An Overview of the Recent Advances of Additive-Improved Mg(BH4)2 for Solid-State Hydrogen Storage Material
Abstract
:1. Introduction
1.1. Renewable and Sustainable Energy System
1.2. Hydrogen as an Ideal Energy Carrier
1.3. Characteristic of Mg(BH4)2 Hydrogen Storage System
1.4. Modification of the Mg(BH4)2 Properties
2. Hydrogen Storage Behaviour of Additive-Enhanced Mg(BH4)2
2.1. Metals
2.2. Metal Oxides
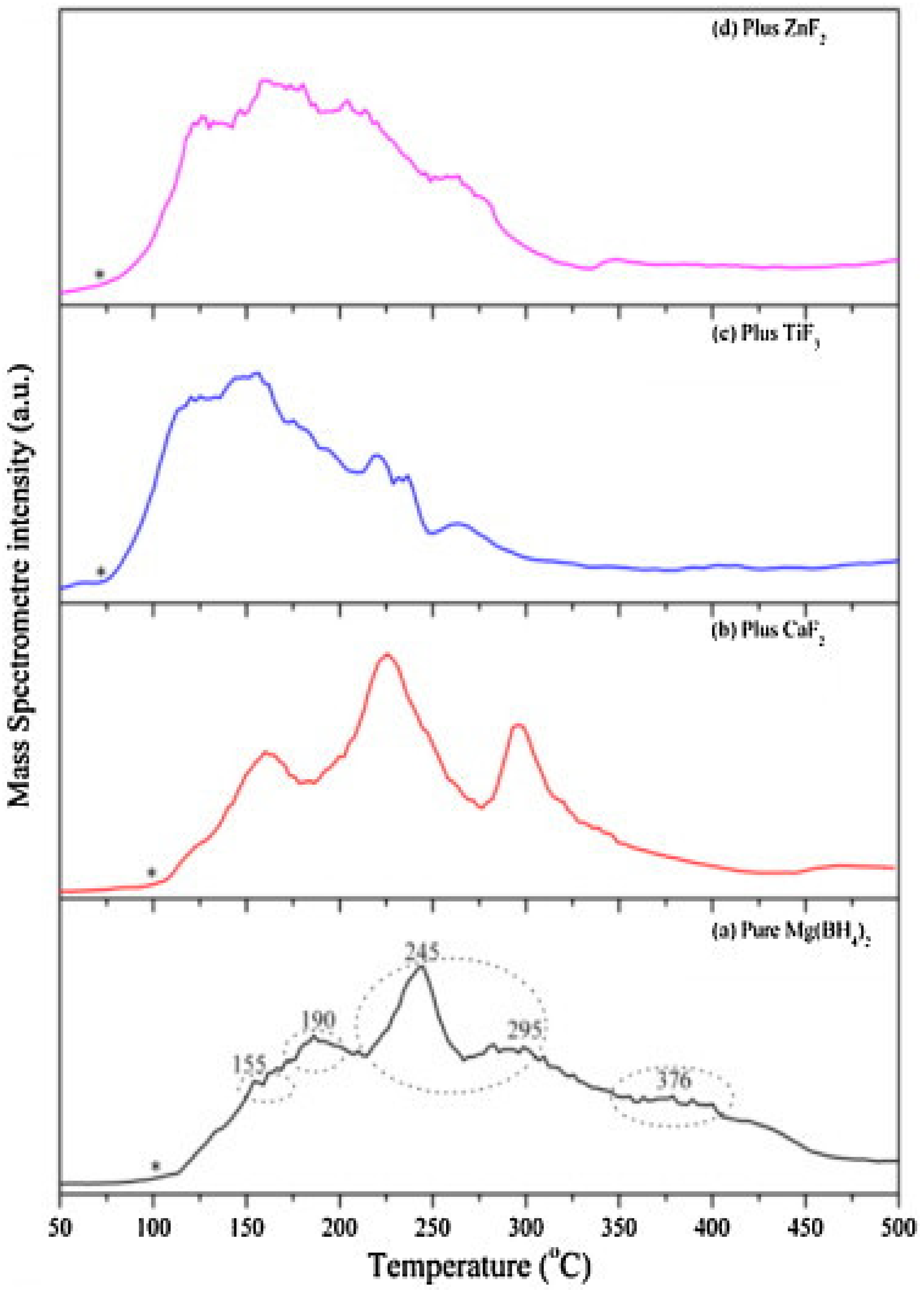
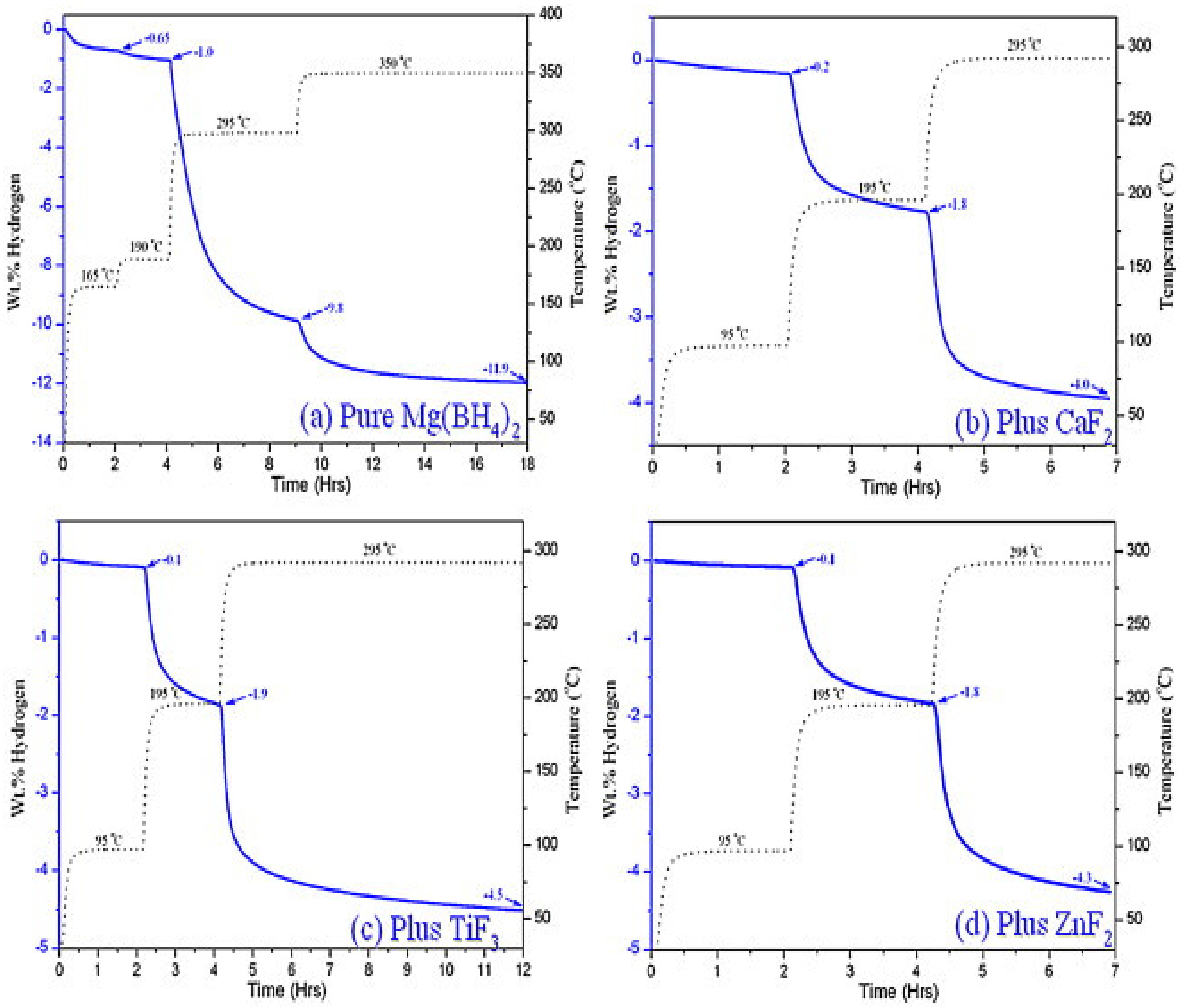
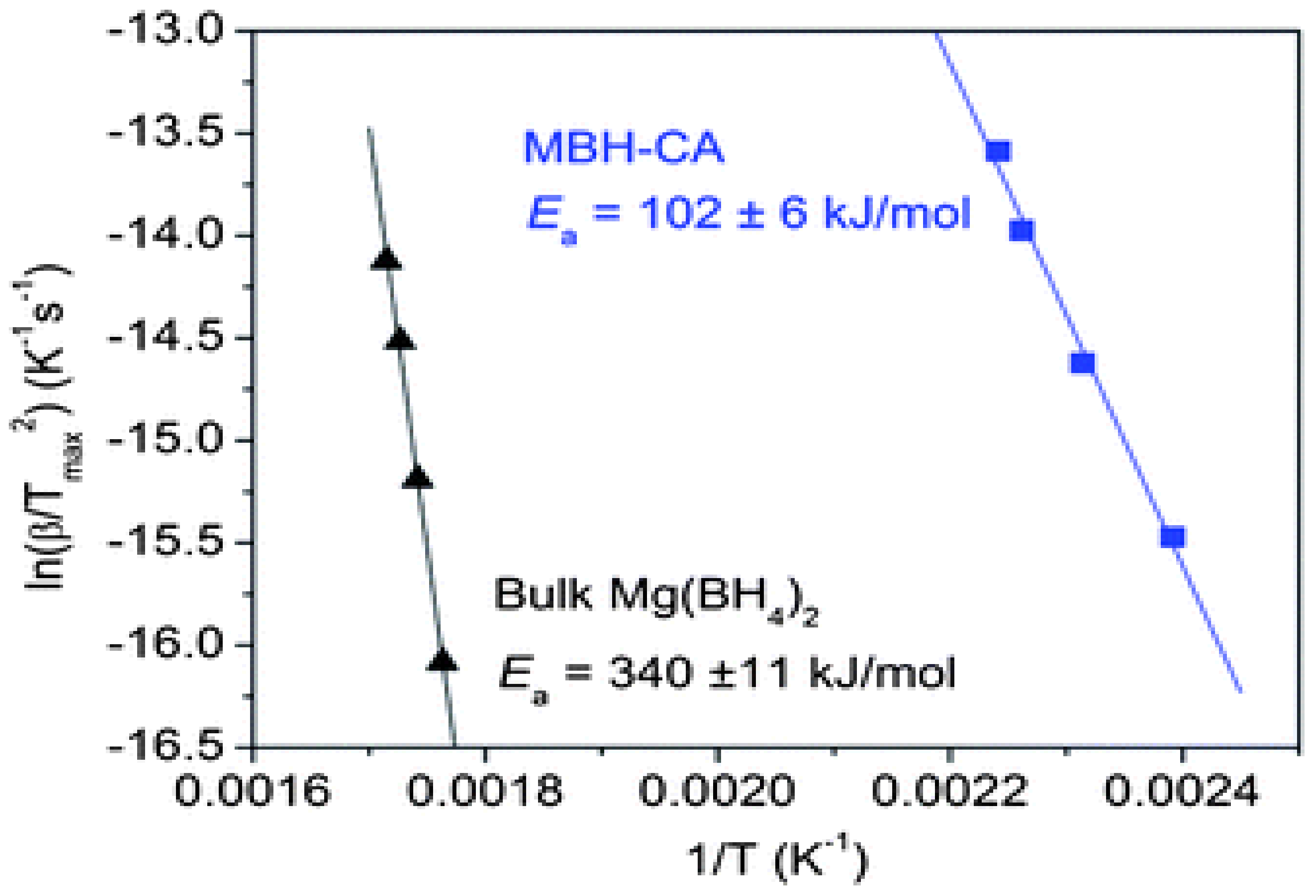
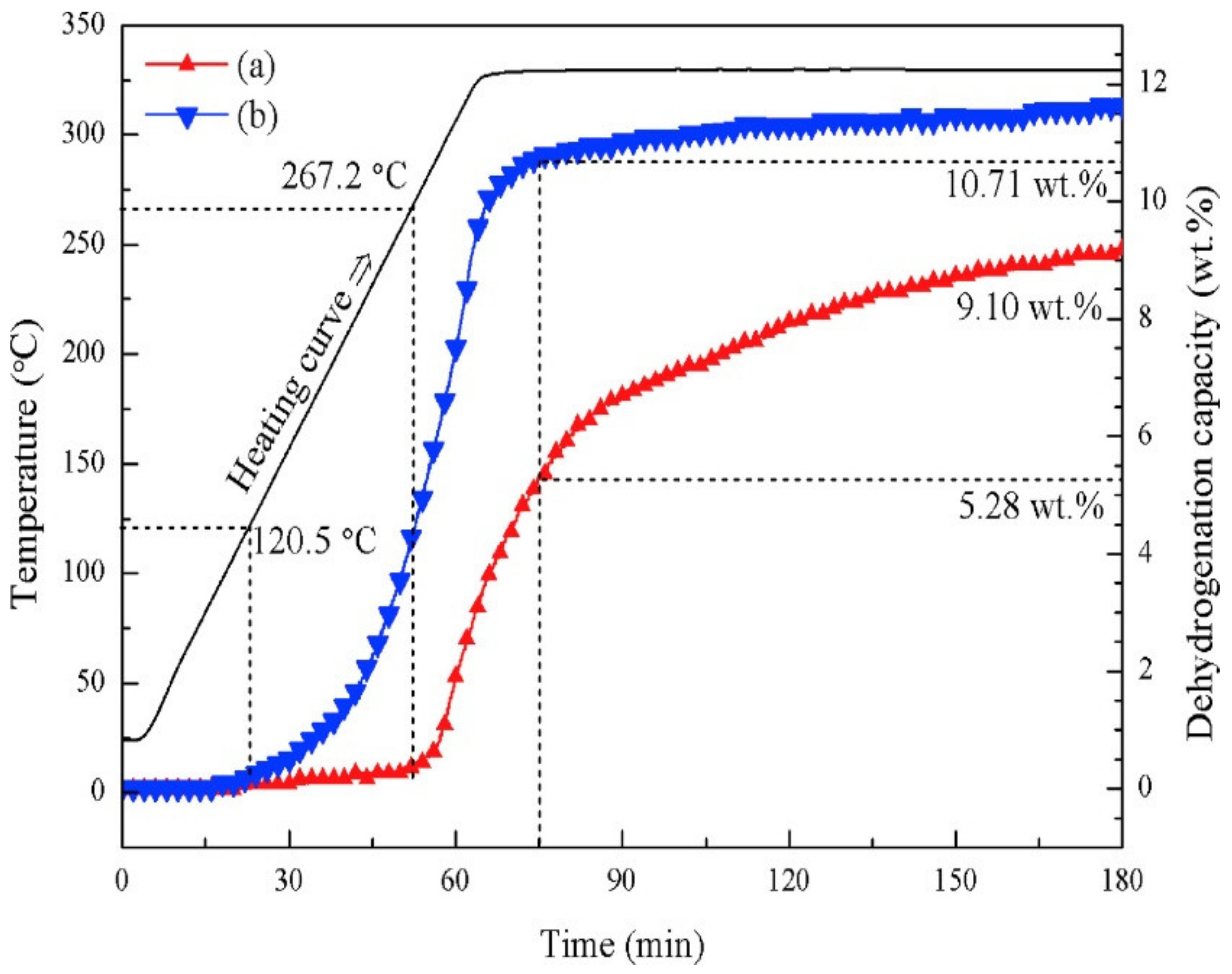
2.3. Metal Halides
2.4. Carbon-Based Materials
3. Future Prospects and Challenges
- (a)
- Reduce the size of the particle of Mg(BH4)2. Nanostructured materials contain a large surface area, which is conducive to the uniform distribution of the catalyst, inducing more defects on the surface of the particles, uniformly mixing different composite hydrides, making the product heterogeneous nucleation and perhaps reducing the enthalpy of the reaction.
- (b)
- Lower the activation barrier, Ea, for the H2 released from Mg(BH4)2, to understand the role of catalyst/additive and the mechanism of destabilization reactions in changing the properties of H2 bonds to achieve a low temperature. The doping of the catalyst/additive changes the reaction route of the mesophase and alternate reactions. By combining with other hydride materials to form new reactions or new compounds, the thermodynamics of the reaction can be adjusted, and a H2 reaction pathway with lower Ea can be generated.
- (c)
- Alter the thermodynamic properties of Mg(BH4)2. Once the kinetic barrier of H2 desorption is overcome, the H2 flux of desorption is proportional to the thermodynamic capability. In principle, the method to reduce the enthalpy of the H2 reaction should also improve the kinetics for H2 desorption under the relevant condition.
- (d)
- The main problem associated with borohydride as an ideal material for solid-state H2 storage is reversible to H2 absorption and desorption under moderate conditions. For the Mg(BH4)2, it was proven that only the second-stage reaction (MgH2 ↔ Mg + H2) is reversible to H2 absorption and desorption under moderate conditions. Thus, a significant breakthrough is needed to ensure the whole system (first and second stages) can absorb and desorb H2. The use of proper catalysts/additives or engineering modification, such as nanoconfinement, might be the solution for the reversibility problem in Mg(BH4)2.
- (e)
- The degradation of H2 storage capacity during the cycling process (rehydrogenation/dehydrogenation process) for the second stage of Mg(BH4)2 is also a crucial problem. It is important to find a suitable catalyst/additive that can prevent the decline in the H2 storage capacity during the cycling process.
- (f)
- The release of diborane gas, which is toxic, might also have a negative effect. To prevent the release of diborane gas, the reactive hydride composite approach (mix of two or three hydride materials) might offer a good solution through the reaction between hydrides during the milling process or dehydrogenation process.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Goldemberg, J. The promise of clean energy. Energy Policy 2006, 34, 2185–2190. [Google Scholar] [CrossRef]
- Omer, A.M. Energy, environment and sustainable development. Renew. Sust. Energy Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Rosen, M.A. Energy efficiency and sustainable development. Int. J. Glob. Energy Issues 2002, 17, 23–34. [Google Scholar] [CrossRef]
- Dincer, I. Technical, environmental and exergetic aspects of hydrogen energy systems. Int. J. Hydrogen Energy 2002, 27, 265–285. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. A worldwide perspective on energy, environment and sustainable development. Int. J. Energy Res. 1998, 22, 1305–1321. [Google Scholar] [CrossRef]
- Ogden, J.M. Prospects for building a hydrogen energy infrastructure. Annu. Rev. Energy Environ. 1999, 24, 227–279. [Google Scholar] [CrossRef]
- Winter, C.-J. Hydrogen energy—Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy 2009, 34, S1–S52. [Google Scholar] [CrossRef]
- Jacobson, M.; Colella, W.; Golden, D. Atmospheric science: Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F. Hydrogen energy. Philos. Trans. R. Soc. A 2007, 365, 1043–1056. [Google Scholar] [CrossRef]
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. Int. J. Hydrogen Energy 2003, 28, 267–284. [Google Scholar] [CrossRef] [Green Version]
- Bockris, J.O.M.; Dunn, S. On hydrogen futures: Toward a sustainable energy system. Author’s reply. Int. J. Hydrogen Energy 2003, 28, 131–135. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sust. Energ. Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Veziroǧlu, T.N. Hydrogen movement and the next action: Fossil fuels industry and sustainability economics. Int. J. Hydrogen Energy 1997, 22, 551–556. [Google Scholar] [CrossRef]
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Goltsov, V.A.; Veziroglu, T.N. From hydrogen economy to hydrogen civilization. Int. J. Hydrogen Energy 2001, 26, 909–915. [Google Scholar] [CrossRef]
- Goltsov, V.A.; Veziroglu, T.N. A step on the road to hydrogen civilization. Int. J. Hydrogen Energy 2002, 27, 719–723. [Google Scholar] [CrossRef]
- Quakernaat, J. Hydrogen in a global long-term perspective. Int. J. Hydrogen Energy 1995, 20, 485–492. [Google Scholar] [CrossRef]
- Midilli, A.; Ay, M.; Dincer, I.; Rosen, M.A. On hydrogen and hydrogen energy strategies: I: Current status and needs. Renew. Sust. Energy Rev. 2005, 9, 255–271. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sust. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y. Complex hydrides for hydrogen storage–New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Rusman, N.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Y. Recent advances in improving performances of the lightweight complex hydrides Li-Mg-NH system. Prog. Nat. Sci. Mater. Int. 2017, 27, 21–33. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.-T.; Jung, S.; Roh, S.-H.; Park, J.-H.; Jung, H.-Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sust. Energ. Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, C.; Shen, S.; Zou, J.; Mao, S.S.; Yao, X. Combination of nanosizing and interfacial effect: Future perspective for designing Mg-based nanomaterials for hydrogen storage. Renew. Sust. Energy Rev. 2015, 44, 289–303. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sust. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Takeichi, N.; Senoh, H.; Yokota, T.; Tsuruta, H.; Hamada, K.; Takeshita, H.T.; Tanaka, H.; Kiyobayashi, T.; Takano, T.; Kuriyama, N. “Hybrid hydrogen storage vessel”, a novel high-pressure hydrogen storage vessel combined with hydrogen storage material. Int. J. Hydrogen Energy 2003, 28, 1121–1129. [Google Scholar] [CrossRef]
- Haaland, A. High-Pressure Conformable Hydrogen Storage for Fuel Cell Vehicles. In Proceedings of the 2000 U.S. DOE Hydrogen Program Review, San Ramon, CA, USA, 9–11 May 2000. [Google Scholar]
- Cowey, K.; Green, K.; Mepsted, G.; Reeve, R. Portable and military fuel cells. Curr. Opin. Solid State Mater. Sci. 2004, 8, 367–371. [Google Scholar] [CrossRef]
- Khafidz, N.Z.A.K.; Yaakob, Z.; Lim, K.L.; Timmiati, S.N. The kinetics of lightweight solid-state hydrogen storage materials: A review. Int. J. Hydrogen Energy 2016, 41, 13131–13151. [Google Scholar] [CrossRef]
- Lahmer, K.; Bessaih, R.; Scipioni, A.; El Ganaoui, M. Simulation of hydrogen absorption in a magnesium hydride tank. Fluid Dyn. Mater. Process. 2014, 10, 149–162. [Google Scholar]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Principi, G.; Agresti, F.; Maddalena, A.; Russo, S.L. The problem of solid state hydrogen storage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- He, T.; Cao, H.; Chen, P. Complex hydrides for energy storage, conversion, and utilization. Adv. Mater. 2019, 31, 1902757. [Google Scholar] [CrossRef]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.-S.; Sun, L.; Zhu, M. Magnesium-based hydrogen storage compounds: A review. J. Alloys Compd. 2020, 832, 154865. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellosta von Colbe, J.M.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Yang, J.; Hirano, S. Improving the hydrogen reaction kinetics of complex hydrides. Adv. Mater. 2009, 21, 3023–3028. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Zuttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 253, 1–9. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356, 515–520. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef]
- Fichtner, M.; Fuhr, O.; Kircher, O. Magnesium alanate—A material for reversible hydrogen storage? J. Alloys Compd. 2003, 356, 418–422. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef]
- Aoki, M.; Miwa, K.; Noritake, T.; Kitahara, G.; Nakamori, Y.; Orimo, S.; Towata, S. Destabilization of LiBH4 by mixing with LiNH2. Appl. Phys. A 2005, 80, 1409–1412. [Google Scholar] [CrossRef]
- Jain, I.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Nakamori, Y.; Orimo, S.-I. Destabilization of Li-based complex hydrides. J. Alloys Compd. 2004, 370, 271–275. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Züttel, A. Material properties of MBH4 (M = Li, Na, and K). Mater. Sci. Eng. B 2004, 108, 51–53. [Google Scholar] [CrossRef]
- Orimo, S.-I.; Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Towata, S.-I.; Züttel, A. Dehydriding and rehydriding reactions of LiBH4. J. Alloys Compd. 2005, 404, 427–430. [Google Scholar] [CrossRef]
- Nakamori, Y.; Orimo, S.-I.; Tsutaoka, T. Dehydriding reaction of metal hydrides and alkali borohydrides enhanced by microwave irradiation. Appl. Phys. Lett. 2006, 88, 112104. [Google Scholar] [CrossRef] [Green Version]
- Züttel, A.; Borgschulte, A.; Orimo, S.-I. Tetrahydroborates as new hydrogen storage materials. Scr. Mater. 2007, 56, 823–828. [Google Scholar] [CrossRef]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Züttel, A. Stability and reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Urgnani, J.; Torres, F.J.; Palumbo, M.; Baricco, M. Hydrogen release from solid state NaBH4. Int. J. Hydrogen Energy 2008, 33, 3111–3115. [Google Scholar] [CrossRef]
- Chlopek, K.; Frommen, C.; Leon, A.; Zabara, O.; Fichtner, M. Synthesis and properties of magnesium tetrahydroborate, Mg(BH4)2. J. Mater. Chem. 2007, 17, 3496–3503. [Google Scholar] [CrossRef] [Green Version]
- Rönnebro, E.; Majzoub, E.H. Calcium borohydride for hydrogen storage: Catalysis and reversibility. J. Phys. Chem. B 2007, 111, 12045–12047. [Google Scholar] [CrossRef]
- Kumar, S.; Kojima, Y.; Dey, G.K. Synergic effect of ZrCl4 on thermal dehydrogenation kinetics of KBH4. J. Alloys Compd. 2017, 718, 134–138. [Google Scholar] [CrossRef]
- Grochala, W.; Edwards, P.P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.; Ohba, N.; Towata, S.-I.; Züttel, A.; Orimo, S.-I. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B 2006, 74, 045126. [Google Scholar] [CrossRef] [Green Version]
- Zavorotynska, O.; El-Kharbachi, A.; Deledda, S.; Hauback, B.C. Recent progress in magnesium borohydride Mg(BH4)2: Fundamentals and applications for energy storage. Int. J. Hydrogen Energy 2016, 41, 14387–14403. [Google Scholar] [CrossRef] [Green Version]
- Severa, G.; Ronnebro, E.; Jensen, C.M. Direct hydrogenation of magnesium boride to magnesium borohydride: Demonstration of >11 weight percent reversible hydrogen storage. Chem. Commun. 2010, 46, 421–423. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, S.F.; Wang, H.; Liu, J.W.; Zhu, M. Feasibility study of the direct synthesis of Mg(BH4)2 complex hydrides by mechanical milling. J. Alloys Compd. 2010, 505, 717–721. [Google Scholar] [CrossRef]
- Matsunaga, T.; Buchter, F.; Mauron, P.; Bielman, M.; Nakamori, Y.; Orimo, S.; Ohba, N.; Miwa, K.; Towata, S.; Züttel, A. Hydrogen storage properties of Mg(BH4)2. J. Alloys Compd. 2008, 459, 583–588. [Google Scholar] [CrossRef]
- Saldan, I. Decomposition and formation of magnesium borohydride. Int. J. Hydrogen Energy 2016, 41, 11201–11224. [Google Scholar] [CrossRef]
- Zavorotynska, O.; Deledda, S.; Li, G.; Matsuo, M.; Orimo, S.-I.; Hauback, B.C. Isotopic exchange in porous and dense magnesium borohydride. Angew. Chem. Int. Ed. 2015, 54, 10592–10595. [Google Scholar] [CrossRef]
- Harrison, D.; Thonhauser, T. Tuning the hydrogen desorption of Mg(BH4)2 through Zn alloying. Phys. Rev. B 2014, 90, 125152. [Google Scholar] [CrossRef] [Green Version]
- Nforbi, L.N.N.; Talekar, A.; Lau, K.H.; Chellapa, R.; Chien, W.M.; Chandra, D.; Hagemann, H.; Filinchuk, Y.; Zhao, J.C.; Levchenko, A. Vapor pressure measurements of Mg(BH4)2 using Knudsen torsion effusion thermo graphic method. Int. J. Hydrogen Energy 2014, 39, 2175–2186. [Google Scholar] [CrossRef]
- Ozolins, V.; Majzoub, E.; Wolverton, C. First-principles prediction of a ground state crystal structure of magnesium borohydride. Phys. Rev. Lett. 2008, 100, 135501. [Google Scholar] [CrossRef]
- Setten, M.J.V.; Wijs, G.A.D.; Fichtner, M.; Brocks, G. A density functional study of α-Mg(BH4)2. Chem. Mater. 2008, 20, 4952–4956. [Google Scholar] [CrossRef]
- Goumri-Said, S.; Ahmed, R.; Kanoun, M.B. Density-functional theory study of high hydrogen content complex hydrides Mg(BH4)2 at low temperature. Renew. Energy 2016, 90, 114–119. [Google Scholar] [CrossRef]
- Pinatel, E.R.; Albanese, E.; Civalleri, B.; Baricco, M. Thermodynamic modelling of Mg(BH4)2. J. Alloys Compd. 2015, 645, S64–S68. [Google Scholar] [CrossRef] [Green Version]
- Paduani, C.; Jena, P. Role of Ti-based catalysts in the dehydrogenation mechanism of magnesium borohydride: A cluster approach. Int. J. Hydrogen Energy 2013, 38, 2357–2362. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Richter, B.; Jensen, T.R.; Dmitriev, V.; Chernyshov, D.; Hagemann, H. Porous and dense magnesium borohydride frameworks: Synthesis, stability, and reversible absorption of guest species. Angew. Chem. 2011, 50, 11162–11166. [Google Scholar] [CrossRef]
- Li, H.W.; Kikuchi, K.; Nakamori, Y.; Miwa, K.; Towata, S.; Orimo, S. Effects of ball milling and additives on dehydriding behaviors of well-crystallized Mg(BH4)2. Scr. Mater. 2007, 57, 679–682. [Google Scholar] [CrossRef]
- Bateni, A.; Scherpe, S.; Acar, S.; Somer, M. Novel approach for synthesis of magnesium borohydride, Mg(BH4)2. Energy Procedia 2012, 29, 26–33. [Google Scholar] [CrossRef]
- Černý, R.; Filinchuk, Y.; Hagemann, H.; Yvon, K. Magnesium Borohydride: Synthesis and Crystal Structure. Angew. Chem. Int. Ed. 2007, 46, 5765–5767. [Google Scholar] [CrossRef] [Green Version]
- Bil, A.; Kolb, B.; Atkinson, R.; Pettifor, D.G.; Thonhauser, T.; Kolmogorov, A.N. Van der Waals interactions in the ground state of Mg(BH4)2 from density functional theory. Phys. Rev. B 2011, 83, 224103. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.-F.; Oganov, A.R.; Qian, G.-R.; Zhu, Q. First-principles determination of the structure of magnesium borohydride. Phys. Rev. Lett. 2012, 109, 245503. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Bao, K.; Duan, D.-F.; Wang, L.-C.; Liu, B.-B.; Cui, T. High volumetric hydrogen density phases of magnesium borohydride at high-pressure: A first-principles study. Chin. Phys. B 2012, 21, 086104. [Google Scholar] [CrossRef]
- Fan, J.; Duan, D.; Jin, X.; Bao, K.; Liu, B.; Cui, T. Structure determination of ultra dense magnesium borohydride: A first-principles study. J. Chem. Phys. 2013, 138, 214503. [Google Scholar] [CrossRef]
- Łodziana, Z.; van Setten, M.J. Binding in alkali and alkaline-earth tetrahydroborates: Special position of magnesium tetrahydroborate. Phys. Rev. B 2010, 81, 024117. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, T.; Buchter, F.; Miwa, K.; Towata, S.; Orimo, S.; Züttel, A. Magnesium borohydride: A new hydrogen storage material. Renew. Energy 2008, 33, 193–196. [Google Scholar] [CrossRef]
- Riktor, M.; Sørby, M.; Chłopek, K.; Fichtner, M.; Buchter, F.; Züttel, A.; Hauback, B. In situ synchrotron diffraction studies of phase transitions and thermal decomposition of Mg(BH4)2 and Ca(BH4)2. J. Mater. Chem. 2007, 17, 4939–4942. [Google Scholar] [CrossRef]
- Li, H.-W.; Kikuchi, K.; Nakamori, Y.; Ohba, N.; Miwa, K.; Towata, S.; Orimo, S. Dehydriding and rehydriding processes of well-crystallized Mg(BH4)2 accompanying with formation of intermediate compounds. Acta Mater. 2008, 56, 1342–1347. [Google Scholar] [CrossRef]
- Li, H.-W.; Kikuchi, K.; Sato, T.; Nakamori, Y.; Ohba, N.; Aoki, M.; Miwa, K.; Towata, S.-I.; Orimo, S.-I. Synthesis and hydrogen storage properties of a single-phase magnesium borohydride Mg(BH4)2. Mater. Trans. 2008, 49, 2224–2228. [Google Scholar] [CrossRef] [Green Version]
- Hanada, N.; Chłopek, K.; Frommen, C.; Lohstroh, W.; Fichtner, M. Thermal decomposition of Mg(BH4)2 under He flow and H2 pressure. J. Mater. Chem. 2008, 18, 2611–2614. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.-W.; Nakamori, Y.; Ohba, N.; Miwa, K.; Towata, S.-I.; Orimo, S.-I. Differential scanning calorimetry measurements of magnesium borohydride Mg(BH4)2. Mater. Trans. 2008, 49, 2751–2752. [Google Scholar] [CrossRef] [Green Version]
- Fichtner, M.; Zhao-Karger, Z.; Hu, J.; Roth, A.; Weidler, P. The kinetic properties of Mg(BH4)2 infiltrated in activated carbon. Nanotechnology 2009, 20, 204029. [Google Scholar] [CrossRef]
- Li, H.; Miwa, K.; Ohba, N.; Fujita, T.; Sato, T.; Yan, Y.; Towata, S.; Chen, M.; Orimo, S. Formation of an intermediate compound with a B12H12 cluster: Experimental and theoretical studies on magnesium borohydride Mg(BH4)2. Nanotechnology 2009, 20, 204013. [Google Scholar] [CrossRef]
- Soloveichik, G.L.; Gao, Y.; Rijssenbeek, J.; Andrus, M.; Kniajanski, S.; Bowman, R.C., Jr.; Hwang, S.-J.; Zhao, J.-C. Magnesium borohydride as a hydrogen storage material: Properties and dehydrogenation pathway of unsolvated Mg(BH4)2. Int. J. Hydrogen Energy 2009, 34, 916–928. [Google Scholar] [CrossRef]
- Ibikunle, A.A.; Goudy, A.J. Kinetics and modeling study of a Mg(BH4)2/Ca(BH4)2 destabilized system. Int. J. Hydrogen Energy 2012, 37, 12420–12424. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Cerny, R.; Hagemann, H. Insight into Mg(BH4)2 with synchrotron X-ray diffraction: Structure revision, crystal chemistry, and anomalous thermal expansion. Chem. Mater. 2009, 21, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Her, J.-H.; Stephens, P.W.; Gao, Y.; Soloveichik, G.L.; Rijssenbeek, J.; Andrus, M.; Zhao, J.-C. Structure of unsolvated magnesium borohydride Mg(BH4)2. Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 561–568. [Google Scholar] [CrossRef]
- Heere, M.; Zavorotynska, O.; Deledda, S.; Sørby, M.H.; Book, D.; Steriotis, T.; Hauback, B.C. Effect of additives, ball milling and isotopic exchange in porous magnesium borohydride. RSC Adv. 2018, 8, 27645–27653. [Google Scholar] [CrossRef] [Green Version]
- Wahab, M.A.; Jia, Y.; Yang, D.; Zhao, H.; Yao, X. Enhanced hydrogen desorption from Mg(BH4)2 by combining nanoconfinement and a Ni catalyst. J. Mater. Chem. A 2013, 1, 3471–3478. [Google Scholar] [CrossRef]
- Clémençon, D.; Davoisne, C.; Chotard, J.N.; Janot, R. Enhancement of the hydrogen release of Mg(BH4)2 by concomitant effects of nano-confinement and catalysis. Int. J. Hydrogen Energy 2019, 44, 4253–4262. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, G.; Zhang, J.; Sun, D.; Guo, Z.; Yu, X. Graphene-tailored thermodynamics and kinetics to fabricate metal borohydride nanoparticles with high purity and enhanced reversibility. Adv. Energy Mater. 2018, 8, 1702975. [Google Scholar] [CrossRef]
- Juahir, N.; Mustafa, N.S.; Halim Yap, F.A.; Ismail, M. Study on the hydrogen storage properties and reaction mechanism of NaAlH4–Mg(BH4)2 (2:1) with and without TiF3 additive. Int. J. Hydrogen Energy 2015, 40, 7628–7635. [Google Scholar] [CrossRef]
- Sulaiman, N.N.; Ismail, M.; Timmiati, S.N.; Lim, K.L. Improved hydrogen storage performances of LiAlH4 + Mg(BH4)2 composite with TiF3 addition. Int. J. Energy Res. 2021, 45, 2882–2898. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Baricco, M. Hydrogen desorption in Mg(BH4)2-Ca(BH4)2 system. Energies 2019, 12, 3230. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, Y.; Xiong, Z.; Wu, G.; Chu, H.; He, T.; Chen, P. Enhanced hydrogen desorption from the Co-catalyzed LiBH4–Mg(BH4)2 eutectic composite. Int. J. Hydrogen Energy 2012, 37, 12425–12431. [Google Scholar] [CrossRef]
- Grube, E.; Jensen, S.R.H.; Nielsen, U.G.; Jensen, T.R. Reactivity of magnesium borohydride–Metal hydride composites, γ-Mg(BH4)2-MHx, M = Li, Na, Mg, Ca. J. Alloys Compd. 2019, 770, 1155–1163. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhou, Y.; Zhang, Y.; Gao, M.; Pan, H. Hydrogen storage properties and mechanisms of the Mg(BH4)2-NaAlH4 system. Int. J. Hydrogen Energy 2012, 37, 17137–17145. [Google Scholar] [CrossRef]
- Pan, H.; Shi, S.; Liu, Y.; Li, B.; Yang, Y.; Gao, M. Improved hydrogen storage kinetics of the Li-Mg-N-H system by addition of Mg(BH4)2. Dalton Trans. 2013, 42, 3802–3811. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, M.; Ley, M.B.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Eutectic melting in metal borohydrides. Phys. Chem. Chem. Phys. 2013, 15, 19774–19789. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Gao, M.; Liu, Y.; Wang, J.; Gu, J.; Pan, H.; Guo, Z. Multi-hydride systems with enhanced hydrogen storage properties derived from Mg(BH4)2 and LiAlH4. Int. J. Hydrogen Energy 2012, 37, 10733–10742. [Google Scholar] [CrossRef]
- Yu, X.B.; Guo, Y.H.; Sun, D.L.; Yang, Z.X.; Ranjbar, A.; Guo, Z.P.; Liu, H.K.; Dou, S.X. A combined hydrogen storage system of Mg(BH4)2-LiNH2 with favorable dehydrogenation. J. Phys. Chem. C 2010, 114, 4733–4737. [Google Scholar] [CrossRef]
- Castilla-Martinez, C.A.; Moury, R.; Ould-Amara, S.; Demirci, U.B. Destabilization of boron-based compounds for hydrogen storage in the solid-state: Recent advances. Energies 2021, 14, 7003. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Shi, B.; Song, Y.; Dai, J.H.; Yu, H.Z. Influence of Ti and Al dopants on the dehydrogenation characteristics of Mg(BH4)2: Electronic structure mechanisms. J. Phys. Chem. C 2012, 116, 12001–12007. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Wang, D.; Yang, F.; Wu, Z.; Wu, L.; Zhang, Z. Role of native defects and the effects of metal additives on the kinetics of magnesium borohydride. Phys. Chem. Chem. Phys. 2019, 21, 11226–11233. [Google Scholar] [CrossRef]
- Jiang, J.; Wei, J.; Leng, H.; Li, Q.; Chou, K.-C. Effect of Al on the hydrogen storage properties of Mg(BH4)2. Int. J. Hydrogen Energy 2013, 38, 10919–10925. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J. Sodium alanates for reversible hydrogen storage. J. Alloys Compd. 2000, 298, 125–134. [Google Scholar] [CrossRef]
- Li, H.-W.; Orimo, S.-I.; Nakamori, Y.; Miwa, K.; Ohba, N.; Towata, S.; Züttel, A. Materials designing of metal borohydrides: Viewpoints from thermodynamical stabilities. J. Alloys Compd. 2007, 446, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xiao, X.; Zheng, J.; Hang, Z.; Lin, W.; Yao, Z.; Zhang, M.; Chen, L. The dehydrogenation kinetics and reversibility improvements of Mg(BH4)2 doped with Ti nano-particles under mild conditions. Int. J. Hydrogen Energy 2021, 46, 23737–23747. [Google Scholar] [CrossRef]
- Saldan, I.; Hino, S.; Humphries, T.D.; Zavorotynska, O.; Chong, M.; Jensen, C.; Deledda, S.; Hauback, B. Structural changes observed during the reversible hydrogenation of Mg(BH4)2 with Ni-based additives. J. Phys. Chem. C 2014, 118, 23376–23384. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sun, Z.; Yao, Z.; Yang, L.; Yan, N.; Lu, X.; Xiao, B.; Zhu, X.; Chen, L. Excellent catalysis of Mn3O4 nanoparticles on the hydrogen storage properties of MgH2: An experimental and theoretical study. Nanoscale Adv. 2020, 2, 1666–1675. [Google Scholar] [CrossRef] [Green Version]
- Zavorotynska, O.; Saldan, I.; Hino, S.; Humphries, T.; Deledda, S.; Hauback, B. Hydrogen cycling in γ-Mg(BH4)2 with cobalt-based additives. J. Mater. Chem. A 2015, 3, 6592–6602. [Google Scholar] [CrossRef] [Green Version]
- Zavorotynska, O.; Deledda, S.; Vitillo, J.G.; Saldan, I.; Guzik, M.N.; Baricco, M.; Walmsley, J.C.; Muller, J.; Hauback, B.C. Combined X-ray and Raman studies on the effect of cobalt additives on the decomposition of magnesium borohydride. Energies 2015, 8, 9173–9190. [Google Scholar] [CrossRef] [Green Version]
- Saldan, I.; Frommen, C.; Llamas-Jansa, I.; Kalantzopoulos, G.N.; Hino, S.; Arstad, B.; Heyn, R.H.; Zavorotynska, O.; Deledda, S.; Sørby, M.H.; et al. Hydrogen storage properties of γ–Mg(BH4)2 modified by MoO3 and TiO2. Int. J. Hydrogen Energy 2015, 40, 12286–12293. [Google Scholar] [CrossRef]
- Saldan, I.; Llamas-Jansa, I.; Hino, S.; Frommen, C.; Hauback, B. Synthesis and Thermal Decomposition of Mg(BH4)2-TMO(TMO= TiO2; ZrO2; Nb2O5; MoO3) Composites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2015; p. 012041. [Google Scholar]
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- Hino, S.; Fonneløp, J.E.; Corno, M.; Zavorotynska, O.; Damin, A.; Richter, B.; Baricco, M.; Jensen, T.R.; Sørby, M.H.; Hauback, B.C. Halide substitution in magnesium borohydride. J. Phys. Chem. C 2012, 116, 12482–12488. [Google Scholar] [CrossRef] [Green Version]
- Al-Kukhun, A.; Hwang, H.T.; Varma, A. NbF5 additive improves hydrogen release from magnesium borohydride. Int. J. Hydrogen Energy 2012, 37, 17671–17677. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, X.; Zheng, J.; Huang, X.; Chen, M.; Chen, L. In-situ synthesis of amorphous Mg(BH4)2 and chloride composite modified by NbF5 for superior reversible hydrogen storage properties. Int. J. Hydrogen Energy 2020, 45, 2044–2053. [Google Scholar] [CrossRef]
- Yan, Y.; Remhof, A.; Rentsch, D.; Züttel, A. The role of MgB12H12 in the hydrogen desorption process of Mg(BH4)2. Chem. Commun. 2015, 51, 700–702. [Google Scholar] [CrossRef] [Green Version]
- Newhouse, R.J.; Stavila, V.; Hwang, S.-J.; Klebanoff, L.E.; Zhang, J.Z. Reversibility and improved hydrogen release of magnesium borohydride. J. Phys. Chem. C 2010, 114, 5224–5232. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.G.; Wang, H.; Liu, J.W.; Zhu, M. Thermal decomposition behaviors of magnesium borohydride doped with metal fluoride additives. Thermochim. Acta 2013, 560, 82–88. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Nakajima, K.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Dey, G.K.; Kojima, Y. Improved hydrogen release from magnesium borohydride by ZrCl4 additive. Int. J. Hydrogen Energy 2017, 42, 22342–22347. [Google Scholar] [CrossRef]
- Bardají, E.G.; Hanada, N.; Zabara, O.; Fichtner, M. Effect of several metal chlorides on the thermal decomposition behaviour of α-Mg(BH4)2. Int. J. Hydrogen Energy 2011, 36, 12313–12318. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, X.; Xiao, X.; Cheng, H.; Zhang, L.; Chen, L. Improved reversible dehydrogenation properties of Mg(BH4)2 catalyzed by dual-cation transition metal fluorides K2TiF6 and K2NbF7. Chem. Eng. J. 2021, 412, 128738. [Google Scholar] [CrossRef]
- Han, M.; Zhao, Q.; Zhu, Z.; Hu, Y.; Tao, Z.; Chen, J. The enhanced hydrogen storage of micro-nanostructured hybrids of Mg(BH4)2–carbon nanotubes. Nanoscale 2015, 7, 18305–18311. [Google Scholar] [CrossRef]
- Jiang, Z.; Yuan, J.; Han, H.; Wu, Y. Effect of carbon nanotubes on the microstructural evolution and hydrogen storage properties of Mg(BH4)2. J. Alloys Compd. 2018, 743, 11–16. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, H.; Jiang, Z.; Lv, Y.; Liu, B.; Zhang, B.; Yan, Y.; Wu, Y. Ni-doped carbon nanotube-Mg(BH4)2 composites for hydrogen storage. ACS Appl. Nano Mater. 2021, 4, 1604–1612. [Google Scholar] [CrossRef]
- Yan, Y.; Au, Y.S.; Rentsch, D.; Remhof, A.; de Jongh, P.E.; Züttel, A. Reversible hydrogen storage in Mg(BH4)2/carbon nanocomposites. J. Mater. Chem. A 2013, 1, 11177–11183. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Cheng, H.; Xiao, X.; Chen, M.; Chen, L. Enhanced low temperature hydrogen desorption properties and mechanism of Mg(BH4)2 composited with 2D MXene. Int. J. Hydrogen Energy 2019, 44, 24292–24300. [Google Scholar] [CrossRef]
- Feng, X.; Yuan, J.; Lv, Y.; Liu, B.; Huang, H.; Zhang, B.; Yan, Y.; Han, S.; Wu, Y. Improvement of desorption performance of Mg(BH4)2 by two-dimensional Ti3C2 MXene addition. Int. J. Hydrogen Energy 2020, 45, 16654–16662. [Google Scholar] [CrossRef]
- Jeong, S.; Heo, T.W.; Oktawiec, J.; Shi, R.; Kang, S.; White, J.L.; Schneemann, A.; Zaia, E.W.; Wan, L.F.; Ray, K.G.; et al. A mechanistic analysis of phase evolution and hydrogen storage behavior in nanocrystalline Mg(BH4)2 within reduced graphene oxide. ACS Nano 2020, 14, 1745–1756. [Google Scholar] [CrossRef] [Green Version]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Thermodynamic changes in mechanochemically synthesized magnesium hydride nanoparticles. J. Am. Chem. Soc. 2010, 132, 5077–5083. [Google Scholar] [CrossRef]
- Gross, A.F.; Vajo, J.J.; Van Atta, S.L.; Olson, G.L. Enhanced hydrogen storage kinetics of LiBH4 in nanoporous carbon scaffolds. J. Phys. Chem. C 2008, 112, 5651–5657. [Google Scholar] [CrossRef]
- Liu, X.; Peaslee, D.; Jost, C.Z.; Baumann, T.F.; Majzoub, E.H. Systematic pore-size effects of nanoconfinement of LiBH4: Elimination of diborane release and tunable behavior for hydrogen storage applications. Chem. Mater. 2011, 23, 1331–1336. [Google Scholar] [CrossRef]
- Sun, N.; Xu, B.; Zhao, S.; Sun, Z.; Li, X.; Meng, L. Influences of Al, Ti and Nb doping on the structure and hydrogen storage property of Mg(BH4)2(001) surface–A theoretical study. Int. J. Hydrogen Energy 2015, 40, 10516–10526. [Google Scholar] [CrossRef]
- Mo, X.; Jiang, W.; Cao, S. First-principles study on the dehydrogenation characteristics of LiBH4 modified by Ti. Results Phys. 2017, 7, 3236–3242. [Google Scholar] [CrossRef]
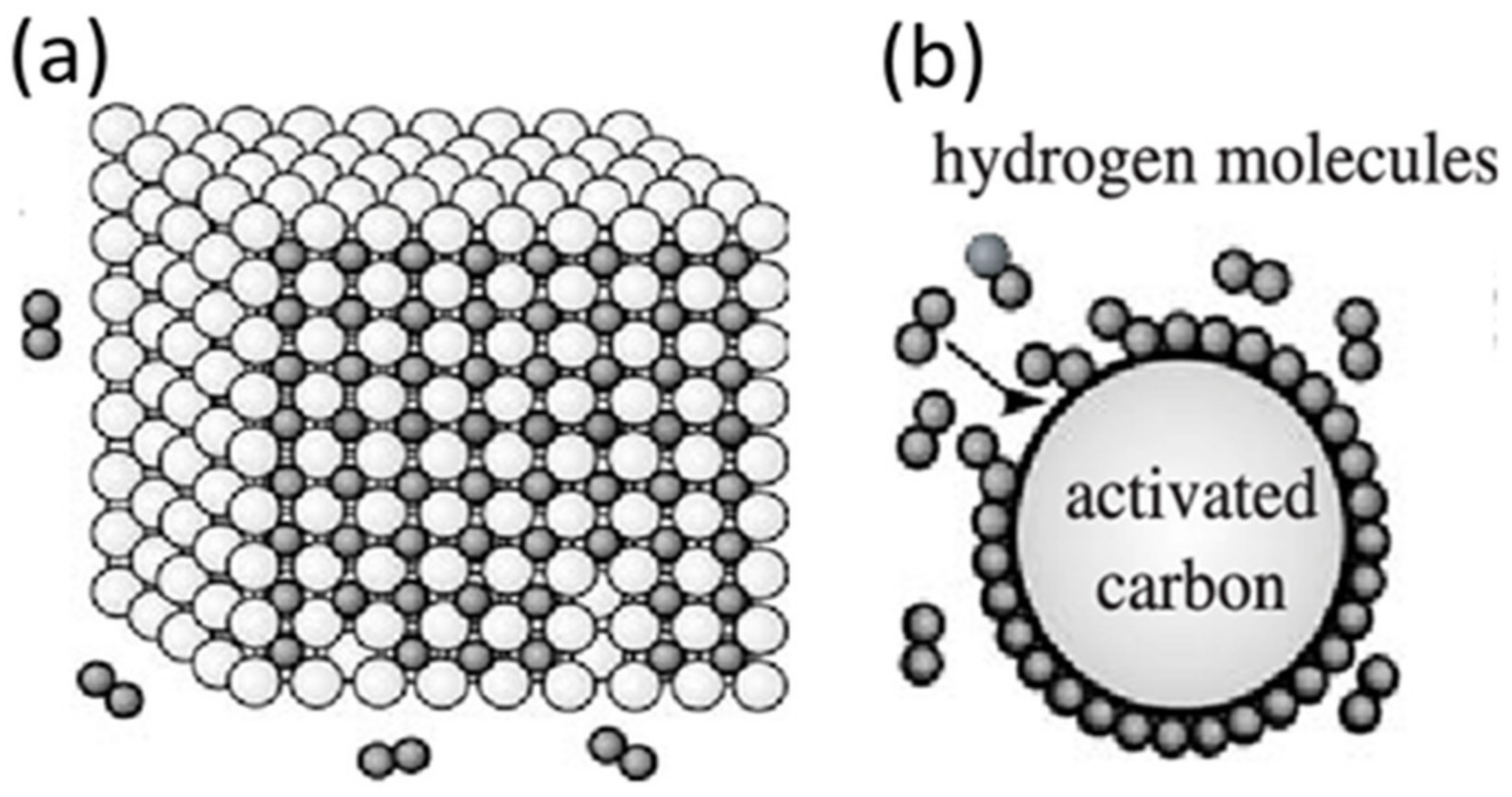

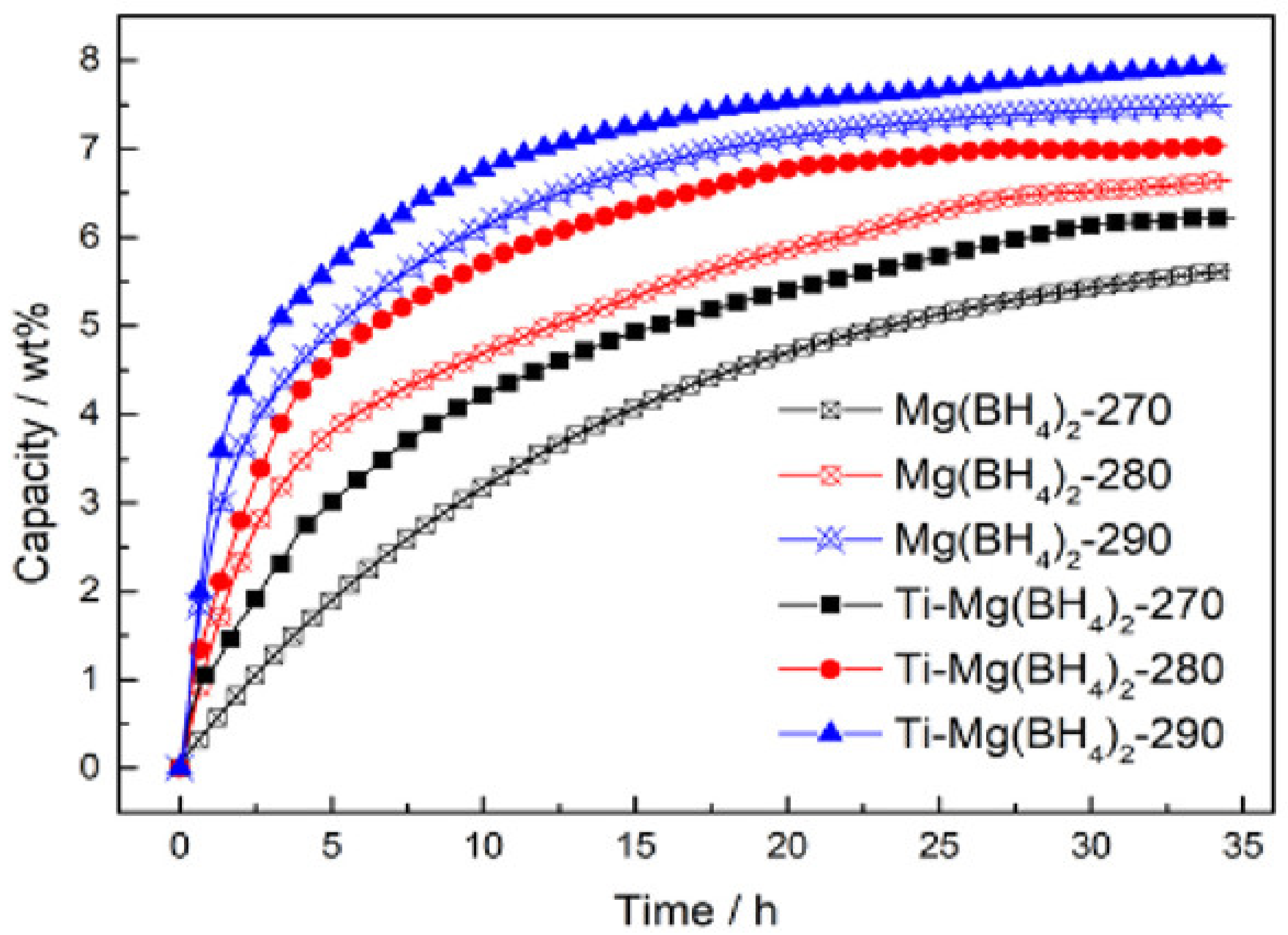
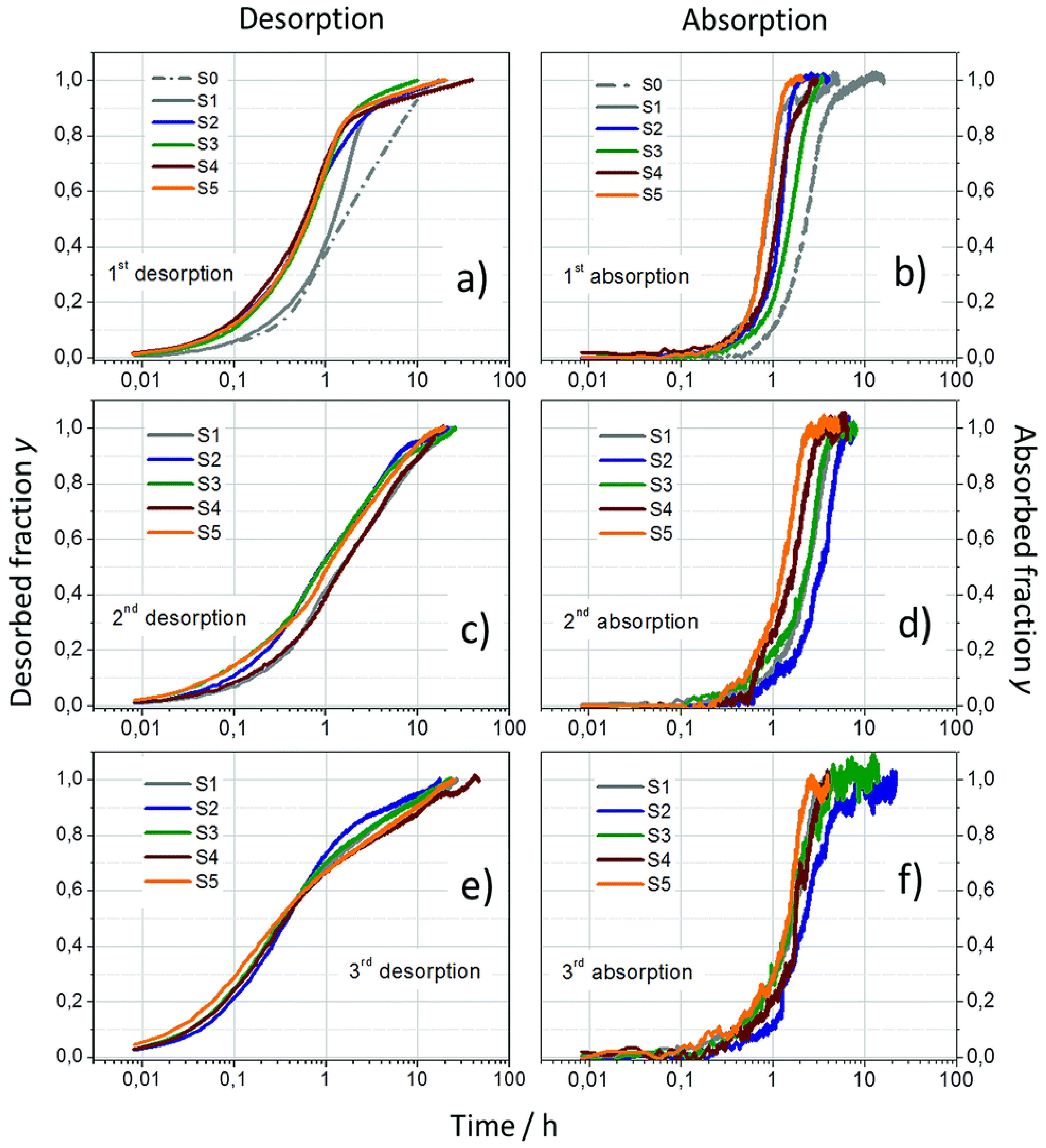

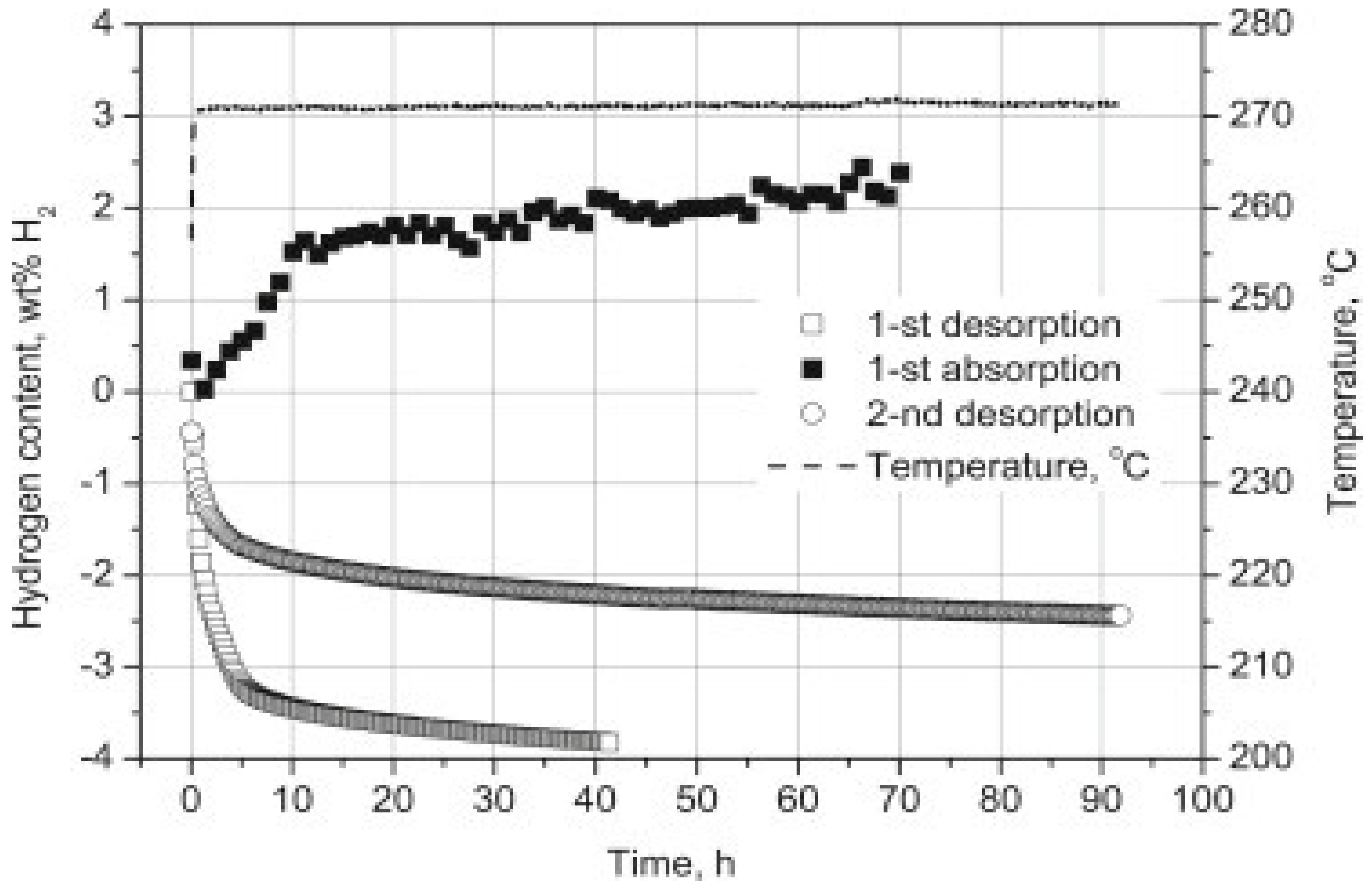

| Formula | Molecular Weight (g mol−1) | Decomposition Temperature (°C) | Hydrogen Storage Capacity (wt%) | Ref. |
|---|---|---|---|---|
| LiBH4 | 21.8 | 320 | 18.5 | [47] |
| NaBH4 | 37.8 | 450 | 10.6 | [54] |
| Mg(BH4)2 | 53.9 | 320 | 14.9 | [55] |
| Ca(BH4)2 | 69.8 | 360 | 11.4 | [56] |
| KBH4 | 53.9 | 550 | 7.5 | [57] |
| Phase | Space Group | ρm, wt% | ρ, gcm−3 | ρv, g L−1 | Ref. |
|---|---|---|---|---|---|
| α-Mg(BH4)2 | P6122 | 14.9 | 0.783 | 117 | [92] |
| β-Mg(BH4)2 | Fddd | 14.9 | 0.761 | 113 | [93] |
| γ-Mg(BH4)2 | Id3a | 14.9 | 0.550 | 82 | [73] |
| γ-Mg(BH4)2-0.80 H2 | Ia3d | 17.4 | 0.565 | 98 | [73] |
| δ-Mg(BH4)2 | P42nm | 14.9 | 0.987 | 147 | [73] |
| Sample | Dehydrogenation | Rehydrogenation | ||
|---|---|---|---|---|
| Temperature Program Desorption (TPD) (wt%) | Desorption at 350 °C (wt%) | Time for 90% H2 Desorption (s) | Absorption at 350 °C (wt%) | |
| Mg(BH4)2 | 12.0 | 10.4 | 1911 | 4.1 |
| (Mg(BH4)2 + Al) | 8.3 | 6.2 | 1173 | 1.7 |
| (Mg(BH4)2 + LiH + Al) | 8.0 | 5.8 | 1699 | 5.1 |
| (Mg(BH4)2 + 1/3(Li3AlH6) + 2Al) | 10.1 | 7.6 | 1277 | 5.0 |
| (Mg(BH4)2 + LiAlH4) | 11.5 | 9.5 | 1316 | 5.7 |
| Additive | Desorption of Mg(BH4)2 | Absorption of Mg(BH4)2 | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Name | Mol. % | Time, min | Temperature, °C | H2, wt% | Time, min | Temperature, °C | H2, wt% | |
| Al | 50 | 125 | 350 | 6.2 | 100 | 350 | 1.7 | [112] |
| Ni | 2 | 3600 | 256 | 2.7 | 1200 | 251 | 1.3 | [116] |
| Ti | - | 900 | 270 | 4.0 | 900 | 270 | 4.23 | [115] |
| Additive | Desorption of Mg(BH4)2 | Absorption of Mg(BH4)2 | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Name | Mol. % | Time, min | Temperature, °C | H2, wt% | Time, min | Temperature, °C | H2, wt% | |
| TiO2 | 2 | 2400 | 271 | 3.8 | 4200 | 271 | 2.4 | [120] |
| Co3O4 | 2 | 1260 | 288 | 3.9 | 120 | 285 | 1.6 | [118] |
| MoO3 | 2 | 1800 | 267 | 5.1 | - | - | - | [120] |
| ZrO2 | 2 | 250 | 299 | - | - | - | - | [121] |
| Nb2O5 | 2 | 250 | 309 | - | - | - | - | [121] |
| Additive | Desorption of Mg(BH4)2 | Absorption of Mg(BH4)2 | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Name | Mol. % | Time, min | Temperature, °C | H2, wt% | Time, min | Temperature, °C | H2, wt% | |
| TiCl3 | 5 | 120 | 300 | 6.1 | - | - | - | [130] |
| CoCl3 | 2 | 2340 | 288 | 4.4 | 180 | 284 | 1.9 | [118] |
| NiCl2 | 2 | 600 | 258 | 2.7 | 900 | 253 | 1.2 | [116] |
| PdCl2 | 5 | 120 | 300 | 5.3 | - | - | - | [130] |
| MoCl3 | 5 | 120 | 300 | 4.2 | - | - | - | [130] |
| NbCl5 | 5 | 120 | 300 | 5.5 | - | - | - | [130] |
| CeCl3 | 5 | 120 | 300 | 3.6 | - | - | - | [130] |
| RuCl3 | 5 | 120 | 300 | 5.0 | - | - | - | [130] |
| VCl3 | 5 | 90 | 300 | 7.3 | - | - | - | [124] |
| CoCl2 | 5 | 90 | 300 | 8.0 | - | - | - | [124] |
| CaF2 | 3 | 420 | 295 | 4.0 | - | - | - | [128] |
| TiF3 | 3 | 420 | 295 | 4.5 | - | - | - | [128] |
| CoF3 | 2 | 1080 | 284 | 3.2 | 240 | 281 | 1.9 | [118] |
| NiF2 | 2 | 1800 | 264 | 6.5 | 600 | 262 | 2.0 | [116] |
| ZnF2 | 3 | 420 | 295 | 4.3 | - | - | - | [128] |
| NbF5 | 5 | 90 | 300 | 10.1 | - | - | - | [124] |
| ZrCl4 | 1 | 60 | 290 | 5.6 | - | - | - | [129] |
| K2TiF6 | 3 | 200 | 280 | 6.4 | 250 | 280 | 2.7 | [131] |
| K2NbF7 | 3 | 200 | 280 | 6.6 | - | - | - | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.A.N.; Sazelee, N.; Ali, N.A.; Ismail, M. An Overview of the Recent Advances of Additive-Improved Mg(BH4)2 for Solid-State Hydrogen Storage Material. Energies 2022, 15, 862. https://doi.org/10.3390/en15030862
Ahmad MAN, Sazelee N, Ali NA, Ismail M. An Overview of the Recent Advances of Additive-Improved Mg(BH4)2 for Solid-State Hydrogen Storage Material. Energies. 2022; 15(3):862. https://doi.org/10.3390/en15030862
Chicago/Turabian StyleAhmad, Muhammad Amirul Nawi, Noratiqah Sazelee, Nurul Amirah Ali, and Mohammad Ismail. 2022. "An Overview of the Recent Advances of Additive-Improved Mg(BH4)2 for Solid-State Hydrogen Storage Material" Energies 15, no. 3: 862. https://doi.org/10.3390/en15030862
APA StyleAhmad, M. A. N., Sazelee, N., Ali, N. A., & Ismail, M. (2022). An Overview of the Recent Advances of Additive-Improved Mg(BH4)2 for Solid-State Hydrogen Storage Material. Energies, 15(3), 862. https://doi.org/10.3390/en15030862









