Influence of the Fertilization Method on the Silphium perfoliatum Biomass Composition and Methane Fermentation Efficiency
Abstract
:1. Introduction
2. Materials and Methods
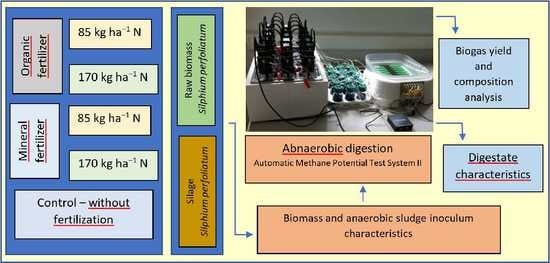
2.1. Organization of Experimental Works
2.2. Feedstock Origin
2.3. Silage Preparation
2.4. Anaerobic Digestion Test
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results and Discussion
3.1. Biomass Characteristics
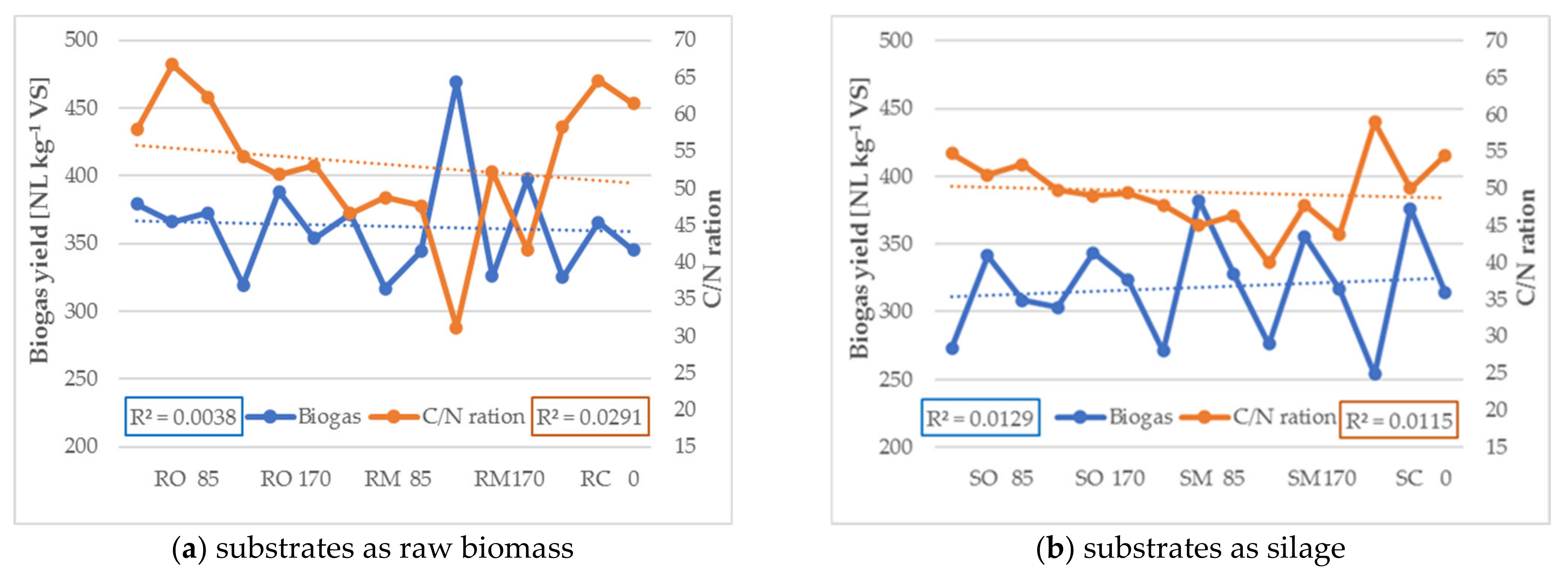
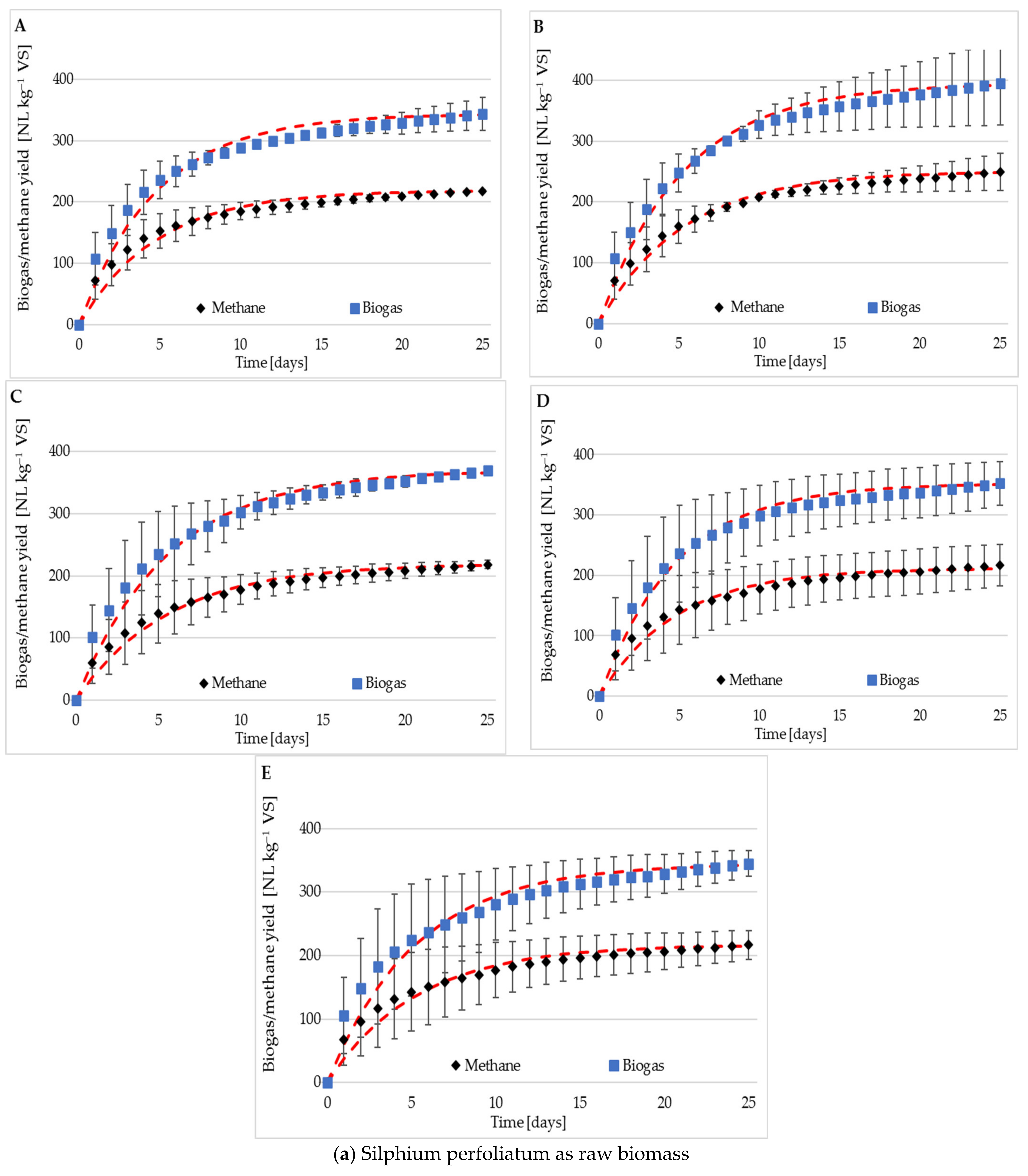
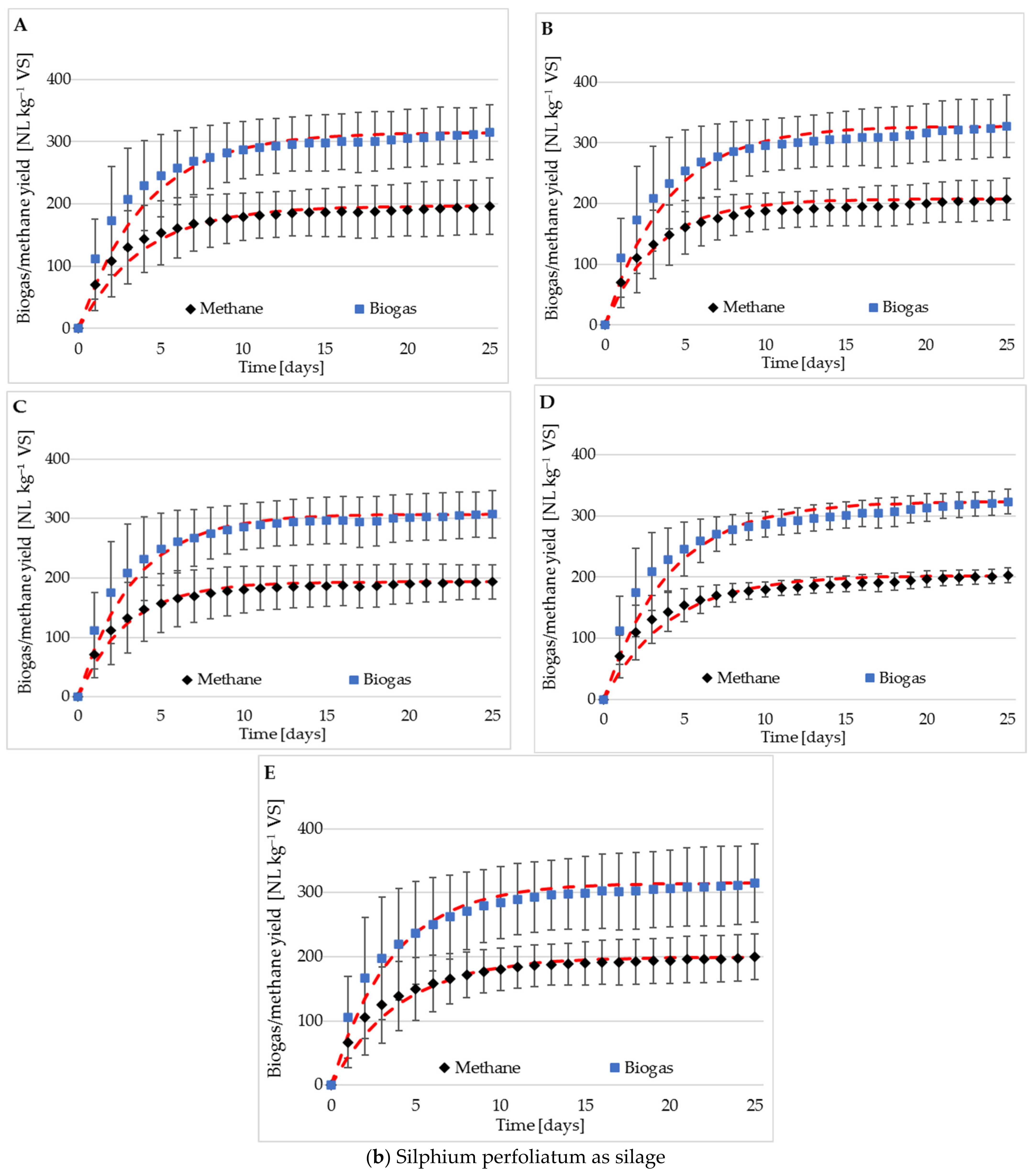
3.2. Methane and Biogas Production
3.3. Methane Content
3.4. Digestate Characteristic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazimierowicz, J.; Dzienis, L. Giant miscanthus as a substrate for biogas production. J. Ecol. Eng. 2015, 16, 139–142. [Google Scholar] [CrossRef]
- Paolini, V.; Petracchini, F.; Segreto, M.; Tomassetti, L.; Naja, N.; Cecinato, A. Environmental impact of biogas: A short review of current knowledge. J. Environ. Sci. Health A 2018, 53, 899–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. IPCC Special Report: Global Warming of 1.5 °C—Summary for Policymakers; World Meteorological Organization: Geneva, Switzerland, 2018; pp. 1–32. [Google Scholar]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. The Effect of Electromagnetic Microwave Radiation on Methane Fermentation of Selected Energy Crop Species. Processes 2022, 10, 45. [Google Scholar] [CrossRef]
- EU Commission. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC. Off. J. Eur. Union 2009, 11, 39–85. [Google Scholar]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Kazimierowicz, J. Organic waste used in agricultural biogas plants. J. Ecol. Eng. 2014, 15, 88–92. [Google Scholar] [CrossRef]
- Lukehurst, C.T.; Frost, P.; Al Seadi, T. Utilisation of Digestate from Biogas Plants as Biofertiliser; IEA Bioenergy: Paris, France, 2010; pp. 1–36. [Google Scholar]
- Thrän, D.; Schaubach, K.; Majer, S.; Horschig, T. Governance of sustainability in the German biogas sector—Adaptive management of the Renewable Energy Act between agriculture and the energy sector. Energy Sustain. Soc. 2020, 10, 3. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Śnieg, M.; Słomińska, E.; Piórkowski, M.; Filipkowski, R. Thermophysical and chemical properties of perennial energy crops depending on harvest period. Int. Agrophys. 2014, 28, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Hijazi, O.; Munro, S.; Zerhusen, B.; Effenberger, M. Review of life cycle assessment for biogas production in Europe. Renew. Sustain. Energy Rev. 2016, 54, 1291–1300. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Zieliński, M.; Dębowski, M. Influence of the Heating Method on the Efficiency of Biomethane Production from Expired Food Products. Fermentation 2021, 7, 12. [Google Scholar] [CrossRef]
- Gołuchowska, B.; Sławiński, J.; Markowski, G. Biomass utilization as a renevable energy source in polish power industry–current status and perspectives. J. Ecol. Eng. 2015, 16, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M.; Olba–Zięty, E.; Akincza, M. Bioenergy technologies and biomass potential vary in Northern European countries. Renew. Sustain. Energy Rev. 2020, 133, 110238. [Google Scholar] [CrossRef]
- Główny Urząd Statystyczny. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/energia/energia-2021-folder,1,9.html (accessed on 30 September 2021).
- Jankowski, K.J.; Dubis, B.; Budzyński, W.S.; Bórawski, P.; Bułkowska, K. Energy efficiency of crops grown for biogas production in a large-scale farm in Poland. Energy 2016, 109, 277–286. [Google Scholar] [CrossRef]
- Biuletyn Informacji Publicznej Krajowego Ośrodka Wsparcia Rolnictwa. Available online: https://bip.kowr.gov.pl/informacje-publiczne/odnawialne-zrodla-energii/biogaz-rolniczy/dane-dotyczace-dzialalnosci-wytworcow-biogazu-rolniczego-w-latach-2011–2019 (accessed on 29 July 2021).
- Zieliński, M.; Rusanowska, P.; Zielińska, M.; Dudek, M.; Nowicka, A.; Purwin, C.; Fijałkowska, M.; Dębowski, M. Influence of preparation of Sida hermaphrodita silages on its conversion to methane. Renew. Energy 2021, 163, 437–444. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of methane fermentation as a valorisation method for food waste products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Peni, D.; Stolarski, M.J.; Bordiean, A.; Krzyżaniak, M.; Dębowski, M. Silphium perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use. Agriculture 2020, 10, 640. [Google Scholar] [CrossRef]
- Siwek, H.; Włodarczyk, M.; Możdżer, E.; Bury, M.; Kitczak, T. Chemical Composition and Biogas Formation potential of Sida hermaphrodita and Silphium perfoliatum. Appl. Sci. 2019, 9, 4016. [Google Scholar] [CrossRef] [Green Version]
- Von Cossel, M.; Amarysti, C.; Wilhelm, H.; Priya, N.; Winkler, B.; Hoerner, L. The replacement of maize (Zea mays L.) by cup plant (Silphium perfoliatum L.) as biogas substrate and its implications for the energy and material flows of a large biogas plant. Biofuels Bioprod. Biorefin. 2020, 14, 152–179. [Google Scholar] [CrossRef] [Green Version]
- Pastukhova, M.A.; Sheliuto, B.V. Cultivation of Silphium perfoaliatum under agricultural crops cover. Bull. Belarus. State Agric. Acad. 2019, 3, 83–87. [Google Scholar]
- Pichard, G. Management, production, and nutritional characteristics of cup-plant (Silphium perfoliatum) in temperate climates of southern Chile. Cienc. Investig. Agrar. 2012, 39, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Guoyan, P.; Ouyang, Z.; Qunying, L.; Qiang, Y.; Jishun, W. Water consumption of seven forage cultivars under different climatic conditions in the North China plain. J. Resour. Ecol. 2011, 2, 74–82. [Google Scholar] [CrossRef]
- Gimbatov, A.S.; Alimirzaeva, G.A. Optimization of technology for the cultivation of new feed crops in the irrigated conditions of the lowland zone of Dagestan. Probl. Dev. Agric. Ind. APK Reg. 2011, 5, 8–11. [Google Scholar]
- Stanford, G. Silphium perfoliatum (cup-plant) as a new forage. In Recapturing a Vanishing Heritage, Proceedings of the 12th North American Prairie Conference, Cedar Falls, IA, USA, 5–9 August 1990; University of Northern Iowa: Cedar Falls, IA, USA, 1990; pp. 33–37. [Google Scholar]
- Kowalski, R. Analysis of lipophilic fraction from leaves, inflorescences and rhizomes of Silphium perfoliatum L. Acta Soc. Bot. Pol. 2005, 74, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.; Kędzia, B. Antibacterial activity of Silphium perfoliatum. Extracts. Pharm. Biol. 2007, 45, 494–500. [Google Scholar] [CrossRef]
- Feng, W.-S.; Pei, Y.-Y.; Zheng, X.-K.; Li, C.-G.; Ke, Y.-Y.; Lv, Y.-Y.; Zhang, Y.-L. A new kaempferol trioside from Silphium perfoliatum. J. Asian Nat. Prod. Res. 2014, 16, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Gansberger, M.; Montgomery, L.F.R.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crop. Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff-Hönninger, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crop. Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Ustak, S.; Munoz, J. Cup-plant potential for biogas production compared to reference maize in relation to the balance needs of nutrients and some microelements for their cultivation. J. Environ. Manag. 2018, 228, 260–266. [Google Scholar] [CrossRef]
- Thompson, W.; Meyer, S. Second generation biofuels and food crops: Co-products or competitors? Glob. Food Secur. 2013, 2, 89–96. [Google Scholar] [CrossRef]
- Gerstberger, P.; Asen, F.; Hartmann, C. Zur Ökonomie und Ökologie der Becherpflanze (Silphium perfoliatum L.) im Vergleich zum Silomais [Economy and ecology of cup plant (Silphium perfoliatum L.) compared with silage maize]. J. Kult. 2016, 68, 372–377. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Niksa, D.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Willow productivity from small-and large-scale experimental plantations in Poland from 2000 to 2017. Renew. Sustain Energy Rev. 2019, 101, 461–475. [Google Scholar] [CrossRef]
- Ogwang, I.; Kasedde, H.; Nabuuma, B.; Kirabira, J.B.; Lwanyaga, J.D. Characterization of Biogas Digestate for Solid Biofuel Production in Uganda. Sci. Afr. 2021, 12, e00735. [Google Scholar] [CrossRef]
- Drosg, B.; Fuchs, W.; Al Seadi, T.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing; IEA Bioenergy: Paris, France, 2015. [Google Scholar]
- Oleszek, M.; Matyka, M. Nitrogen fertilization level and cutting affected lignocellulosic crops properties important for biogas production. BioResources 2017, 12, 8565–8580. [Google Scholar] [CrossRef]
- Oleszek, M.; Matyka, M. Determination of the efficiency and kinetics of biogas production from energy crops through nitrogen fertilization levels and cutting frequency. BioResources 2018, 13, 8505–8528. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zieliński, M. The effects of Microalgae Biomass Co-Substrate on Biogas Production from the Common Agricultural Biogas Plants Feedstock. Energies 2020, 13, 2186. [Google Scholar] [CrossRef]
- Schnurer, A.; Jarvis, A. Microbiological Handbook for Biogas Plants. Swedish Waste Management U2009:03 Wedish Gas Centre Report 207; Avfall Sverige and Svenskt Gastekniskt Center AB: Malmö, Sweden, 2009; pp. 1–74. [Google Scholar]
- Drosg, B.; Braun, R.; Bochmann, G.; Al Saedi, T. Analysis and characterisation of biogas feedstocks. In The Biogas Handbook: Science, Production and Applications; Wellinger, A., Murphy, J., David, B., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 52–84. [Google Scholar] [CrossRef]
- Schoo, B.; Kage, H.; Schittenhelm, S. Radiation use efficiency, chemical composition, and methane yield of biogas crops under rainfed and irrigated conditions. Eur. J. Agron. 2017, 87, 8–18. [Google Scholar] [CrossRef]
- Wever, C.; Höller, M.; Becker, L.; Biertümpfel, A.; Köhler, J.; van Inghelandt, D.; Westhoff, P.; Pude, R.; Pestsova, E. Towards high-biomass yielding bioenergy crop Silphium perfoliatum L.: Phenotypic and genotypic evaluation of five cultivated populations. Biomass Bioenergy 2019, 124, 102–113. [Google Scholar] [CrossRef]
- Ruf, T.; Emmerling, C. Site-adapted production of bioenergy feedstocks on poorly drained cropland through the cultivation of perennial crops. A feasibility study on biomass yield and biochemical methane potential. Biomass Bioenergy 2018, 119, 429–435. [Google Scholar] [CrossRef]
- Von Cossel, M.; Steberl, K.; Hartung, J.; Pereira, L.A.; Kiesel, A.; Lewandowski, I. Methane yield and species diversity dynamics of perennial wild plant mixtures established alone, under cover crop maize (Zea mays L.), and after spring barley (Hordeum vulgare L.). GCB Bioenergy 2019, 11, 1376–1391. [Google Scholar] [CrossRef] [Green Version]
- Ţîţei, V. The evaluation of biomass of the Sida hermaphrodita and Silphium perfoliatum for renewable energy in Moldova. Sci. Pap. Ser. A Agron. 2017, 60, 534–540. [Google Scholar]
- Mazurkiewicz, J.; Marczuk, A.; Pochwatka, P.; Kujawa, S. Maize Straw as a Valuable Energetic Material for Biogas Plant Feeding. Materials 2019, 12, 3848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, Z.; Visvanathan, C. Evaluation of anaerobic digestate for greenhouse gas emissions at various stages of its management. Int. Biodeterior. Biodegrad. 2014, 95, 167–175. [Google Scholar] [CrossRef]
| Stage 1 | ||||
| Raw biomass Silphium perfoliatum | Control | |||
| Series | ||||
| Organic fertilizer | Mineral fertilizer | Without fertilization | ||
| Variant | Variant | Variant | ||
| 85 | 170 | 85 | 170 | 0 |
| Symbol | ||||
| RO85(A) | RO170(B) | RM85(C) | RM170(D) | RC0(E) |
| Stage 2 | ||||
| Silage Silphium perfoliatum | Control | |||
| Series | ||||
| Organic fertilizer | Mineral fertilizer | Without fertilization | ||
| Variant | Variant | Variant | ||
| 85 | 170 | 85 | 170 | 0 |
| Symbol | ||||
| SO85(A) | SO170(B) | SM85(C) | SM170(D) | SC0(E) |
| Item | Inoculum |
|---|---|
| DM | 7.1 ± 0.3 |
| ODM | 70.6 ± 2.9 |
| Ash | 29.4 ± 2.9 |
| Carbon | 40.4 ± 1.3 |
| Nitrogen | 3.9 ± 0.1 |
| C/N ratio | 10.2 ± 0.1 |
| O85 | O170 | M85 | M170 | C0 | ||
|---|---|---|---|---|---|---|
| DM | Raw material | 22.9 ± 0.8 | 23.1 ± 0.7 | 25.3 ± 0.8 | 22.5 ± 0.6 | 22.9 ± 0.1 |
| Silage | 21.4 ± 0.0 | 21.8 ± 1.1 | 21.1 ± 2.6 | 20.6 ± 1.9 | 20.6 ± 1.5 | |
| ODM | Raw material | 90.7 ± 0.2 | 90.3 ± 0.8 | 91.0 ± 1.5 | 92.0 ± 0.1 | 91.4 ± 0.8 |
| Silage | 89.5 ± 0.1 | 89.7 ± 0.6 | 90.5 ± 1.1 | 91.9 ± 0.4 | 89.7 ± 0.1 | |
| Ash | Raw material | 9.3 ± 0.2 | 9.7 ± 0.8 | 9.0 ± 1.5 | 7.9 ± 0.1 | 8.6 ± 0.8 |
| Silage | 10.5 ± 0.1 | 10.3 ± 0.6 | 9.5 ± 1.1 | 8.1 ± 0.4 | 10.3 ± 0.1 | |
| Carbon | Raw material | 43.7 ± 0.5 | 44.4 ± 1.1 | 43.6 ± 1.1 | 44.8 ± 1.0 | 43.7 ± 0.7 |
| Silage | 45.1 ± 0.9 | 45.5 ± 0.0 | 44.9 ± 2.5 | 46.8 ± 1.1 | 45.4 ± 1.0 | |
| Nitrogen | Raw material | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.9 ± 0.0 | 1.1 ± 0.3 | 0.7 ± 0.0 |
| Silage | 0.9 ± 0.0 | 0.9 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.1 | 0.8 ± 0.1 | |
| C/N ratio | Raw material | 62.3 ± 4.4 | 53.0 ± 1.2 | 47.6 ± 1.1 | 41.7 ± 10.6 | 61.4 ± 3.1 |
| Silage | 53.3 ± 1.4 | 49.4 ± 0.4 | 46.4 ± 1.4 | 43.8 ± 3.9 | 54.5 ± 4.5 |
| Source of Variation | Organic Dry Matter | Ash Content | Dry Matter | C Content | N Content | C/N Ratio | CO2% | CH4% | Methane Production | Biogas Production |
|---|---|---|---|---|---|---|---|---|---|---|
| Substrate type | 0.005 * | 0.005 * | 0.115 | 0.000 * | 0.027 * | 0.005 * | 0.164 | 0.164 | 0.021 * | 0.006 * |
| Fertilization type | 0.002 * | 0.002 * | 0.019 * | 0.560 | 0.000 * | 0.000 * | 0.603 | 0.603 | 0.801 | 0.659 |
| Nitrogen (N) dose | 0.208 | 0.208 | 0.232 | 0.068 | 0.021 * | 0.017 * | 0.704 | 0.704 | 0.584 | 0.645 |
| Substrate type × Fertilization type | 0.058 | 0.058 | 0.719 | 0.879 | 0.205 | 0.078 | 0.936 | 0.936 | 0.894 | 0.830 |
| Substrate type × N dose | 0.692 | 0.692 | 0.381 | 0.877 | 0.455 | 0.307 | 0.984 | 0.984 | 0.715 | 0.736 |
| Fertilization type × N dose | 0.067 | 0.067 | 0.031 * | 0.238 | 0.089 | 0.175 | 0.949 | 0.949 | 0.763 | 0.767 |
| Substrate type × Fertilization type × N dose | 0.956 | 0.956 | 0.801 | 0.829 | 0.719 | 0.732 | 0.965 | 0.965 | 0.466 | 0.368 |
| Item | Organic Dry Matter | Ash Content | Dry Matter | C Content | N Content | C/N Ratio | Methane Production | Biogas Production | CO2 % | CH4 % |
|---|---|---|---|---|---|---|---|---|---|---|
| Organic dry matter | 1.00 | |||||||||
| Ash content | −1.00 * | 1.00 | ||||||||
| Dry matter | 0.03 | −0.03 | 1.00 | |||||||
| C content | 0.08 | −0.08 | −0.58 * | 1.00 | ||||||
| N content | 0.27 | −0.27 | −0.02 | 0.33 * | 1.00 | |||||
| C/N ratio | −0.20 | 0.20 | −0.20 | −0.23 | −0.93 * | 1.00 | ||||
| Methane production | −0.03 | 0.03 | 0.40 * | −0.48 * | 0.20 | −0.16 | 1.00 | |||
| Biogas production | 0.11 | −0.11 | 0.41 * | −0.54 * | 0.26 | −0.22 | 0.96 * | 1.00 | ||
| CO2% | 0.49 * | −0.49 * | 0.08 | −0.27 | 0.15 | −0.18 | −0.08 | 0.20 | 1.00 | |
| CH4% | −0.49 * | 0.49 * | −0.08 | 0.27 | −0.15 | 0.18 | 0.08 | −0.20 | −1.00 * | 1.00 |
| Item | Substrate Type | O85 | O170 | M85 | M170 | C0 |
|---|---|---|---|---|---|---|
| DM (%) | Raw material | 8.1 ± 0.7 | 8.2 ± 0.7 | 8.2 ± 0.7 | 8.1 ± 0.6 | 8.1 ± 0.6 |
| Silage | 7.5 ± 0.0 | 7.6 ± 0.0 | 7.6 ± 0.1 | 7.6 ± 0.1 | 7.6 ± 0.1 | |
| ODM (% d.m.) | Raw material | 68.9 ± 0.0 | 68.9 ± 0.0 | 68.8 ± 0.1 | 68.9 ± 0.0 | 68.9 ± 0.0 |
| Silage | 74.3 ± 2.0 | 74.3 ± 2.0 | 74.2 ± 2.1 | 74.4 ± 2.0 | 74.3 ± 2.0 | |
| Ash (% d.m.) | Raw material | 31.1 ± 0.0 | 31.1 ± 0.0 | 31.2 ± 0.1 | 31.1 ± 0.0 | 31.1 ± 0.0 |
| Silage | 25.7 ± 2.0 | 25.7 ± 2.0 | 25.8 ± 2.1 | 25.6 ± 2.0 | 25.7 ± 2.0 | |
| Carbon (%d.m.) | Raw material | 39.3 ± 1.1 | 39.3 ± 1.1 | 39.2 ± 1.1 | 39.2 ± 1.1 | 39.3 ± 0.1 |
| Silage | 41.8 ± 2.0 | 41.9 ± 1.9 | 41.8 ± 2.1 | 41.9 ± 2.0 | 41.9 ± 2.0 | |
| Nitrogen (% d.m.) | Raw material | 3.7 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.2 | 3.7 ± 0.2 |
| Silage | 3.9 ± 0.0 | 3.9 ± 0.0 | 3.9 ± 0.0 | 3.9 ± 0.0 | 3.9 ± 0.0 | |
| C/N ratio | Raw material | 12.8 ± 0.5 | 12.3 ± 0.3 | 11.9 ± 0.3 | 11.7 ± 0.8 | 12.7 ± 0.4 |
| Silage | 12.5 ± 0.4 | 12.3 ± 0.4 | 11.9 ± 0.4 | 12.0 ± 0.6 | 12.5 ± 0.6 |
| Substrate Type | O85 | O170 | M85 | M170 | C0 | |
|---|---|---|---|---|---|---|
| Raw material | r [cm3 d–1] | 66.6 | 70.4 | 72.2 | 75.1 | 65.5 |
| k [l d–1] | 0.18 | 0.20 | 0.21 | 0.19 | 0.19 | |
| Silage | r [cm3 d–1] | 89.1 | 80.8 | 81.7 | 90.0 | 88.2 |
| k [l d–1] | 0.29 | 0.25 | 0.25 | 0.29 | 0.28 | |
| Raw material | CH4 [%] | 60.9 ± 2.5 | 61.5 ± 3.5 | 61.8 ± 4.1 | 62.2 ± 3.2 | 62.4.6 ± 2.9 |
| CO2 [%] | 39.1 ± 2.5 | 38.5 ± 3.5 | 38.2 ± 4.1 | 37.8 ± 3.2 | 37.6 ± 2.9 | |
| Silage | CH4 [%] | 62.7 ± 2.3 | 62.7 ± 0.2 | 62.4± 1.0 | 63.2 ± 0.8 | 63.6 ± 1.0 |
| CO2 [%] | 37.3 ± 2.3 | 37.3 ± 0.2 | 37.6 ± 1.0 | 36.8 ± 0.8 | 36.4 ± 1.0 |
| Item | Substrate Type | O85 | O170 | M85 | M170 | C0 | I.A.A.D. |
|---|---|---|---|---|---|---|---|
| DM | Raw material | 6.8 ± 0.6 | 6.7 ± 0.4 | 6.9 ± 0.5 | 6.8 ± 0.5 | 6.8 ± 0.4 | 6.7 ± 0.6 |
| Silage | 6.3 ± 0.3 | 6.2 ± 0.3 | 6.4 ± 0.4 | 6.2 ± 0.2 | 6.3 ± 0.2 | 5.7 ± 0.0 | |
| ODM | Raw material | 71.2 ± 1.0 | 69.8 ± 1.8 | 72.1 ± 0.7 | 71.5 ± 0.8 | 71.7 ± 0.8 | 70.4 ± 1.4 |
| Silage | 69.7 ± 2.3 | 69.9 ± 2.5 | 71.1 ± 1.5 | 70.8 ± 2.4 | 70.5 ± 1.9 | 70.0 ± 2.1 | |
| Ash | Raw material | 28.8 ± 1.0 | 30.2 ± 1.8 | 27.9 ± 0.7 | 28.6 ± 0.8 | 28.4 ± 0.8 | 29.6 ± 1.4 |
| Silage | 30.3 ± 2.3 | 30.1 ± 2.5 | 28.9 ± 1.5 | 29.2 ± 2.4 | 29.5 ± 1.9 | 30.0 ±2.1 | |
| Carbon | Raw material | 39.9 ± 0.6 | 38.9 ± 1.3 | 40.2 ± 0.9 | 39.7 ± 0.9 | 39.5 ± 1.0 | 39.4 ± 1.4 |
| Silage | 40.8 ± 1.6 | 41.1 ± 2.1 | 40.6 ± 0.7 | 41.2 ± 1.4 | 41.5 ± 1.9 | 40.1 ± 2.0 | |
| Nitrogen | Raw material | 3.2 ± 0.2 | 3.1 ± 0.3 | 3.3 ± 0.1 | 3.2 ± 0.2 | 3.1 ± 0.3 | 3.5 ± 0.2 |
| Silage | 3.1 ± 0.2 | 3.2 ± 0.1 | 3.2 ± 0.3 | 3.3 ± 0.1 | 3.1 ± 0.1 | 3.4 ± 0.0 | |
| C/N ratio | Raw material | 12.7 ± 0.7 | 12.1 ± 0.7 | 12.4 ± 0.1 | 12.6 ± 0.6 | 12.8 ± 0.9 | 11.3 ± 0.1 |
| Silage | 11.9 ± 1.1 | 11.7 ± 1.0 | 11.5 ± 1.1 | 11.6 ± 0.8 | 12.3 ± 1.2 | 11.1 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peni, D.; Dębowski, M.; Stolarski, M.J. Influence of the Fertilization Method on the Silphium perfoliatum Biomass Composition and Methane Fermentation Efficiency. Energies 2022, 15, 927. https://doi.org/10.3390/en15030927
Peni D, Dębowski M, Stolarski MJ. Influence of the Fertilization Method on the Silphium perfoliatum Biomass Composition and Methane Fermentation Efficiency. Energies. 2022; 15(3):927. https://doi.org/10.3390/en15030927
Chicago/Turabian StylePeni, Dumitru, Marcin Dębowski, and Mariusz Jerzy Stolarski. 2022. "Influence of the Fertilization Method on the Silphium perfoliatum Biomass Composition and Methane Fermentation Efficiency" Energies 15, no. 3: 927. https://doi.org/10.3390/en15030927
APA StylePeni, D., Dębowski, M., & Stolarski, M. J. (2022). Influence of the Fertilization Method on the Silphium perfoliatum Biomass Composition and Methane Fermentation Efficiency. Energies, 15(3), 927. https://doi.org/10.3390/en15030927