Recent Advances in the Multifunctional Natural Gum-Based Binders for High-Performance Rechargeable Batteries
Abstract
1. Introduction
2. Physicochemical Properties of Various Types of Gums
3. Applications in Rechargeable Batteries
3.1. Li-Ion Batteries
3.2. Li-S Batteries
3.3. Na-Ion Batteries
4. Summary and Conclusions
- Natural gum-based binders lead to better mechanical properties, hence resulting in better adhesion between the current collector and active materials (nanoscratch test, force-displacement test, Fourier-transform infrared spectroscopy (FTIR), scanning probe microscopy (SPM), scratch test, peeling test, scanning electron microscopy (SEM), proton nuclear magnetic resonance (1H-NMR), optical microscopy, adherence test, and nanoindentation test).
- Natural gum-based binders lead to enhanced electronic conductivity, as it shows good adhesion between the active materials and conductive material (e.g., Super P carbon black), conductive material and current collector, and current collector and active material (Kelvin probe force microscope, electrochemical impedance spectroscopy (EIS) analysis).
- Natural gum-based binders contribute to the formation of stable solid electrolyte interphase (SEI) layer, which allows more stable interface between the electrode and electrolyte (SEM).
- Natural gum-based binders prevent the electrode corrosion from electrolyte. Particularly, for XG, such phenomenon takes place due to abundant hydroxyl/carboxylate functional groups as well as the special double helix structure (photographs of binders in electrolytes, FTIR, X-ray photoelectron spectroscopy (XPS), and 1H-NMR).
- Natural gum-based binders demonstrate good thermal stability, leading to the safety of the rechargeable batteries (thermogravimetry analysis, differential scanning calorimetry analysis).
- Natural gum-based binders show good flowability and allow effective dispersion of active materials and conductive materials, leading to uniform distribution of each component for slurry (rheology analysis).
- Natural gum-based binders have fast transfer of Li ions and good ionic conductivity (cyclic voltammetry and others).
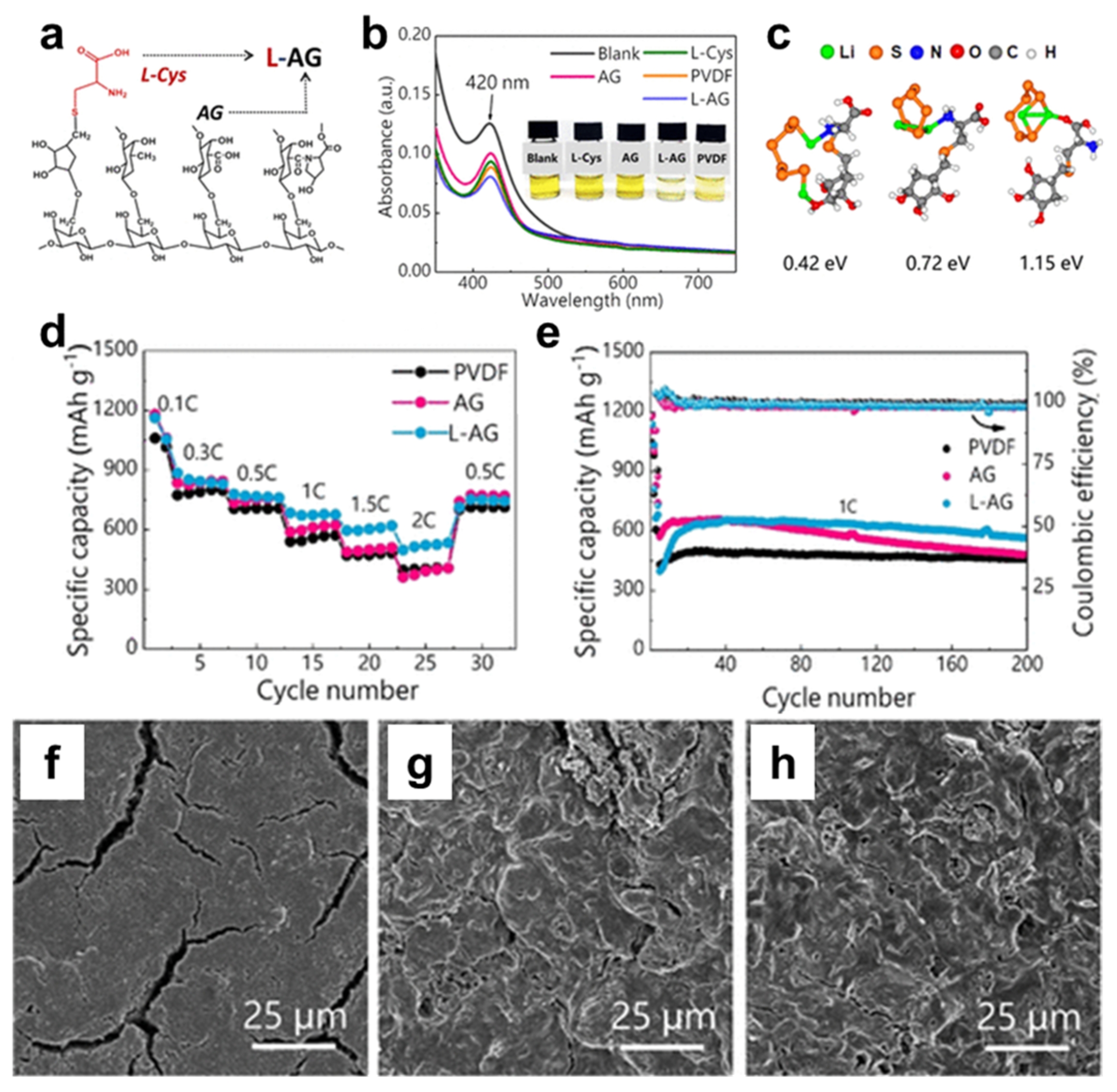
- For Li-S batteries, natural gum-based binders are capable of capturing polysulfides, mitigating the shuttling effect arising from polysulfide dissolution (photographs, UV-visible spectrometer, simulation, XPS, and FTIR).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.X.; Chen, L.Q.; Huang, X.J. Research on Advanced Materials for Li-ion Batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Irmale, T.C.; Kale, B.B.; Varma, A.J. A review on the cellulose and lignin based binders and electrodes: Small steps towards a sustainable lithium ion battery. Int. J. Biol. Macromol. 2017, 103, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Bridel, J.-S.; Azaïs, T.; Morcrette, M.; Tarascon, J.-M.; Larcher, D. Key Parameters Governing the Reversibility of Si/Carbon/CMC Electrodes for Li-Ion Batteries. Chem. Mater. 2010, 22, 1229–1241. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward Efficient Binders for Li-Ion Battery Si-Based Anodes: Polyacrylic Acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Xuan, Y.; Zhou, L.; Wang, D.; Li, Z.; Lin, H.; Tretiak, S.; Wang, H.; Wang, L.; et al. Multifunctional Cellulose Nanocrystals as a High-Efficient Polysulfide Stopper for Practical Li-S Batteries. ACS Appl. Mater. Interfaces 2020, 12, 17592–17601. [Google Scholar] [CrossRef]
- Su, A.; Pang, Q.; Chen, X.; Dong, J.; Zhao, Y.; Lian, R.; Zhang, D.; Liu, B.; Chen, G.; Wei, Y. Lithium poly-acrylic acid as a fast Li+ transport media and a highly stable aqueous binder for Li3V2(PO4)3 cathode electrodes. J. Mater. Chem. A 2018, 6, 23357–23365. [Google Scholar] [CrossRef]
- Yi, H.; Lan, T.; Yang, Y.; Zeng, H.; Zhang, T.; Tang, T.; Wang, C.; Deng, Y. A robust aqueous-processible polymer binder for long-life, high-performance lithium sulfur battery. Energy Storage Mater. 2019, 21, 61–68. [Google Scholar] [CrossRef]
- Ling, L.; Bai, Y.; Wang, Z.; Ni, Q.; Chen, G.; Zhou, Z.; Wu, C. Remarkable Effect of Sodium Alginate Aqueous Binder on Anatase TiO2 as High-Performance Anode in Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5560–5568. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ha, S.; Prakash, J.; Dees, D.W.; Lu, W. Investigations on high energy lithium-ion batteries with aqueous binder. Electrochim. Acta 2013, 114, 1–6. [Google Scholar] [CrossRef]
- Chen, S.; Ling, H.Y.; Chen, H.; Zhang, S.; Du, A.; Yan, C. Development of cross-linked dextrin as aqueous binders for silicon based anodes. J. Power Sources 2020, 450, 227671. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Zhong, H.; Ding, J.; Zhang, L. Cyanoethylated Carboxymethyl Chitosan as Water Soluble Binder with Enhanced Adhesion Capability and electrochemical performances for LiFePO4 Cathode. Electrochim. Acta 2015, 182, 900–907. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Chang, J.H.; Cho, S.-H.; Jung, J.-W.; Kim, C.; Dae, K.S.; Yuk, J.M.; Kim, I.-D. High-rate formation cycle of Co3O4 nanoparticle for superior electrochemical performance in lithium-ion batteries. Electrochim. Acta 2019, 295, 7–13. [Google Scholar] [CrossRef]
- Phanikumar, V.V.N.; Rikka, V.R.; Das, B.; Gopalan, R.; Appa Rao, B.V.; Prakash, R. Investigation on polyvinyl alcohol and sodium alginate as aqueous binders for lithium-titanium oxide anode in lithium-ion batteries. Ionics 2019, 25, 2549–2561. [Google Scholar] [CrossRef]
- Sun, J.; Ren, X.; Li, Z.; Tian, W.; Zheng, Y.; Wang, L.; Liang, G. Effect of poly(acrylic acid)/poly(vinyl alcohol) blending binder on electrochemical performance for lithium iron phosphate cathodes. J. Alloys Compd. 2019, 783, 379–386. [Google Scholar] [CrossRef]
- Kim, S.; Cho, M.; Lee, Y. Multifunctional Chitosan-rGO Network Binder for Enhancing the Cycle Stability of Li-S Batteries. Adv. Funct. Mater. 2020, 30, 1907680. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, X.; Wang, X.; Gu, A.; Zhang, L.; Chen, X.; Li, J.; Yu, Z. Chitosan-g-Poly(acrylic acid) Copolymer and Its Sodium Salt as Stabilized Aqueous Binders for Silicon Anodes in Lithium-Ion Batteries. ACS Chem. Eng. 2019, 7, 16274–16283. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, Y.K.; Wang, Y.; Lee, H.; Choi, J.W. A “Sticky” Mucin-Inspired DNA-Polysaccharide Binder for Silicon and Silicon-Graphite Blended Anodes in Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1707594. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, T.-W.; Coskun, A.; Choi, J.W. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 2017, 357, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Huang, T.; Yu, A. Carboxymethylated tamarind polysaccharide gum as a green binder for silicon-based lithium-ion battery anodes. Electrochem. Commun. 2022, 136, 107241. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macrmol. 2016, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S. Advanced green materials: An overview. Adv. Green Mater. 2021, 1–13. [Google Scholar] [CrossRef]
- Wang, A.; Wang, W. Gum-g-Copolymers: Synthesis, Properties, and Applications. In Polysaccharide Based Graft Copolymers; Springer: Berlin/Heidelberg, Germany, 2013; pp. 149–203. [Google Scholar]
- Kohajdová, Z.; Karovičová, J. Application of hydrocolloids as baking improvers. Chem. Pap. 2009, 63, 26–38. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Fahmideh-Rad, E. Fenugreek seed gum: Biological properties, chemical modifications, and structural analysis—A review. Int. J. Biol. Macromol. 2019, 138, 386–393. [Google Scholar] [CrossRef]
- Chauhan, G.; Verma, A.; Das, A.; Ojha, K. Rheological studies and optimization of Herschel-Bulkley flow parameters of viscous karaya polymer suspensions using GA and PSO algorithms. Rheol. Acta 2018, 57, 267–285. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Esmaeili, M.S.; Varzi, Z.; Eivazzadeh-Keihan, R.; Maleki, A.; Shalan, A.E. Facile route to synthesize Fe3O4@acacia–SO3H nanocomposite as a heterogeneous magnetic system for catalytic applications. RSC Adv. 2020, 10, 40055–40067. [Google Scholar] [CrossRef]
- Maji, B.; Maiti, S. Chemical modification of xanthan gum through graft copolymerization: Tailored properties and potential applications in drug delivery and wastewater treatment. Carbohydr. Polym. 2021, 251, 117095. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Jiang, B.; Chen, J. A review of the enzymatic, physical, and chemical modification techniques of xanthan gum. Int. J. Biol. Macromol. 2021, 186, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.W.M.; Laurentino, L.D.S.; Alves, C.R.; Bastos, M.D.S.R.; Costa, J.M.C.d.; Canuto, K.M.; Furtado, R.F. Chemical modification of gum arabic and its application in the encapsulation of Cymbopogon citratus essential oil. J. Appl. Polym. 2015, 132, 41519. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kumar, V.; Soni, P.L. Carbamoylethylation of guar gum. Carbohydr. Polym. 2005, 58, 449–453. [Google Scholar] [CrossRef]
- Gering, C.; Rasheed, A.; Koivisto, J.T.; Párraga, J.; Tuukkanen, S.; Kellomäki, M. Chemical modification strategies for viscosity-dependent processing of gellan gum. Carbohydr. Polym. 2021, 269, 118335. [Google Scholar] [CrossRef]
- Krishnappa, P.B.; Badalamoole, V. Karaya gum-graft-poly(2-(dimethylamino)ethyl methacrylate) gel: An efficient adsorbent for removal of ionic dyes from water. Int. J. Biol. Macromol. 2019, 122, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Nickzare, M.; Zohuriaan-Mehr, M.J.; Yousefi, A.A.; Ershad-Langroudi, A. Novel Acrylic-modified Acacia Gum Thickener: Preparation, Characterization and Rheological Properties. Starch 2009, 61, 188–198. [Google Scholar] [CrossRef]
- Liu, R.; Wang, L.; Liu, Y.; Wu, T.; Zhang, M. Fabricating soy protein hydrolysate/xanthan gum as fat replacer in ice cream by combined enzymatic and heat-shearing treatment. Food Hydrocoll. 2018, 81, 39–47. [Google Scholar] [CrossRef]
- Pagano, C.; Luzi, F.; Ricci, M.; Michele, A.D.; Puglia, D.; Ceccarini, M.R.; Beccari, T.; Blasi, F.; Cossignani, L.; Schoubben, A.; et al. Wound Dressing: Combination of Acacia Gum/PVP/Cyclic Dextrin in Bioadhesive Patches Loaded with Grape Seed Extract. Pharmaceutics 2022, 14, 485. [Google Scholar] [CrossRef]
- Sethi, S.; Kaith, B.S.; Kaur, M.; Sharma, N.; Khullar, S. Study of a cross-linked hydrogel of Karaya gum and Starch as a controlled drug delivery system. J. Biomater. Sci. Polym. Ed. 2019, 30, 1687–1708. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Jo, C.; Choi, S.; Ramalingam, S.; Lee, J. Flexible Ternary Combination of Gellan Gum, Sodium Carboxymethyl Cellulose, and Silicon Dioxide Nanocomposites Fabricated by Quaternary Ammonium Silane: Rheological, Thermal, and Antimicrobial Properties. ACS Omega 2020, 5, 28767–28775. [Google Scholar] [CrossRef]
- Chu, M.; Feng, N.; An, H.; You, G.; Mo, C.; Zhong, H.; Pan, L.; Hu, D. Design and validation of antibacterial and pH response of cationic guar gum film by combining hydroxyethyl cellulose and red cabbage pigment. Int. J. Biol. Macromol. 2020, 162, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.K.; Kwon, T.-w.; Lee, I.; Kim, T.-S.; Coskun, A.; Choi, J.W. Millipede-inspired structural design principle for high performance polysaccharide binders in silicon anodes. Energy Environ. Sci. 2015, 8, 1224. [Google Scholar] [CrossRef]
- Ling, M.; Xu, Y.; Zhao, H.; Gu, X.; Qiu, J.; Li, S.; Wu, M.; Song, X.; Yan, C.; Liu, G.; et al. Dual-functional gum arabic binder for silicon anodes in lithium ion batteries. Nano Energy 2015, 12, 178–185. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Zhang, T.; Li, J.-T.; Huang, L.; Sun, S.-G. A Robust Ion-Conductive Biopolymer as a Binder for Si Anodes of Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 3599–3605. [Google Scholar] [CrossRef]
- Kuruba, P.; Datta, M.K.; Damodaran, K.; Jampani, P.H.; Gattu, B.; Patel, P.P.; Shanthi, P.M.; Damle, S.; Kumta, P.N. Guar gum: Structural and electrochemical characterization of natural polymer based binder for silicon–carbon composite rechargeable Li-ion battery anodes. J. Power Sources 2015, 298, 331–340. [Google Scholar] [CrossRef]
- Bie, Y.; Yang, J.; Nuli, Y.; Wang, J. Natural karaya gum as an excellent binder for silicon-based anodes in high-performance lithium-ion batteries. J. Mater. Chem. A 2017, 5, 1919. [Google Scholar] [CrossRef]
- Qiu, L.; Shen, Y.; Fan, H.; Yang, X.; Wang, C. Carboxymethyl fenugreek gum: Rehological characterization and as a novel binder for silicon anode of lithium-ion batteries. Int. J. Biol. Macromol. 2018, 115, 672–679. [Google Scholar] [CrossRef]
- Hu, S.; Cai, Z.; Huang, T.; Zhang, H.; Yu, A. A Modified Natural Polysaccharide as a High-Performance Binder for Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 4311–4317. [Google Scholar] [CrossRef]
- Zhao, T.; Meng, Y.; Yin, H.; Guo, K.; Ji, R.; Zhang, G.; Zhang, Y. Beneficial effect of green water-soluble binders on SiOx/graphite anode for lithium-ion batteries. Chem. Phys. Lett. 2020, 742, 137145. [Google Scholar] [CrossRef]
- Bai, J.; Li, X.; Liu, G.; Qian, Y.; Xiong, S. Unusual Formation of ZnCo2O4 3D Hierarchical Twin Microspheres as a High-Rate and Ultralong-Life Lithium-Ion Battery Anode Material. Adv. Funct. Mater. 2014, 24, 3012–3020. [Google Scholar] [CrossRef]
- Koo, W.-T.; Jang, H.-Y.; Kim, C.; Jung, J.-W.; Cheong, J.Y.; Kim, I.-D. MOF derived ZnCo2O4 porous hollow spheres functionalized with Ag nanoparticles for a long-cycle and high-capacity lithium ion battery anode. J. Mater. Chem. A 2017, 5, 22717–22725. [Google Scholar] [CrossRef]
- Gao, G.; Wu, H.B.; Dong, B.; Ding, S.; Lou, X.W.D. Growth of Ultrathin ZnCo2O4 Nanosheets on Reduced Graphene Oxide with Enhanced Lithium Storage Properties. Adv. Sci. 2015, 2, 1400014. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xuan, Y.; Galpaya, D.G.D.; Gu, Y.; Lin, Z.; Zhang, S.; Yan, C.; Feng, S.; Wang, L. A high-volumetric-capacity and high-areal-capacity ZnCo2O4 anode for Li-ion batteries enabled by a robust biopolymer binder. J. Mater. Chem. A 2018, 40, 19455. [Google Scholar] [CrossRef]
- Zhou, K.; Kang, M.; He, X.; Hong, Z.; Huang, Z.; Wei, M. A multi-functional gum arabic binder for NiFe2O4 nanotube anodes enabling excellent Li/Na-ion storage performance. J. Mater. Chem. A 2017, 5, 18138. [Google Scholar] [CrossRef]
- Wang, Z.; Dang, G.; Zhang, Q.; Xie, J. Xanthan Gum as a Potential Binder for Graphite Anode in Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2017, 12, 7457–7468. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Tian, J.; Ma, X.; Wang, B.; Li, W.; Wang, Q. Adhesive nanocomposites of hypergravity induced Co3O4 nanoparticles and natural gels as Li-ion battery anode materials with high capacitance and low resistance. RSC Adv. 2017, 7, 21061. [Google Scholar] [CrossRef]
- Zhang, G.; Qiu, B.; Xia, Y.; Wang, X.; Gu, Q.; Jiang, Y.; He, Z.; Liu, Z. Double-helix-superstructure aqueous binder to boost excellent electrochemical performance in Li-rich layered oxide cathode. J. Power Sources 2019, 420, 29–37. [Google Scholar] [CrossRef]
- He, J.; Zhong, H.; Wang, J.; Zhang, L. Investigation on xanthan gum as novel water soluble binder for LiFePO4 cathode in lithium-ion batteries. J. Alloys Compd. 2017, 714, 409–418. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, X.; Ji, R.; Xie, S.; Huang, X.; Zheng, Y.; Chang, L.; Zhao, T. Xanthan gum with double-helix structure as a novel aqueous binder to stabilize lithium-rich cathode. Chem. Phys. Lett. 2022, 801, 139700. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.-T.; Liu, J.; Deng, Y.-P.; Wu, Z.-G.; Yin, Z.-W.; Guo, D.; Huang, L.; Sun, S.-G. Suppressing the voltage-fading of layered lithium-rich cathode materials via an aqueous binder for Li-ion batteries. Chem. Commun. 2016, 52, 4683. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, J.; Hwang, J.-Y.; Sun, Y.-K. Recent research trends in Li-S batteries. J. Mater. Chem. A 2018, 6, 11582–11605. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Sun, Y.-K. Recent progress of advanced binders for Li-S batteries. J. Power Sources 2018, 396, 19–32. [Google Scholar] [CrossRef]
- Qi, Q.; Deng, Y.; Gu, S.; Gao, M.; Hasegawa, J.-y.; Zhou, G.; Lv, X.; Lv, W.; Yang, Q.-H. L-Cysteine-Modified Acacia Gum as a Multifunctional Binder for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 47956–47962. [Google Scholar]
- Lu, Y.-Q.; Li, J.-T.; Peng, X.-X.; Zhang, T.; Deng, Y.-P.; Wu, Z.-Y.; Deng, L.; Huang, L.; Zhou, X.-D.; Sun, S.-G. Achieving high capacity retention in lithium-sulfur batteries with an aqueous binder. Electrochem. Commun. 2016, 72, 79–82. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Xie, L.; Yang, J.; Nuli, Y.; Wang, J. Guar gum as a novel binder for sulfur composite cathodes in rechargeable lithium batteries. Chem. Commun. 2016, 52, 13479. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ling, M.; Ye, Y.; Li, Z.; Guo, J.; Yao, Y.; Zhu, J.; Lin, Z.; Zhang, S. Acacia-Senegal-Inspired Bifunctional Binder for Longevity of Lithium-Sulfur Batteries. Adv. Energy Mater. 2015, 5, 1500878. [Google Scholar] [CrossRef]
- Liu, J.; Galpaya, D.G.D.; Yan, L.; Sun, M.; Lin, Z.; Yan, C.; Liang, C.; Zhang, S. Exploiting a robust biopolymer network binder for an ultrahigh-areal-capacity Li-S battery. Energy Environ. Sci. 2017, 10, 750–755. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Xu, L.; Sitinamaluwa, H.; Li, H.; Qiu, J.; Wang, Y.; Yan, C.; Li, H.; Yuan, S.; Zhang, S. Low cost and green prepartion process for α-Fe2O3@gum arabic electrode for high performance sodium ion batteries. J. Mater. Chem. A 2017, 5, 2102–2109. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhang, S.-J.; Li, J.-T.; Wang, K.; Zhang, Y.-C.; Liu, Q.; Xie, R.-S.; Pei, Y.-R.; Huang, L.; Sun, S.-G. Improvement of electrochemical properties of P2-type Na2/3Mn2/3Ni1/3O2 sodium ion battery cathode material by water-soluble binders. Electrochim. Acta 2019, 298, 496–504. [Google Scholar]
- Luo, C.; Fan, X.; Ma, Z.; Gao, T.; Wang, C. Self-Healing Chemistry between Organic Material and Binder for Stable Sodium-Ion Batteries. Chem 2017, 3, 1050–1062. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Nazir, M.S.; Tao, H.; Cao, S.; Ji, R.; Jiang, M.; Yao, L. Integration of energy storage system and renewable energy sources based on artificial intelligence: An overview. J. Energy Storage 2021, 40, 102811. [Google Scholar] [CrossRef]
- Lv, Z.; Li, W.; Wei, J.; Ho, F.; Cao, J.; Chen, X. Autonomous Chemistry Enabling Environment-Adaptive Electrochemical Energy Storage Devices. CCS Chem. 2022, 1–19. [Google Scholar] [CrossRef]
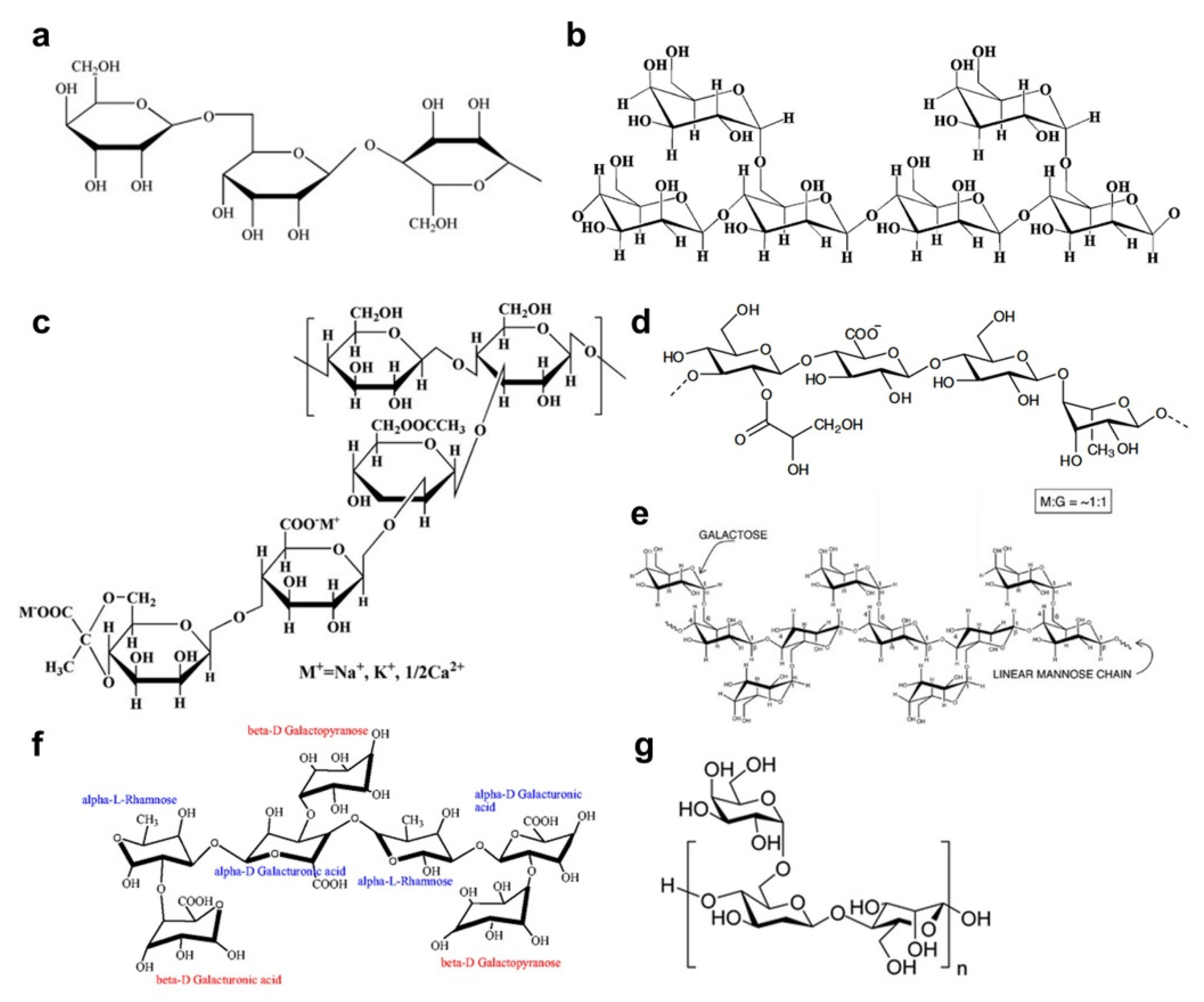
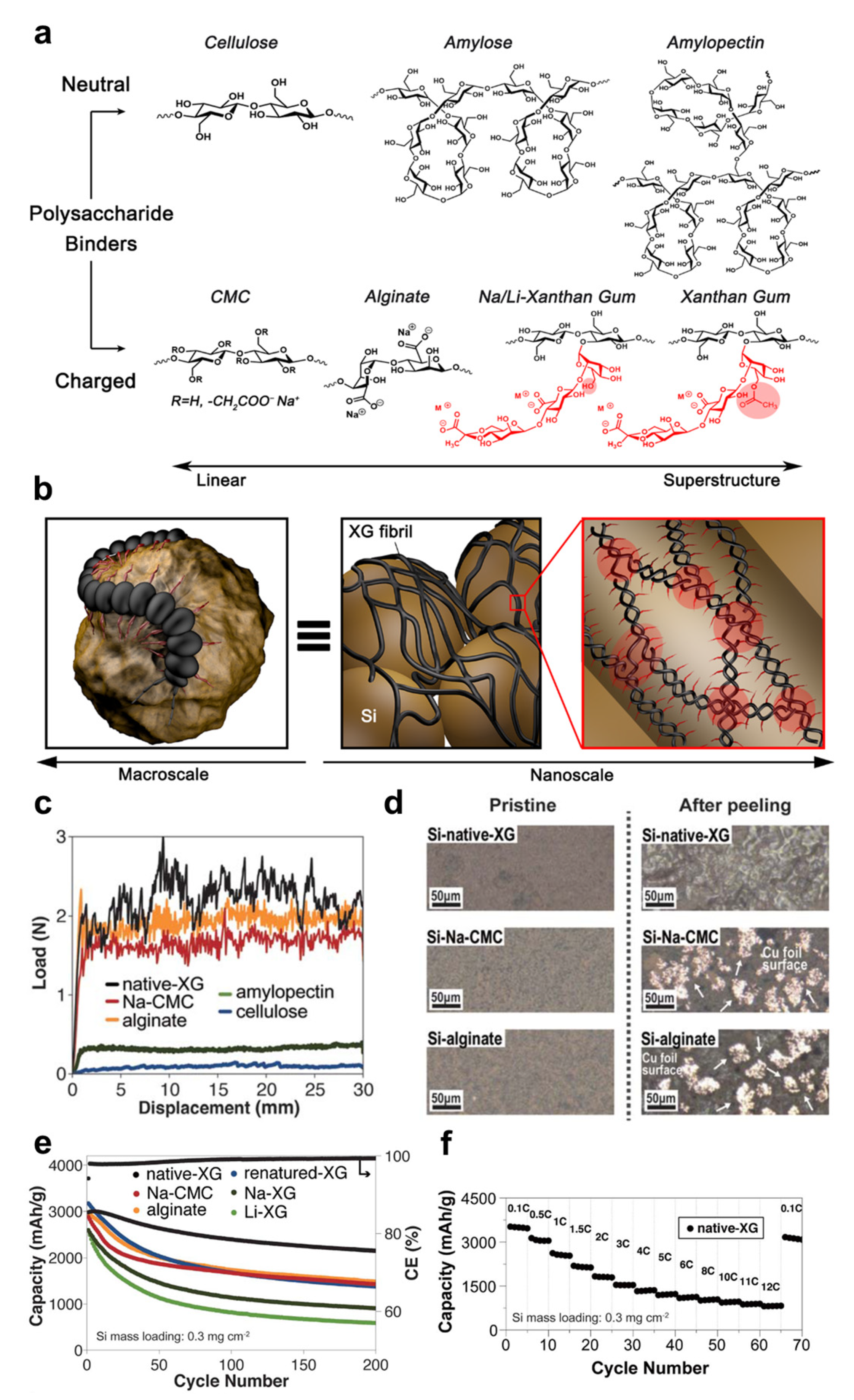
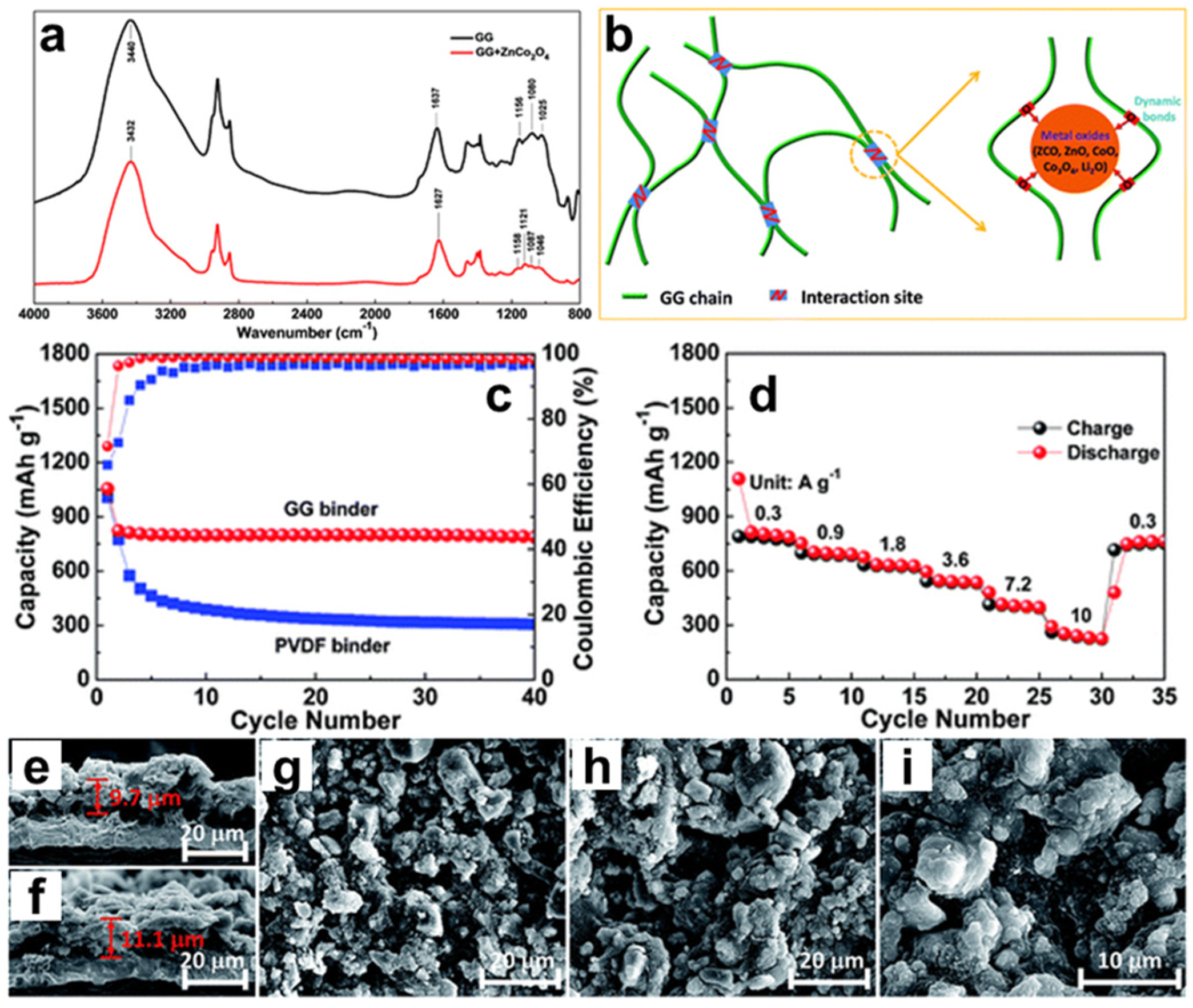
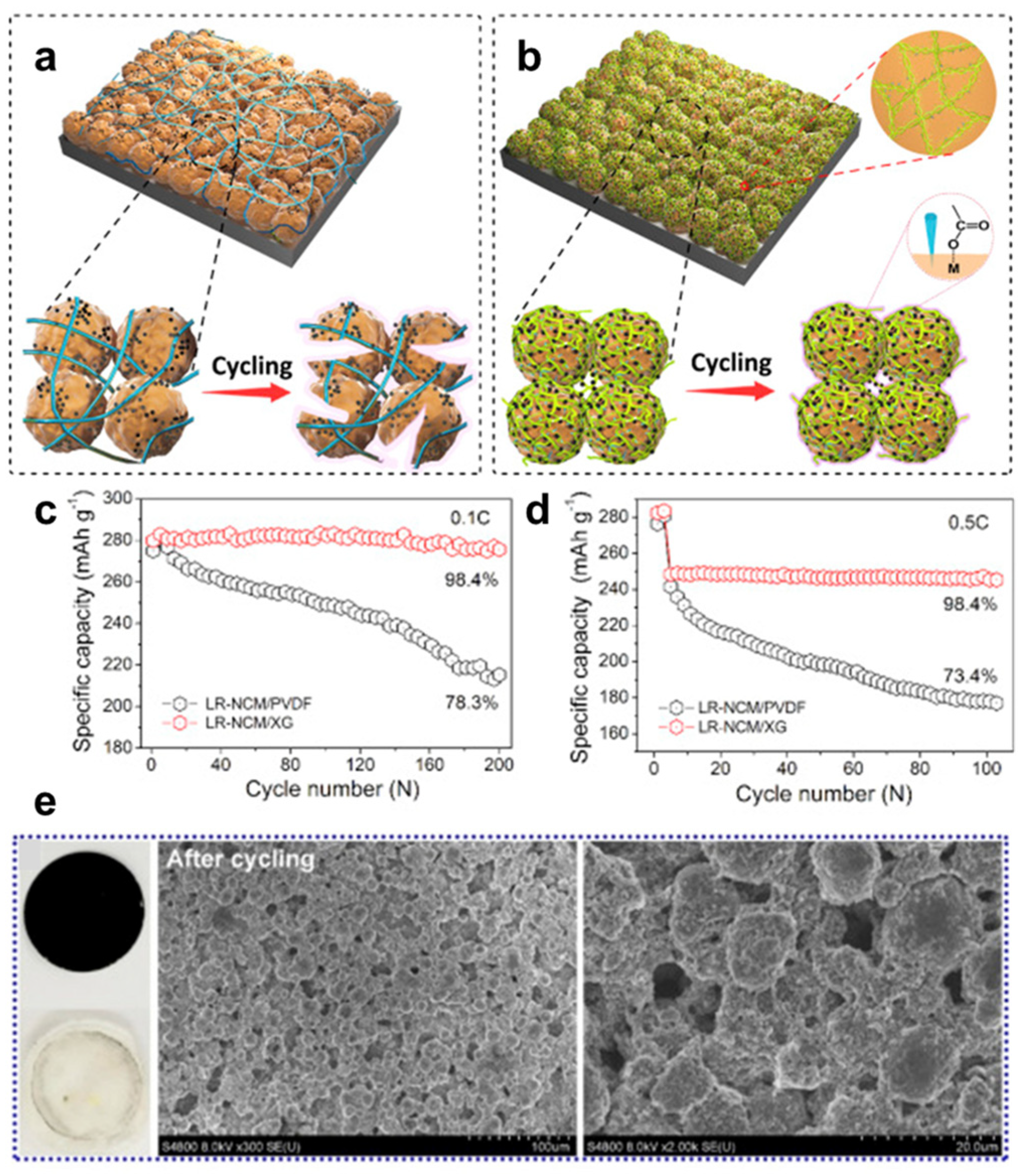

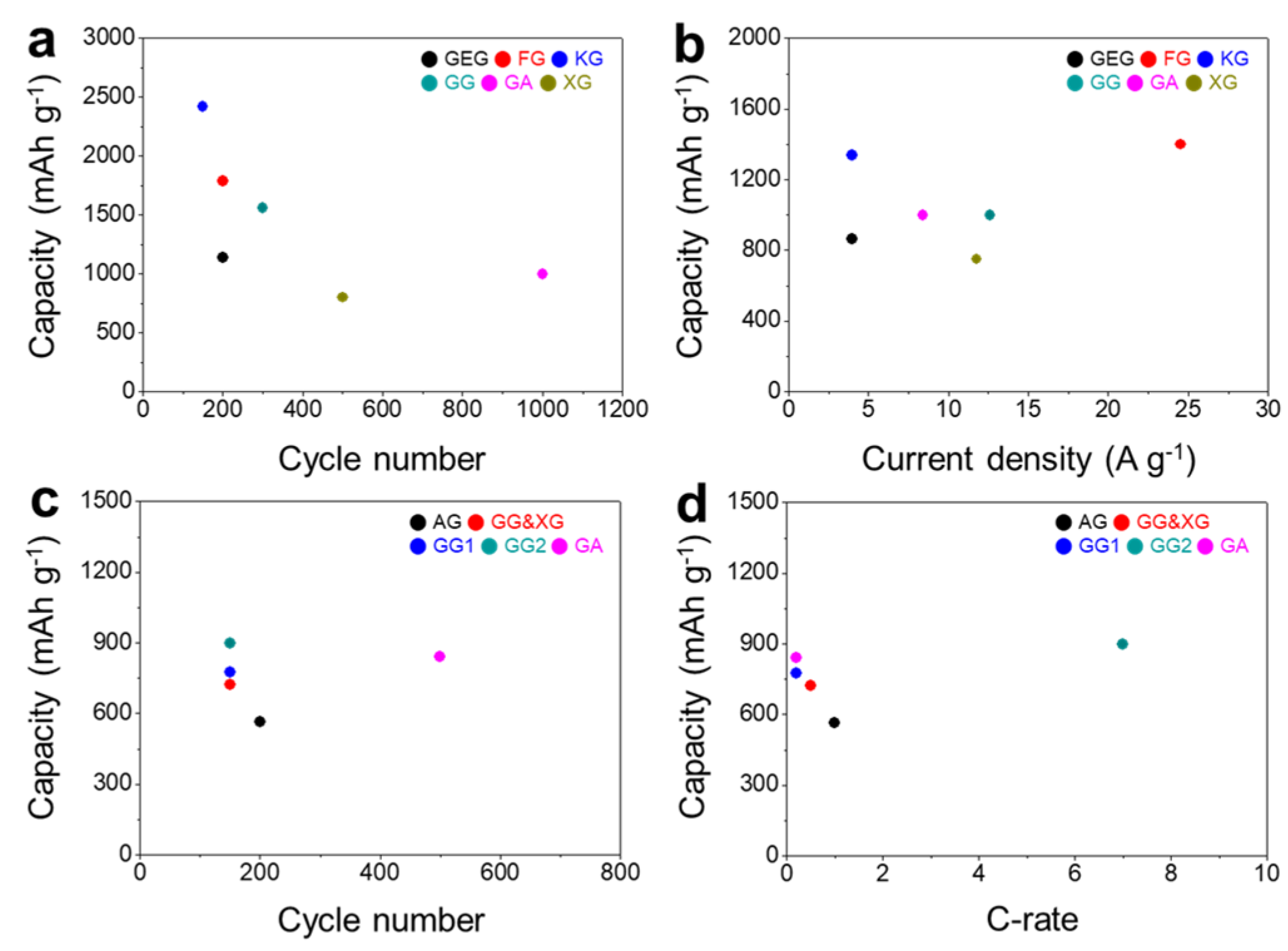
| Types of Gums | Loading Amount | Active Material | Cycle Performance | Rate Capabilities | Ref. |
|---|---|---|---|---|---|
| XG | 20 wt% | Si | ~800 mAh g−1 at 6.0 C after 500 cycles | ~750 mAh g−1 at 12 C | [43] |
| GA | 25 wt% | Si | 1000 mAh g−1 at 1.0 C after 1000 cycles | 1000 mAh g−1 at 2.0 C | [44] |
| GG | 15 wt% | Si | 1561 mAh g−1 at 2.1 A g−1 after 300 cycles | ~1000 mAh g−1 at 12.6 A g−1 | [45] |
| GG | 8 wt% | Si-C | ~600 mAh g−1 at 0.2 A g−1 after 111 cycles | N/A | [46] |
| KG | 20 wt% | Si | 2421 mAh g−1 at 1.5 A g−1 after 150 cycles | 1336 mAh g−1 at 4.0 A g−1 | [47] |
| FG | 5 wt% | Si | 1790 mAh g−1 at 1.0 A g−1 after 200 cycles | ~1400 mAh g−1 at 10 C | [48] |
| GEG | 20 wt% | Si | 1138 mAh g−1 at 1.0 A g−1 after 200 cycles | 865 mAh g−1 at 4.0 A g−1 | [49] |
| GG | 1 wt% | SiOx/graphite | 385.7 mAh g−1 after 100 cycles | N/A | [50] |
| GG | 10 wt% | ZnCo2O4 | 412 mAh g−1 at 1.2 A g−1 after 600 cycles | 248 mAh g−1 at 10 A g−1 | [54] |
| GA | 15 wt% | NiFe2O4 | 770 mAh g−1 at 5.0 A g−1 after 500 cycles | 421 mAh g−1 at 5.0 A g−1 | [55] |
| XG | 6 wt% | Graphite | 250 mAh g−1 at 0.5 C after 180 cycles | 275 mAh g−1 at 5.0 C | [56] |
| XG | 15 wt% | Co3O4 | 742.5 mAh g−1 at 0.5 C after 50 cycles | ~750 mAh g−1 at 1.0 C | [57] |
| XG | 10 wt% | Li[Li0.144Ni0.136Co0.136Mn0.544]O2 | 275.6 mAh g−1 at 0.1 C after 200 cycles | 261 mAh g−1 at 0.5 C | [58] |
| XG | 5 wt% | LiFePO4 | 151.1 mAh g−1 at 0.2 C after 100 cycles | 87 mAh g−1 at 5.0 C | [59] |
| XG | 5 wt% | Li[Li0.2Co0.13Ni0.13Mn0.54]O2 | 203.2 mAh g−1 at 0.2 C after 50 cycles | N/A | [60] |
| GG | 10 wt% | Li1.14Ni0.18 Mn0.62O2 | ~180 mAh g−1 at 0.1 A g−1 after 250 cycles | N/A | [61] |
| AG | 22.7 wt% | S | 564.7 mAh g−1 at 1.0 C after 200 cycles | 500 mAh g−1 at 2.0 C | [64] |
| GG | 15 wt% | S | 777 mAh g−1 at 0.2 C after 150 cycles | N/A | [65] |
| GG | 10 wt% | S | ~900 mAh g−1 at 7.0 C after 150 cycles | ~1000 mAh g−1 at 10.0 C | [66] |
| GA | 20 wt% | S | 841 mAh g−1 at 0.2 C after 500 cycles | 460 mAh g−1 at 10.0 C | [67] |
| GG and XG | 10 wt% | S | 724 mAh g−1 at 0.5 C after 150 cycles | 737 mAh g−1 at 5.0 C | [68] |
| GA | 15 wt% | α-Fe2O3 | 492 mAh g−1 at 5.0 A g−1 after 500 cycles | 372 mAh g−1 at 15.0 A g−1 | [70] |
| XG | 10 wt% | Na2/3Mn2/3Ni1/3O2 | 115.9 mAh g−1 at 0.2 C after 80 cycles | N/A | [71] |
| XG | 15 wt% | sodium rhodizonate dibasic | ~150 mAh g−1 at 0.05 A g−1 after 200 cycles | N/A | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padil, V.V.T.; Cheong, J.Y. Recent Advances in the Multifunctional Natural Gum-Based Binders for High-Performance Rechargeable Batteries. Energies 2022, 15, 8552. https://doi.org/10.3390/en15228552
Padil VVT, Cheong JY. Recent Advances in the Multifunctional Natural Gum-Based Binders for High-Performance Rechargeable Batteries. Energies. 2022; 15(22):8552. https://doi.org/10.3390/en15228552
Chicago/Turabian StylePadil, Vinod V. T., and Jun Young Cheong. 2022. "Recent Advances in the Multifunctional Natural Gum-Based Binders for High-Performance Rechargeable Batteries" Energies 15, no. 22: 8552. https://doi.org/10.3390/en15228552
APA StylePadil, V. V. T., & Cheong, J. Y. (2022). Recent Advances in the Multifunctional Natural Gum-Based Binders for High-Performance Rechargeable Batteries. Energies, 15(22), 8552. https://doi.org/10.3390/en15228552








