1. Introduction
Since the 20th century and the beginning of the 21st century, the generation of alternative clean technologies has been increasing to counteract the increase in pollutants and energy consumption due to the increasing global population [
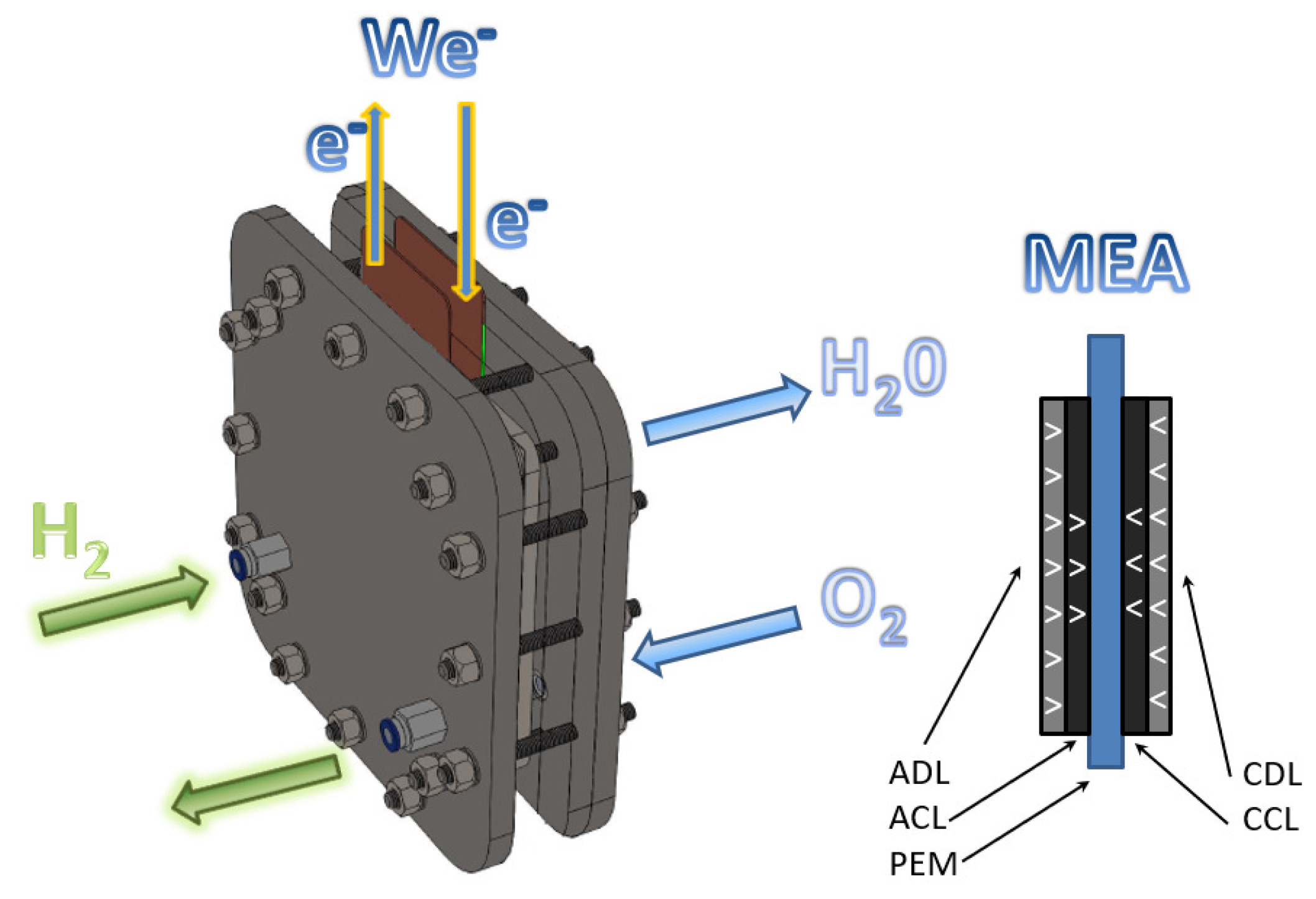
1]. The PEM fuel cells are clean devices that directly generate electrical energy, water, and heat through electrochemical reactions in their cathodic and anodic compartments, as shown in
Figure 1. The electrochemical reactions in a PEMFC are represented in Equations (
1) to (
3) [
2,
3]:
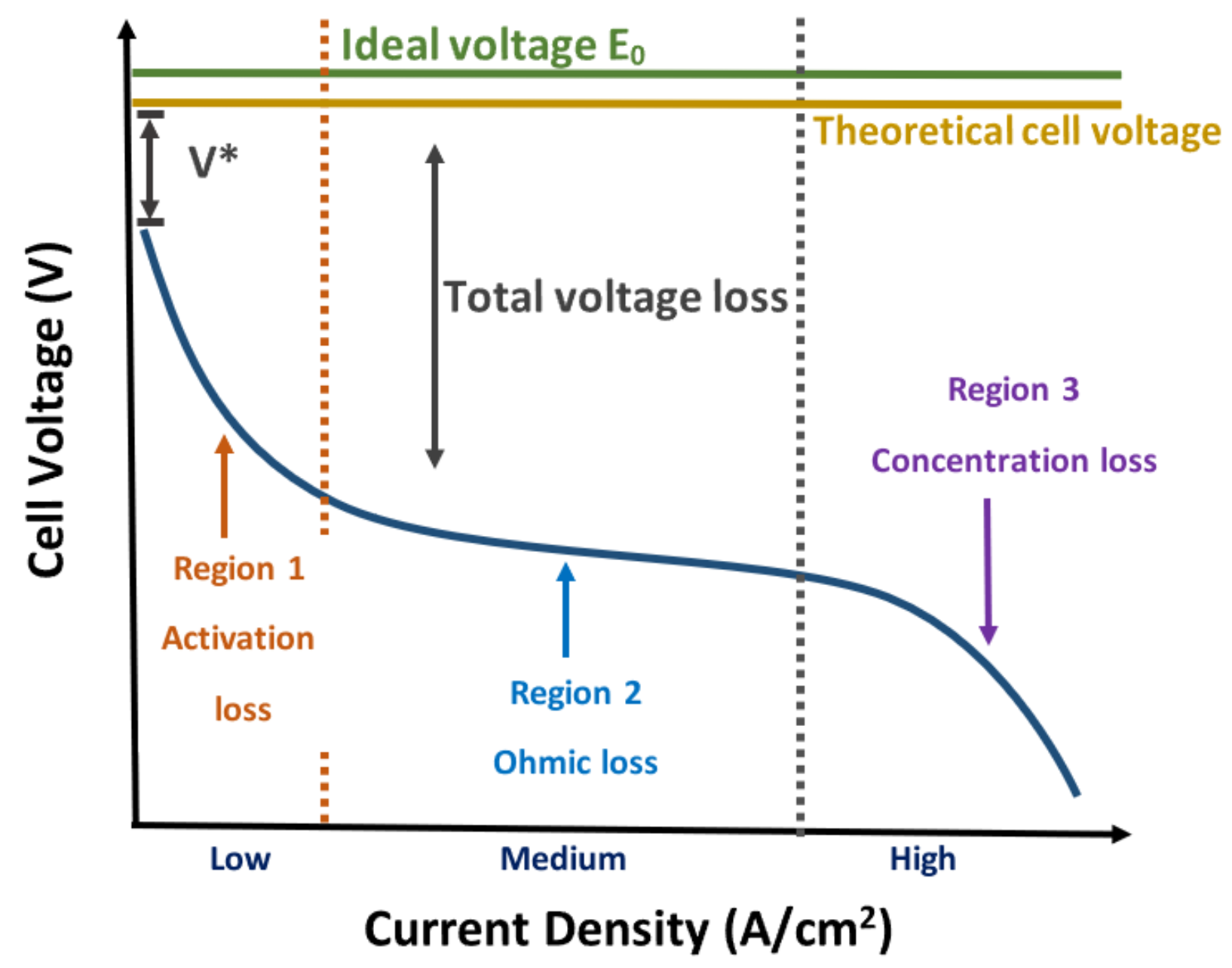
The mathematical modeling of fuel cells allows us to study the energy conversion processes inside of a PEM fuel cell; analyze designs, structures, or material composition; and improve electrical energy generation. Several mathematical models have been implemented and validated through laboratory experimentation in the literature. Among the models, the following stand out: (a) Empirical and semi-empirical models: These models are based on algebraic expressions obtained from physical relations present in the PEMFC. They model the behavior of the polarization curves using a set of empirical or semi-empirical parameters through non-linear regression methods [
2,
4,
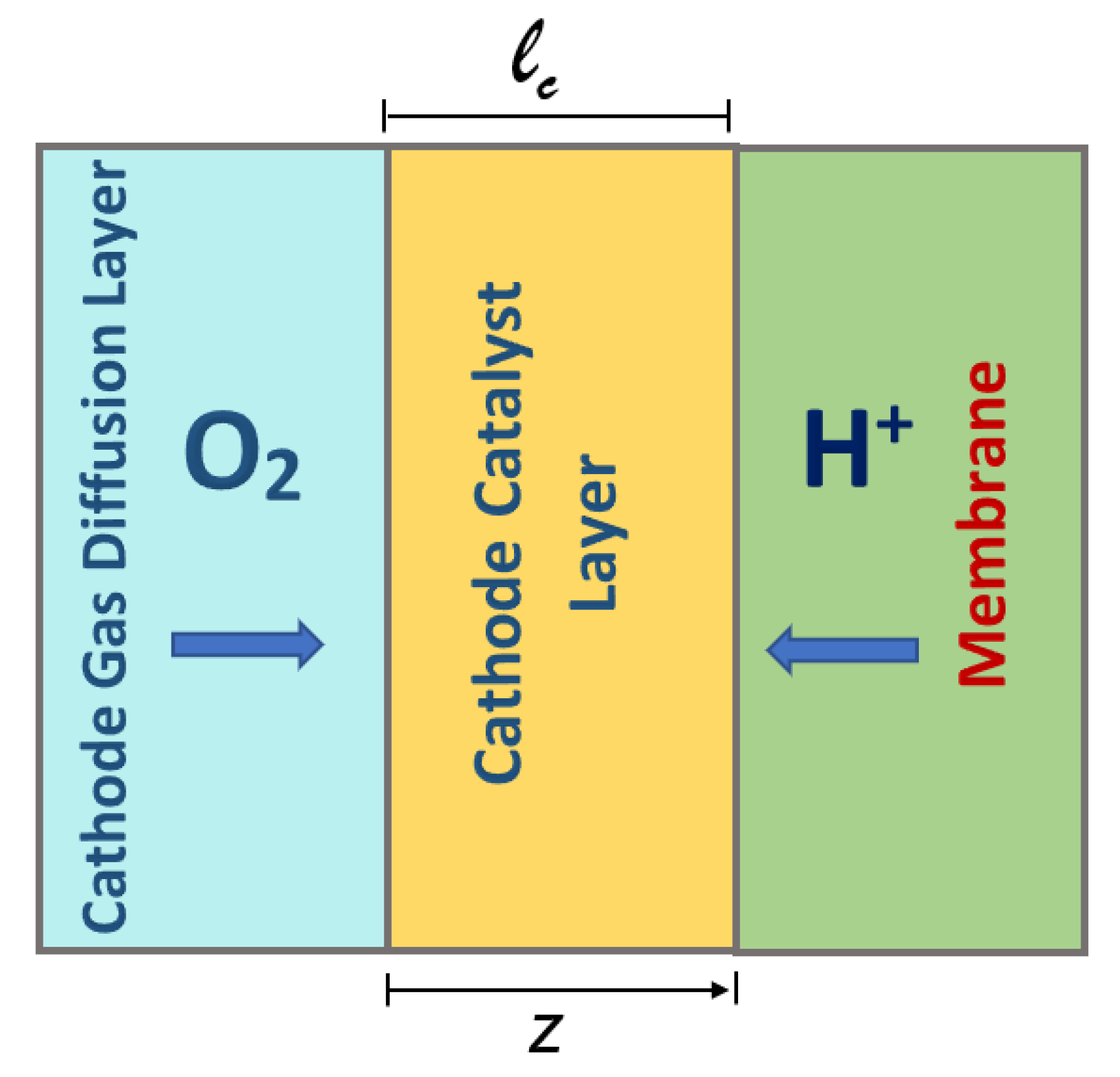
5]. (b) Interface models: These models’ diffusion processes occur through the anode, membrane, and cathode using diffusion differential equations, assuming that the catalytic layer is negligible [
6,
7,
8]. (c) Macro-homogeneous models: These consist of a set of non-linear ordinary differential equations based on the assumption that the main reactions occur in the catalytic layer of the cathode and analyze the physical compositions of the materials and their properties [
9,
10,
11,
12,
13,
14]. (d) Agglomerated models: These consist of partial differential equations, model diffusion processes, and involve a description of the physical composition of the cathodic catalytic layer, its porosity, or catalyst conglomerates [
15,
16,
17].
In the modeling and simulation of PEMFC, mathematical models require a set of parameters that are not obtained experimentally, such as diffusion coefficients; hence, they need to be estimated [
2,
12,
13,
15]. The parameter estimation problem is an optimization problem with an objective function defined by the error minimization of the fitting experimental data and the model output [
5,
18,
19,
20]. Algorithms for solving the parameter estimation problem in PEMFC are particle swarm optimization (PSO) [
21,
22], the artificial immune system [
23], simulated annealing [
2,
18], the genetic algorithm (GA) [
5,
24], the RNA-genetic algorithm [
19], the differential evolution algorithm [
25,
26], the artificial bee swarm [
27], fuzzy logic control [
28], harmonic search in [
29], JAYA-NM [
30], the Grasshopper Optimization Algorithm (GOA) [
31], the Salp Swarm Optimizer (SSO) [
32], moth–flame optimization [
33], and UMDA
[
34], among others. In general, most of them report acceptable solutions; nevertheless, they vary in the consistency of their results, that is, in delivering similar estimated parameters for the same conditions in several independent executions. UMDA
[
34] has shown a reliable balance between the precision of the approximation and the computational cost and consistency. Consequently, it was used in this work for the estimation task.
In this context, the contributions of this article are various. First, we unified three high-performing models to demonstrate how to simulate the same cell with all of them; from our point of view, the main advantage of these multiple simulations is that the models corroborate that the parameters are the correct ones and deliver information about the cell performance, which is not possible to measure but is obtained from the simulation. Second, we estimated parameters in one model using a few experimental data, and then we used the other models to increase the precision of the estimation and to corroborate that the parameters reproduce the cell performance. Third, we reproduced incomplete experimental information. Once the parameters had been estimated, we were able to reproduce the full polarization curves and concentration profiles. Fourth, we presented the assembly of numerical techniques as a complete method for parameter estimation and multimodel simulation of the PEMFC.
The paper is organized as follows:
Section 2 presents an overview of the three selected mathematical models; we describe their advantages and disadvantages. In
Section 3, we present the parameter estimation problem. Our proposed self-validating methodology is presented in
Section 4. In
Section 5, we show the numerical and comparative results of our proposal with those reported in the literature. Finally, a conclusion of this work is presented in
Section 6.
3. Parameter Estimation Problem
In a parameter estimation problem, regardless of the algorithm to be used, it is necessary to define a function that is minimized or maximized. This function is called the objective function. For PEMFC, different researchers apply various objective functions; see [
44]. In this work, given a set of unknown parameters
, we defined the objective function,
, as the sum of the squared error between the experimental data of the polarization curve and the output of the model:
where
N is the number of experimental data;
and
are the j-th experimental datum and the output of the model, respectively, at point
. The problem consists of determining the set of parameters
that returns a minimum value for
.
4. Self-Validating Methodology
In
Section 2, we explained the benefits of the three selected mathematical models. In this section, we propose a methodology to unify the models using the advantages of each model to solve the disadvantages of the others. An advantage of our proposal is that it works with a few experimental data, since the three models complement and provide information to one another.
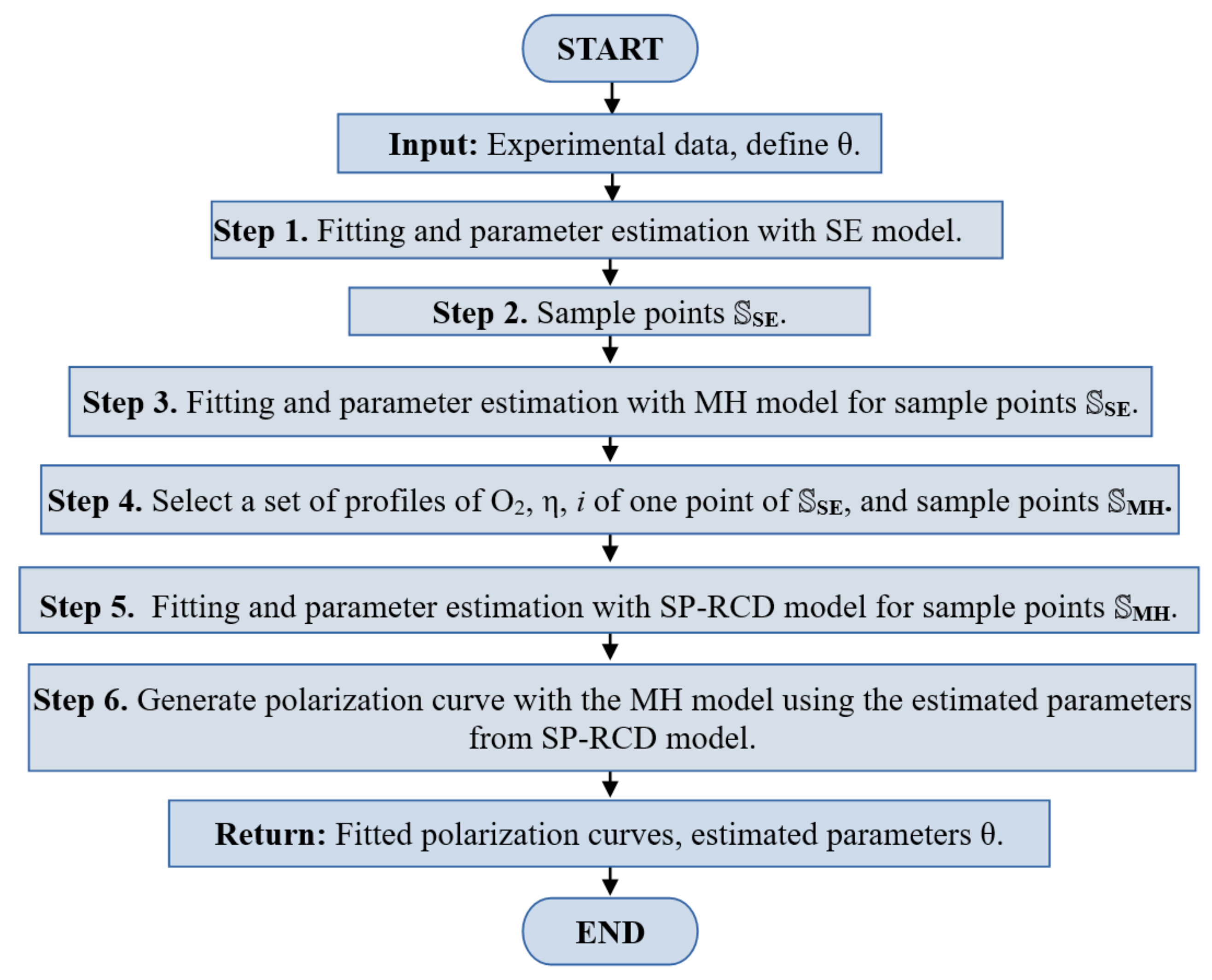
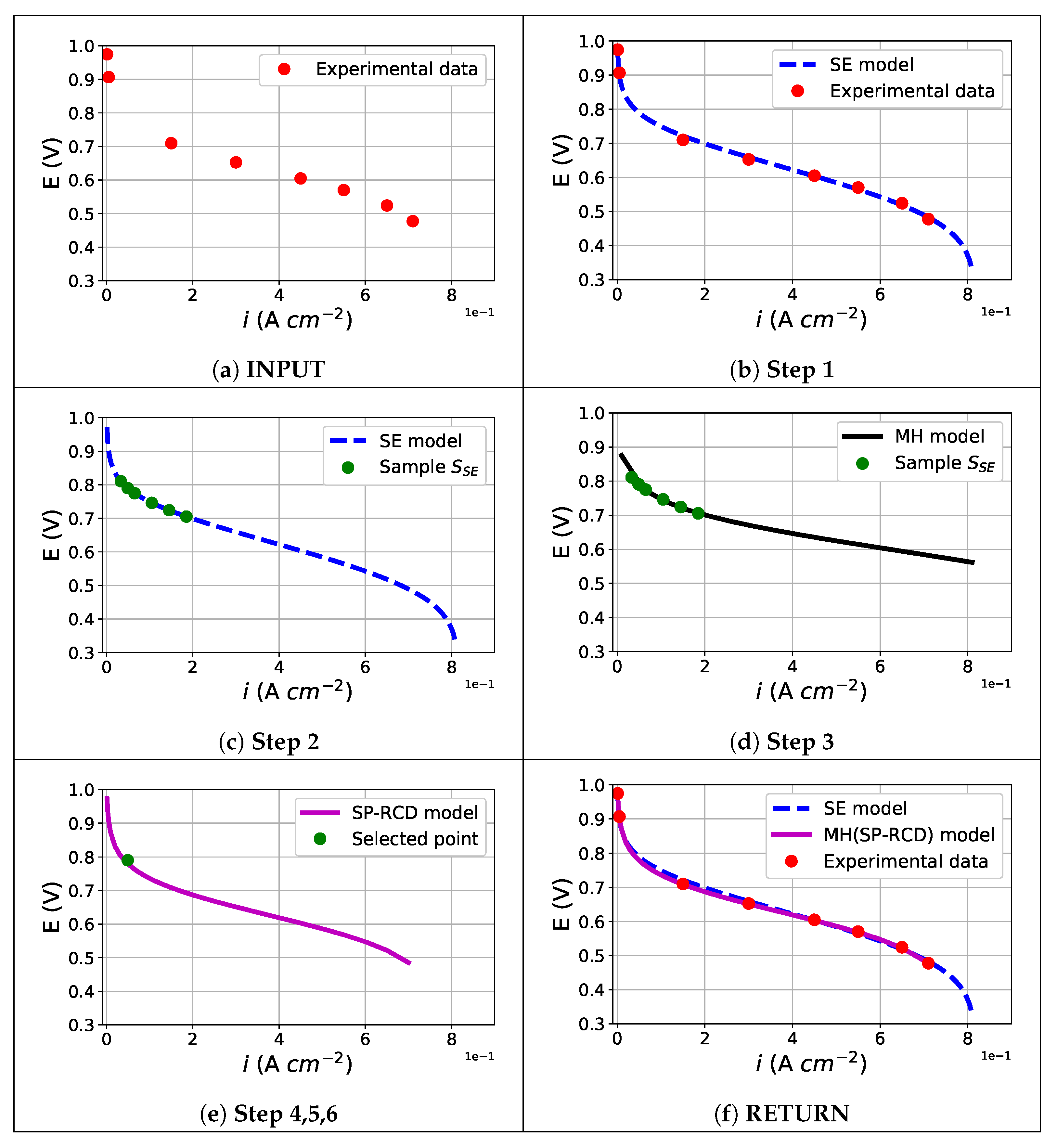
The algorithm begins with an experimental data set of a polarization curve and returns a set of estimated parameters and the fitting of the polarization curve, following steps of the scheme in
Figure 4. In this initial step, it is necessary to declare the set of parameters
to be estimated. Using the semi-empirical model, we obtained a complete polarization curve, including the concentration losses region. The SE-parameter estimation fitted the model’s output to the experimental data, providing the first set of estimated parameters
. As we explain in
Section 2.1, this model is incapable of describing the specific physical properties of the catalyst layer.
The macro-homogeneous model describes the missing characteristics that the semi-empiric model is unable to describe; we propose to use a part of the polarization curve simulated by the semi-empiric model, close to the activation losses region, to sample a set of points, called
, which was used to estimate a second set of parameters
from the macro-homogeneous model. It is important to note that in this region, the macro-homogeneous model is well-posed and provides correct solutions, as noted in [
13]. With a set of solutions from the macro-homogeneous model corresponding to one point from
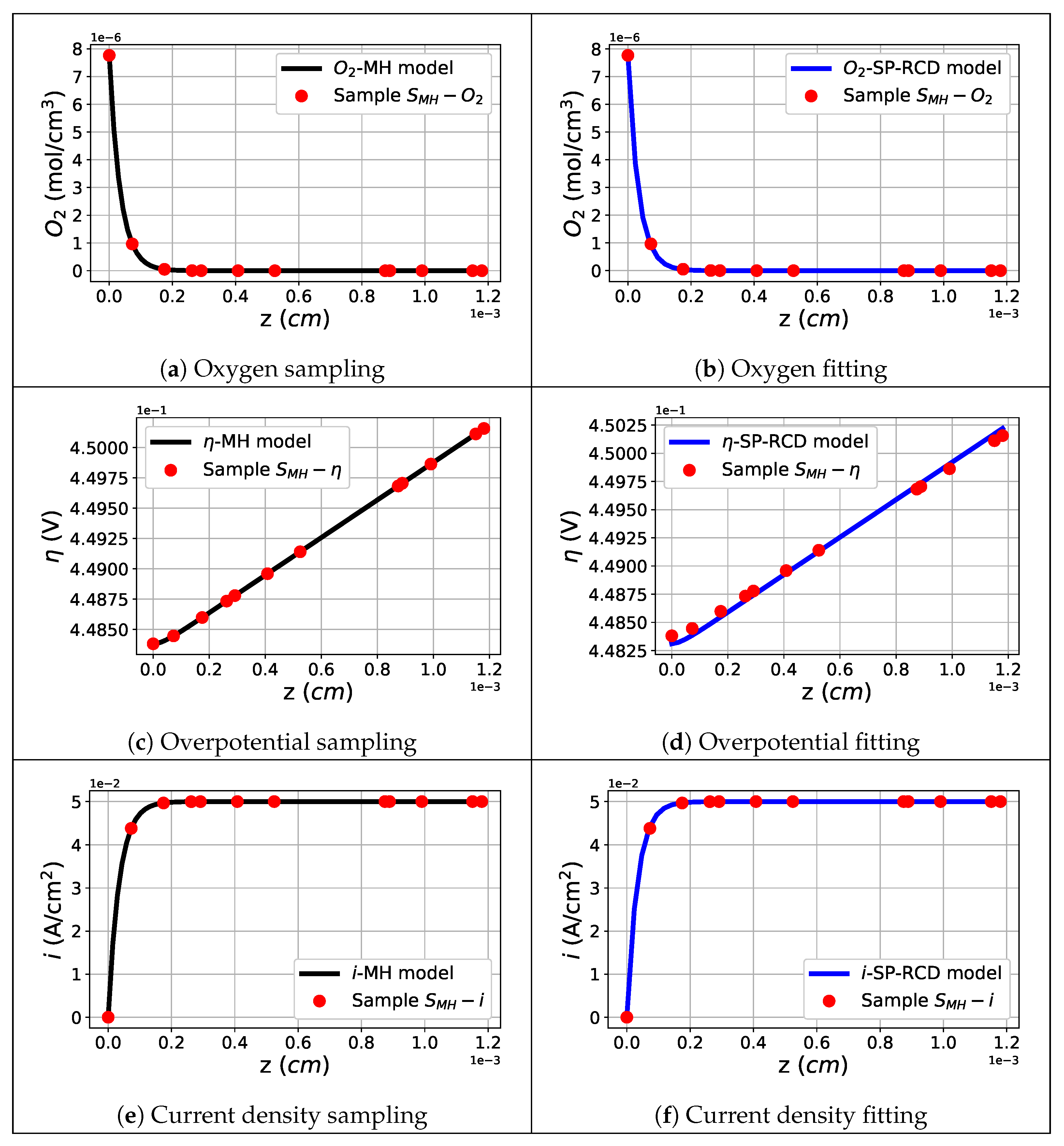
, the oxygen concentration profiles, overpotentials, and current densities were obtained. It is clear that the estimation of the macro-homogeneous model was correct for the points
, but it could not be correct for the complete polarization curve. To improve this, we used the SP-RCD model. Then, we generated a set of sample points named
from the obtained oxygen concentration, overpotential, and current density profiles and used them as data for the parameter estimation with the SP-RCD model. In this step, we re-estimated the previously estimated parameters,
, and defined
as the set of boundary conditions found by the shooting method. Finally, with the new re-estimated parameters
, we reproduced the complete polarization curve with the macro-homogeneous model.
This methodology is independent of the parameter estimation problem algorithm and is described as an algorithm that follows the steps below (graphs in
Figure 5 and
Figure 6), which should be followed in the same way as the methodology steps:
The proposed methodology is a self-validating strategy as the principal objective of the coupling of the three mathematical models is to generate simulated polarization curves to describe the experimental data of a PEMFC.
5. Results
To validate the proposal, we used two experimental datasets of PEMFC. General information about the reference initial experimental PEMFC can be found in [
45]; the authors used Nafion
117 with humidified air and prototech-brand electrodes (20 wt. % Pt) onto which they sputtered platinum with an equivalent thickness of 50 nm, corresponding to a total Pt loading of 0.45 mg/cm
. Complementary base operating conditions used by [
12,
13] are presented in
Table 4.
The optimization algorithm to minimize
is an Estimation of the Distribution Algorithm (EDA) named the Univariate Marginal Distribution Algorithm with Gaussian models (UMDA
) [
46,
47,
48,
49,
50,
51]. It is a stochastic algorithm that computes, in each iteration, the
l-dimensional joint probability function, factored as a product of
l univariate and independent probability functions. This probability function evolves until a stopping criterion is reached. Details of this algorithm and its implementation are shown in [
34,
51]. The UMDA
uses a population of 200 individuals and 1000 generations with a tolerance of
, for the semi-empirical model. For the macro-homogeneous and the SP-RCD models, it uses a population of 100 individuals and 500 generations with a tolerance of
In the context of evolutionary algorithms, the number of individuals in the population is usually considered dependent on the number of parameters to estimate. Thus, for the semi-empirical model, we needed to estimate nine parameters; for the macro-homogeneous model, we needed to estimate three parameters; and for the SP-RCD model, we re-estimated six parameters used in the macro-homogeneous model. Then, the search space for the semi-empirical model should have included more individuals to reach an adequate approximation; meanwhile, the other two models required fewer individuals.
5.1. Dataset 1
We carried out simulations with Dataset 1 (
Table 4) and selected 8 points of the polarization curve, which was necessary to obtain the profile of the curvature of the reported polarization curve in [
12]. For the semi-empirical model, we selected the set of parameters
,
,
,
, B,
, A,
,
}. The search space to fit the semi-empirical model with upper and lower bounds is presented in
Table 5. These values are based on previous research [
2,
23,
30,
34,
37].
With the UMDA
, we obtained the estimated parameters for the set
, as presented in
Table 6—Dataset 1, and we generated the simulated initial polarization curve
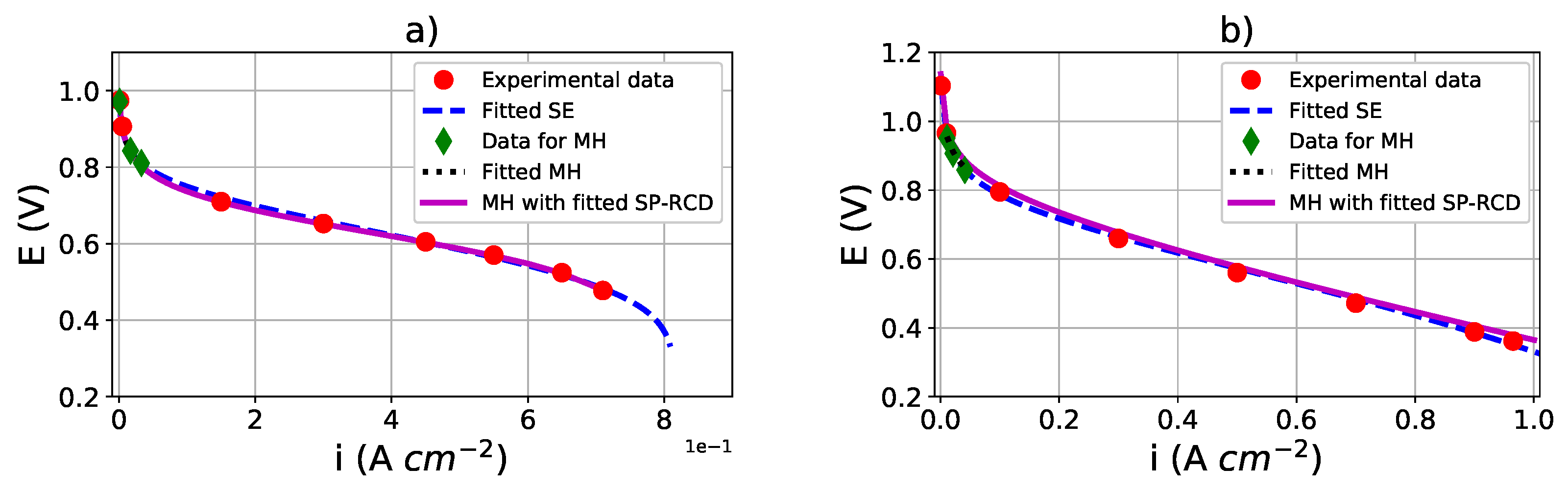
, as shown by the dashed blue line in
Figure 7a. Next, we sampled three points on
to obtain
into the activation losses region, as shown by the green points in
Figure 7a, and we used them to obtain the set of estimated parameters
with the macro-homogeneous model using the search space presented in
Table 7, based on [
12,
13,
14]; the results are reported in
Table 8—Dataset 1, in which we also present the estimated boundary conditions obtained by a shooting method.
With the results of the macro-homogeneous model, we selected information from the oxygen concentration, overpotential, and current density profiles corresponding to
Acm
; we sampled
uniform points on the profiles and carried out a parameter estimation with the SP-RCD model using the set of parameters
with
; the search limits were obtained by perturbing 5% of the estimated values reached with the shooting method in the previous step using the macro-homogeneous model. In
Table 8—Dataset 1, we show the estimated values compared to those reported in the literature and to the previously estimated values from the macro-homogeneous model. In
Figure 7a, the continuous magenta line represents the simulated polarization curve generated with the macro-homogeneous model using the estimated parameters of the SP-RCD model; the simulated polarization curves are similar and closer together, producing self-validating results.
5.2. Dataset 2
A second dataset was used with the baseline parameters in
Table 4—Dataset 2; we selected eight points of the experimental polarization curve, and the reported experimental data are presented in [
13]. For the semi-empirical model, we used the same set of parameters and the search space in
Table 2, except for
; we increased the interval to
following the reported values in [
18] to preserve the profile of the experimental polarization curve. The estimations are presented in
Table 6—Dataset 2. In
Figure 7b, the dashed blue line represents the simulated polarization curve
obtained from the semi-empirical model. We sampled
points on
to obtain
, as shown by the green points in
Figure 7b. For the macro-homogeneous model, we estimated the set of parameters
, similar to that for Dataset 1. We report the estimated parameters in
Table 8—Dataset 2. For parameter estimation with the SP-RCD model, we selected the profiles of oxygen concentration, overpotential, and current density corresponding to
Acm
; we sampled
uniform points on the profiles, overpotential, and current density. In the estimation process, we included
,
,
. We compared the estimated values with those reported in [
13], and with estimated values from the macro-homogeneous model in
Table 8—Dataset 2. We present the simulated polarization curve generated by the macro-homogeneous model using the estimated values with SP-RCD in
Figure 7b, as shown by the continuous magenta line.
The results of the numerical experiments in this work provide us with coherent and similar results to those obtained in previous works [
12,
13,
34] (see
Table 6 and
Table 8). The simulated polarization curves with the semi-empirical or macro-homogeneous models using the estimated parameters of the SP-RCD are similar and close together, showing that our methodology is adequate to describe PEMFC performances.
6. Conclusions
In this article, we described a proposal of a self-validating methodology using three different mathematical models: semi-empirical (SE), macro-homogeneous (MH), and singularly perturbed reaction–convection–diffusion (SP-RCD). The set of linked parameter estimation problems, solved by the UMDA, uses the benefits and advantages of each model to circumvent the disadvantages of the others. With the proposal in this work, it is possible to analyze the performance of a PEMFC, from the operational and construction parameters to the structural and composition parameters in the catalyst layer. The semi-empirical model describes the operational and construction parameters, such as temperature, pressure, and membrane properties; meanwhile, the macro-homogeneous and the SP-RCD models are useful to determine the composition parameters, such as platinum mass loading . The coupling of the models serves to take advantage of the polarization curve region that is better fitted by each one; for instance, the SE model reproduces the catalyst activation region; then, the MH takes the SE output to produce a gross approximation of the boundary conditions of the concentration profiles and a set of parameters. The actual boundary conditions and parameters are re-estimated with the SP-RCD model, producing more precise solutions. This last precise solution is inputted into the MH model to obtain a correction of the polarization curve.
The results are comparable with those reported in the literature; the simulated polarization curves of the semi-empirical and macro-homogeneous models obtained using the estimated parameters of the SP-RCD are similar and close together, validating the approximations between the models. The power of the method is to estimate parameters that are difficult or impossible to measure, starting from experimental information. Nevertheless, once we estimate the parameters that reproduce the experimental data, one can change a few parameters to describe the performance of a different design. Furthermore, one can use the model to optimize the performance of a PEMFC by testing parameters in a physically plausible interval of values. This strategy equips the practitioners with complementary information about parameters that are difficult to measure experimentally in a laboratory and increases the confidence of the estimated parameters that consistently reproduce the experimental data. Future work contemplates the optimization of the parameters. Notice that a first step to optimizing a PEMFC is to have a reliable numerical simulation that reproduces the actual polarization curve and concentration species profiles. Once the adequate parameters for the models have been determined by means of the proposed methodology, it is possible to vary any parameter, not only the estimated ones, to find an optimized value for improving the cell performance or cost. For example, it could be the platinum mass loading , for which optimization could reduce the cost while maintaining the performance of the original design, or the operational parameters to increase the electricity generation with the same design.