Abstract
Chemical looping combustion (CLC) is one of the most advanced technologies allowing for the reduction in CO2 emissions during the combustion of solid fuels. The modified method combines chemical looping with oxygen uncoupling (CLOU) and in situ gasification chemical looping combustion (iG-CLC). As a result, an innovative hybrid chemical looping combustion came into existence, making the above two technologies complementary. Since the complexity of the CLC is still not sufficiently recognized, the study of this process is of a practical significance. The paper describes the experiences in the modelling of complex geometry CLC equipment. The experimental facility consists of two reactors: an air reactor and a fuel reactor. The paper introduces the fuzzy logic (FL) method as an artificial intelligence (AI) approach for the prediction of SO2 and NOx (i.e., NO + NO2) emissions from coal and biomass combustion carried out in air-firing; oxyfuel; iG-CLC; and CLOU conditions. The developed model has been successfully validated on a 5 kWth research unit called the dual fluidized bed chemical looping combustion of solid fuels (DFB-CLC-SF).
1. Introduction
The fluidized bed combustion of coal or a co-combustion with the biomass under the oxyfuel conditions, as well as chemical looping combustion (CLC) or chemical-looping with oxygen uncoupling combustion processes (CLOU), seem to be the most promising technologies, with a high application potential which meets the requirements for the reduction in gaseous pollutant emissions [1,2,3]. Carbon dioxide sequestration (CCS) technologies reduce CO2 emissions to the atmosphere. The following are distinguished among CCS technologies: pre-combustion capture, oxyfuel combustion, and post-combustion capture. The chemical looping combustion is classified as a CCS technology. Due to the high concentration of CO2 in the exhaust gas, the CLC process is less energy-consuming and more effective than other CCS technologies. This is because there is no need to build additional installations for CO2 separation [4,5]. Two processes are distinguished depending on the type of solid oxygen carrier: CLOU (chemical looping with oxygen uncoupling) and iG-CLC (in situ gasification chemical looping combustion). The CLOU-type oxygen carriers do not require a direct contact with the fuel to release oxygen (Figure 1) [6]. Under the operating conditions of a fuel reactor, they can release gaseous oxygen. As a result of the reduction in the oxygen carrier in the fuel reactor, oxygen appears in addition to carbon dioxide (a gas mixture consisting of CO2 and O2 is formed) [7,8].
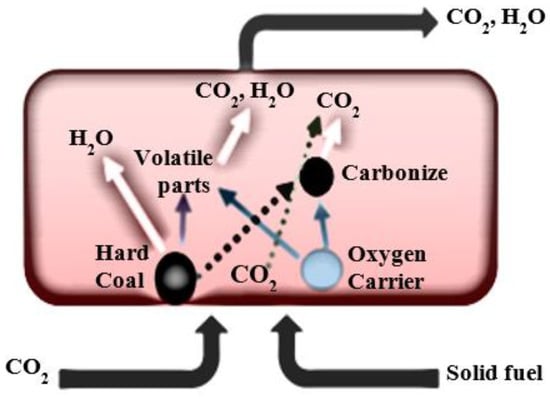
Figure 1.
The scheme of the oxygen release process from the CLOU type of the oxygen carrier in the fuel reactor.
However, in the iG-CLC process, which involves combustion in chemical looping with a fuel gasification in a fuel reactor, the oxygen carrier requires a direct contact with the fuel (Figure 2) [6].
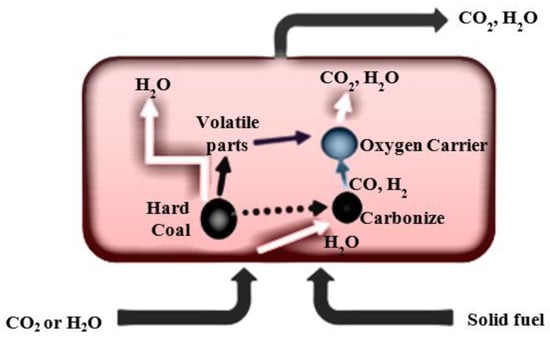
Figure 2.
The scheme of oxygen release in the fuel reactor in the iG-CLC process.
Therefore, in the first step, the metal oxide is mixed with the fuel after it enters the combustion chamber. Only then does the oxygen carrier react with the volatile components and gaseous products resulting from the gasification of the fuel. The gasifying agent, and gas fluidising the fuel reactor bed, is CO2 or H2O. However, carbon dioxide is often used for economic reasons, which can also be recirculated from flue gas [9].
Moreover, with its fuel flexibility feature, fluidised bed technology is suitable for co-firing the coal and biomass, which is considered to be a renewable energy source [10,11]. Finally, different combustion atmospheres can be applied in fluidized bed systems, i.e., the air-firing mode, when the combustion runs in air or in oxy-combustion conditions; that is, the mixture consists of oxygen and carbon dioxide or oxygen with the flue gas mixture. The exclusion of nitrogen from inlet gas in oxyfuel combustion generates exhaust gas consisting mostly of CO2 and H2O. This exhaust composition is suitable for industrial reuse or geological storage [12]. Similar results are obtained with the CLC technology, which uses oxygen transported by solid oxygen carriers [6]. The operation of such systems uses the cyclic processes of oxidation and reduction in solid oxygen carriers (metal oxides). The oxidation and reduction processes occur in the air and fuel reactor, respectively (Figure 3) [13].
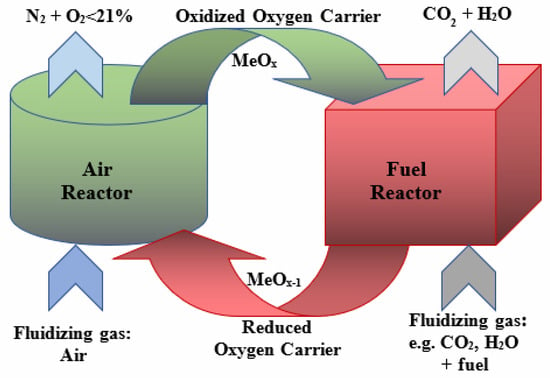
Figure 3.
The scheme of the oxidation and reduction in the oxygen carrier in the reactors.
The above-listed combustion methods belong to so-called clean combustion technologies. They can offer combustion processes with a reduced fuel consumption and zero or almost zero unwanted pollutants [14]. The chemical looping combustion process takes place in the fluidized bed regime; therefore, this combustion does not require such high temperatures as conventional combustion. Usually, the chemical looping combustion process occurs at temperatures of 800–900 °C. This prevents the formation of nitrogen oxides from the atmospheric air. The exhaust gases have a high CO2 concentration (above 95%) and are not diluted with air, as in the case of conventional combustion. Therefore, the flue gases from the CLC process can be directly stored or used in industry after the steam condensation. In addition, the operating parameters of the CLC system need to be adequately selected to ensure that the fuel is completely burned, reducing its consumption in the combustion process [9].
The composition of solid fuels includes sulphur and nitrogen, which are the sources of NOx (i.e., NO + NO2) and SO2 emissions from combustion, i.e., the major gaseous pollutants, and their emissions should be considered when a combustion technology is implemented. Many factors influence NOx and SO2 formation, including the fuel properties, combustion conditions, and construction of the combustor [15]. The CLC technology produces NOx from the fuel, which means that this technology does not produce thermal nitrogen oxides from the air, as in conventional combustion. Additionally, other factors, such as the temperature and amount of oxygen transported by oxygen carriers, impact fuel combustion. A too low temperature and small amounts of oxygen in the fuel reactor result in incomplete combustion of the fuel, which results in lower NOx and SOx emissions [12].
Many papers have dealt with the topic of solid fuel combustion in fluidized bed boilers [8]. The modelling methods can generally be classified into programmed computing and artificial intelligence (AI) approaches. The programmed computing approach is based on mathematical descriptions of the considered processes [10,16,17,18,19]. A review of the CFB combustor models can be found in [17]. The author defined the two main approaches to performance modelling. The first one, i.e., the furnace approach, describes what happens in the furnace, but the second, the so-called system approach, is focused on system integration. In the furnace approach, the author distinguishes three levels of the model advancement: level 1:1D is the plug flow/stirred tank, using simple mass and energy balance; level 2:1.5D is the core-annulus models; and level 3:3D is the models which are based on the Navier–Stokes Equation with chemical kinetics and physical processes. A similar classification of the existing models was provided by Gungor and Eskin [20].
Adanez and Abad [21] selected the mechanistic and process models as the two main approaches in CLC modelling. In the first one, the fluid dynamics, the reactions’ kinetics, and the heat transfer in each reactor can be considered separately. The fluid dynamics modelling works can be further classified into two categories: computational fluid dynamic (CFD) models and macroscopic ones. The first one is the most complex and computationally advanced, requiring an extensive computation effort. On the other hand, the macroscopic models employ empirical equations and/or correlations describing the gas and particles’ characteristic flows in the fluidized beds, making them, therefore, simpler to use but still making them effective and reliable.
The process models allow for defining the design and operating conditions, so they are valuable tools in optimising the CLC performance processes [21].
Finally, the AI approach employs nature-inspired artificial intelligence methods. Comprehensive literature reviews of the AI methods in the Energy sector can be found in [22,23,24]. Among other main AI modelling methods are artificial neural networks, fuzzy logic, and genetic algorithms.
Even though much work has been done, the complex mechanisms of the formation of and the reduction in NOx and SO2 emissions during combustion in oxyfuel, iG-CLC, and CLOU technologies are still not sufficiently recognized [21].
Some recent studies reported in the literature have demonstrated the reliable and effective utilization of machine learning models for the modelling, predicting, and optimising of coal-based energy devices and power generation systems. An artificial intelligence modelling-based operational strategy was built to optimize the SO2, Hg, NOx, and dust emissions from the wet flue gas desulphurization system simultaneously. Similarly, comprehensive utilization of the machine learning models like artificial neural networks and support vector machines was presented for optimizing the coal-fired power plant performance parameters from the component, system, and strategic levels. Recently, artificial intelligence-based models were developed for the effective power production from a 660 MW power plant for optimizing the efficiency of industrial-scale steam turbines that support the net-zero goal in terms of a higher energy efficiency and a reduced emissions load [25].
This paper introduces the fuzzy logic (FL) method as an artificial intelligence (AI) approach to predict the NOx and SO2 emissions from the biomass and bituminous coal combustion in air-firing, oxyfuel, iG-CLC, CLOU, and air-firing conditions (as a reference). The proposed model has been validated using the experimental data from the existing combustion facility.
2. Experimental Procedures and Methods
The measurements of the gas emissions (NOx and SO2) were carried out on the existing 5 kWth dual fluidized bed chemical looping combustion of solid fuels (DFB-CLC-SF) facility located at the Czestochowa University of Technology, Poland. In the Fuel Reactor (FR), the oxygen carriers (OC) release oxygen, resulting in the combustion process. Then, the reduced metal oxides are transported to the air reactor (AR) for the oxidation processes [15]. A schematic diagram of the FB-CLC-SF system is shown in Figure 4.
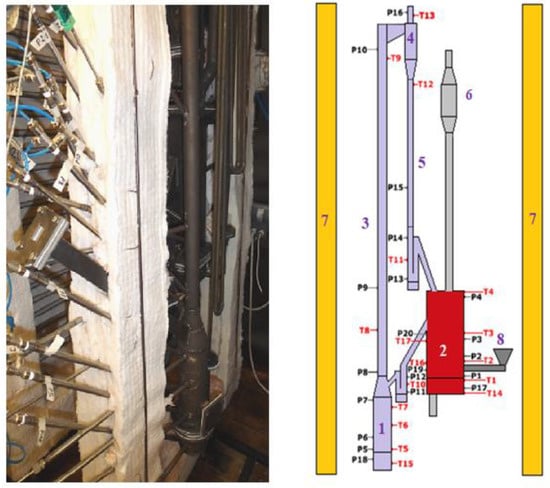
Figure 4.
The hot FB-CLC-SF facility; 1—air reactor, 2—fuel reactor, 3—riser, 4—cyclone, 5—downcomer, 6—particle’s collector, 7—heaters, 8—screw feeder, P1—P19—pressure transducers, and T1–T16—thermocouples [15].
The total height of the air reactor is 2.52 m, and the fuel reactor is 0.5 m, while the air reactor’s and the riser’s diameters are 0.098 m and 0.04 m, respectively. A more detailed description of the discussed research unit can be found in other papers [12].
The experimental tests were carried out on a research unit in the temperature range from 1079 to 1157 K. Two different solid fuels were used during these investigations, i.e., wood chips and bituminous coal from a Polish coal mine (Table 1).

Table 1.
Properties of fuels (as received).
A wide variety of working conditions were used in the present study. Seven tests involving different fuels, gaseous atmospheres in the FR, and combustion modes were carried out. The first one, called Test 0, considers a conventional coal combustion in the air in the atmosphere. Test 1 corresponds to oxyfuel conditions when the combustion process occurs in a suitable gas mixture of CO2 and O2.
Solid oxygen carriers (OCs) acting as oxygen sources, and CO2 as fluidizing gas, were applied during the other five tests 2–6. Three different types of OCs were considered: calcined ilmenite (OC1) in tests 2, 5, and 6, CuO (60% wt.) enriched with the copper tailing support (OC2) in test 3, as well as CuO (60% wt.) with the support of ilmenite (20% wt.) and fly ash (OC3) in test 4. Ilmenite is a natural mineral and belongs to the group of iG-CLC oxygen carriers, while the CuO-based oxygen carriers belong to the CLOU functional group. A detailed description of all the oxygen carriers used in this work can be found in [12,15]. The flue gas components were measured at the outlet of the fuel reactor of the FB-CLC-SF facility using gas analysers based on infrared detection (GASMET DX-4000). The measurement technique is FTIR (Fourier transform infrared spectroscopy). A dedicated software Calcmet was used for the data acquisition and further analyses. The sampling frequency was set as 1 Hz. The measurement ranges were 0–1000 ppm for SO2, 0–1000 ppm for NO, and 0–100 ppm for NO2, respectively. The NO and NO2 concentrations were recalculated into the NOx in this study. The measurement accuracy is 1% of the measurement range for all the flue gas components. The detailed operating conditions and results of the measurements are summarized in Table 2.

Table 2.
The operating parameters (ad—air-dried basis).
Since the complexity of the CLC process is still not sufficiently recognized, especially in such CLC equipment of a sophisticated geometry, the fuzzy logic (FL) approach, as one of the main artificial intelligence (AI) methods, was introduced to the research presented in this paper [22]. Together with artificial neural networks and evolutionary algorithms, the FL approach belongs to the main AI techniques [26]. The fuzzy logic-based methods employ linguistic variables and fuzzy sets to evaluate the considered process, enabling a qualitative judgment to be applied to the quantitative parameters and dealing with imprecise, vague, and uncertain information [27,28]. The following four components make an FL model: the fuzzifier, a fuzzy rule base, an inference engine, and a defuzzifier [29,30,31]. The first stage of building a model consists of expressing the input variables by fuzzy sets, where a numeric value input is assigned to a value of a membership function (ranging from 0 to 1) [30]. This is the fuzzification stage since transforming a crisp input value into a fuzzy set occurs. The IF-THEN fuzzy rule base allows the system to generate fuzzy outputs. Finally, the defuzzification process produces crisp outputs corresponding to the crisp inputs supplied to the model. The Qtfuzzylite fuzzy logic control application [32] is used for this research to predict the SO2 and NOx emissions from coal and biomass combustion in air-firing, oxyfuel, iG-CLC, and CLOU conditions in a complex geometry fluidized bed furnace.
This approach is a valuable modelling technique suitable for describing complex systems, which allows for overcoming the shortcomings of the programmed computing approach and measurements during the experimental research [33]. The introduced strategy can be considered an alternative to the above data processing techniques, taking into account the complexity of analytical and numerical methods and the high cost of empirical experiments [34]. The FL approach is practical, especially when the subjective knowledge and experience of the expert are significant in defining the objective function and decision variables [33,35]. It is also an approach to map the input into the output space, involving the so-called membership functions to define how each input value is mapped to a membership one [35].
On the other hand, the low data availability of expert knowledge and a small number of input variables are the main limitations of this technique. Intelligent hybrid neuro-fuzzy systems may overcome these shortcomings [34].
3. Results and Discussion
The Qtfuzzylite fuzzy logic control application [32] was used as a computational tool to make the presented model. Nine inputs influencing the SO2 and NOx emissions were employed to establish the model, i.e., (1) the IDmode tag defining the combustion mode (Air1, Air2 for air-fired combustion, Oxy for oxyfuel conditions, and CLC_CLOU for iG-CLC and CLOU functionalities), (2) the kind of oxygen carrier OC (symbols OC0 and OC4 for air-fired conditions, indicating that no oxygen carriers are used, OC1 for ilmenite, OC2 for CuO (60% wt.) enriched with the copper tailing support, and OC3 for CuO (60% wt.) enriched with ilmenite (20% wt.) and fly ash (20% wt.) supports, (3) excess oxygen OE in the fuel reactor, (4) the average fuel reactor temperature T, (5) the FCad./VMad ratio (ad—air-dried basis), (6) the Nad/Cad molar ratio, (7) the sulphur content Sad and (8) ash content Aad in the fuel (coal and biomass), and 9. the IDfuel tag defines the fuel type, 1 being for coal and 2 being for wood chips (Table 3).

Table 3.
Parameters of entry conditions.
Thus, the model has the unique ability to consider the diverse combinations of solid fuels combustion modes and atmospheres as well as various OCs mixtures, which is very effective from a practical point of view [12,15]. The developed model can generalize the information about the process behaviour. This comprehensive tool provides innovative abilities for design considerations and the performance of iG-CLC and CLOU systems. Such selected inputs allowed for the description of the outputs, i.e., the SO2 and NOx emissions from the FB-CLC-SF unit. The model uses triangular and constant terms for both the inputs and outputs, allowing for a mapping of the inputs to the outputs domains. Sugeno-type fuzzy inference systems were applied in this study [35].
The screenshot of the developed system is shown in Figure 5, whereas the IF-THEN rule-base is depicted in Table 4.
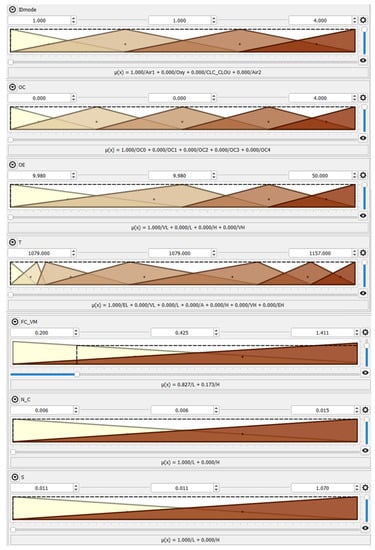
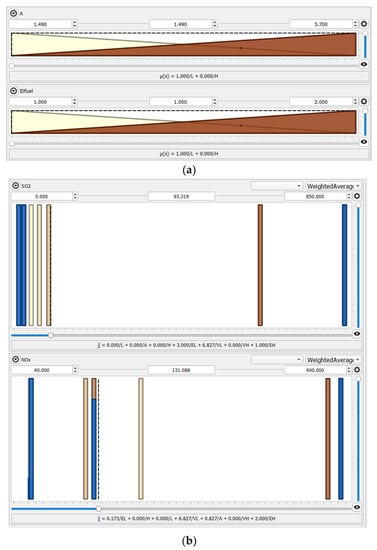
Figure 5.
The FL model with graphical representations of input (a) and output (b) variables.

Table 4.
The IF-THEN rule-base of the model for (a) SO2 and (b) NOx.
The developed model may serve as a novel and convenient tool to predict the influence of the selected input parameters on the SO2 and NOx emissions from advanced biomass and bituminous coal combustion under oxyfuel, iG-CLC, and CLOU conditions as well as from conventional air-firing, which was carried out with complex geometry fluidized bed equipment.
The validation procedure of the presented model was successfully carried out using the experimental data from the 5 kWth FB-CLC-SF facility (Figure 6).
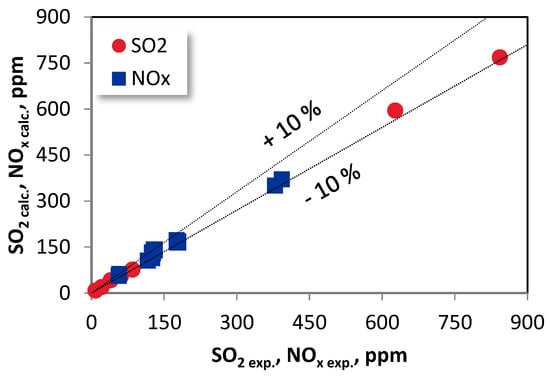
Figure 6.
Validation of the model.
The results depicted in Figure 6 correspond to a variety of operating parameters. A good accuracy of the developed model was achieved. The maximum relative percentage error between the measured values and predicted emissions of SO2 and NOx is lower than 10% and hence stands for a reliable basis for the possible use of the developed system in practice.
The SO2 and NOx emissions from wood chips and bituminous coal combustion under different combustion modes for OE = 10% and T = 1080 K, predicted by the model, are shown in Figure 7.
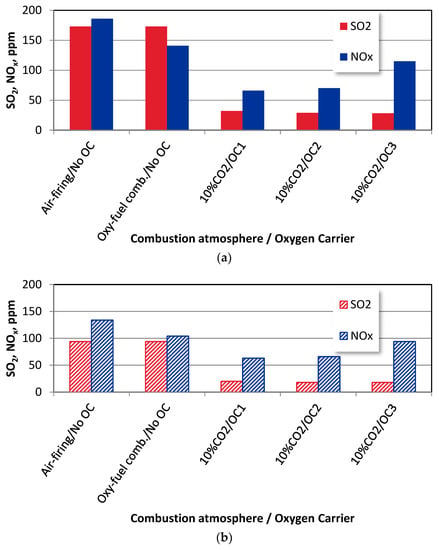
Figure 7.
The SO2 and NOx emissions from (a) bituminous coal and (b) biomass combustion under different combustion modes, where OE = 10% and T = 1080 K.
The SO2 and NOx emissions are the lowest for the CLOU and iG-CLC combustion modes, with OC1 and OC2 oxygen carriers. This behaviour corresponds to the first observations reported during the experiments [12]. Since the use of ilmenite (OC1) was accompanied by the relatively low efficiency of the combustion process, the emissions of SO2 and NOx are also low. The rather good oxygen transport abilities of OC2 at the beginning (iG-CLC feature), reported in [12], were gradually worsened during the experiments.
Moreover, the numerous sinters observed in the fuel reactor may contribute to a further deterioration of the oxygen transport. This oxygen carrier expressed only about half of its oxygen transport capabilities [12]. Such a behaviour resulted in low SO2 and NOx concentrations in the exhaust gas.
These results also comply with the conclusions withdrawn from the comparison of the SO2 and NOx emissions during the coal and biomass combustion, shown in Figure 7a,b. Since the sulphur and nitrogen contents in the bituminous coal are higher than those for the biomass, the SO2 and NOx emissions from the coal combustion are also higher. Moreover, the DeNOx mechanism is favoured by H and OH radicals from the higher volume of volatiles released from the biomass. This can be reported for all tests, including the air-firing and oxyfuel combustion modes and CLOU and iG-CLC combustion with OC1, OC2, and OC3 oxygen carriers.
As the fuel-bound sulphur is the only source of SO2 in this study, replacing the O2/N2 (air-firing conditions) with an O2/CO2 (oxyfuel) atmosphere results in the same SO2 emissions when burning the same fuel. On the other hand, a high concentration of CO2 under the oxyfuel mode favours a higher CO content in the fuel reactor’s atmosphere due to the Bouduoard reaction, leading to decreased NOx emissions which are observed when comparing Figure 7a,b [2]. In addition, CO oxidation inhibition occurs in the presence of high concentrations of SO2 [15]. The increase in the carbon conversion to CO in the presence of SO2 [9] also decreases the NO emissions.
The lower fuel burn-out arising from poor oxygen transport by the OCs results in comparable low levels of SO2 emissions [36].
A similar behaviour was reported for the NOx emissions with OC1 and OC2, as the reduction character of the atmosphere deteriorates the NOx formation mechanisms.
Different observations were made for the fuel combustion with the OC3 oxygen carrier. Relatively good oxygen transport capabilities characterized this oxygen carrier. Thus, the released oxygen deteriorates the DeNOx mechanism and allows for converting the volatiles N to NOx, limiting the N2 formation and ultimately leading to an increase in the NOx emissions.
The effect of the oxygen excess on the SO2 and NOx emissions is given in Figure 8.
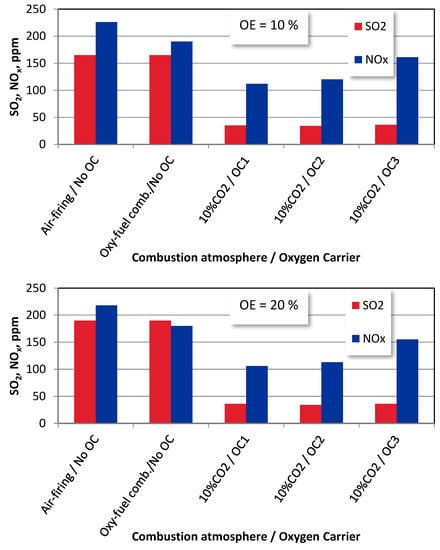
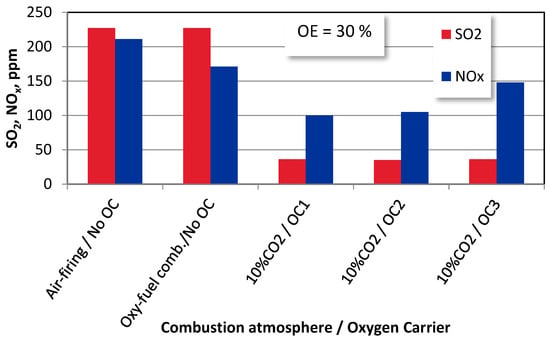
Figure 8.
The effect of oxygen excess on SO2 and NOx emissions from bituminous coal combustion under different combustion modes (T = 1100 K).
The increase in the oxygen excess is better expressed in air-firing and oxyfuel combustion atmospheres where no oxygen carriers are employed, and the sulphur evolution is enhanced in such conditions [36]. The increase in the SO2 concentrations in these combustion conditions leads to a decrease in the NOx emissions. This dependence can be explained by the well-known CO oxidation inhibition effect that occurs in the presence of SO2, which results in the NO emissions lowering with the SO2 formation [37].
Due to the replacement of the gaseous oxidized with solid oxygen carriers OC1, OC2, and OC3, the access to oxygen has become limited, which deteriorates the SO2 and NOx formation mechanisms [12]. A similar behaviour can be registered for wood chips combustion (Figure 9). Due to the lack of CLOU functionality, the additional dilution effect makes the lowest emissions for OC1 (ilmenite). The CuO content (OC2 and OC3 carriers), characterized by the CLOU functionality, facilitates the release and transport of oxygen, causing an increase in the NOx emissions. At the same time, the dilution effects keep the SO2 emissions at similar levels.
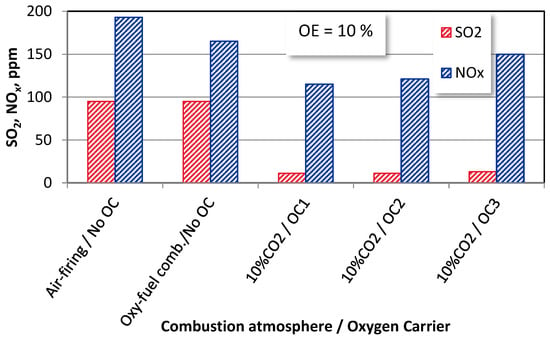
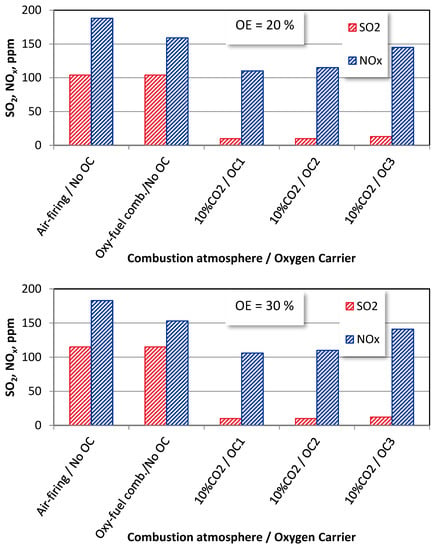
Figure 9.
The effect of oxygen excess on SO2 and NOx emissions wood chips combustion under different combustion modes (T = 1100 K).
The effects of the average fuel reactor temperatures on the SO2 and NOx emissions from bituminous coal and wood chips under various combustion atmospheres are shown in Figure 10 and Figure 11. The average fuel reactor temperature is an essential operating parameter strongly influencing the SO2 and NOx emissions. The increase in the temperature leads to the combustion processes intensifications, resulting in the increase in sulphur and nitrogen oxides in all the combustion environments.
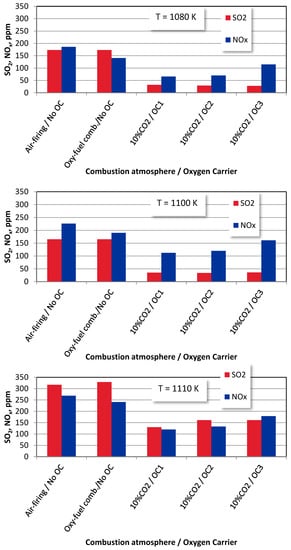
Figure 10.
The average fuel reactor temperature effect on SO2 and NOx emissions from bituminous coal combustion under different combustion modes (OE = 10%).
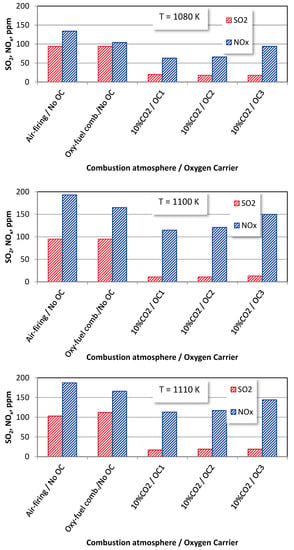
Figure 11.
The effect of average fuel reactor temperature on SO2 and NOx emissions from wood chips combustion under different combustion modes (OE = 10%).
Similar behaviour can be observed for biomass combustion in temperatures of 1080 K and 1100 K.
For a temperature of 1110 K, the NOx concentrations in the flue gas are lower than for a temperature of 1100 K. It may be caused by high SO2 emissions inhibiting the oxidation of CO, which is the active agent in the DeNOx mechanisms. Furthermore, the already mentioned DeNOx mechanism is favoured by H and OH radicals from the higher volume of volatiles in the biomass material.
4. Conclusions
The paper introduces an AI approach for predicting the SO2 and NOx emissions from coal and biomass advanced combustion under oxyfuel, iG-CLC, and CLOU conditions and conventional air firing.
A new fuzzy logic-based model was developed and presented in this article. The model was successfully validated using the experimental data from the existing 5 kWth dual fluidized bed chemical looping combustion of solid fuels unit.
The developed system achieved satisfying accuracy. The maximum relative errors between the measured values and predicted by the developed model SO2 and NOx emissions are lower than 10%.
Based on the obtained results, the following main conclusions can be drawn:
- Simulations confirmed that biomass combustion generates lower SO2 and NOx emissions than coal burning for all combustion modes.
- The SO2 and NOx emissions strongly depend on the properties of the oxygen carriers used in the systems and operating parameters.
- The fuzzy logic-based method constitutes a practical AI approach for modelling gaseous pollutants emissions from advanced and complex combustion systems.
Author Contributions
Conceptualization, J.K. and T.C.; methodology, J.K.; software, J.K.; validation, J.K., T.C. and A.Z.; formal analysis, M.S., D.S., K.G., A.K., W.N., K.S., W.M.A. and Y.G.; investigation, J.K., T.C. and A.Z.; resources, T.C., A.Z. and J.K.; data curation, J.K., T.C. and A.Z., writing—original draft preparation, J.K.; writing—review and editing, T.C., A.Z., M.S. and D.S.; visualization, J.K., A.Z. and T.C.; supervision, W.N. and T.C.; project administration, T.C. and W.N. All authors have read and agreed to the published version of the manuscript.
Funding
The Project “Innovative Idea for Combustion of Solid Fuels via Chemical Looping Technology” (Agreement No. POL-NOR/235083/104/2014) is funded by Norway Grants Polish-Norwegian Research Programme operated by the National Center for Research and Development, Poland. CCS 2013 Call “New innovative solutions for CO2 capture.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lyngfelt, A. 20-Chemical Looping Combustion (CLC). In Fluidized Bed Technologies for Near-Zero Emission Combustion and Gasification; Scala, F., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 895–930. ISBN 978-0-85709-541-1. [Google Scholar]
- Lyngfelt, A.; Linderholm, C. Chemical-Looping Combustion of Solid Fuels—Status and Recent Progress. Energy Procedia 2017, 114, 371–386. [Google Scholar] [CrossRef]
- Imtiaz, Q.; Hosseini, D.; Müller, C.R. Review of Oxygen Carriers for Chemical Looping with Oxygen Uncoupling (CLOU): Thermodynamics, Material Development, and Synthesis. Energy Technol. 2013, 1, 633–647. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent Developments on Carbon Capture and Storage: An Overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Rackley, S.A. Carbon Capture and Storage; Butterworth-Heinemann: Oxford, UK, 2017; ISBN 978-0-12-812042-2. [Google Scholar]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming Technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Cormos, C.-C. Chemical Looping with Oxygen Uncoupling (CLOU) Concepts for High Energy Efficient Power Generation with near Total Fuel Decarbonisation. Appl. Therm. Eng. 2017, 112, 924–931. [Google Scholar] [CrossRef]
- de Souza-Santos, M.L. Solid Fuels Combustion and Gasification: Modeling, Simulation, and Equipment Operations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-4749-3. [Google Scholar]
- Czakiert, T.; Krzywanski, J.; Zylka, A.; Nowak, W. Chemical Looping Combustion: A Brief Overview. Energies 2022, 15, 1563. [Google Scholar] [CrossRef]
- Al Asfar, J.J.; AlShwawra, A.; Sakhrieh, A.; Hamdan, M.A. Combustion Characteristics of Solid Waste Biomass, Oil Shale, and Coal. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 335–342. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Y.; Wang, X.; Jin, B. Experimental and Kinetics Investigations of Separated-Gasification Chemical Looping Combustion of Char with an Iron Ore as the Oxygen Carrier. Fuel Process. Technol. 2020, 210, 106554. [Google Scholar] [CrossRef]
- Zylka, A.; Krzywanski, J.; Czakiert, T.; Idziak, K.; Sosnowski, M.; Grabowska, K.; Prauzner, T.; Nowak, W. The 4th Generation of CeSFaMB in Numerical Simulations for CuO-Based Oxygen Carrier in CLC System. Fuel 2019, 255, 115776. [Google Scholar] [CrossRef]
- Liu, F.; Liu, J.; Yang, Y. Review on the Theoretical Understanding of Oxygen Carrier Development for Chemical-Looping Technologies. Energy Fuels 2022, 36, 9373–9384. [Google Scholar] [CrossRef]
- Moghtaderi, B. Review of the Recent Chemical Looping Process Developments for Novel Energy and Fuel Applications. Energy Fuels 2012, 26, 15–40. [Google Scholar] [CrossRef]
- Krzywanski, J.; Czakiert, T.; Nowak, W.; Shimizu, T.; Zylka, A.; Idziak, K.; Sosnowski, M.; Grabowska, K. Gaseous Emissions from Advanced CLC and Oxyfuel Fluidized Bed Combustion of Coal and Biomass in a Complex Geometry Facility:A Comprehensive Model. Energy 2022, 251, 123896. [Google Scholar] [CrossRef]
- Basu, P. Circulating Fluidized Bed Boilers; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-06172-6. [Google Scholar]
- Basu, P. Combustion of Coal in Circulating Fluidized-Bed Boilers: A Review. Chem. Eng. Sci. 1999, 54, 5547–5557. [Google Scholar] [CrossRef]
- Al Asfar, J.; AlShwawra, A.; Shaban, N.A.; Alrbai, M.; Qawasmeh, B.R.; Sakhrieh, A.; Hamdan, M.A.; Odeh, O. Thermodynamic Analysis of a Biomass-Fired Lab-Scale Power Plant. Energy 2020, 194, 116843. [Google Scholar] [CrossRef]
- Al Asfar, J.J.; Hammad, A.; Sakhrieh, A.; Hamdan, M.A. Two-Dimensional Numerical Modeling of Combustion of Jordanian Oil Shale. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1189–1196. [Google Scholar] [CrossRef]
- Gungor, A.; Eskin, N. Two-Dimensional Coal Combustion Modeling of CFB. Int. J. Therm. Sci. 2008, 47, 157–174. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A. Chemical-Looping Combustion: Status and Research Needs. Proc. Combust. Inst. 2019, 37, 4303–4317. [Google Scholar] [CrossRef]
- Lyu, W.; Liu, J. Artificial Intelligence and Emerging Digital Technologies in the Energy Sector. Appl. Energy 2021, 303, 117615. [Google Scholar] [CrossRef]
- Ahmad, T.; Zhang, D.; Huang, C.; Zhang, H.; Dai, N.; Song, Y.; Chen, H. Artificial Intelligence in Sustainable Energy Industry: Status Quo, Challenges and Opportunities. J. Clean. Prod. 2021, 289, 125834. [Google Scholar] [CrossRef]
- Kalogirou, S.A. Artificial Intelligence for the Modeling and Control of Combustion Processes: A Review. Prog. Energy Combust. Sci. 2003, 29, 515–566. [Google Scholar] [CrossRef]
- Muhammad Ashraf, W.; Moeen Uddin, G.; Muhammad Arafat, S.; Krzywanski, J.; Wang, X. Strategic-level performance enhancement of a 660 MWe supercritical power plant and emissions reduction by AI approach. Energy Convers. Manag. 2021, 250, 114913. [Google Scholar] [CrossRef]
- Esen, H.; Inalli, M.; Sengur, A.; Esen, M. Artificial Neural Networks and Adaptive Neuro-Fuzzy Assessments for Ground-Coupled Heat Pump System. Energy Build. 2008, 40, 1074–1083. [Google Scholar] [CrossRef]
- Dragojlovic, Z.; Kaminski, D.A.; Ryoo, J. Tuning of a Fuzzy Rule Set for Controlling Convergence of a CFD Solver in Turbulent Flow. Int. J. Heat Mass Transf. 2001, 44, 3811–3822. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Z.; Zhu, L.; Chen, H.; Zhang, L. Fuzzy Estimation for Temperature Distribution of Furnace Inner Surface. Int. J. Therm. Sci. 2012, 51, 84–90. [Google Scholar] [CrossRef]
- Pospíchal, J. Fuzzy Sets and Fuzzy Logic: Theory and Applications. By George J. Klir and Bo Yuan. Prentice Hall: Upper Saddle River, NJ, 1995. 574 pp. ISBN 0-13-101171-5. J. Chem. Inf. Comput. Sci. 1996, 36, 619. [Google Scholar] [CrossRef]
- Kucukali, S.; Baris, K. Turkey’s Short-Term Gross Annual Electricity Demand Forecast by Fuzzy Logic Approach. Energy Policy 2010, 38, 2438–2445. [Google Scholar] [CrossRef]
- Kılıç, B. Optimisation of Refrigeration System with Two-Stage and Intercooler Using Fuzzy Logic and Genetic Algorithm. Int. J. Eng. Appl. Sci. 2017, 9, 42–54. [Google Scholar] [CrossRef]
- Available online: www.fuzzylite.com (accessed on 26 September 2022).
- Ross, T.J. Fuzzy Logic with Engineering Applications, 3rd ed.; John Wiley: Chichester, UK, 2010; ISBN 978-0-470-74376-8. [Google Scholar]
- Krzywanski, J. Heat Transfer Performance in a Superheater of an Industrial CFBC Using Fuzzy Logic-Based Methods. Entropy 2019, 21, 919. [Google Scholar] [CrossRef]
- Mohd Adnan, M.R.; Sarkheyli, A.; Mohd Zain, A.; Haron, H. Fuzzy Logic for Modeling Machining Process: A Review. Artif. Intell. Rev. 2015, 43, 345–379. [Google Scholar] [CrossRef]
- Krzywanski, J.; Blaszczuk, A.; Czakiert, T.; Rajczyk, R.; Nowak, W. Artificial intelligence treatment of NOX emissions from CFBC in air and oxy-fuel conditions. In Proceedings of the CFB-11—11th International Conference on Fluidized Bed Technology, Beijing, China, 14–17 May 2014; pp. 619–624. [Google Scholar]
- Hildor, F.; Leion, H.; Mattisson, T. Steel Converter Slag as an Oxygen Carrier—Interaction with Sulfur Dioxide. Energies 2022, 15, 5922. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).