Study on the Effect of Acid Corrosion on Proppant Properties
Abstract
1. Introduction
2. Experiment Description
2.1. Experimental Samples
2.2. Experimental Methods
2.2.1. Proppant Acid Solubility Test
2.2.2. Proppant Compressive Strength Test
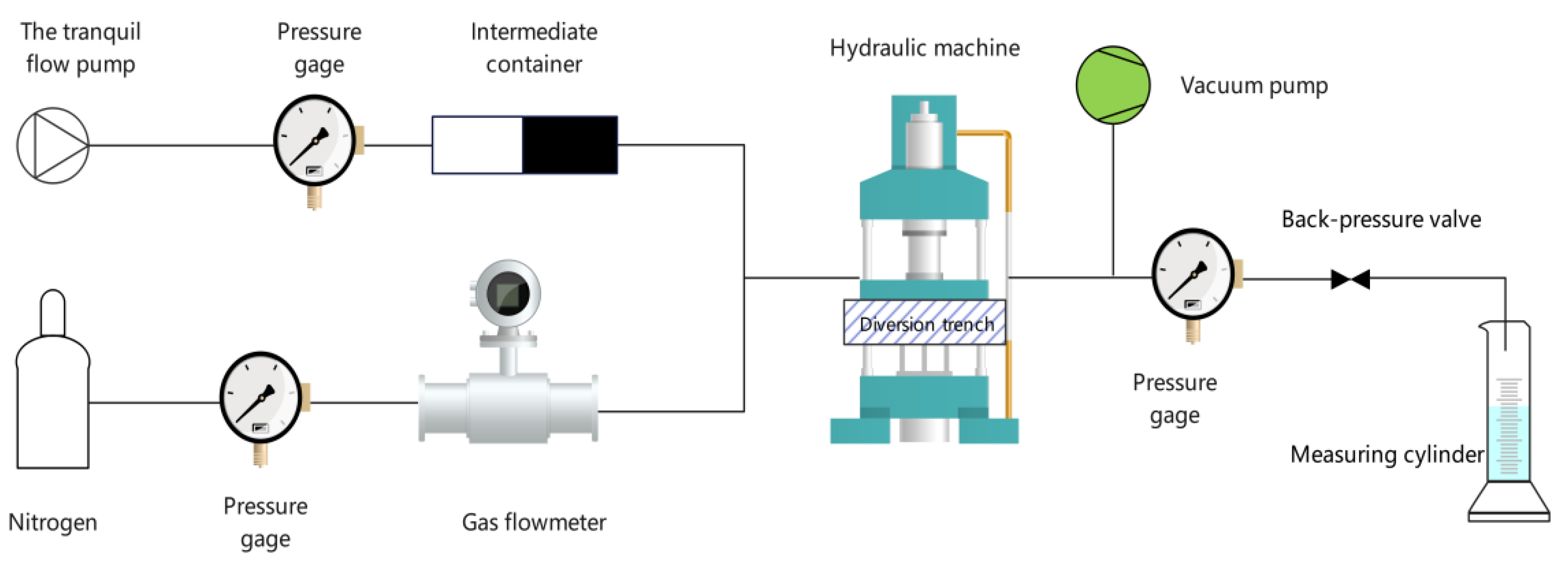
2.2.3. Proppant Conductivity Test
3. Discussion of Experimental Results
3.1. Proppant Acid Solubility Test
3.1.1. Effect of Temperature on Acid Solubility
3.1.2. Acid Solubility of Different Types of Proppants
3.1.3. Effect of Proppant Particle Size on Acid Solubility
3.1.4. Effect of Acid Concentration on Acid Solubility
3.1.5. Effect of Contact Reaction Time on Acid Solubility
3.2. Damage to Proppant Compressive Strength
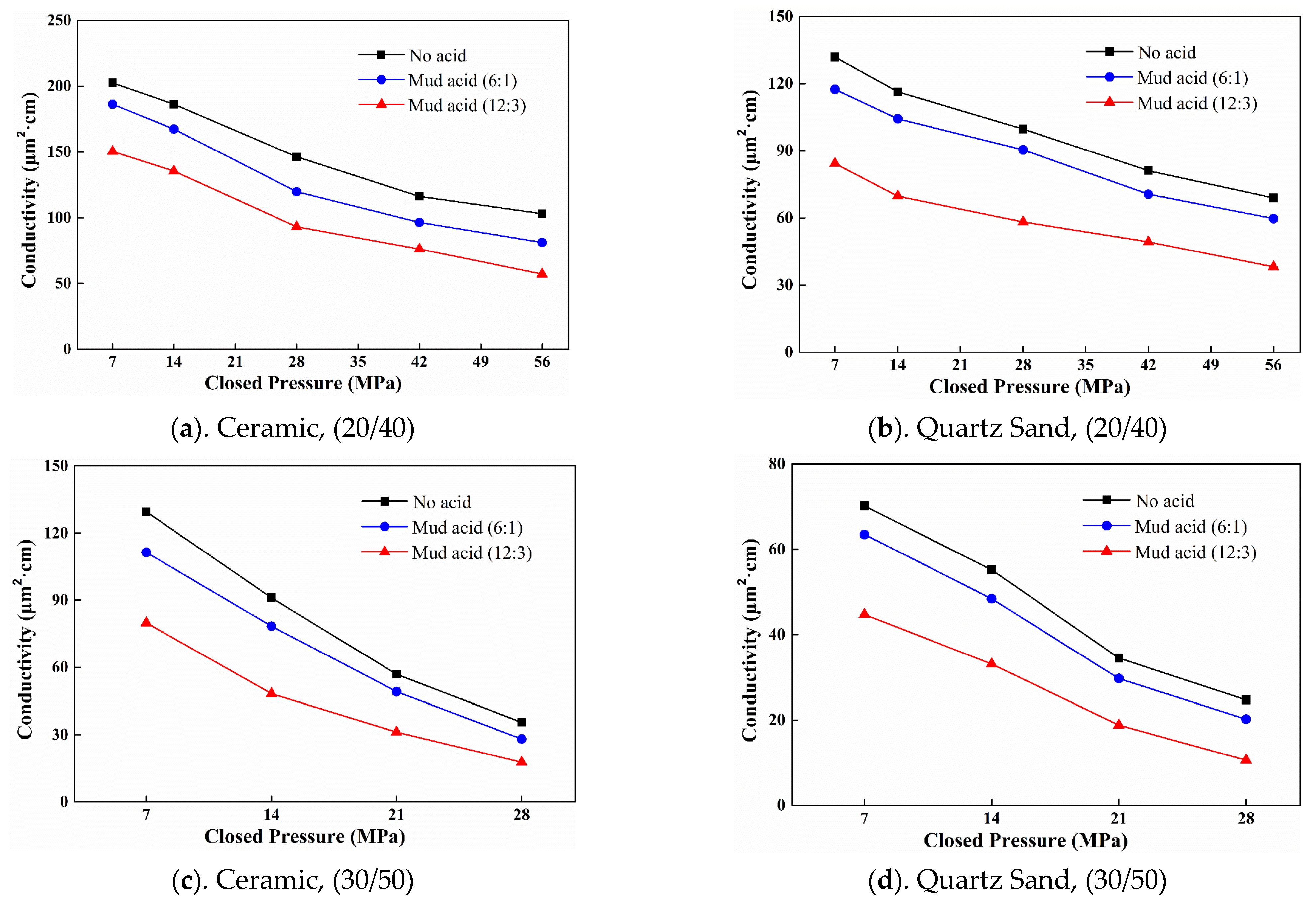
3.3. Damage to Conductivity
4. Conclusions
- (1)
- Acid concentration and contact reaction time are the most dominant factors affecting the solubility of proppant in acid. When conducting pre-acid operation, it is not advisable to use a high concentration of acid. Alternatively, it is desirable to inject a certain amount of fracturing fluid to dilute the acid concentration before injecting proppant. Another measure is to conduct the flowback as soon as possible to reduce the damage to the proppant performance.
- (2)
- The larger contact area of the small particle proppant with the acid solution leads to larger solubility compared to that for proppant of a larger particle size. However, the compressive strength of smaller size proppant is superior to that of larger size and the degree of damage to conductivity is also small. Therefore, a small particle proppant is preferred under the requirement of a certain conductivity.
- (3)
- The quartz sand proppant presents high stability under acidic conditions although its compressive strength is weak. In shallow formations with a closure pressure less than 28 MPa, the pre-acidification combined with fracturing using quartz sand proppant may produce superior performance.
Author Contributions
Funding
Conflicts of Interest
References
- Wei, X.; Peng, Y.; Luo, S.; Ren, J.; Shao, Y. Pyramid structure of tight sandstone reservoirs in the Upper Paleozoic of the Ordos Basin. Unconv. Oil Gas 2016, 3, 1–7. [Google Scholar]
- Li, Q.; Gao, S.; Ye, L. Seepage mechanism and development technology of tight sandstone gas reservoirs. Sci. Technol. Eng. 2014, 14, 79–87. [Google Scholar]
- Zhang, T.; Sun, S.; Song, H. Flow Mechanism and Simulation Approaches for Shale Gas Reservoirs: A Review. Transp. Porous Media 2018, 126, 655–681. [Google Scholar] [CrossRef]
- Assem, A.I.; Nasr-El-Din, H.A. Interactions between mud acid and sand proppants. In Proceedings of the SPE North Africa Technical Conference and Exhibition, Cairo, Egypt, 14–16 September 2015. [Google Scholar] [CrossRef]
- Liang, H. Technical analysis of collaborative operation of acidification and sand fracturing. China Pet. Chem. Stand. Qual. 2011, 31, 153. [Google Scholar]
- Jennings, A.R. Fracture Acidizing Sandstone Formations. U.S. Patent 4807703, 28 February 1989. [Google Scholar]
- Xian, X.; Zhu, Z.; Wang, X. Discussion on acidification technology combined with sand fracturing technology. Chem. Eng. Equip. 2010, 64–65. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, D.; Huang, Y. New technology for sandstone reservoir stimulation-acid fracturing. Fault-Block Oil Gas Field 2006, 78–80. [Google Scholar] [CrossRef]
- Stephens, W.T.; Schubarth, S.K.; Dickson, K.R.; Snyder, E.M.; Doles, K.; Herndon, D.C. Behavior of proppants under cyclic stress. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, College Station, TX, USA, 29 January 2007. [Google Scholar]
- Yang, Y. Feasibility Study on Acid Fracturing of Sandstone Reservoirs D; Southwest Petroleum Institute: Chengdu, China, 2004. [Google Scholar]
- Weaver, J.D.; Nguyen, P.D. Hydrophobic filming reduces frac gel and mineral scale damage. In Proceedings of the SPE Eastern Regional Meeting, Morgantown, WV, USA, 13 October 2010. [Google Scholar]
- Liu, H.; Zhao, L.; Liu, P.; Chen, J.; Zhao, W. Mechanism analysis of pre-acid to improve hydraulic fracturing effect. J. Oil Gas Technol. 2008, 30, 332–334. [Google Scholar]
- Terracina, J.M.; Turner, J.M.; Collins, D.H.; Spillars, E. Proppant selection and its effect on the results of fracturing treatments performed in shale formations. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 19 September 2010. [Google Scholar]
- LaFollette, R.F.; Carman, P.S. Proppant diagenesis: Results so far. In Proceedings of the SPE Unconventional Gas Conference, Pittsburgh, PA, USA, 23 February 2010. [Google Scholar] [CrossRef]
- Raysoni, N.; Weaver, J. Long-term hydrothermal proppant performance. SPE Prod. Oper. 2013, 28, 414–426. [Google Scholar] [CrossRef]


| Type | Particle Size (Mesh) | Soaking Time (h) | Temperature (°C) | Dissolved Proppant (%) | |
|---|---|---|---|---|---|
| Mud Acid (6:1) | Mud Acid (12:3) | ||||
| Ceramic | 20/40 | 0.5 | 65 | 1.09 | 4.02 |
| Quartz Sand Ceramic | 0.85 | 1.97 | |||
| 85 | 1.37 | 5.58 | |||
| Quartz Sand | 1.16 | 2.79 | |||
| Proppant | Mesh | T, (°C) | Soaking Time (h) | Weight Before (g) | Mud Acid (6:1) | Mud Acid (12:3) | ||
|---|---|---|---|---|---|---|---|---|
| Weight After (g) | Proppant Solubility (%) | Weight After (g) | Proppant Solubility (%) | |||||
| Ceramic | 20/40 | 65 | 0.5 | 10 | 9.89 | 1.09 | 9.60 | 4.02 |
| 1 | 10 | 9.75 | 2.54 | 9.18 | 8.17 | |||
| 2 | 10 | 9.70 | 2.96 | 9.06 | 9.38 | |||
| 30/50 | 65 | 0.5 | 10 | 9.87 | 1.35 | 9.51 | 4.94 | |
| 1 | 10 | 9.65 | 3.52 | 9.04 | 9.63 | |||
| 2 | 10 | 9.58 | 4.23 | 8.85 | 11.46 | |||
| Quartz Sand | 20/40 | 65 | 0.5 | 10 | 9.92 | 0.85 | 9.80 | 1.97 |
| 1 | 10 | 9.84 | 1.64 | 9.66 | 3.41 | |||
| 2 | 10 | 9.80 | 1.97 | 9.60 | 4.05 | |||
| 30/50 | 65 | 0.5 | 10 | 9.90 | 1.04 | 9.76 | 2.43 | |
| 1 | 10 | 9.81 | 1.92 | 9.55 | 4.47 | |||
| 2 | 10 | 9.79 | 2.10 | 9.48 | 5.17 | |||
| Type | Mesh | Pressure (MPa) | Crushing Rate (%) | ||
|---|---|---|---|---|---|
| No Acid | Mud Acid (6:1) | Mud Acid (12:3) | |||
| Ceramic | 20/40 | 52 | 1.4 | 2.5 | 5.9 |
| 30/50 | 52 | 1.1 | 1.9 | 5.2 | |
| Quartz Sand | 20/40 | 35 | 3.6 | 5.2 | 8.3 |
| 30/50 | 35 | 3.2 | 4.7 | 7.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Yao, K.; Li, D.; Xu, D.; Yang, H. Study on the Effect of Acid Corrosion on Proppant Properties. Energies 2022, 15, 8368. https://doi.org/10.3390/en15228368
Xu F, Yao K, Li D, Xu D, Yang H. Study on the Effect of Acid Corrosion on Proppant Properties. Energies. 2022; 15(22):8368. https://doi.org/10.3390/en15228368
Chicago/Turabian StyleXu, Feng, Kuai Yao, Desheng Li, Dongjin Xu, and Huan Yang. 2022. "Study on the Effect of Acid Corrosion on Proppant Properties" Energies 15, no. 22: 8368. https://doi.org/10.3390/en15228368
APA StyleXu, F., Yao, K., Li, D., Xu, D., & Yang, H. (2022). Study on the Effect of Acid Corrosion on Proppant Properties. Energies, 15(22), 8368. https://doi.org/10.3390/en15228368






