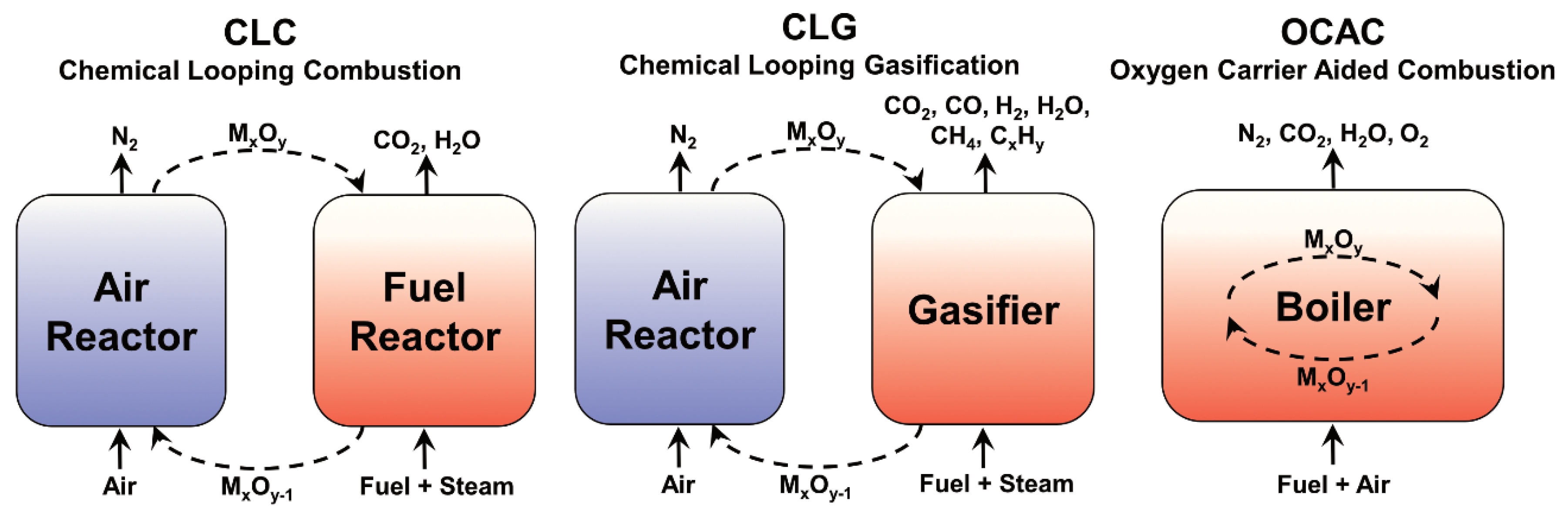
1. Introduction
Low-cost oxygen carriers, such as ores or waste products, have been evaluated for thermochemical biomass conversion techniques such as chemical-looping combustion (CLC), chemical-looping gasification (CLG), and oxygen carrier aided combustion (OCAC) [
1,
2,
3]. These techniques can be used to decrease emissions, but also, in the case of CLC, can be used to separate CO
2 from the flue gases.
Figure 1 presents the layout of these processes. If biomass is used and the separated CO
2 is stored, the overall carbon flow to the atmosphere will be negative, i.e., bio-energy with carbon capture and storage (BECCS) [
4,
5]. However, for this process to be efficient, the oxygen carrier needs to be both efficient and have the chemical and physical strength to enable a lifetime of hundreds of hours of operation [
6,
7].
Low-cost oxygen carriers are expected to be needed for biomass conversion in these processes, but biomass contains ash that will be mixed with the oxygen carrier and could be hard to separate. Iron-based oxygen carriers are to be preferred since these are nonhazardous to handle and are more environmentally friendly [
8]. Among the most promising iron-based low-cost oxygen carriers are ilmenite, an iron-titanium ore that is available in large quantities. Ilmenite has sufficient fuel conversion, as well as good chemical and physical properties, thus currently making ilmenite the bench-mark oxygen carrier [
2,
9,
10,
11,
12,
13]. However, since ilmenite is about 6–8 times more expensive than sand, which is used today for fluidized bed boilers, other oxygen carriers with lower costs are of interest [
3,
14].
Steel converter slag, also called Linz-Donawitz (LD) slag, is a byproduct of the steel manufacturing industry that has been investigated as an oxygen carrier [
15]. Since the demand is low, the price of this material is also expected to be low and more similar to sand [
14]. LD slag has been investigated as an oxygen carrier and has been suggested to have sufficient chemical and physical properties in addition to having similar fuel reactivity as ilmenite [
15,
16,
17].
During OCAC operation with LD slag, relatively high CO emissions were generated. It was suggested that this unconverted CO was a result of the alkali interaction with the slag. It was suspected that higher amounts of alkali, in the form of KOH and KCl in the boiler, resulted in a lower burnout [
14,
15]. Sulfur is known to influence alkali chemistry and boiler operation during combustion. If sulfur is present, alkali will react and form sulfates resulting in, e.g., decreased superheater corrosion. Therefore, co-combustion with sulfur-rich fuel, or the addition of sulfur, is commonly used to decrease corrosion [
18,
19,
20]. When LD slag was used as bed material in the Chalmers CFB boiler, sulfur was added with two different methods: (I) ammonium sulfate was added to the cyclone resulting in decreased CO emission, as expected; (II) elementary sulfur was added directly to the bed together with the fuel. However, elementary sulfur only had a temporary effect on decreasing CO emissions [
14]. Thus, it is expected that co-combustion with sulfur-rich fuels will not be as efficient to decrease CO emission or corrosion for longer periods when using LD slag as an oxygen carrier. Lime in the LD slag supposably reacted with the sulfur and formed CaSO
4. From SEM-EDX analysis, it was also indicated that sulfur was accumulated not only on the surface of the particles, but also inside the bed particles [
15]. Projecting this observed interaction and the knowledge that CaSO
4 has a larger crystal structure than CaO and CaCO
3, the formation of CaSO
4 might also result in decreased surface area of the particles since pores can be plugged. This could result in decreased reactivity of the oxygen carrier since gas transport through the particle is decreased [
21].
Previous studies with oxygen carriers and sulfur interaction have concluded that oxygen access via the oxygen carrier is essential for the forms of sulfur which are generated in a chemical-looping process. From both thermodynamic calculations [
22,
23] and experiments [
23,
24,
25,
26], it has been observed that mostly H
2S is formed if the oxygen access is under-stochiometric, as in the fuel reactor of a CLG process. When lower fuel to oxygen carrier ratios are used, the larger oxygen access will result in mostly SO
2 being formed. From these studies, it has been concluded that most sulfur is released in the fuel reactor. However, none of these investigated oxygen carriers contained calcium, which is known to react with sulfur at combustion boiler temperatures. In one study, Arjmand et al. investigated calcium manganite with methane and SO
2, and here it was found that calcium played a significant role in binding the sulfur to different compounds [
27]. Ilmenite that had been used under OCAC conditions has also been evaluated regarding sulfur interaction, finding that the calcium from ash has a significant role in the sulfur chemistry also for other oxygen carriers [
28].
This study aims to investigate how sulfur affects the properties of the calcium-rich oxygen carrier LD slag under conditions relevant for CLC and CLG. It is expected that sulfur affects the gasification rate of the char, conversion of gaseous species, and composition of the outgoing gases, hence it is significant for understanding the process.
Theoretical Background
The formation of CaSO
4 from limestone in boilers for high-temperature sulfur capturing processes was studied intensely during the 1960s and 1970s. These studies were mostly directed toward the combustion of sulfur-containing coal [
29,
30]. Additionally, more recently there have been investigations of utilizing lime together with iron ore oxygen carriers to remove sulfur from coal in a laboratory fluidized bed [
31]. Thermodynamically, the optimal sulfur capture temperature for limestone is around 880 °C, at which temperature CaCO
3 calcines to CaO according to Reaction (R1) [
32]. CaO, in turn, reacts with SO
2 under oxidizing conditions, forming CaSO
4 according to Reaction (R2).
However, due to the shifting reducing and oxidizing conditions in fluidized bed boilers, part of the CaSO
4 is reduced, forming CaS or CaO, as well as gaseous species such as H
2S, COS, and SO
2 [
21,
29,
33]. In comparison to conventional boilers, the fuel reactor in CLG and CLC are moderate-to-very reducing, and will most likely generate sulfur in only the more reduced forms, such as H
2S [
23,
34]. When using materials such as ilmenite in CLC operation with sulfur-containing fuel, it has been observed that most of the sulfur exits from the fuel reactor as SO
2 and H
2S [
23]. These are the most thermodynamic stable gaseous compounds of sulfur under reducing conditions. For materials with oxygen uncoupling properties, it is instead expected that sulfur is emitted as mainly SO
2 from the fuel reactor [
23,
24,
25].
LD slag contains plenty of calcium in different phases. The slag used in this study contains roughly 30 wt.% of calcium, whereof 2–4 wt.% is in the form of lime (CaO or free calcium). This means that the overwhelming majority of the calcium is bound as calcium silicates or in metal-containing structures [
15]. Even though the amount of free calcium is low, the catalytic properties of CaO towards the water gas shift reaction (Reaction (R3)) are important for gasification processes using lime-containing beds, such as LD slag [
35,
36]. If CaO is sulfated in a gasifier using LD slag as an oxygen carrier, this may affect both the gasification process, as well as the catalytic properties of the bed material.
If CaSO
4 is formed, Reaction (R4) or (R5) can occur to generate CaO or CaS. The CaO can, in turn, react under reducing conditions, forming CaS according to Reaction (R6). In both CLG and CLC with solid fuel, steam will most likely be used in the gasifier/fuel reactor to increase the gasification rate of the solid fuel. CaS can react in these cases with steam according to Reaction (R7), forming H
2S [
21]. During oxidation, it can form CaSO
4 or SO
2 according to Reactions (R8) and (R9). CaS might also react with CaSO
4 at temperatures relevant for boilers according to Reaction (R10) [
37]. In addition, sulfur has been found to deposit as elementary sulfur inside chemical-looping reactors as a result of the equilibrium Reaction (R11), called the Claus reaction [
23].
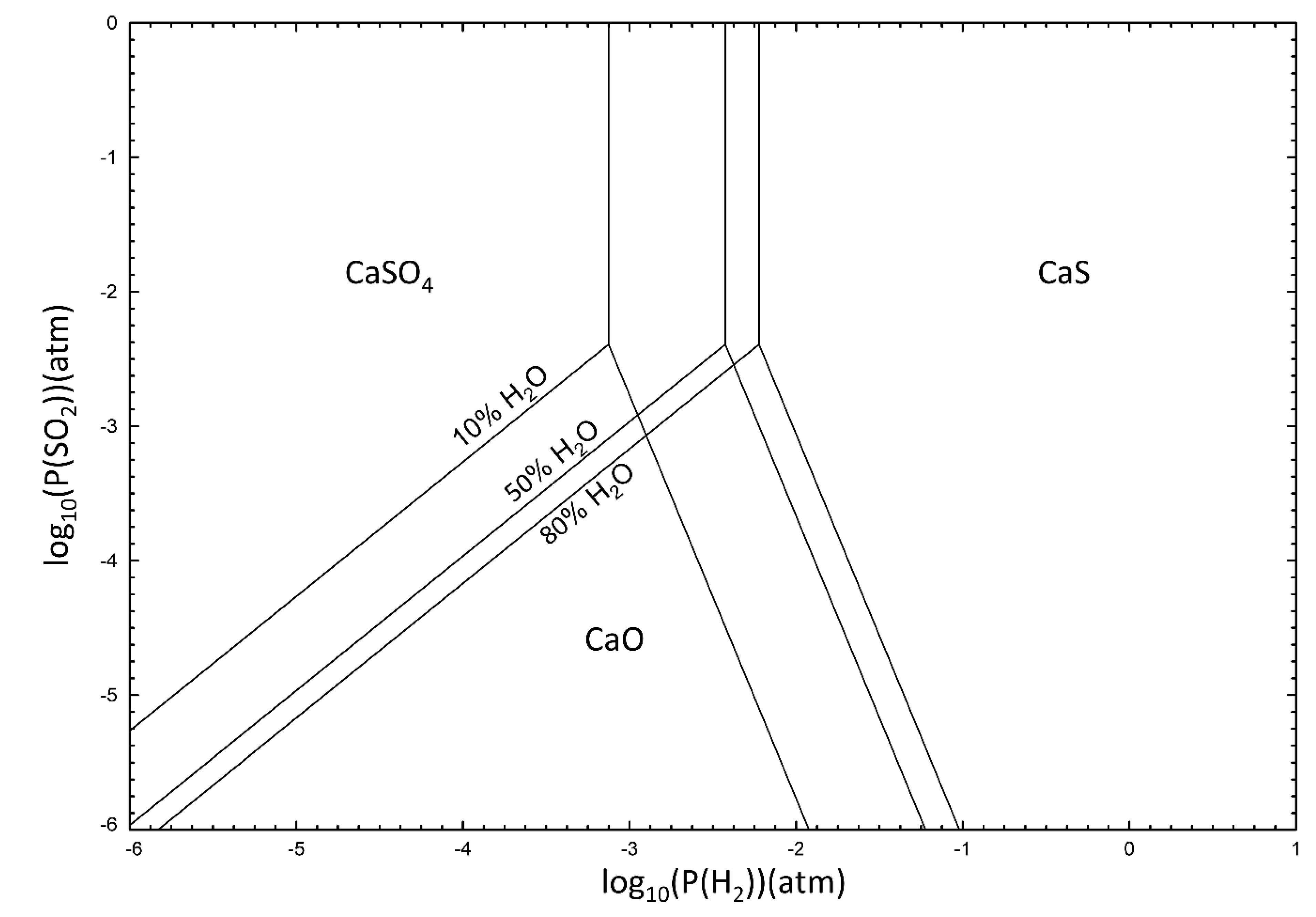
According to thermodynamic calculations, it has been shown in an oxidative environment that CaSO
4-CaS-CaO is dependent on temperature, as well as the SO
2 and O
2 partial pressure [
21]. During reducing conditions, as with H
2, a similar phase diagram of the stable solid phases at different temperatures can be calculated (see
Figure 2).
The partial pressure of steam is important for the oxidizing potential in the gasifier/fuel reactor. This is particularly interesting for LD slag since this material generates high hydrogen concentrations via the water-splitting reaction (Reaction (R12)). “A” in the reaction is Mn and Mg that are present in the oxygen carrying phase of LD slag [
36]. How steam partial pressure affects the sulfonation of calcium can be seen in
Figure 3.
From
Figure 2 and
Figure 3, it can be concluded that even with a small partial pressure of sulfur and hydrogen, the most stable phase will be CaS. This means that in CLG and CLC, the phase of free calcium in presence of sulfur is most likely CaS, due to the reducing conditions, especially since significant fractions of hydrogen are expected to be present when using LD slag as an oxygen carrier for gasification [
36]. It is important to note that these reactions mentioned above are only related to CaO. The CaO content in LD slag is around 1%; the majority of Ca in LD slag is found in phases like (CaO)
xSiO
2 and CaFe
2-xA
xO
5 [
15]. However, CaS and CaSO
4 can also be formed from these phases at a low partial pressure of SO
2 at 850 °C and reducing conditions (see
Supplementary Materials for calculated equilibriums).
Although LD slag was the main focus of this work, the benchmark oxygen carrier ilmenite was also used for comparison. It has been observed for ilmenite that the reaction rate towards methane [
23] and coal pyrolysis gas [
38] increases in the presence of sulfur dioxide. This was not only during the experiments with sulfur dioxide added in the gas phase, but there also seemed to be a lasting effect on the oxygen carrier. Even afterward, when sulfur was not added with the fuel, continued experiments showed an increased reactivity due to bound sulfur in the particles.
The scope of this study is to investigate how sulfur interacts with LD slag during reducing conditions. The fate of sulfur will be investigated and the potential formation of CaS and CaSO4 explored. The effect of sulfur on the gasification properties and whether sulfur will be released during oxidizing or reducing conditions are other aspects that will be explored. The conversion behavior of benzene, an important tar precursor, together with sulfur, was also investigated in this work.
2. Experiments
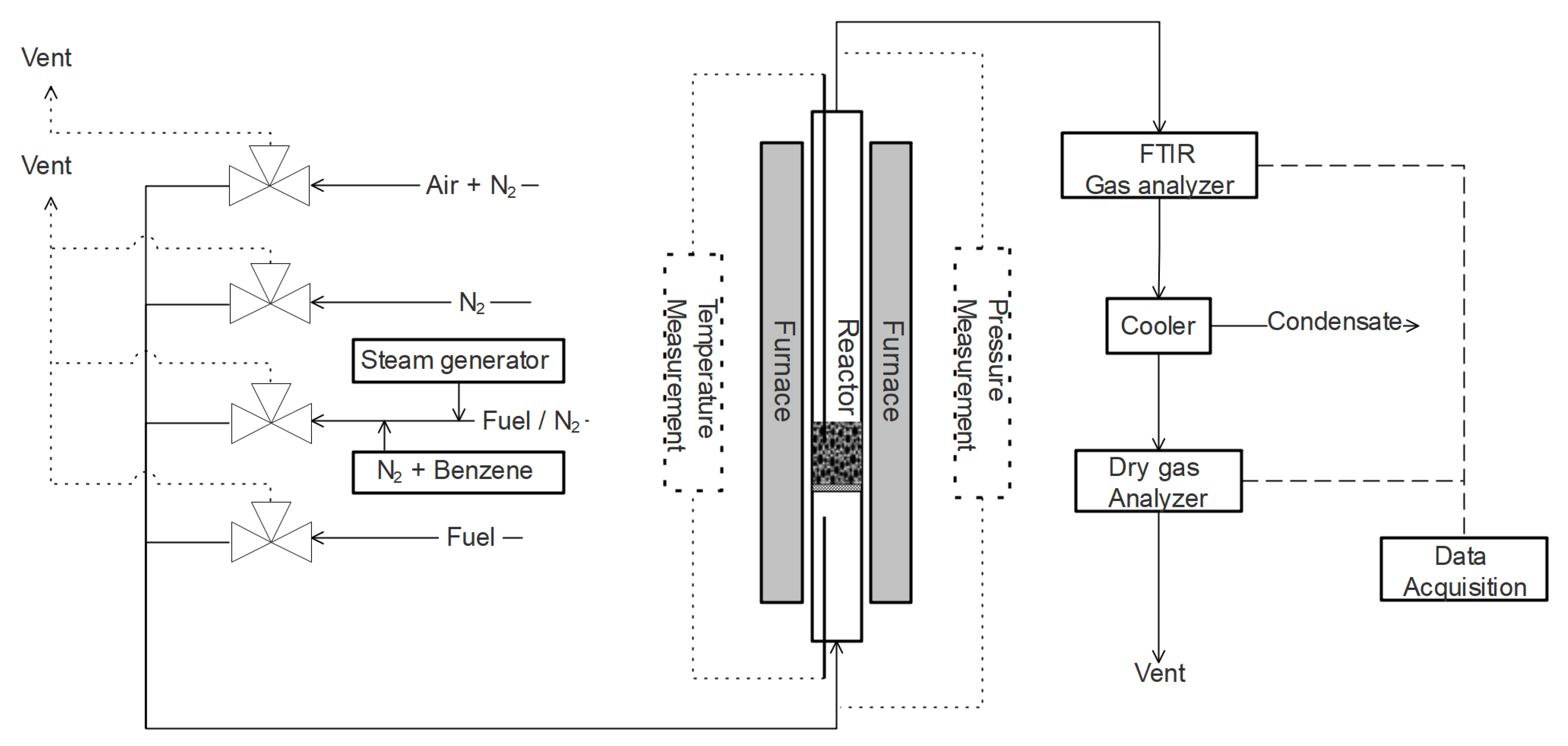
The experiments in this work were performed in a batch laboratory fluidized bed reactor (see
Figure 4 for a schematic overview of the unit). Here, gases were selected and inserted from the bottom of the reactor. The reactor, with an inner diameter of 22 mm, was constructed of quartz glass and mounted inside a furnace. In the quartz reactor, a porous plate was fixed where particles were placed for exposure. Temperature was measured both underneath the bed and inside the bed of particles. Pressure fluctuations over the bed were measured using a Honeywell pressure transducer with a frequency of 20 Hz for observation of bed fluidization behavior or interruptions in the fluidization.
The wet outgoing gases were analyzed using an FTIR during gaseous fuel experiments to measure benzene. After a cooler and a condensation trap, one of two five-channel Rosemount NGA 2000 instruments analyzed the gases. The first four channels of both analyzers were equipped with NDIR (nondispersive infrared) sensors for CO
2, CO, and CH
4, and a paramagnetic sensor for O
2 measurement. The two analyzers differed on the fifth channel where either an SO
2 NDIR detector or a thermal conductivity detector for H
2 was installed. Dräger tubes for H
2S detection and SO
2 were also used to estimate the H
2S/SO
2 formation. Gases for Dräger tubes were collected after the dry gas analyzer in air-tight 1 L aluminum bags and measured afterward. It is important to note that SO
2 dissolves in water and was partly dissolved in the condensation trap, identified by both the smell and the increased acidity of the water. For experiments with solid fuel, the FTIR was bypassed, in order to protect the FTIR instrument from tars. For these experiments, the components in the outgoing gas were only analyzed with one of the Rosemount analyzers at the time. More details regarding the system can be found elsewhere [
39].
The oxygen carrier material used in these experiments was LD slag received from SSAB, sieved to 150–400 µm. The same batch of slag has been used in other studies [
14,
15,
40,
41]. The ilmenite used was a Norwegian rock ilmenite received from Titania, sieved between 100–300 µm. This batch has also been used in other studies [
2,
15,
42]. Both oxygen carriers were heat-treated in air at 950 °C for 24 h. The sand was extra pure sea sand received from Merck, sieved between 180–250 µm. The elemental composition of these bed materials can be seen in
Table 1. Elemental composition was determined using ICP-SFMS according to SS EN ISO 17294-2: 2016 on LD slag and sand, and XRF according to the SP method 4343 on ilmenite.
The solid fuel used in the laboratory fluidized bed reactor was produced by inert heat treatment of wood pellets. Wood pellets were devolatilized in a fluidized silica bed at 820 °C under an N
2 atmosphere for more than 20 min. After devolatilization and cooling down, the sand was sieved. The devolatilized wood pellets were then crushed and sieved to obtain the desired size range of 180–300 µm. The volatile mass fraction of the original pellets was calculated to be 82 wt.%. The elemental composition of the wood pellets and the devolatilized fuel can be seen in
Table 2.
In addition to the wood pellets, another reference fuel was used called German wood char. This was delivered already as char and was only crushed and sieved to obtain the desired size range of 180–300 µm.
Prior to experiments in the batch fluidized bed reactor, the bed was activated using syngas (50% CO in H
2). These activation cycles were performed with 900 mL/min syngas for 20 s in every cycle, followed by oxidization with 5% O
2. The oxidation was performed until the sample was fully oxidized and the outgoing gases reached 5% O
2 again. Between these reducing and oxidizing atmospheres, inert N
2 with a flow of 1000 mL/min was used to purge the reactor for 180 s. These activation cycles were performed until a constant conversion of syngas could be observed for the sample, normally 7–20 cycles depending on the mass of the oxygen carrier. This activation was performed since there is a known increase in reactivity for these oxygen carriers, and after activation, the conversion of fuels is rather stable [
12]. These types of cycles were also used before every experiment as a reference.
2.1. Solid Fuel Experiments
For the solid fuel experiments, a sample of 40 g of oxygen carrier bed material was used. First, the oxygen carriers were activated using syngas. After the activation cycles, the solid fuel experiments were performed. Here, devolatilized and crushed wood char pellets were inserted from the top of the reactor together with a stream of 500 mL/min N2. A sample of 0.2 g of devolatilized char in the size range of 180–300 µm was used. During solid fuel cycles without sulfur addition, the bed material was fluidized using 50% steam in N2 with a total flow of 1000 Nml/min. For experiments with SO2, a total flow of 1000 Nml/min was used with a concentration of 50% steam, and was mixed with SO2 in N2, meaning that the inlet concentration of SO2 was 0.2 or 0.4% of the total inlet flow.
A summary of all the experiments can be seen in
Table 3. The experiment matrix was performed with both fuels, the devolatilized wood pellets and German wood char. There were differences in the setup with the analyzers detecting either H
2 or SO
2. Dräger tubes for SO
2 and H
2S were mainly used for 0.4% SO
2 experiments with German wood char. For experiments with German wood char, complementary reference experiments were performed after all SO
2 experiments to detect releases of sulfur from the used material using Dräger tubes.
This oxidation–inert–reducing–inert series, or cycle, was repeated three times at each experimental condition. The results were averaged over these cycles. Experiments were performed at 870 °C and 970 °C. The same sample (LD slag, ilmenite or sand) was used for all experiments, first for all experiments without sulfur, and then for the experiments with sulfur.
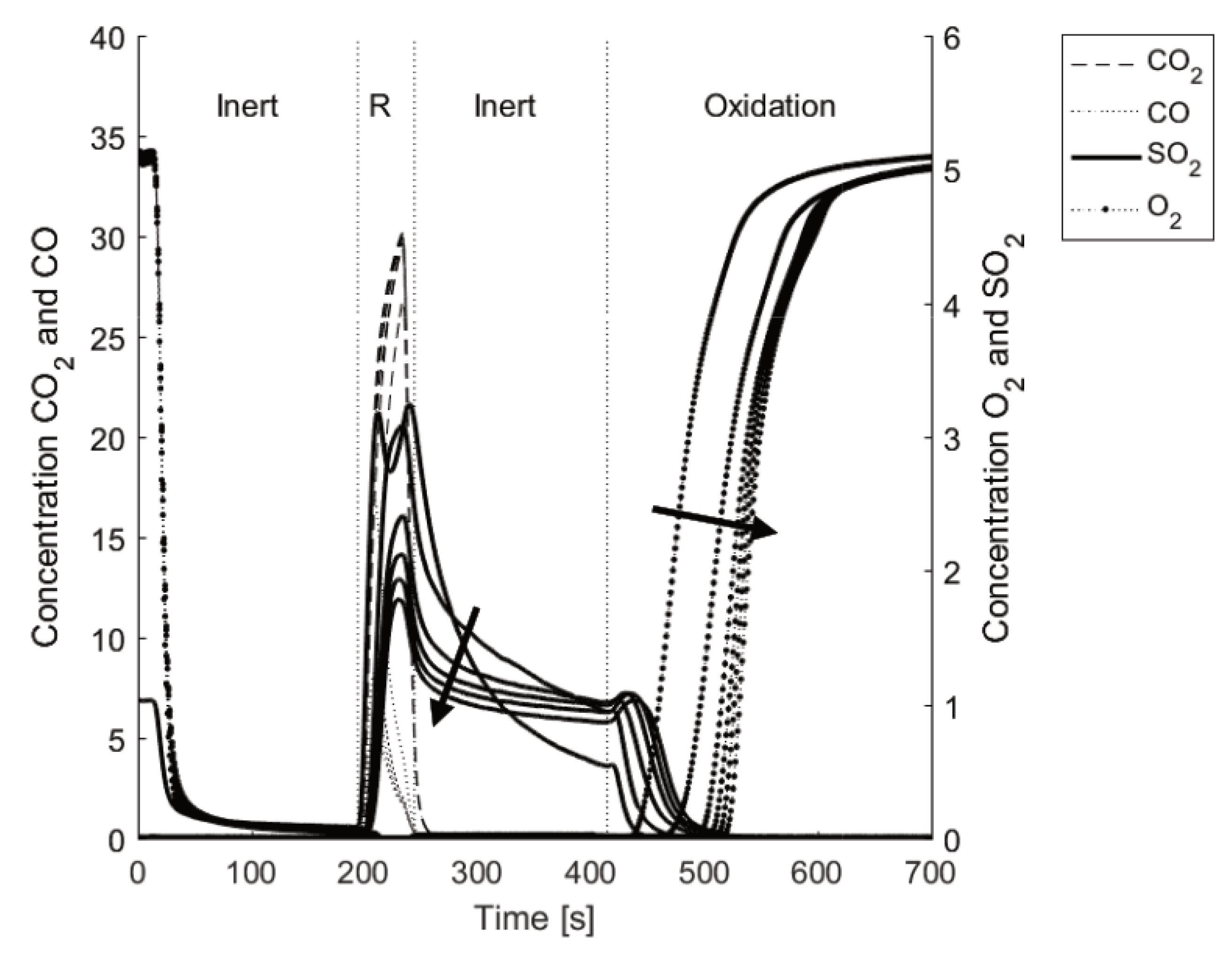
Figure 5 shows one typical cycle of solid fuel gasification with LD slag at 870 °C. When the 5% oxygen in nitrogen is replaced with inert gas at time = 0 s, the oxygen concentration rapidly drops as the oxygen is flushed from the system. The solid fuel, fluidization gas containing steam, N
2, and for the specific experiment 0.4% SO
2, was introduced at 180 s. The gasification of the fuel proceeds, as indicated by the rising concentrations of CO
2, CO, and H
2. After 900–3000 s when all carbon in the bed has been converted, another inert period of 180 s was performed before reoxidation of the oxygen carriers. During the oxidation period, 5% oxygen in nitrogen was first fully reacting with the particles, clearly seen as no gas-phase O
2 exits from the reactor initially. At about 2500 s in the figure, the oxygen increases as the oxygen carriers become saturated with oxygen. At the same time as gas-phase O
2 was observed, a small amount of CO
2 was generated. This CO
2 originates from the combustion of unconverted char and char that may be trapped on the walls of the upper part of the reactor tube [
43]. In the figure, the concentration of SO
2 is multiplied by a factor of 10 to improve readability. Two things should be noted regarding the concentration of SO
2: (i) SO
2 does not decrease to zero when SO
2 was removed from the fluidizing gas during the inert period after the fuel cycle; and (ii) SO
2 never reaches the ingoing concentration since some sulfur is absorbed in the cooler and bed material.
Data Evaluation
For the solid fuel experiments, the total molar gas yield for each component from the gasification of the char was calculated with Equation (1) between time
t1 to time
t2. This was done for either a full cycle or for a part of a cycle. The total molar flow was calculated assuming ideal gas using Equation (2),
P—pressure (Pa),
R—gas constant,
T—temperature (K),
V—volume (m
3) and
xA—concentration. This was done with the assumption that the only outgoing gases of significant volume in the non-condensed gas were CO
2, CO, H
2, and CH
4. SO
2 was excluded due to the limited concentration and since SO
2 could not be measured at the same time as H
2.
The fraction of char converted,
Xc, is defined as the cumulative carbon released at a certain time divided by the total carbon emitted from the converted char during the reduction period. These are defined in Equations (3) and (4). The integral in Equation (4) is evaluated from the time of char insertion (
t1 =
tred, start) to the end of the reduction when the atmosphere in the reactor is changed to inert (
t2 =
tred, end).
The gasification rate (
rw) was calculated using Equation (5) where
was the mass-based rate of conversion of carbon. The gasification rate was then normalized using Equation (6). In this work, a mean value for the gasification rate was normally extracted from the gasification rate between a char conversion of 0.3 <
Xc < 0.7. The reason for this conversion span is that the gasification rate is more stable in this region and easier to compare to other experiments [
44].
In addition to the char conversion, the gas phase conversion is also very important. Two parameters have been used in this work to evaluate the gas conversion: (i) the conversion of carbon species to CO, and (ii) the H
2/CO molar ratio. The former is defined as Equation (7). Here the molar yield of each component is calculated using Equation (1), averaged over 0.3 <
Xc < 0.7.
Re-oxidation after the reduction cycle, correlated to the omega value at the end of the cycle, was calculated using Equation (8). A reference O
2 curve using inert sand in the same experimental conditions was used to determine the point of transition between inert and oxidizing conditions. The difference between the actual uptake using an oxygen carrier and the reference cycle with inert sand was then judged to be a result of O
2 uptake of the oxygen carrier.
Since there are both traces of soot and some remaining char, the final O2 consumed by the oxygen carrier is calculated by subtracting the molar CO2 generated during oxidation from the total O2 uptake during the same period.
The equilibrium constant
Keq for the WGS Reaction (R6) is defined as in Equation (9) when gases are at equilibrium. The temperature dependence of the equilibrium at atmospheric pressure is shown in the same equation [
45,
46]. The reaction quotient
Qi is defined similarly as the equilibrium, and calculated for the measured outgoing gases from the reactor at the time
i (see Equation (10)).
Qi is used to estimate how far the concentrations of the gases are from the equilibrium. If
Qi =
Keq, the gases are at equilibrium with respect to Reaction (R6).
2.2. Gaseous Fuel Experiments
Two different setups for gaseous fuel experiments were conducted for this study: (I) benzene experiments to evaluate how and if sulfur affects the conversion of the tar component benzene, and (II) the effects of SO2 on the conversion of syngas (CO+H2). Here, it is important to study possible interactions with sulfur with the bed material with lasting effects on the conversion of the fuel.
2.2.1. Effect of SO2 on Benzene Conversion
Benzene tests were executed with 5 g oxygen carrier (ilmenite or LD slag) diluted with 10 g silica sand at 850 °C. This dilution was necessary to avoid full conversion of the gaseous fuel species. The samples were activated as described in
Section 2.1. After activation, the same cyclic schedule was used, oxidation–inert–reduction–inert, with the same flow during inert and oxidation as described in
Section 2.1. For benzene tests, a flow of 500 mL/min steam and 300 mL/min N
2/benzene mixture was introduced during the reduction phase in the cycles. The N
2/benzene mixture was N
2 saturated with benzene at 6 °C, resulting in a benzene concentration of 1.46% in the ingoing flow. The sulfur content was regulated to obtain a total content of 0%, 0.2%, and 0.4% SO
2 in the fluidization gas according to
Table 4. Reducing gas was inserted for 60 s every cycle. Experiments were performed at 850 °C to be more comparable to previous results [
15,
47].
2.2.2. Effect of SO2 on CO Conversion and Structure
To investigate if and how SO
2 binds into or onto the LD slag, a sequence of separate reduction cycles was performed with subsequent analysis. The experiments were conducted with CO so the SO
2 effect on the reactivity towards CO could be observed at the same time. Here, 15 g of oxygen carrier was used, without any sand. After activation at 850 °C (described in
Section 2.1), the same cyclic oxidation–inert–reduction–inert was used also for the experiments, but at 870 °C instead. Oxidation using 1000 mL/min 5% O
2 in N
2 and inert for 180 s using 1000 mL/min N
2. First, reference experiments with reduction using 900 mL/min 25% CO and 25% H
2 in N
2 for 40 s were committed. After this, samples were reduced for 40 s with a flow of 25% CO, 25% H
2, and 1% SO
2 in N
2. This was done for six cycles at 870 °C. After the final reduction, the sample was oxidized using 5% O
2 and 1.5% SO
2 in N
2 instead of only diluted air, and this was done for over 90 min. This was to investigate how SO
2 was absorbed in the structure of the particles. After this loading of sulfur, the samples were again reduced, this time as the first reference experiments using a flow of 900 mL/min 25% CO and 25% H
2 in N
2 for 40 s. This was also done 6 times. A summary of these experiments can be seen in
Table 5.
2.2.3. Data Evaluation
Equation (1) was used to determine the cumulative molar flow of the outgoing gas species, and the conversion over the entire cycle was calculated by dividing the amount of outgoing gas species by the amount of ingoing. The molar flow was assumed to be unchanged for benzene and CO experiments. For benzene, the expected gas expansion is small compared to the total flow considering the low concentration of ingoing benzene.
2.3. Material Characterization
SEM-EDX (scanning electron microscopy equipped with energy dispersive X-ray spectroscopy) analysis was done with an FEI Quanta 200 FEG ESEM. Bed material was mounted in epoxy and polished, exposing a cross-section of the particles, thus making it possible to investigate the inside of the particles with SEM-EDX. The crystalline phases in the bed material were determined by powder X-ray diffraction (XRD). The XRD was a Brucker D8 ADVANCE equipped with a Cu Kα radiation source. BET surface area was measured using a TriStar 3000. The 0.5 g samples were degassed for 1 h at 90 °C, followed by 18 h at 250 °C. The surface area was measured using nitrogen and was cooled using liquid nitrogen.
4. Discussion
It is clear from these experiments that the possible reaction paths for sulfur are many and complex. In addition to gaseous species that are not measured, such as COS and SO3, some sulfur species are lost in the condensed water in the gas treatment system, and most likely elemental sulfur is deposited in the colder areas after the reactor. Additionally, the interaction with calcium in LD slag adds more complexity. Therefore, a total sulfur balance over the system is in this work not possible.
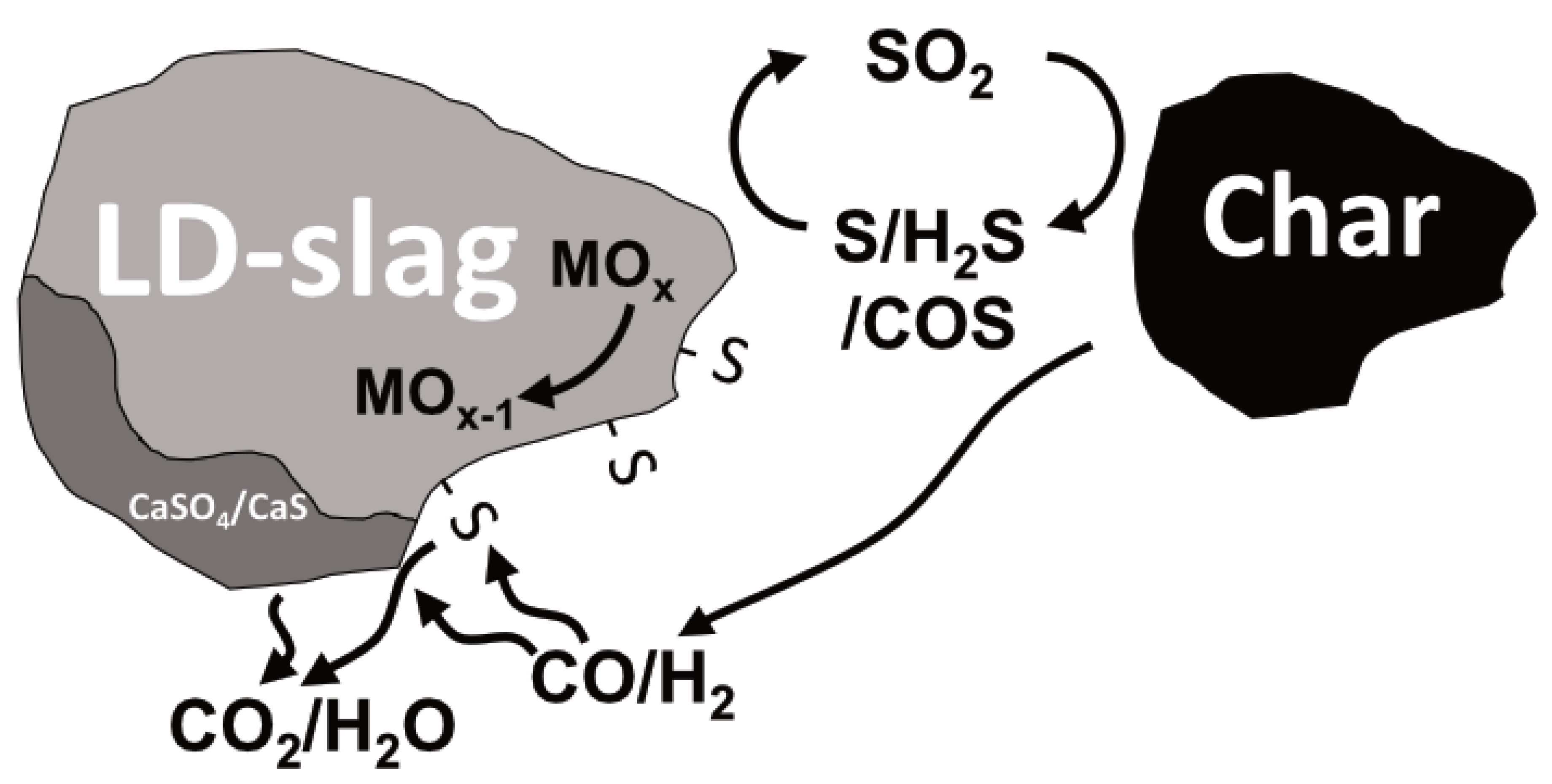
A summary of the reaction paths and effects of sulfur on LD slag can be seen in
Figure 17. There are three important reactions path that are emphasized in the figure, from the left: (i) formation of CaSO
4/CaS; (ii) surface-active sulfur increasing the reaction rate towards, e.g., CO; and (iii) increased gasification rate of char due to oxygen transfer via SO
2 from OC to char.
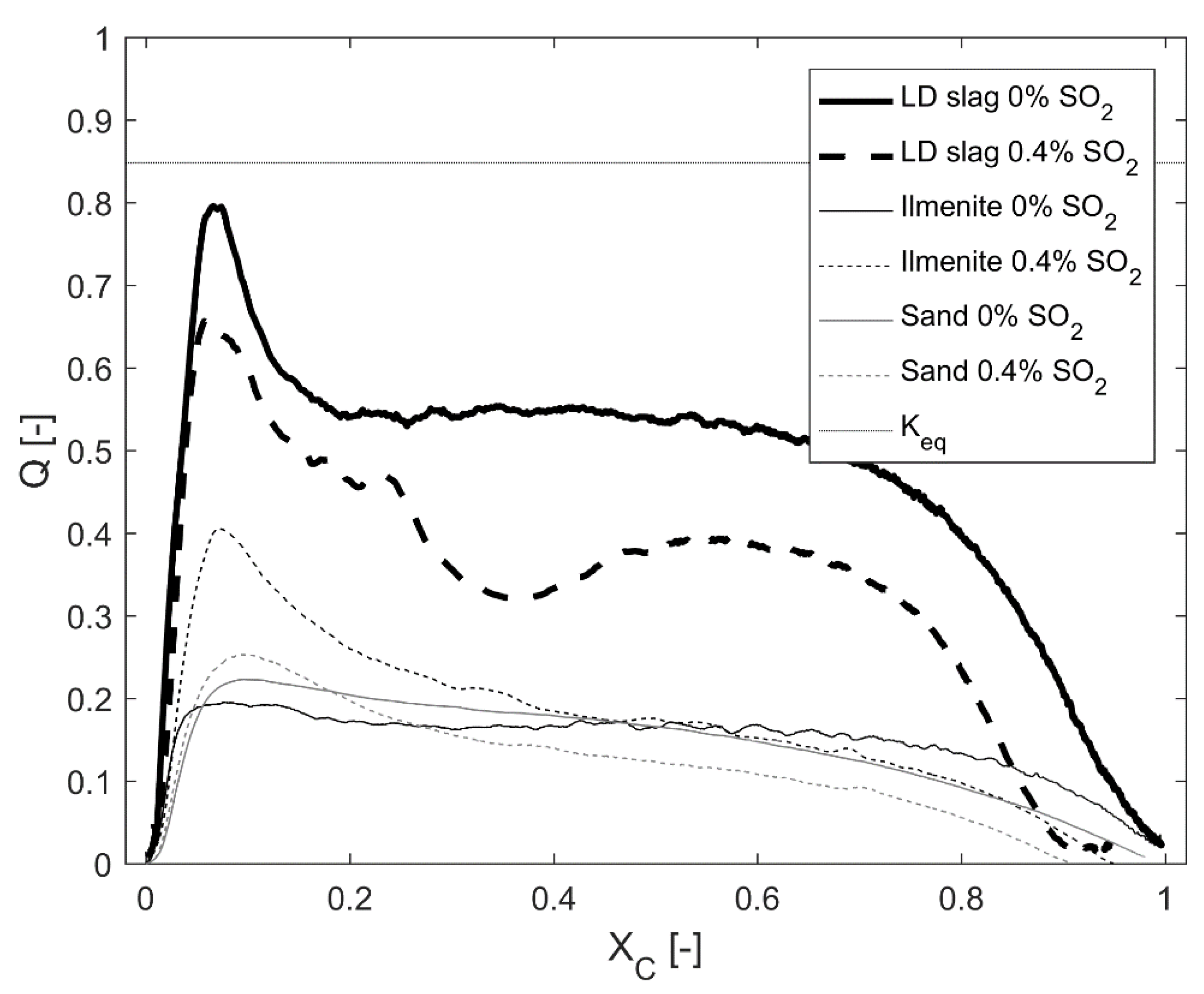
For LD slag, one of the significant interactions of CaO with SO
2 is the formation of CaS and CaSO
4. This was expected from the thermodynamic calculations presented in
Figure 2 and
Figure 3. CaO is a known catalyst and can explain why LD slag has a reaction quotient
Q closer to the equilibrium compared to the other tested materials in
Figure 11 [
35,
36,
49]. Forming CaS or CaSO
4, less CaO in the bed can catalyse the WGS reaction. As could be observed in
Figure 11, the sulfur content in the ingoing gas decreased the reaction quotient
Q for LD slag. The same effect of sulfur could not be observed for the other tested materials. However, this effect could only be detected at 870 °C. At 970 °C, no significant effect of sulfur regarding the reaction quotient
Q could be observed. This suggests that neither CaS nor CaSO
4 was formed at this partial pressure of SO
2, as compared with
Figure 2 to see the effect of temperature on equilibrium for the CaS/CaSO
4/CaO system.
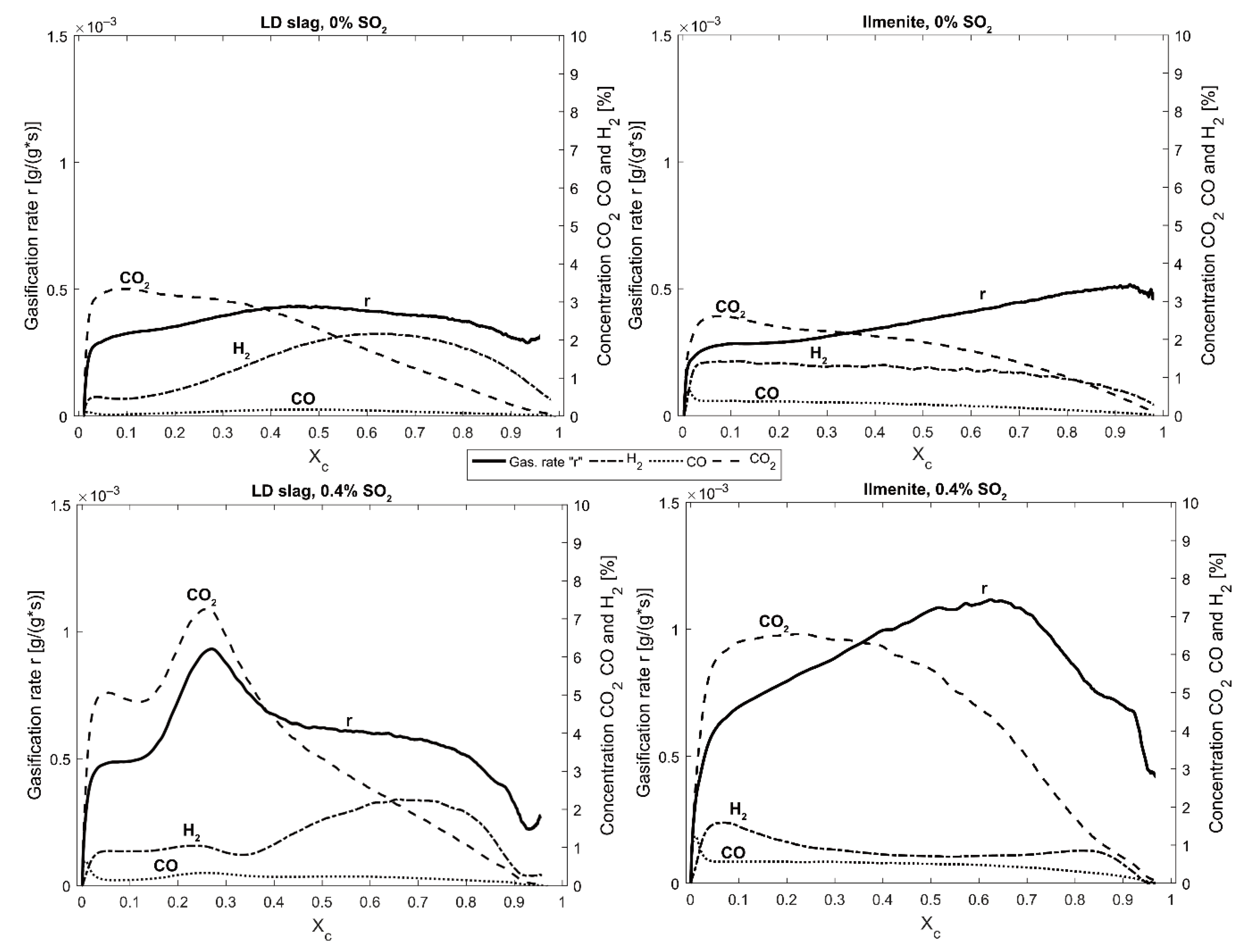
SO
2 and H
2S in the outgoing gas are highly affected by the bed material used. This can be observed in
Figure 13 and
Table 6. For both ilmenite and LD slag, the initial part of the char conversion (0 <
Xc < 0.1) was characterized by high amounts of mostly H
2S that were detected with Dräger tubes. Almost no SO
2 was present in this initial phase. This is most likely due to the high amount of H
2 that was released with the volatiles from the char. Likely, H
2 reacts with SO
2, forming H
2S that is more thermodynamic stable with high amounts of H
2. It is after this initial char conversion (
Xc > 0.1) where LD slag and ilmenite perform differently. For ilmenite, the outgoing concentration of H
2 is stable, as well as the SO
2, until the solid fuel is fully converted. However, when LD slag is reduced, more H
2 is formed due to water splitting [
36]. This was observed as a simultaneous increase in H
2 concentration in
Figure 8, and as a decrease in SO
2 in
Figure 13. Thermodynamic calculations using the concentrations in these figures also suggest the formation of H
2S. The presence of H
2S and SO
2 in the same gas mixture enables elemental sulfur to be formed according to the Claus reaction (Reaction (R11)). Elemental sulfur can stick to the walls of the reactor cooler areas. When oxidation of the oxygen carrier starts, all oxygen is absorbed into the bed. When oxygen breaks through, it might react with the sulfur that is stuck to the walls. This could be the reason for the SO
2 peak that occurs in
Figure 14 just before the oxygen breakthrough.
Sulfur was observed to absorb into the LD slag. Loading a large amount of sulfur over a long period of time under oxidative conditions resulted in a clear decrease in surface area and reactivity (see
Table 5 and
Figure 14). Parts of the sulfur that were loaded into the LD slag particle were then observed to be released during the following reduction cycles without SO
2 in the ingoing gas. This could mean that CaS forms during the reduction and then reacts with the CaSO
4, according to Reaction (R10). This reaction path is also strengthened from the observation of the SO
2 concentration profile over the cycle, seen in
Figure 15. SO
2 concentration in the outgoing gases peaks during the reduction and starts to decrease when the inert phase starts. However, the SO
2 concentration drops first to zero, then oxidation starts and all remaining CaS is oxidized to CaSO
4. Even after seven reduction cycles, the SO
2 release during reduction was significant, and sulfur-containing phases were detected in the cross-section of the particles using SEM-EDX. Only CaSO
4, and no CaS, could be detected in these samples using XRD. If CaS was formed, the amounts were very small and could be expected to react fast with CaSO
4 that is likely closely located in the same calcium-containing phases in the LD slag. Further, the SO
2 peak that was observed directly when oxygen was introduced indicates that CaS was formed and partly oxidized to SO
2, according to Reaction (R9). This occurred in gaseous fuel experiments after and before the loading of sulfur in the sample. Additionally, in gasification experiments, sulfur interacted accordingly with sulfur release during inert phases and a small release when oxygen was introduced (see, e.g.,
Figure 5). In similar experiments with ilmenite, this behavior was not observed. Interpolating this behavior to an industrial scale indicates that SO
2 could be released in loop seals and in the air reactor in a CLC or CLG plant using LD slag as an oxygen carrier. However, when using biofuel as fuel, calcium is known to be deposited on ash layers of ilmenite and inside the structure as well [
11,
50]. For ilmenite, these ash layers have been observed to be important for the sulfur interaction [
28], since calcium is identified as the main component for this interaction path that might transport sulfur to the loop seal or air reactor. Further studies including calcium from the fuel are required to estimate the importance of the ingoing calcium in LD slag compared to ilmenite in a full-scale application.
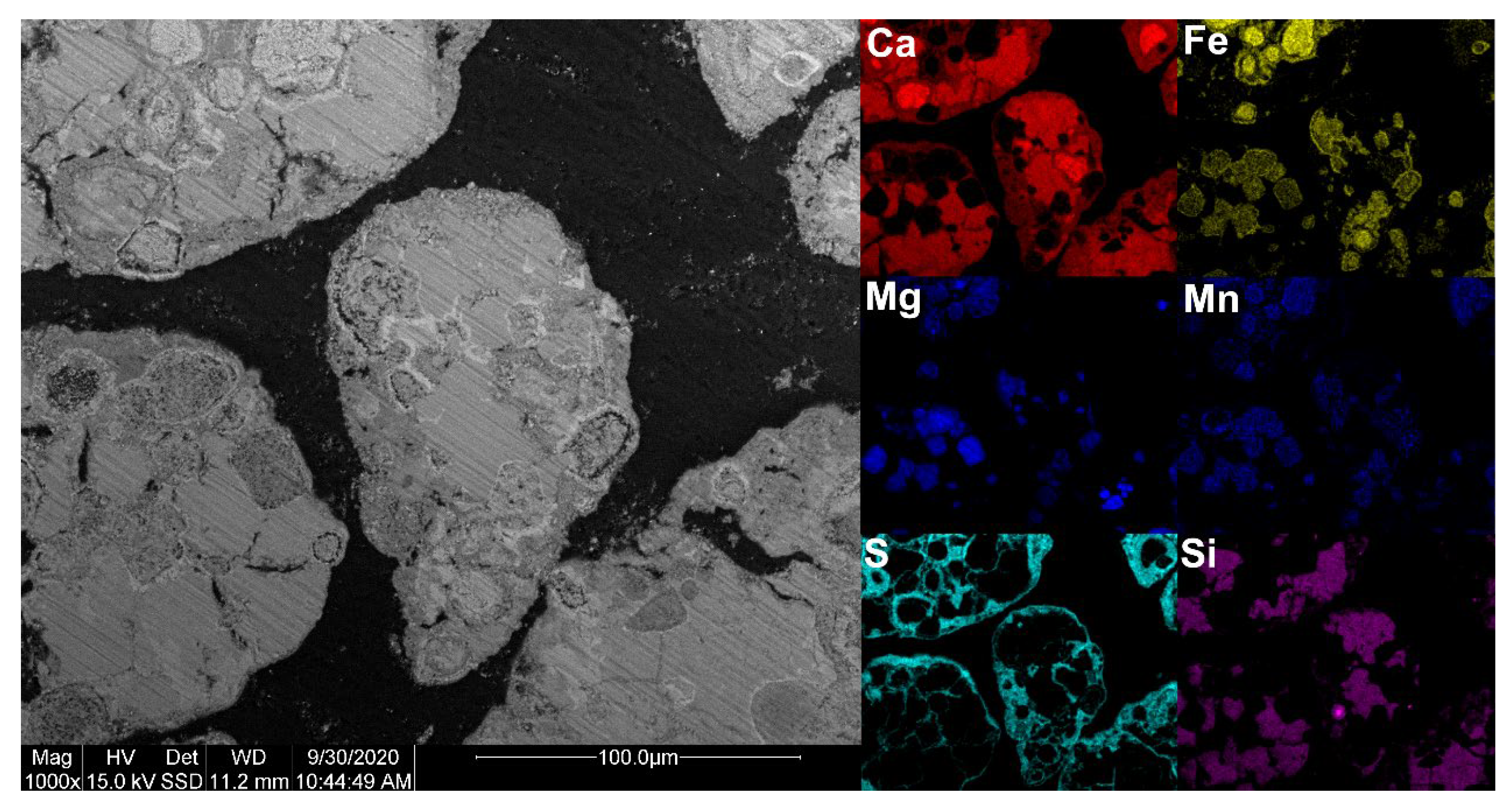
In comparison to previous studies where LD slag has been used in a boiler [
15], it can be observed that the sulfur from the fuel was located on the surface of the particles. In this study, it was observed that sulfur was present throughout the entire sample (see
Figure 16). This could be due to the lack of available calcium from the fuel ash that can form on the surface, or because the amount of sulfur introduced to the sample was high, resulting in saturation on the surface. From the SEM-EDX analysis, no migration limitation/border of sulfur could be observed, indicating an inward-going migration of sulfur in the cross-section. However, since LD slag is so heterogeneous, a clear migration trend is hard to detect. From previous studies, it has been found that sulfur penetration can increase significantly under alternating oxidizing and reducing conditions [
21].
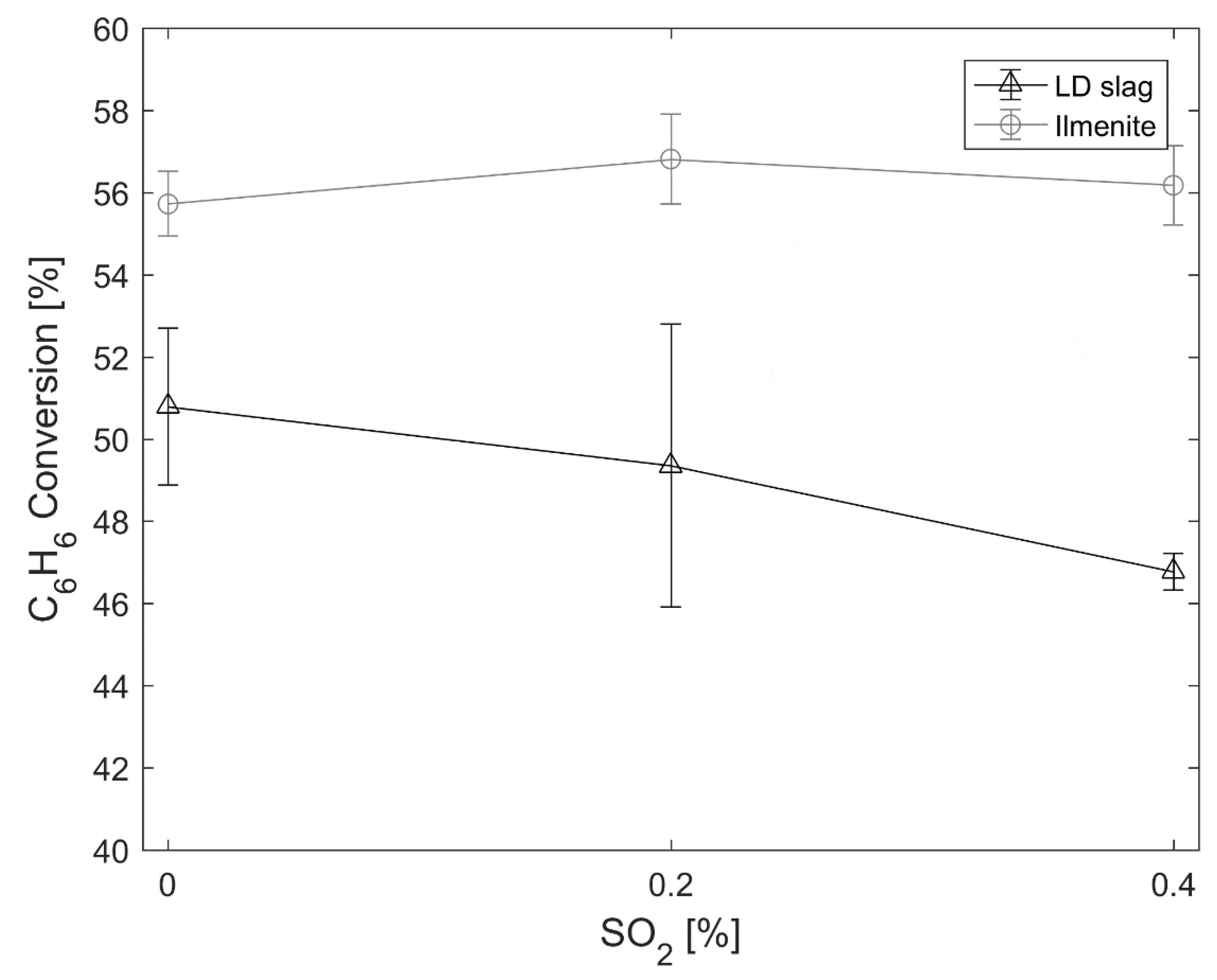
The effect of sulfur towards reactions of larger hydrocarbons was tested using benzene. For the conversion of benzene, the investigated concentrations of SO
2 have minimal or no effect on the conversion. It is important to note that the particles were oxidized prior to the benzene experiments. Therefore, the extra oxygen provided by the small amount of SO
2 should have minimal effect. This suggests that the oxidation reaction towards benzene is unaffected by sulfur or the reactions with LD slag forming CaS or CaSO
4. However, the minor decrease in reactivity that can be seen comparing 0% to 0.4% SO
2 could be due to the decreased surface area of the particles. This means that the same mechanism observed to increase the conversion of methane in the presence of sulfur observed in previous studies [
23] might not be applicable for benzene. Since no change is observed, SO
2 does not seem to affect the rate-limiting step of activating benzene, i.e., destabilizing the benzene ring [
51].
The increased reactivity of LD slag with SO
2 could be partly be a result of the oxygen transport from CaSO
4 forming CaS; i.e., a new oxygen-carrying phase in LD slag is formed [
52]. However, since ilmenite does not contain Ca, this is not contributing to the changed reactivity of ilmenite; rather, this occurs by the direct effect of oxygen transfer from SO
2 reduced to H
2S. However, the direct addition of SO
2 as a source of oxygen can only have a limited effect due to the low concentrations. This is obvious from the experiments with sand where only H
2S could be detected during the main part of gasification and the oxygen provided by SO
2 is consumed during the gasification with limited effect on the overall gasification rate. This indicates that sulfur does not interact with the char directly to increase the reaction rate, in the same manner as sulfur does not interact with the destabilization of the benzene ring. Therefore, another mechanism is expected to be the origin of the observed reaction rate for both the investigated oxygen carriers.
Hydrogen inhibition of char during gasification is a well-known effect that decreases the gasification rate [
53,
54,
55]. This has also been observed to be important for LD slag, since H
2 is generated from water-splitting of steam when LD slag is reduced and this affects the gasification rate [
36]. One hypothesis of the increased gasification rate observed in this study could be that SO
2 reacts with H
2, forming H
2S, and that this decreases the H
2 concentration, and ultimately, the hydrogen inhibition. It is unlikely, however, that this effect is dominant. In the sand samples, there is a small effect on the H
2 concentration and the H
2/CO ratio (see
Figure 10 and
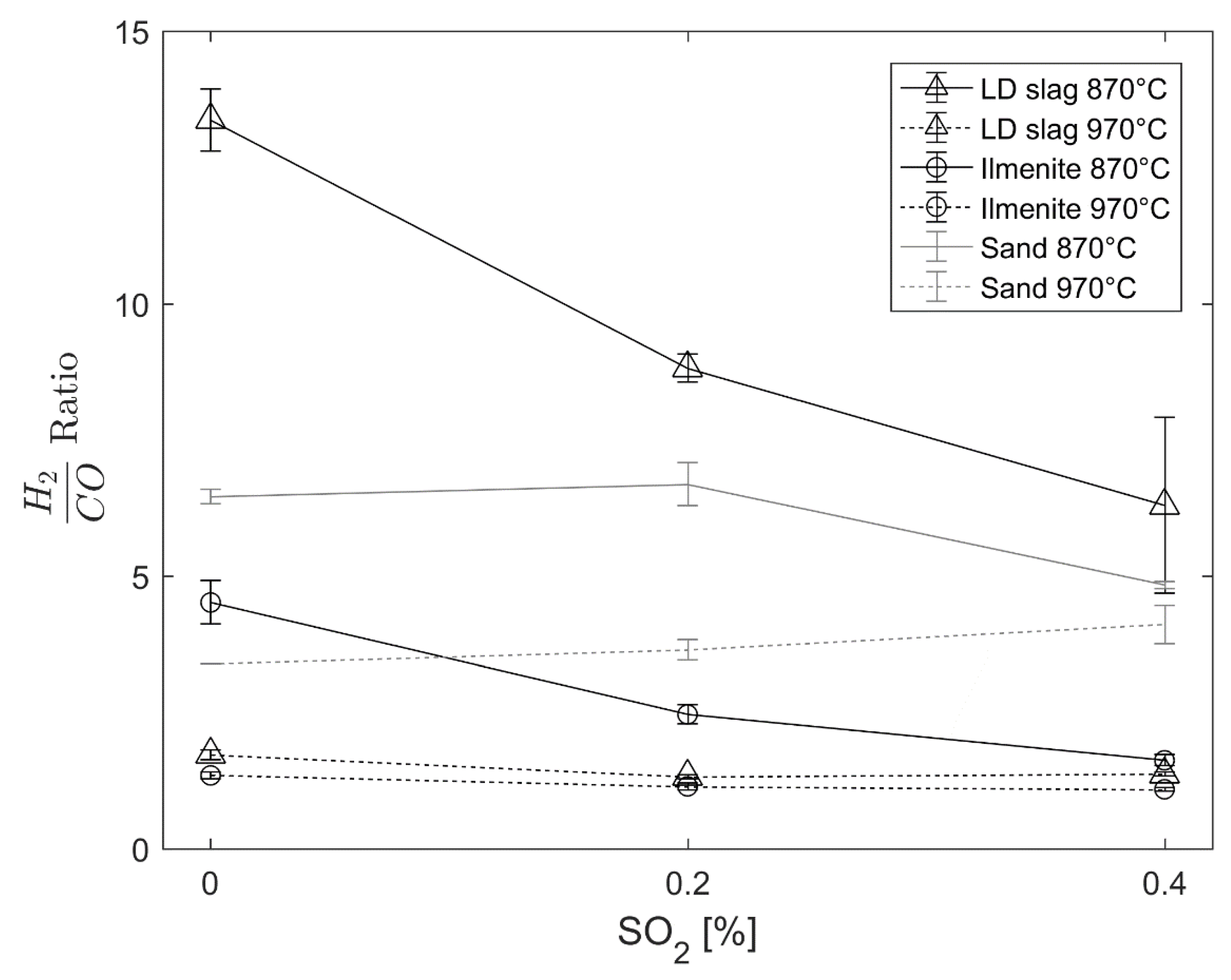
Supplementary Materials figures). However, the effect of the gasification rate is negligible for sand. Moreover, for LD slag and ilmenite, there is an effect on the H
2/CO ratio at 870 °C but not at 970 °C; still, the gasification rate is increased in the presence of SO
2 at both temperatures. Further, the increased CO conversion observed in this study for LD slag and the increased methane conversion for ilmenite with sulfur [
23] could not be related to hydrogen inhibition since no char is present.
More likely is that sulfur interacts as an intermediate to transport oxygen from the oxygen carrier to the char. SO
2 reacts with char forming H
2S, COS, or S, that then, in turn, is oxidized to SO
2 by the oxygen carrier. Thermodynamic calculations regarding the conversion of char from SO
2 compared to H
2S can be seen in the
Supplementary Materials. Similar reaction schemas have been suggested previously for the observed increased reaction rate towards petroleum coke of iron-based oxygen carriers [
43]. This could also explain why the experiments with sand did not show any significant gasification rate change. In sand, the oxygen from SO
2 can only be transferred once to the char and then is not oxidized again. This would explain the mixture of H
2S and SO
2 in the outgoing gases during the char conversion for LD slag and ilmenite (
Figure 13 and
Table 6) compared to sand (see
Supplementary Materials). The oxygen transport also increases with the amount of SO
2 added, which the increased gasification rate seen in
Figure 7 at both 870 °C and 970 °C indicates. Furthermore, the absorbed sulfur that was released in the following experiments without SO
2 addition also increased the gasification rate. This also suggests that it is the gas phase sulfur transporting the oxygen.
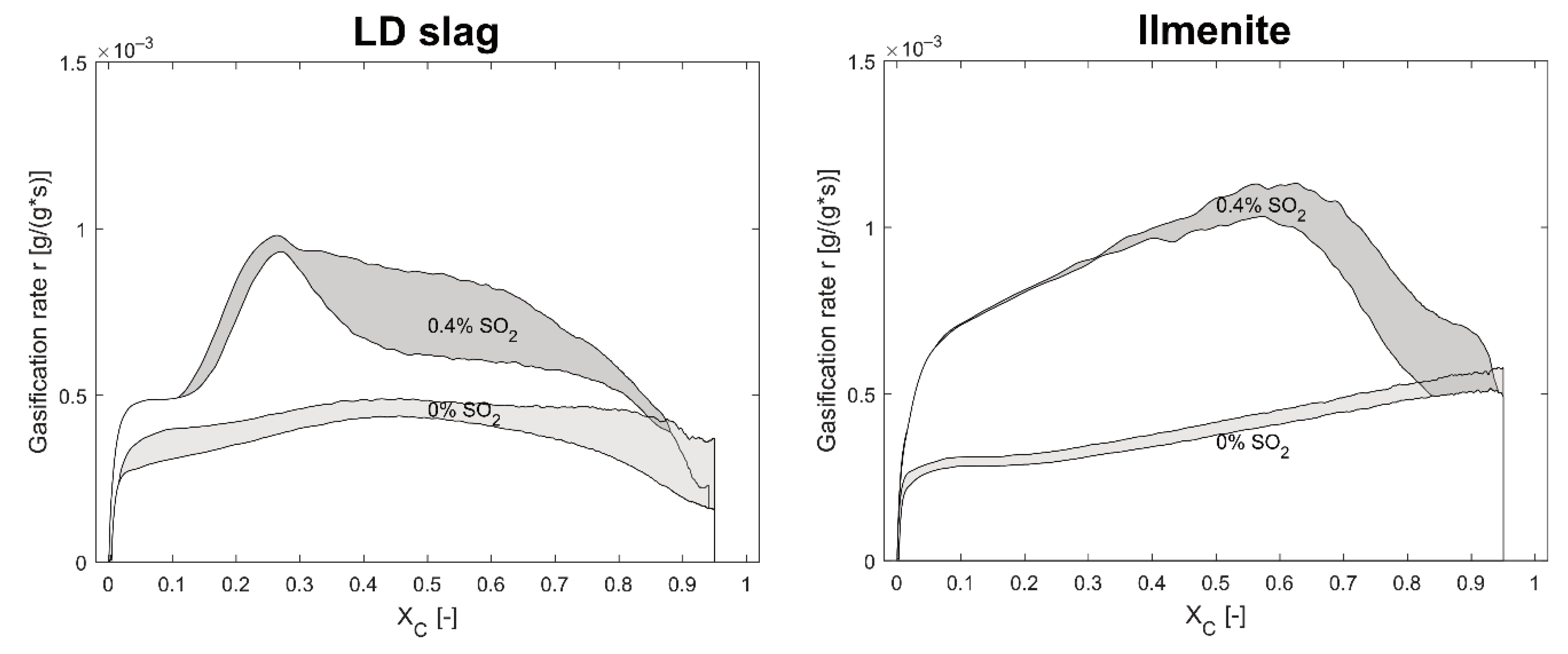
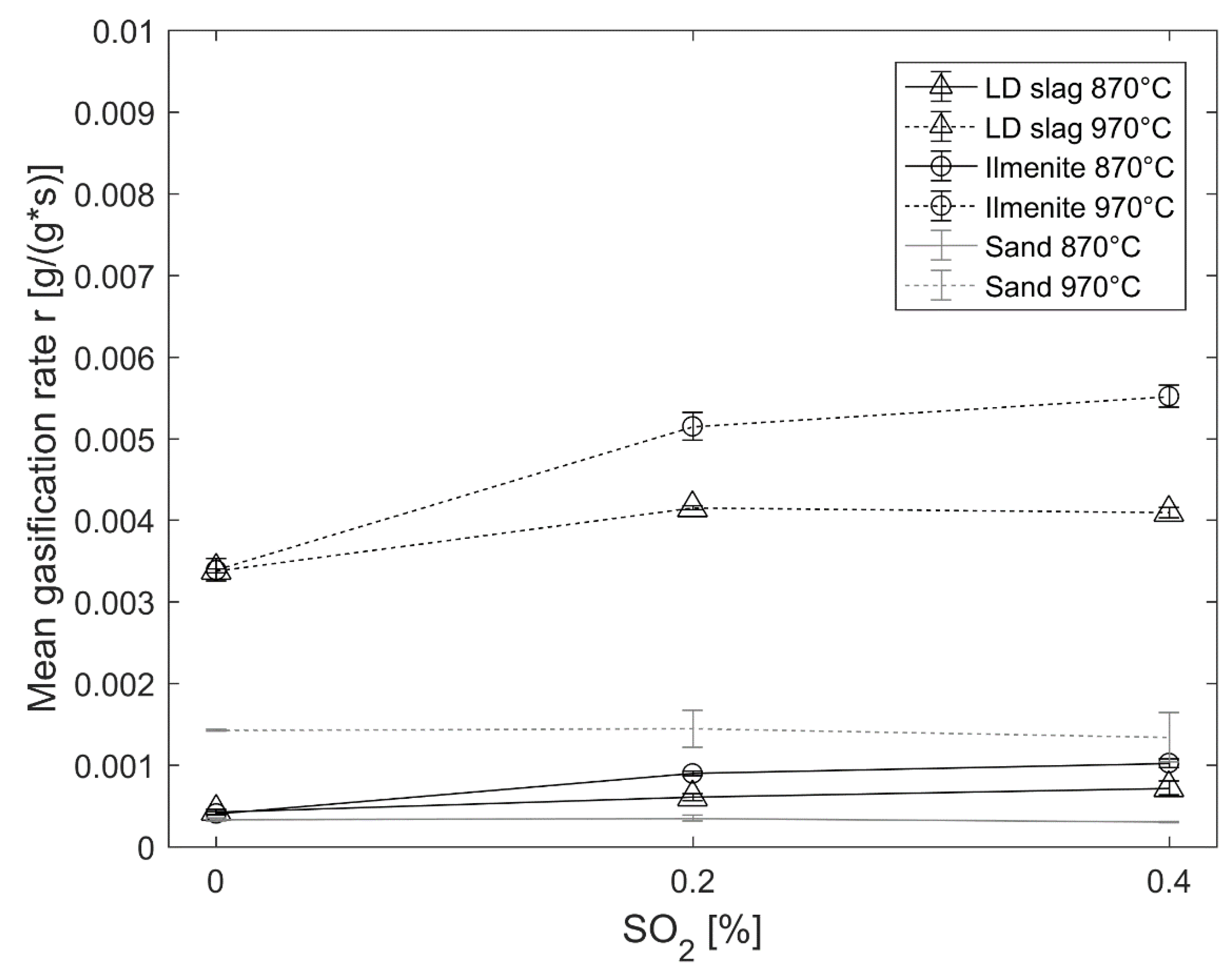
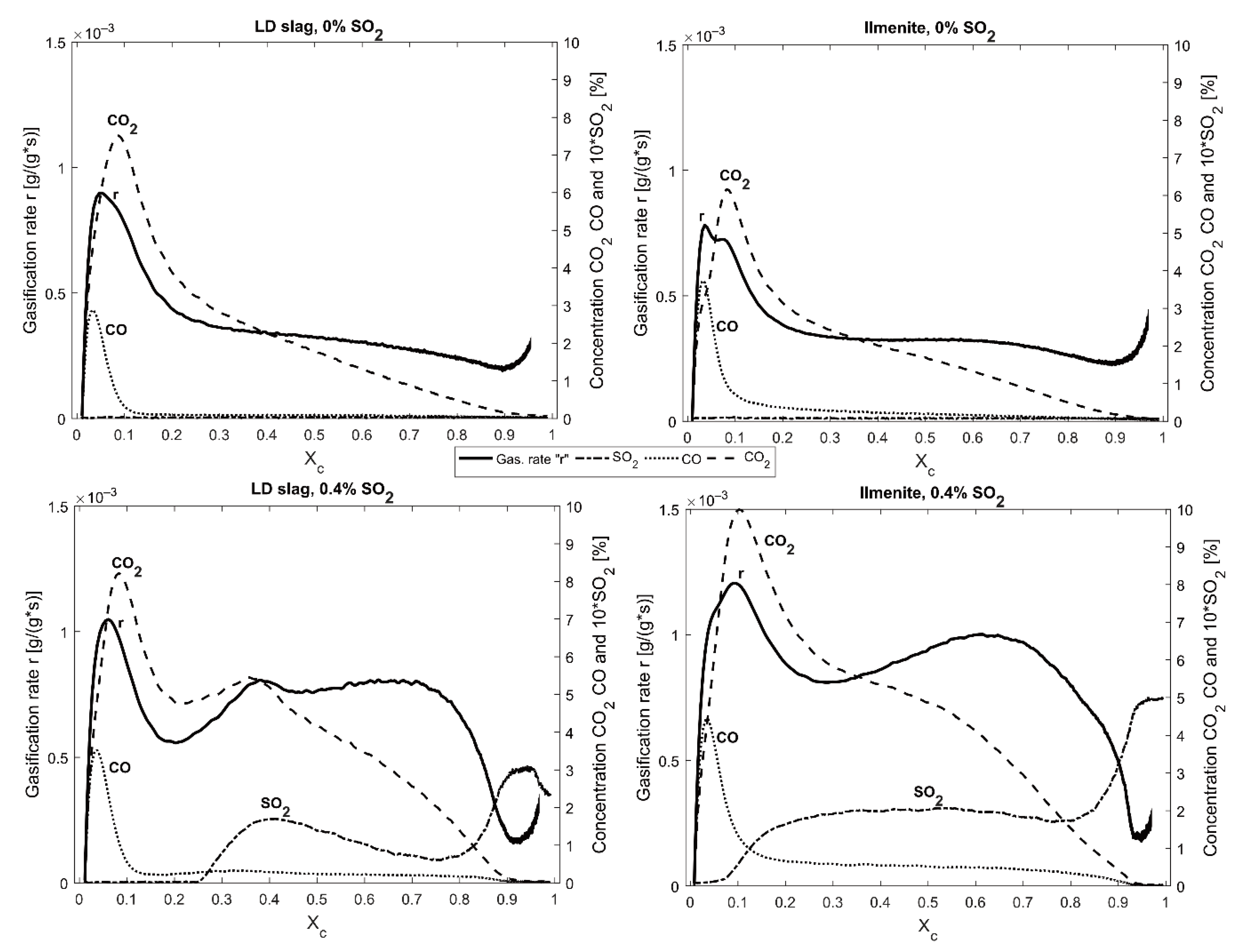
The characteristic peak at
Xc ≈ 0.25 with the increased gasification rate during the solid fuel experiments with LD slag is seen in
Figure 6,
Figure 8 and
Figure 13. This type of peak was not observed at 970 °C, suggesting that this correlated to the sulfur interaction with calcium in the LD slag. In
Figure 8 and
Figure 13, it can be observed that the peak is correlated to low H
2 and high SO
2. High SO
2 could be correlated to a phase change that increases the amount of sulfur in the gas phase. Compared with the phase diagram in
Figure 2, at a lower partial pressure of SO
2, changing the partial pressure of H
2 converts CaS to CaSO
4 via the formation of CaO. Before the peak, it can be observed in
Table 6 that the first bag sample for LD slag contained much less H
2S + SO
2 than ilmenite. This could suggest that sulfur is first absorbed into the structure, and then at one point partly released when the LD slag is reduced to a specific degree. This release causes an increased gasification rate and an observed high SO
2 concentration.
Another hypothesis for the increased reaction rate towards CO and char could be that sulfur is surface-active. It is known that sulfur affects catalysts both as poison and promotors [
56]. In addition, reactions including iron and iron oxide are known to be promoted by low amounts of sulfur [
57,
58]. Since the effect of sulfur seems to be lasting (at least for a few cycles) both for ilmenite [
23] and LD slag, it could be suspected that surface reactions are promoted by low amounts of sulfur. Small amounts of sulfur have also been observed to be absorbed on the surface of iron oxygen carriers [
59] and in other studies to increase reactivity towards coal pyrolysis gas [
38]. This could explain the increased reactivity towards gaseous species, and also suggest that the catalytic cracking of benzene is not active with the same reaction mechanism affected by sulfur. However, the solid-solid reaction between oxygen carrier and char has been expected to be low in fluidized beds [
9,
60]. Nevertheless, studies of iron oxygen carriers in non-fluidized beds have indicated that the solid-solid interaction might be of significance due to the low reactivity towards reducing gasses for iron compared to, e.g., Ni [
61,
62,
63]. Sulfur attached to the surface iron of LD slag and ilmenite might therefore increase the gasification rate of the char. This could also explain the lack of effect that sulfur has on sand, since sand cannot transport oxygen via a solid-solid reaction with the char. Moreover, since ilmenite has more iron at the surface after activation [
12], more sulfur can interact with the surface iron. This could be contributing to the relatively higher gasification rate increase observed for ilmenite compared to LD slag in the presence of sulfur. In the experiments for this study, the fluidization velocity is relatively low, roughly four times the minimum fluidization velocity at 870 °C. Even so, this low fluidization velocity should make the solid-solid interactions less pronounced.