] [CrossRef]
- Liu, B.; He, Q.; Jiang, Z.; Xu, R.; Hu, B. Relationship between Coal Ash Composition and Ash Fusion Temperatures. Fuel 2013, 105, 293–300. [Google Scholar] [CrossRef]
- Sobieraj, J.; Gądek, W.; Jagodzińska, K.; Kalisz, S. Investigations of Optimal Additive Dose for Cl-Rich Biomasses. Renew. Energy 2021, 163, 2008–2017. [Google Scholar] [CrossRef]
- Kalisz, S.; Ciukaj, S.; Mroczek, K.; Tymoszuk, M.; Wejkowski, R.; Pronobis, M.; Kubiczek, H. Full-Scale Study on Halloysite Fireside Additive in 230 t/h Pulverized Coal Utility Boiler. Energy 2015, 92, 33–39. [Google Scholar] [CrossRef]
- Wejkowski, R.; Kalisz, S.; Tymoszuk, M.; Ciukaj, S.; Maj, I. Full-Scale Investigation of Dry Sorbent Injection for NOx Emission Control and Mercury Retention. Energies 2021, 14, 7787. [Google Scholar] [CrossRef]
- Yang, T.; Ma, J.; Li, R.; Kai, X.; Liu, F.; Sun, Y.; Pei, L. Ash Melting Behavior during Co-Gasification of Biomass and Polyethylene. Energy Fuels 2014, 28, 3096–3101. [Google Scholar] [CrossRef]
- Munir, S. Potential Slagging and Fouling Problems Associated with Biomass-Coal Blends in Coal-Fired Boilers. J. Pak. Inst. Chem. Eng. 2010, 38, 1–11. [Google Scholar]
- Tortosa Masiá, A.A.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising Ash of Biomass and Waste. Fuel Processing Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Yang, M.; Li, W. Content of Heavy Metals in Animal Feeds and Manures from Farms of Different Scales in Northeast China. Int. J. Environ. Res. Public Health 2012, 9, 2658–2668. [Google Scholar] [CrossRef] [Green Version]
- Carlon, C. Derivation Methods of Soil Screening Values in Europe. A Review and Evaluation of National Procedures towards Harmonisation; European Commission, Joint Research Centre: Ispra, Italy, 2007. [Google Scholar]
- De Vos, W.; Tarvainen, T. Geochemical Atlas of Europe. Part 2; Geological Survey of Finland: Espoo, Finland, 2006. [Google Scholar]
- Fernández-Martínez, A.; Charlet, L. Selenium Environmental Cycling and Bioavailability: A Structural Chemist Point of View. Rev. Environ. Sci. Bio/Technol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Warsaw, Poland, 2000; ISBN 9780429191121. [Google Scholar]
- Anderson, A.J. Molybdenum as a Fertilizer; Commonwealth Scientific and Industrial Research Organization: Canberra, Australia, 1956. [Google Scholar]
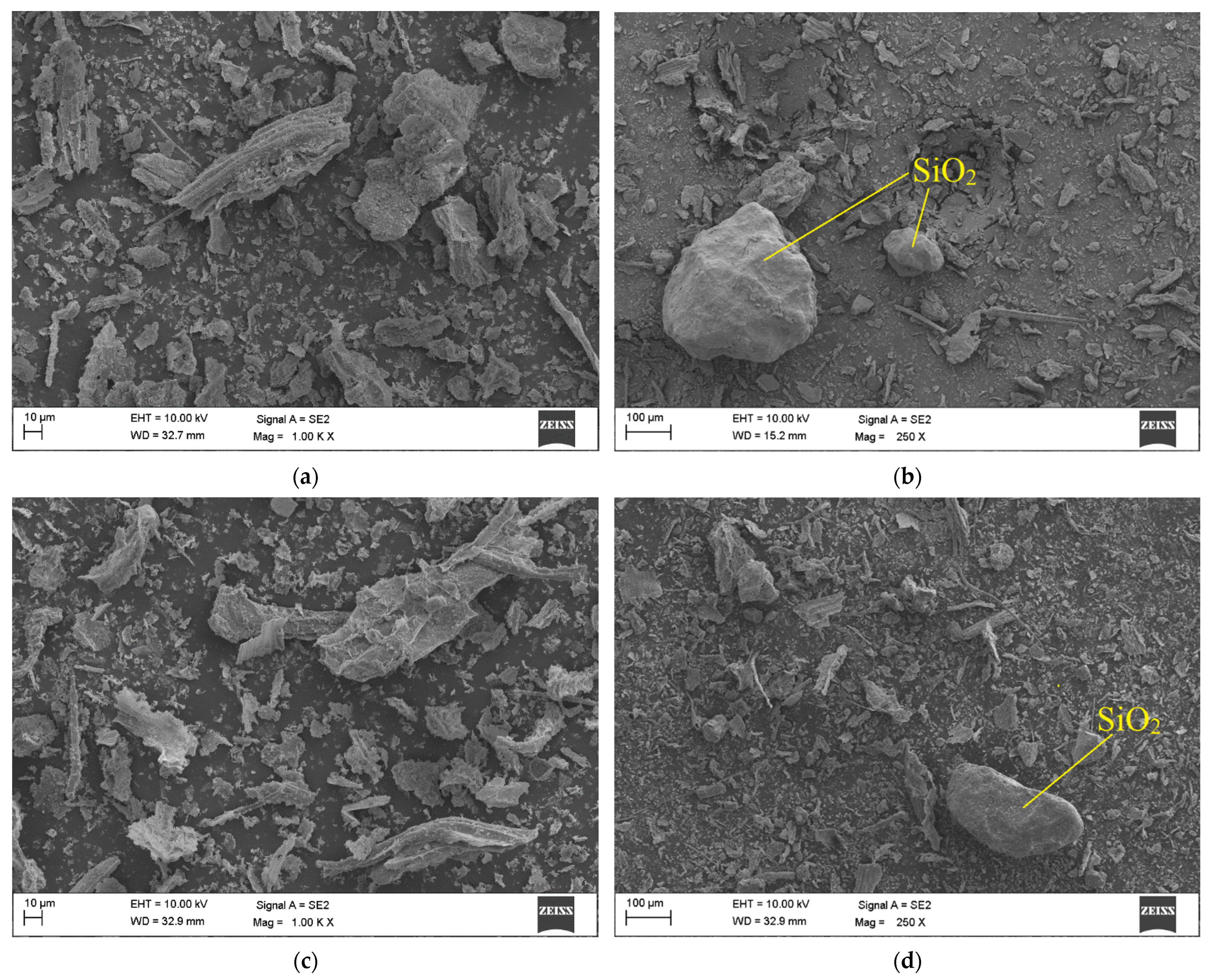
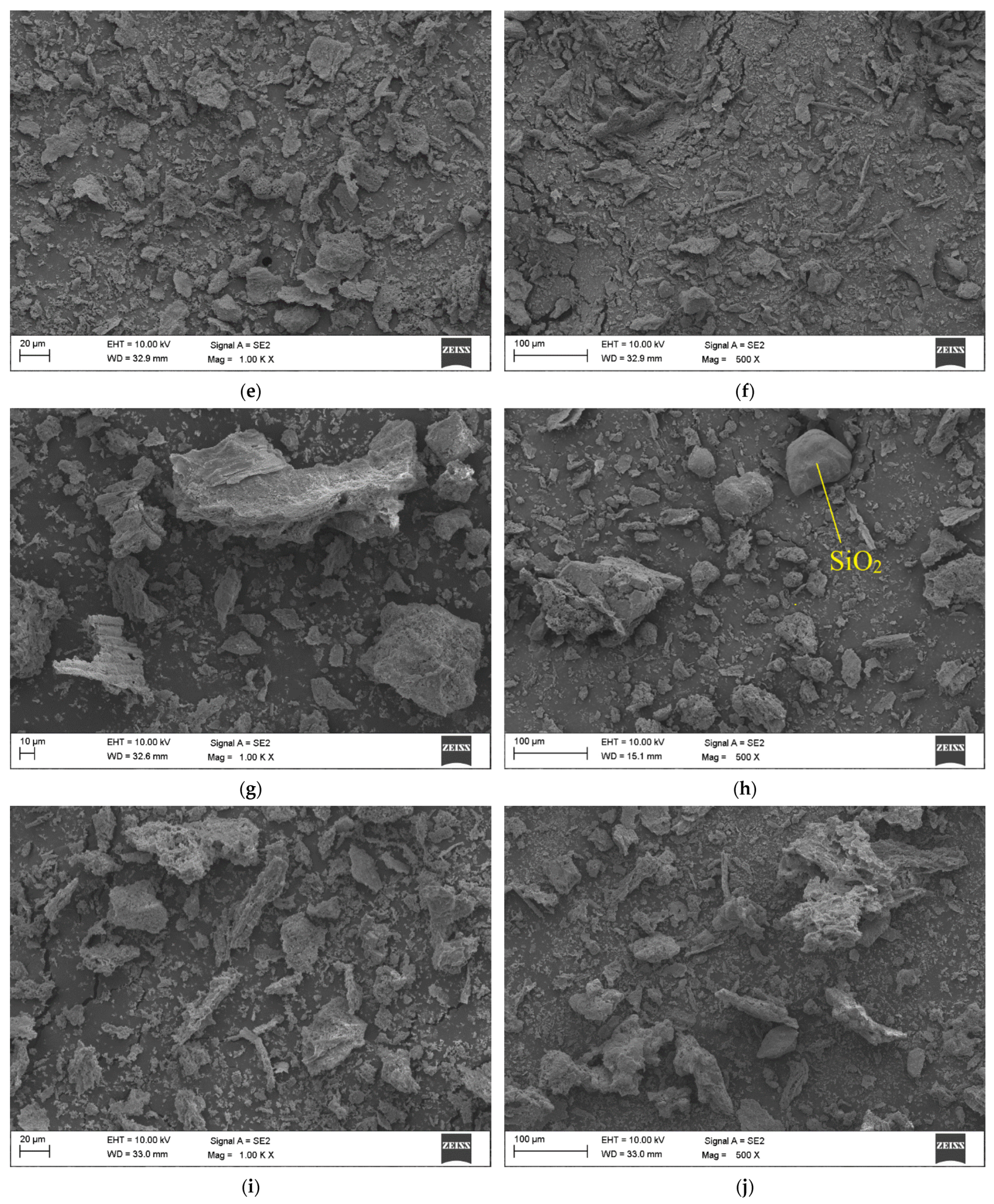
| Parameter | Basis | Unit | Cow Manure | Chicken Litter | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD1_FR | CD2_FR | CD3_IF | CD4_IF [36] | CL1_FR | CL2_IF | CL3_IF | CL4_IF | CL5_IF | |||
| Moisture | a.r. | wt% | 11.4 | 8.4 | 11.1 | 15.5 | 12.10 | 11.1 | 26.7 | 21.9 | 38.8 |
| Ash | d.b. | wt% | 21.4 | 22.06 | 16.99 | 13.86 | 30.10 | 17.09 | 9.31 | 10.70 | 7.10 |
| HHV | d.b. | MJ/kg | 17.26 | 16.93 | 17.91 | 19.04 | 12.22 | 17.22 | 15.60 | 16.90 | 16.60 |
| a.r | MJ/kg | 15.49 | 15.5 | 15.92 | 16.09 | 10.97 | 15.31 | 11.40 | 13.20 | 10.20 | |
| LHV | d.b. | MJ/kg | 15.78 | 15.86 | 16.72 | 17.84 | 11.32 | 16.02 | 15.20 | 15.80 | 16.10 |
| a.r. | MJ/kg | 13.98 | 14.32 | 14.59 | 14.69 | 10.08 | 13.97 | 10.60 | 11.86 | 8.38 | |
| Cl | d.b. | wt% | 0.086 | 0.33 | 1.02 | 0.54 | 0.11 | 0.99 | 0.96 | 0.66 | 0.82 |
| C | d.b. | wt% | 41.94 | 38.93 | 44.07 | 45.26 | 31.19 | 41.85 | 39.10 | 40.3 | 37.7 |
| H | d.b. | wt% | 5.38 | 4.89 | 5.45 | 5.53 | 3.91 | 5.5 | 5.10 | 5.40 | 5.20 |
| N | d.b. | wt% | 2.59 | 1.61 | 2.5 | 2.79 | 2.90 | 4.89 | 4.70 | 4.80 | 4.70 |
| S | d.b. | wt% | 0.34 | 0.32 | 0.47 | 0.32 | 0.50 | 0.97 | 0.73 | 0.75 | 0.31 |
| Parameter | Unit | Cow Manure | Chicken Litter | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD1_FR | CD2_FR | CD3_IF | CD4_IF [36] | CL1_FR | CL2_IF | CL3_IF | CL4_IF | CL5_IF | ||
| SO3 | wt% | 0.88 | 1.18 | 4.45 | 2.63 | 1.02 | 9.68 | 0.94 | 1.01 | 0.82 |
| K2O | wt% | 3.19 | 8.64 | 18.6 | 5.56 | 2.61 | 25.20 | 13.04 | 20.01 | 16.54 |
| SiO2 | wt% | 59.63 | 75.60 | 33.60 | 18.30 | 57.11 | 3.66 | 10.30 | 7.13 | 13.26 |
| Fe2O3 | wt% | 1.52 | 1.11 | 1.24 | 1.06 | 1.94 | 0.92 | 4.10 | 4.11 | 1.84 |
| Al2O3 | wt% | 4.28 | 2.65 | 1.95 | 1.31 | 4.15 | 0.48 | 1.66 | 1.11 | 2.81 |
| Mn3O4 | wt% | 0.18 | 0.12 | 0.16 | 0.51 | 0.13 | 0.63 | 1.75 | 1.92 | 1.86 |
| TiO2 | wt% | 0.21 | 0.24 | 0.15 | 0.09 | 0.23 | 0.04 | 0.63 | 0.51 | 0.43 |
| CaO | wt% | 11.85 | 2.11 | 13.6 | 30.60 | 13.55 | 18.3 | 34.07 | 28.18 | 26.61 |
| MgO | wt% | 2.72 | 1.56 | 5.55 | 8.14 | 2.15 | 7.45 | 6.73 | 6.48 | 5.72 |
| P2O5 | wt% | 8.21 | 4.09 | 10.8 | 17.50 | 7.81 | 21.00 | 19.23 | 22.49 | 23.74 |
| Na2O | wt% | 3.57 | 0.73 | 2.66 | 3.20 | 3.21 | 3.87 | 6.80 | 6.26 | 5.74 |
| BaO | wt% | 0.02 | 0.04 | 0.05 | 0.03 | 0.02 | 0.03 | 0.15 | 0.17 | 0.14 |
| SrO | wt% | 0.02 | 0.02 | 0.03 | 0.04 | 0.02 | 0.05 | 0.34 | 0.39 | 0.31 |
| Cl | wt% | 0.65 | 7.56 | 6.55 | 2.57 | 0.90 | 5.67 | - | - | - |
| Ash Fusion Temperatures in reducing/oxidizing atmosphere | ||||||||||
| initial deformation temperature (IDT) | °C | 910/1020 | 1270/1260 | 1140/1130 | 1160/1230 | 1060/1140 | 1330/1400 | -/1357 | -/1254 | -/1303 |
| softening temperature (ST) | °C | 1150/1240 | 1310/1290 | 1170/1170 | 1210/1270 | 1170/1210 | 1380/1470 | -/1500 | -/1500 | -/1439 |
| hemisphere temperature (HT) | °C | 1370/1340 | 1460/1420 | 1200/1200 | 1320/1300 | 1300/1330 | 1430/>1500 | - | - | - |
| flow temperature (FT) | °C | 1410/1370 | >1500/>1500 | 1310/1310 | 1430/1440 | 1320/1360 | 1490/>1500 | - | - | - |
| Index | Unit | CD1_FR | CD2_FR | CD3_IF | CD4_IF [36] | CL1_FR | CL2_IF | CL3_IF | CL4_IF | CL5_IF | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SiO2 | % | 59.63 | 75.6 | 33.6 | 18.3 | 57.11 | 3.66 | 10.3 | 7.13 | 13.26 |
| 2 | Cl | % d.b. | 0.086 | 0.33 | 1.02 | 0.54 | 0.11 | 0.99 | 0.96 | 0.66 | 0.82 |
| 3 | B/A | - | 0.48 | 0.23 | 1.47 | 3.35 | 0.50 | 18.36 | 6.67 | 10.00 | 4.86 |
| 4 | BAI | - | 0.22 | 0.12 | 0.06 | 0.12 | 0.33 | 0.03 | 0.21 | 0.16 | 0.08 |
| 5 | Rs | - | 0.16 | 0.07 | 0.69 | 1.07 | 0.25 | 17.81 | 4.87 | 7.50 | 1.51 |
| 6 | Fu | - | 3.27 | 2.18 | 31.23 | 29.37 | 2.94 | 533.69 | 132.32 | 262.79 | 108.28 |
| 7 | Sr | - | 78.75 | 94.05 | 62.23 | 31.50 | 76.40 | 12.07 | 18.66 | 15.53 | 27.96 |
| 8 | kg/GJ | 0.44 | 0.31 | 0.17 | 0.46 | 0.50 | 0.13 | 0.36 | 0.23 | 0.39 | |
| 9 | Initial deformation temperature IT | °C | 1020 | 1260 | 1130 | 1230 | 1140 | 1400 | 1357 | 1254 | 1303 |
| 10 | Softening temperature ST | °C | 1240 | 1290 | 1170 | 1270 | 1210 | 1470 | 1500 | 1500 | 1439 |
| 11 | AFI | °C | 1084 | 1292 | 1144 | 1244 | 1178 | 1420 | 1086 | 1003 | 1042 |
| Unit | CD1_FR | CD2_FR | CD3_IF | CL1_FR | CL2_IF | CL3_IF | CL4_IF | CL5_IF | EU Regulation 2019/1009 1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| P2O5 | wt% | 8.21 | 4.09 | 10.8 | 7.81 | 21.00 | 19.23 | 22.49 | 23.74 | |
| P 2 | wt% | 3.58 | 1.78 | 4.71 | 3.41 | 9.16 | 8.38 | 9.81 | 10.35 | |
| Zn | mg/kg | 980 | 493 | 938 | 846 | 2787 | 3400 | 2700 | 2700 | 1500 3 |
| Hg | mg/kg | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 1 |
| Cu | mg/kg | 138 | 35 | 129 | 109 | 612 | 232 | 321 | 280 | 600 3 |
| Cr | mg/kg | 22 | 178 | 52 | 24 | 36 | 73 | 65 | 44 | 2 4 |
| As | mg/kg | 8.86 | 1.78 | 1.38 | 9.99 | <1.0 | <1.0 | <1.0 | <1.0 | 40 |
| Cd | mg/kg | 4.35 | 2.23 | 2.43 | <0.05 | 2.93 | 0.19 | 0.13 | 0.17 | 3 5 |
| Cdrecalc | mg/kg P2O5 | 52.98 | 54.52 | 22.50 | <0.64 | 13.95 | 0.99 | 0.58 | 0.72 | 60 6 |
| Ni | mg/kg | 18.1 | 14.1 | 22.6 | 16.0 | 41.8 | 99.0 | 75.0 | 58.0 | 100 |
| Pb | mg/kg | 40.00 | 16.40 | 18.80 | 49.30 | 5.24 | 4.26 | 2.37 | 3.39 | 120 |
| Unit | CD1_FR | CD2_FR | CD3_IF | CL1_FR | CL2_IF | CL3_IF | CL4_IF | CL5_IF | Concentration in European/USA Soils | |
|---|---|---|---|---|---|---|---|---|---|---|
| V | mg/kg | <0.05 | 29.7 | 15.1 | 34 | 13.4 | 88 | 49 | 36 | median 60 [62] |
| Sb | mg/kg | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | 0.02–31.1 median 0.60 [63] |
| Se | mg/kg | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | up to 600 [64] |
| Sn | mg/kg | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 | <2–106 median 3.0 [63] |
| Mo | mg/kg | 5.59 | 10.90 | 11.80 | 6.25 | 46.50 | 43.00 | 31.00 | 40.00 | <0.1–17.2 median 0.62 [63] |
| Co | mg/kg | 6.07 | 2.97 | 4.57 | 7.61 | 3.66 | 31.00 | 10.70 | 7.03 | 0.1–7.0 [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maj, I.; Kalisz, S.; Ciukaj, S. Properties of Animal-Origin Ash—A Valuable Material for Circular Economy. Energies 2022, 15, 1274. https://doi.org/10.3390/en15041274
Maj I, Kalisz S, Ciukaj S. Properties of Animal-Origin Ash—A Valuable Material for Circular Economy. Energies. 2022; 15(4):1274. https://doi.org/10.3390/en15041274
Chicago/Turabian StyleMaj, Izabella, Sylwester Kalisz, and Szymon Ciukaj. 2022. "Properties of Animal-Origin Ash—A Valuable Material for Circular Economy" Energies 15, no. 4: 1274. https://doi.org/10.3390/en15041274
APA StyleMaj, I., Kalisz, S., & Ciukaj, S. (2022). Properties of Animal-Origin Ash—A Valuable Material for Circular Economy. Energies, 15(4), 1274. https://doi.org/10.3390/en15041274








