Microwave-Assisted Pyrolysis of Leather Waste
(This article belongs to the Section D1: Advanced Energy Materials)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Materials
2.2. Pyrolysis Equipment
2.3. Analysis Methodology
2.3.1. Calorimetry
2.3.2. Experimental Design
2.3.3. GC-MS Analysis
2.3.4. Karl Fischer (KF)
2.3.5. Elemental Analysis and Calorific Value Determination
2.3.6. X-ray Fluorescence Spectrometry
2.3.7. Specific Surface Area Determination
3. Results
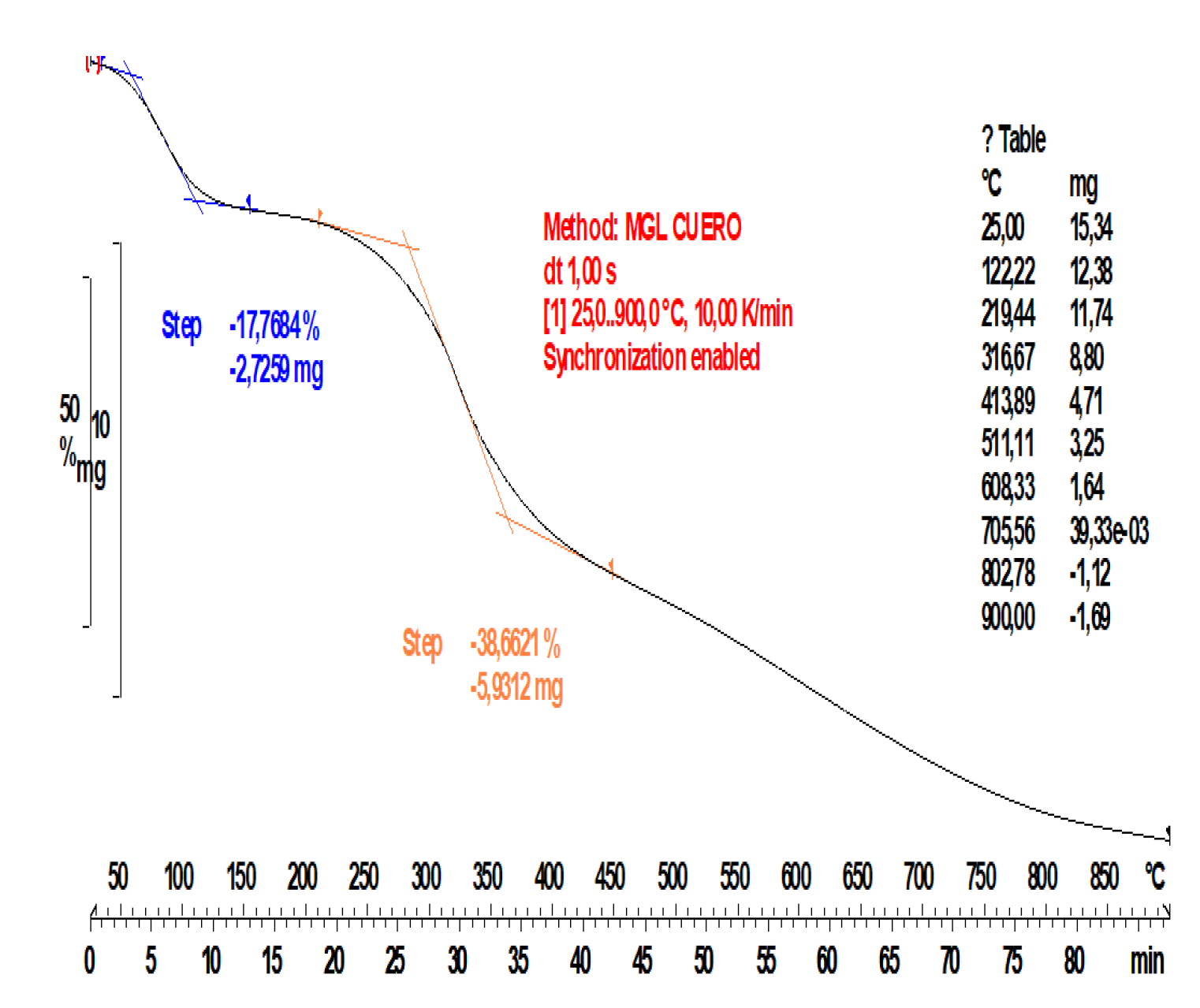
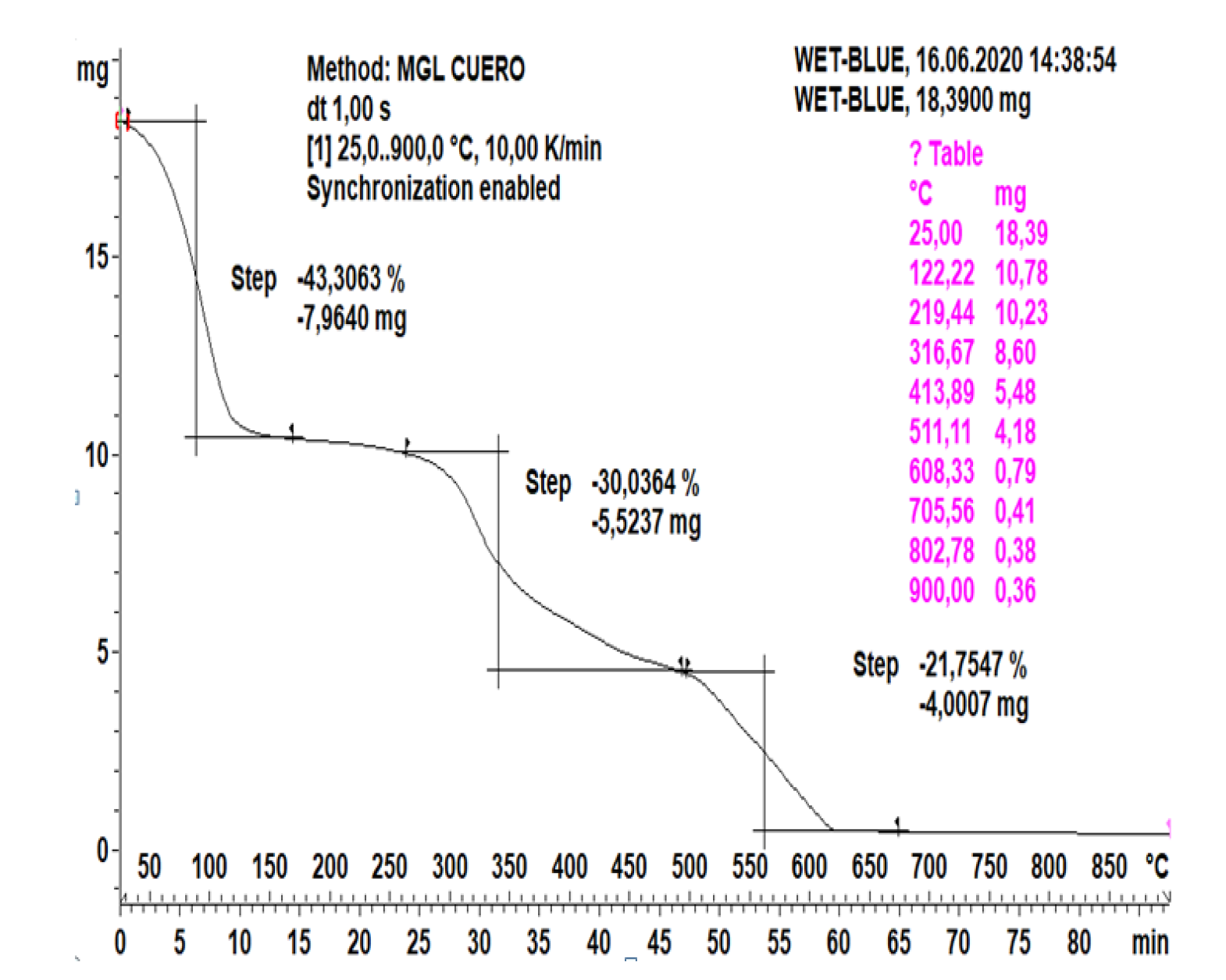
3.1. Raw Material Study
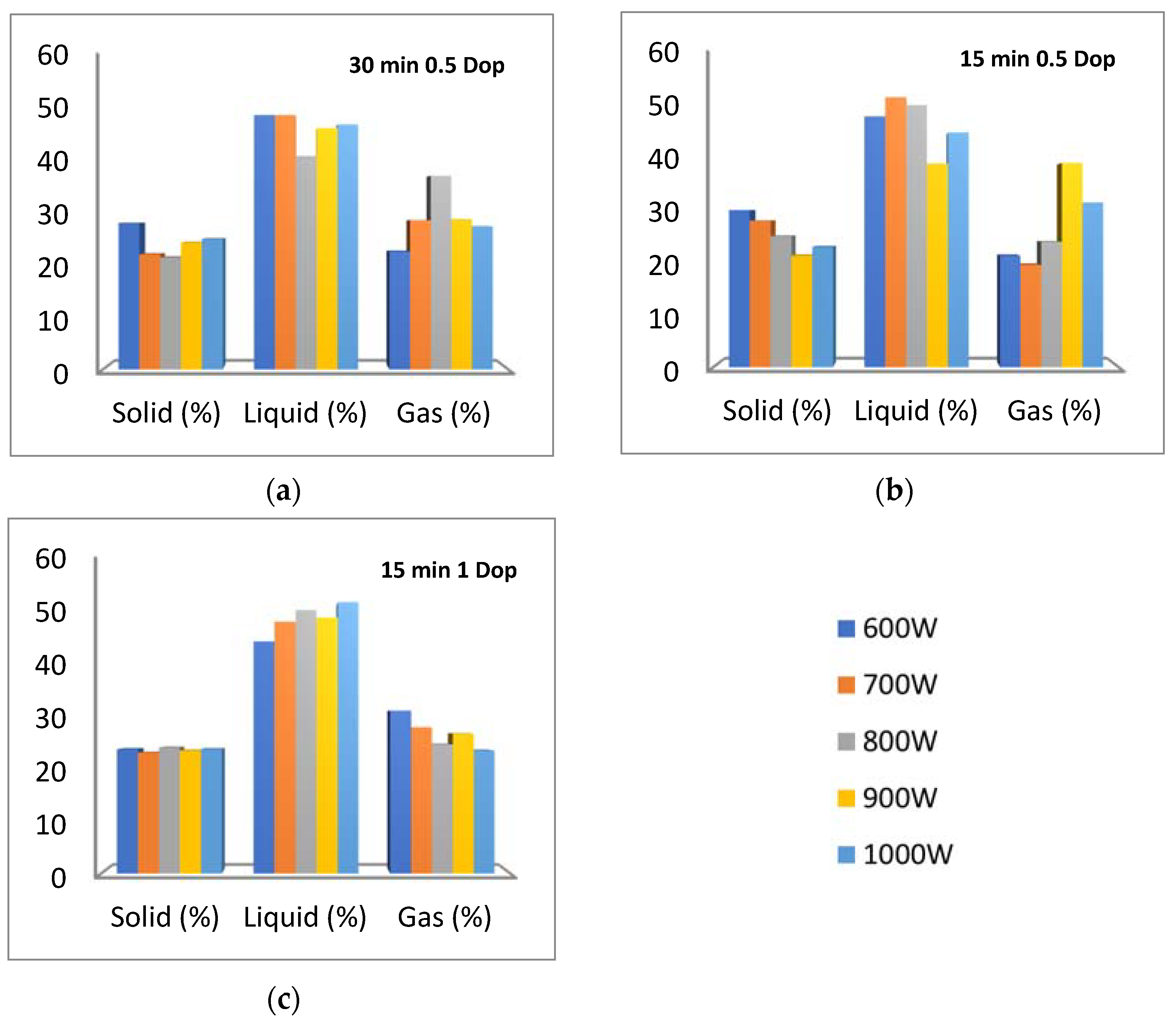
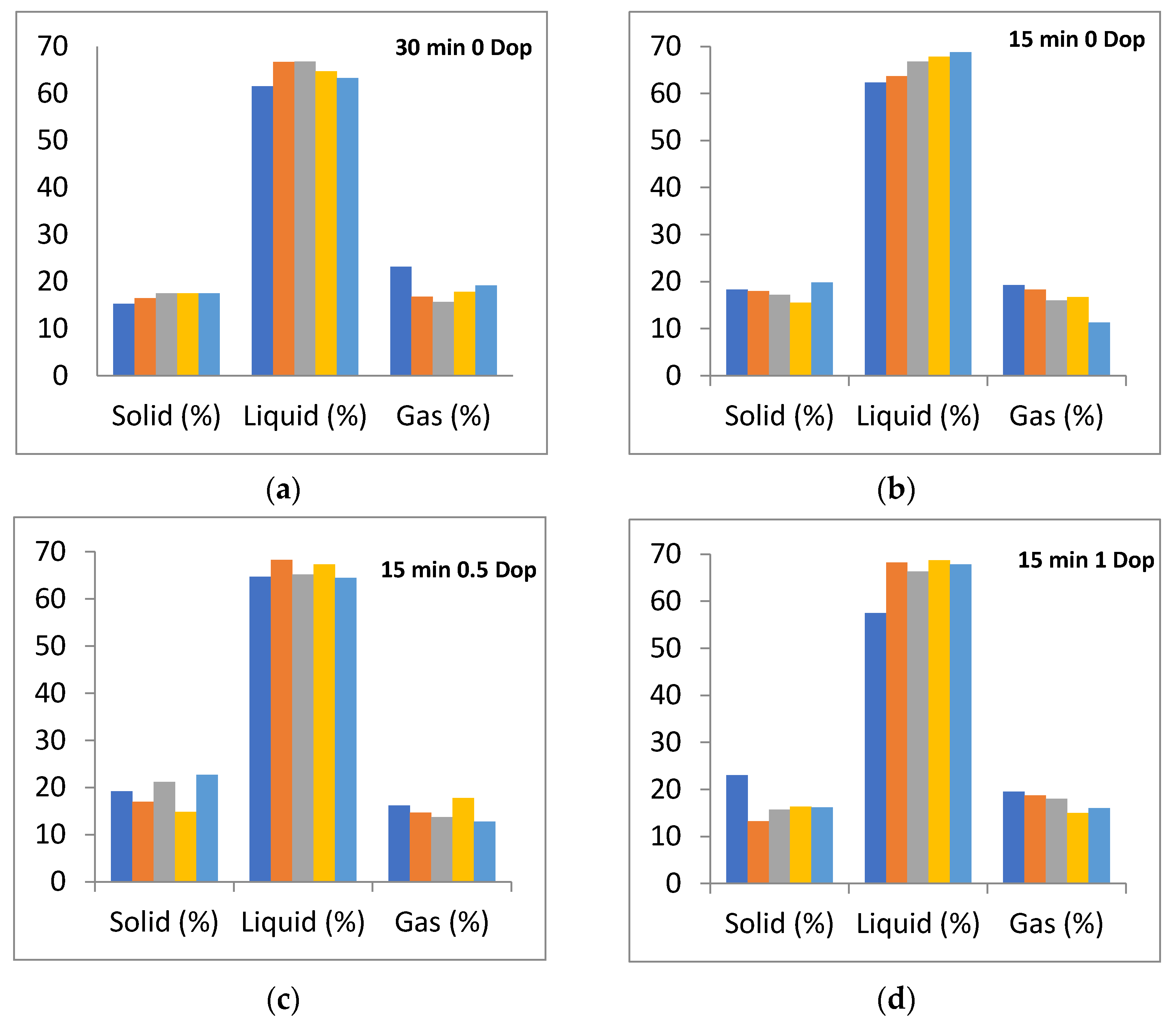
3.2. Distribution of Pyrolysis Products
3.3. Characterisation of the Liquid Fraction
3.4. Characterisation of the Solid Fraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gil, R.R. Aprovechamiento Integral de Residuos Sólidos de Curtición. Implicaciones Medioambient. 2014. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=89768 (accessed on 6 February 2022).
- Engineers, N.B.C. Leather Processing & Tanning Technology Handbook; NIIR Projet Consultancy Services: Delhi, Indonesia, 2011; ISBN 9788190568593. [Google Scholar]
- Kanagaraj, J.; Senthilvelan, T.; Panda, R.C.; Kavitha, S. Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: A comprehensive review. J. Clean. Prod. 2015, 89, 1–17. [Google Scholar] [CrossRef]
- Bermúdez, J.; Dominguez, P.; Arenillas, A.; Cot, J.; Weber, J.; Luque, R. CO2 separation and capture properties of porous carbonaceous materials from leather residues. Materials 2013, 6, 4641–4653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabeza, L.F.; Taylor, M.M.; DiMaio, G.L.; Brown, E.M.; Marmer, W.N.; Carrio, R.; Celma, P.J.; Cot, J. Processing of leather waste: Pilot scale studies on chrome shavings. Isolation of potentially valuable protein products and chromium. Waste Manag. 1998, 18, 211–218. [Google Scholar] [CrossRef]
- Rao, J.R.; Thanikaivelan, P.; Sreeram, K.J.; Nair, B.U. Green route for the utilization of chrome shavings (chromium-containing solid waste) in tanning industry. Environ. Sci. Technol. 2002, 36, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Aylón, E.; Fernández-Colino, A.; Murillo, R.; Navarro, M.V.; García, T.; Mastral, A.M. Valorisation of waste tyre by pyrolysis in a moving bed reactor. Waste Manag. 2010, 30, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, M.L.; Mkhize, N.M. Characterization and pyrolysis of post-consumer leather shoe waste for the recovery of valuable chemicals. Detritus 2021, 14, 92. [Google Scholar] [CrossRef]
- López, L.A.; De Becerra, J.O.; Sánchez, M.A.M.; Palacios, J.F.; Alicante, E. Combustión límpia de residuos de piel. CIEMAT INESCOP 2008, 1–23. [Google Scholar]
- Alptekin, E.; Canakci, M.; Sanli, H. Evaluation of leather industry wastes as a feedstock for biodiesel production. Fuel 2012, 95, 214–220. [Google Scholar] [CrossRef]
- Özgünay, H.; Çolak, S.; Zengin, G.; Sari, Ö.; Sarikahya, H.; Yüceer, L. Performance and emission study of biodiesel from leather industry pre-fleshings. Waste Manag. 2007, 27, 1897–1901. [Google Scholar] [CrossRef]
- Luis, M.; Damaris, C. Estudio mecánico del asfalto modificado con polímeros y cueros que son utilizados en la elaboración del calzado. Rev. L’Estrit Ingénieux 2014, 5. [Google Scholar]
- El-Sabbagh, S.H.; Mohamed, O.A. Recycling of chrome-tanned leather waste in acrylonitrile butadiene rubber. J. Appl. Polym. Sci. 2011, 121, 979–988. [Google Scholar] [CrossRef]
- Baena-González, J.; Santamaria-Echart, A.; Aguirre, J.L.; González, S. Chemical recycling of plastic waste: Bitumen, solvents, and polystyrene from pyrolysis oil. Waste Manag. 2020, 118, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Mahari, W.A.W.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of microalgae residues after lipid extraction: Pyrolysis characteristics for biofuel production. Biochem. Eng. J. 2021, 179, 108330. [Google Scholar] [CrossRef]
- Li, G.; Bai, X.; Huo, S.; Huang, Z. Fast pyrolysis of LERDADEs for renewable biofuels. IET Renew. Power Gener. 2020, 14, 959–967. [Google Scholar] [CrossRef]
- Muralidhara, H.S.; Maggin, B.; Phipps, H., Jr. Conversion of tannery waste to useful products. Resour. Conserv. 1982, 8, 43–59. [Google Scholar] [CrossRef]
- Muralidhara, H.S. Recovery of Potential Energy and Chromium Values from Leather Tannery Wastes. U.S. Patent No 4,332,584, 1982. [Google Scholar]
- Font, R.; Caballero, J.A.; Esperanza, M.M.; Fullana, A. Pyrolytic products from tannery wastes. J. Anal. Appl. Pyrolysis 1999, 49, 243–256. [Google Scholar] [CrossRef]
- Yılmaz, O.; Kantarli, I.C.; Yuksel, M.; Saglam, M.; Yanik, J. Conversion of leather wastes to useful products. Resour. Conserv. Recycl. 2007, 49, 436–448. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Guerreiro, M.C.; Gonçalves, M.; Oliveira, D.Q.L.; Costa, L.C.M. Preparation of activated carbon from leather waste: A new material containing small particle of chromium oxide. Mater. Lett. 2008, 62, 3710–3712. [Google Scholar] [CrossRef]
- Sethuraman, C.; Srinivas, K.; Sekaran, G. Double pyrolysis of chrome tanned leather solid waste for safe disposal and products recovery. Int. J. Sci. Eng. Res. 2013, 4, 61–67. [Google Scholar]
- Gil, R.R.; Girón, R.P.; Lozano, M.S.; Ruiz, B.; Fuente, E. Pyrolysis of biocollagenic wastes of vegetable tanning. Optimization and kinetic study. J. Anal. Appl. Pyrolysis 2012, 98, 129–136. [Google Scholar] [CrossRef]
- Gil, R.R.; Ruiz, B.; Lozano, M.S.; Fuente, E. The role of crosslinking treatment on the pore structure and water transmission of biocollagenic materials. J. Appl. Polym. Sci. 2013, 130, 1812–1822. [Google Scholar] [CrossRef]
- Marcilla, A.; León, M.; García, A.N.; Bañón, E.; Martínez, P. Upgrading of tannery wastes under fast and slow pyrolysis conditions. Ind. Eng. Chem. Res. 2012, 51, 3246–3255. [Google Scholar] [CrossRef]
- Gürel, K.; Magalhães, D.; Kazanç, F. The effect of torrefaction, slow, and fast pyrolysis on the single particle combustion of agricultural biomass and lignite coal at high heating rates. Fuel 2022, 308, 122054. [Google Scholar] [CrossRef]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. Gcb Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of slow and fast pyrolysis for converting biomass into fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Amdouni, S.; Trabelsi, A.B.H.; Elasmi, A.M.; Chagtmi, R.; Haddad, K.; Jamaaoui, F.; Khedhira, H.; Chérif, C. Tannery fleshing wastes conversion into high value-added biofuels and biochars using pyrolysis process. Fuel 2021, 294, 120423. [Google Scholar] [CrossRef]
- El-Hout, S.I.; Attia, S.Y.; Mohamed, S.G.; Abdelbasir, S.M. From waste to value-added products: Evaluation of activated carbon generated from leather waste for supercapacitor applications. J. Environ. Manag. 2022, 304, 114222. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Coura, C.V.Z.; Guimarães, I.R.; Gonçalves, M. Removal of organic dyes using Cr-containing activated carbon prepared from leather waste. J. Hazard. Mater. 2011, 192, 1094–1099. [Google Scholar] [CrossRef]
- Kluska, J.; Ochnio, M.; Kardaś, D.; Heda, Ł. The influence of temperature on the physicochemical properties of products of pyrolysis of leather-tannery waste. Waste Manag. 2019, 88, 248–256. [Google Scholar] [CrossRef]
- Kaur, R.; Rani, V.; Abbot, V. Recent synthetic and medicinal perspectives of pyrroles: An overview. J. Pharm. Chem. Chem. Sci. 2017, 1, 17–32. [Google Scholar]
- Getachew, P.; Getachew, M.; Joo, J.; Choi, Y.S.; Hwang, D.S.; Hong, Y.-K. The slip agents oleamide and erucamide reduce biofouling by marine benthic organisms (diatoms, biofilms and abalones). Toxicol. Environ. Health Sci. 2016, 8, 341–348. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Oasmaa, A.; Solantausta, Y. Power generation using fast pyrolysis liquids from biomass. Renew. Sustain. Energy Rev. 2007, 11, 1056–1086. [Google Scholar] [CrossRef]
- Francescato, V.; Antonini, E.; Bergomi, L.Z. Manual de Combustibles de Madera Producción Requisitos de Calidad Comercialización. AIEL Italian Agriforestry Energy Association. 2008. Available online: https://docplayer.es/11944307-Manual-de-combustibles-de-madera-produccion-requisitos-de-calidad-comercializacion.html (accessed on 6 February 2022).
- Tôrres Filho, A.; Lange, L.C.; de Melo, G.C.B.; Praes, G.E. Pyrolysis of chromium rich tanning industrial wastes and utilization of carbonized wastes in metallurgical process. Waste Manag. 2016, 48, 448–456. [Google Scholar] [CrossRef]
- Konikkara, N.; Kennedy, L.J.; Vijaya, J.J. Preparation and characterization of hierarchical porous carbons derived from solid leather waste for supercapacitor applications. J. Hazard. Mater. 2016, 318, 173–185. [Google Scholar] [CrossRef]
- Kong, J.; Yue, Q.; Wang, B.; Huang, L.; Gao, B.; Wang, Y.; Li, Q. Preparation and characterization of activated carbon from leather waste microwave-induced pyrophosphoric acid activation. J. Anal. Appl. Pyrolysis 2013, 104, 710–713. [Google Scholar] [CrossRef]
- Choudhury, T.R.; Naher, U.H.B.; Akter, S.; Begum, B.A.; Rahman, M.S. Chromium (III) Removal from Synthetic Wastewater Using Biochar Produced from Vegetable Tanned Leather Shaving Dust. J. Sci. Res. Rep. 2020, 26, 68–80. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Zhao, J.; Du, Z.; Song, T.; Chu, M.; Wang, L.; Xie, W. Synthesis of floatable magnetic iron/biochar beads for the removal of chromium from aqueous solutions. Environ. Technol. Innov. 2020, 19, 100907. [Google Scholar] [CrossRef]
- Huang, X.; Yu, F.; Peng, Q.; Huang, Y. Superb adsorption capacity of biochar derived from leather shavings for Congo red. RSC Adv. 2018, 8, 29781–29788. [Google Scholar] [CrossRef] [Green Version]
- Wells, H.C.; Sizeland, K.H.; Edmonds, R.L.; Aitkenhead, W.; Kappen, P.; Glover, C.; Johannessen, B.; Haverkamp, R.G. Stabilizing chromium from leather waste in biochar. ACS Sustain. Chem. Eng. 2014, 2, 1864–1870. [Google Scholar] [CrossRef]
- Martínez-Casillas, D.C.; Alonso-Lemus, I.L.; Mascorro-Gutiérrez, I.; Cuentas-Gallegos, A.K. Leather waste-derived biochar with high performance for supercapacitors. J. Electrochem. Soc. 2018, 165, A2061. [Google Scholar] [CrossRef]

| Vegetable Tannins | Chromium Salt | |
|---|---|---|
| Elemental analysis | ||
| C (%wt) | 53.13 | 44.71 |
| H (%wt) | 6.29 | 5.83 |
| N (%wt) | 13.9 | 17.6 |
| S (%wt) | 2.07 | 1.09 |
| O (%wt) | 23.8 | 23.7 |
| Proximate analysis | ||
| Ash (%wt) | 0.79 | 7.16 |
| Moisture (wt%) | 13.3 | 36.9 |
| Calorific value (MJ kg−1) | 16–17 | 12–14 |
| Tanning with Vegetable Tannin | |||||
|---|---|---|---|---|---|
| Light Fraction | Heavy Fraction | ||||
| Tr (min) | Compound | Area Sum (%) (a) | Tr (min) | Compound | Area Sum (%) |
| 9.25 | Pyridine | 3.14–12.1 | 13.33 | Phenol | 9.86–21.2 |
| 10.01 | Pyrrole | 15.6–38.7 | 14.00 | 3-Methylphenol (cresol) | 5.16–10.5 |
| 13.02 | Aniline | 15.0–24.1 | 14.28 | m-Cresyl methylcarbamate | 3.90–8.44 |
| 13.33 | Phenol | 9.46–26.6 | 17.56 | 1H-Pyrrole-2-carboxamide | 4.41–14.6 |
| 14.00 | 3-Methylphenol (cresol) | 3.87–12.9 | 17.93 | 2,4-Imidazolidinedione, 5,5-dimethyl- | 2.05–11.7 |
| Tanning with Chromium Salts | |||||
| Tr (min) | Compound | Area Sum (%) | |||
| 9.25 | Pyridine | 1.53–6.92 | |||
| 10.01 | Pyrrole | 18.0–28.1 | |||
| 11.26 | 3-Methylpyrrol | 2.38–6.98 | |||
| 15.94 | Succinimide | 2.45–7.00 | |||
| 17.56 | 1H-Pyrrole-2-carboxamide | 3.89–10.3 | |||
| 17.93 | 2,4-Imidazolidinedione, 5,5-dimethyl- | 4.74–8.38 | |||
| Vegetable Tannins | Chromium Salt | |||||
|---|---|---|---|---|---|---|
| Power (W) | 600 | 800 | 1000 | 600 | 800 | 1000 |
| LHV (MJ/kg) | 26–28 | 20–23 | ||||
| BET (m2/g) | 0.8–15.3 | 0.2–104.9 | 2.0–5.4 | 0.3–2.4 | 0.8–119.8 | 2.9–87.8 |
| Element Concentration (ppm) | Vegetable Tannins | Chromium Salt |
|---|---|---|
| Al | 600–880 | 170–620 |
| Si | 1700–6600 | 0–310 |
| P | 930–1900 | 118–450 |
| S | 1800–3500 | 3600–7600 |
| Cl | - | 11,900–31,600 |
| K | 65–90 | 0–13 |
| Ca | 500–630 | 1000–2100 |
| V | - | 18–42 |
| Cr | - | 18,000–33,000 |
| Mn | - | 80–173 |
| Fe | 100–250 | 395–450 |
| Zn | 2–100 | 1.3–10 |
| Br | 0–310 | 790–5100 |
| Sr | 320–610 | 300–530 |
| Zr | 670–1030 | 310–1900 |
| Sn | 0–8 | 0–7.7 |
| Sb | 50–100 | 20–60 |
| I | 38–74 | 0–53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Lucas, M.; Peinado, M.; Vaquero, J.J.; Nozal, L.; Aguirre, J.L.; González-Egido, S. Microwave-Assisted Pyrolysis of Leather Waste. Energies 2022, 15, 1273. https://doi.org/10.3390/en15041273
González-Lucas M, Peinado M, Vaquero JJ, Nozal L, Aguirre JL, González-Egido S. Microwave-Assisted Pyrolysis of Leather Waste. Energies. 2022; 15(4):1273. https://doi.org/10.3390/en15041273
Chicago/Turabian StyleGonzález-Lucas, María, Manuel Peinado, Juan J. Vaquero, Leonor Nozal, Juan Luis Aguirre, and Sergio González-Egido. 2022. "Microwave-Assisted Pyrolysis of Leather Waste" Energies 15, no. 4: 1273. https://doi.org/10.3390/en15041273
APA StyleGonzález-Lucas, M., Peinado, M., Vaquero, J. J., Nozal, L., Aguirre, J. L., & González-Egido, S. (2022). Microwave-Assisted Pyrolysis of Leather Waste. Energies, 15(4), 1273. https://doi.org/10.3390/en15041273






