Abstract
Biodiesel is a clean-burning, alternative diesel replacement fuel that may be used in existing diesel engines in either pure or blended form without or with modest modifications. In some countries, biodiesel is recommended as a potential alternative to diesel fuel since it is a renewable energy source that is environmentally benign. The main problems with the widespread commercialization of biodiesel are its high viscosity and its limited feedstock, due to which complete replacement of diesel fuel is not possible and the use of blends of biodiesel and petrodiesel are being used increasingly worldwide. The paper presents a behavioral study of the petro-based diesel, and their blend (B20, B40, B60, B80) with Pongamia and Jatropha biodiesel. The results reveal a considerable viscosity lowering due to the dilution effect of increasing diesel concentration in both the cases. In addition, improvements in oxidation stability in both cases have also been observed. The research shows that as the biodiesel concentration increases, the stability of blends decreases. In blending Jatropha curcus methyl ester with EURO-III and EURO-IV HSD, the ester’s viscosity decreased as the diesel level in the blends increased, and blends comprised up to 80 percent biodiesel remained below the viscosity limit. Pongamia pinnata blends with both fuels above 60% diesel; however, exceeds the stipulated viscosity limit of 4.50 cSt at 40 °C.
1. Introduction
Biofuels made from vegetable oil are a worthy alternative to fossil fuels since they are recyclable, biodegradable, easy to manufacture, nontoxic, and benzene-free [1,2,3] Direct application of raw/virgin vegetable oils in the existing diesel engines is, at best, possible in light service diesel engines such as stationary applications [4]. However, for severe applications (high-speed engines in road transport), the viscosity of vegetable oils must be lowered, which can be accomplished using a variety of techniques such as cracking/pyrolysis, micro emulsification, oil blending, and transesterification [5]. Among the mentioned methods, transesterification [6] is commonly used to modify the properties of the virgin oils to match petro-diesel characteristics, [7] which otherwise create problems in operation [8].
Biodiesel is miscible with conventional diesel at all blend levels since it is defined as vegetable oils’ alkyl ester (usually methyl esters). These alkyl esters are environmentally benign liquid fuels with combustion qualities similar to petro-diesel. They biodegrade significantly more quickly and easily [9]. The promotion of esters as an alternative fuel is driven by India’s agriculture-based economy, depleting petroleum reserves, and rising environmental concerns. Implementing biodiesel in India will benefit our agricultural and rural economies, reduce our reliance on imported crude oil, and improve air pollution control.
Regarding the use of biodiesel in CI engines, the problems are its poor cold flow properties, poor stability, volatility, heat of combustion, and ignition temperature. All of the concerns listed are being researched globally to find a solution. Mixing/blending [10], phase shifting, transesterification, and other physical and chemical processes are employed to improve the low-temperature performance of biodiesel [11]. The greater viscosity of biodiesel fuels is the primary cause of substantial downsides. As a result, improving viscosity is a major difficulty when using biodiesel in CI engines. According to ASTM guidelines, blending diesel with biodiesel is the best way to increase biodiesel quality. CI engines also do not require modifications for biodiesel blends of up to 30% in diesel fuel. However, oxidation stability is one of the most significant properties in neat methyl esters and their blends, which must be maintained within strict ASTM guidelines. The oxidation stability limit for mixes was set at 20 h by EN 14214 and prEN16091, while for biodiesel, it was set at 8 h by ASTM-D 7545-09. According to the literature, oxidation stability is connected to the overall number and location of allylic and bis-allylic carbons proximal to the double bond rather than the total amount of double bonds [12]. Antioxidants can be used to control all of the concerns listed above. Their use will slow down oxidation processes and, to some extent, increase fuel stability [13]. Antioxidants improve fuel stability by preventing the reaction between oxygen and fatty acids, slowing down the oxidation of biodiesel [14]. The stability of diesel biodiesel mixes has been studied in several papers. Sharafutdinov I et al. [15] found that adding commercially available FAMEs to petroleum diesel lowers the cold filter plugging threshold (100 percent rape seed oil; 70 percent soya and 30 percent palm oil; 50 percent rape seed oil and 50 percent sunflower oil) (CFPP). Karavalakis [16] have studied the impact of using different diesel (variable sulfur content) on the oxidation stability of biodiesel blends. They observed that synthetic antioxidants could improve the oxidative stability of various types of biodiesels and that binary antioxidant formulations (TBHQ and PY) have a synergistic effect on biodiesel oxidative stability. The effect of adding antioxidants was studied by Lamba et al. [17] BHT (butylatedhydroxy toluene), BHA (butylated hydroxy anisole), DPA (diphenylamine), and TBHQ (tert-butylhydroxyquinone) on Jatropha and EURO-III HSD blends. They concluded that adding an antioxidant to diesel fuel blends improved the majority of the key fuel attributes.
Yang et al. [18] investigated the effects of several antioxidants and found that pyrogallol (PY) was the best at enhancing IP at concentrations less than 3000 ppm, whereas TBHQ was the best at concentrations greater than 3000 ppm, followed by propyl gallate PG, BHA, BHT, and -tocopherol. Christensen et al. [19] reported the long-term stability of biodiesel and its blends, concluding that treating aged biodiesel was also efficient at restoring stability; however, antioxidant efficiency was reduced compared to fresh biodiesel.
Serrano et al. [20] analyzed the oxidation stability of several biodiesel fuels using three commercial synthetic and one natural antioxidant. Antioxidants can help with oxidative stability, and their effectiveness is proportional to their concentration. Using citric acid (0.1 M) rather than distilled water in the purification step enhanced the oxidative stability of biodiesel.
Karavalakis et al. [21] experimented with the effect of several phenolic antioxidants on biodiesel blends. They found that BHT and BHA had the lowest effectiveness in neat biodiesel but had a higher stabilizing potential in biodiesel blends. The additives PG and PA were found to be effective in both pure biodiesel and blends with HSD.
Joshi et al. [22] investigated the effects of BHA, BHT, PL, PG, TBHQ, and DPA additives on Jatropha biodiesel-conventional diesel blends sold at retail and discovered that the antioxidants TBHQ, PG, and PL were the most effective and had greater blend stabilizing potential among the antioxidants studied. Rawat et al. [23] have studied the effects of different antioxidants on Pongamia biodiesel blends, such as BHA, BHT, TBHQ, PrG, and PY antioxidants. The density, oxidation stability, and kinematic viscosity of blends all improved significantly. PY, PrG, and BHA were shown to be the most effective antioxidants, with increased stabilizing capability when used in diesel/biodiesel mixes.
On biodiesel blends of Jatropha and Pongamia with conventional diesel, Rawat et al. [24] investigated the synergy of antioxidants utilizing 500 ppm, 600 ppm, and 700 ppm of pyrogallol: butylated hydroxyanisole (PY:BHA), pyrogallol: propyl gallate (PY: PrG), and pyrogallol: tert-butyl hydroquinone (PY:TBHQ). The binary systems of PY: PrG and PY: TBHQ, in a 1:3 weight ratio, demonstrated the highest antioxidant synergy, whereas the binary mixture of PY:BHA resulted in full antagonism. The binary system’s efficiency on oxidation stability was determined to be 1:3/3:1 > 1:2/2:1 > 1:1 > 1:9/9:1. The study also discovered that higher binary mixture mixes resulted in negative synergy.
Using the Petrotest PetrOxymeter, Lamba et al. [25] studied Jatropha methyl ester (JOME), Pongamia methyl ester (KOME), and their 5%, 10%, 20%, and 40% blends with low sulfur EURO-IV high-speed diesel for storage stability with additives BHA, TBHQ, PrG, BHT, and PY. For JOME and its EURO-IV blends, PY is the most effective antioxidant, while for KOME and its EURO-IV HSD mixes, PrG is the most effective antioxidant. The optimal concentration for both antioxidants was found to be 500 ppm.
Lamba et al. [26] used several antioxidants to investigate the oxidation behavior of Jatropha biodiesel with EURO IV HSD. TBHQ was determined to be the most potent antioxidant among all antioxidants tested, with plain methyl esters and lesser blends of JB-5 and JB-10.
Karavalakis et al. [27] explored the effects of several synthetic phenolic antioxidant additions on the oxidation stability of many neat biodiesels, focusing on diesel/biodiesel blend stability. BHT and BHA had the lowest efficiency in neat methyl esters of the antioxidants tested, but their application in biodiesel blends had a higher stabilizing potential. It was also discovered that additives that promote biodiesel stability might function as pro-oxidants in biodiesel blends.
The influence of nanoparticles and antioxidants combined with biodiesel on diesel performance and characteristics was investigated by Reddy K.N.S [28]. They found that using biodiesel blends with additives reduces carbon monoxide, unburned hydrocarbon, and NOx emissions while also improving brake-specific fuel consumption and thermal efficiency.
According to Krishna Kumar S et al. [29], adding graphene to biodiesel–diesel blends improves performance and emissions while increasing nitrogen oxide emissions. However, adding antioxidants reduces nitrogen oxide emissions.
Atabani E. A. et al. [30] studied the B5 blend of Manketti (Ricinodendron rautonemii) methyl ester (MME) with diesel and concluded that the blends resulted in significant improvements in the kinematic viscosity, calorific value and density of MME.
Because the availability of significant amounts of biodiesel as a full replacement for high-speed diesel is still a long way off, the first step in this direction is to replace it partially with biodiesel, which is now available nearly everywhere. In light of this, experiments are being conducted at various locations/laboratories across India to compare the performance and emissions of various blends (biodiesel and petrodiesel) to diesel alone. [31].
According to the Indian government’s auto fuel policy [32], HSD (high-speed diesel) corresponding to EURO-III specification is already implemented from early 2009 onward in 13–15 major cities of the country. While EUROpe has already moved to EURO-V since late 2008, India also introduced EURO-IV in 2010. As the availability of large volumes of biodiesel as a full substitute for high-speed diesel is still a very long-term possibility, its partial replacement with biodiesel practically all over the world is the first step in this direction. Keeping this in view and looking at the pace of development of biodiesel production in India, the paper present a study of these petro-based diesels, corresponding to EURO-III and EURO-IV specifications and their blend (B20, B40, B60, B80) characteristics with the two methyl esters produced from both Jatropha curcus and Pongamia pinnata.
2. Selection and Characterization of Feedstock
Developed nations such as the USA and Western EUROpe have primarily based their biodiesel production on edible vegetable oils such as soybean microalgae biomass [33] and waste cooking oil [34] and sunflower because they are available in large quantities. Given the situation in our country, the previous condition is no longer applicable due to the widespread need for edible oils for food preparation and their high per-liter cost.
Fortunately, India is rich in non-edible oil, yielding plants that grow on waste/marginal lands. A scan of such plants by various agencies in our country, such as Alternate Energy Resources, Central Pollution Control Board, National oil seeds and vegetable oil development (NOVOD) board India, the Associated Chambers of Commerce and Industry in India, and many Indian Agricultural Universities has led to a short listing of the following non-edible oil sources (Table 1) in India which could possibly be considered for non-edible oil sources for conversion to biodiesel [35].

Table 1.
Non-edible oil sources in India.
The composition and qualities of the oil feedstock are generally linked to biodiesel attributes [36]. The oil from all the above plants has been studied in detail in various labs/universities to arrive at a definite process scheme of conversion into their respective esters/biodiesel.
Based on the study of botanical and chemical features of the oils from the above two plants, the order of priority is Jatropha curcus and Pongamia pinnata for further exploitation and extensive studies. Jatropha curcus, as compared to Pongamia pinnata plant matures fast, and yields a higher quantity of seeds per hectare with ease of de-shelling and higher oil yield. The oil yield per hectare in the case of Jatropha curcus is 1892 L, while in the case of Pongamia pinnata it is 3600–4800 L [37]. Of this oil content, Indian mills, on average, extract around 25–30% of oil for commercial sales.
Triglycerides/fatty acids are the main constituents of both oils. Table 2 gives the detailed composition percentage. While Jatropha curcus contains four major fatty acids with linoleic (C18:2), oleic (C18:1), stearic (C18:0), and palmitic (C16:0) acids as the major constituents, the spread in the case of Pongamia pinnata is much large (nine fatty acids) with oleic (C18:1) acid being maximum 51.5%. Their unsaturated-to-saturated ratios are 3.42 and 2.52, respectively.

Table 2.
Composition (weight %) of fatty acids in raw/virgin Jatropha and Pongamia oils [38].
A comparison of raw/virgin oil properties of both oils with typical diesel properties (Table 3) shows that:

Table 3.
Characteristic features of Jatropha and Pongamia raw oil and average property of diesel.
- Both oils are more viscous (have high viscosity), have high density, and are practically free from sulfur.
- The two oils have poor flow characteristics, i.e., they solidify at much higher temperatures than petro-based diesel.
- Pongamia pinnata is rich in free fatty acid content, up to 7% max, compared to Jatropha curcus, which has an average value of 1.3% FFA. This FFA concentration directly affects their further processing into esters.
- Both oils have higher cetane index values indicating these to be of better combustion characteristics. The calorific values are close, well within a reasonable range.
The above comparative characteristics indicate that the two virgin/raw oils can be utilized as a diesel fuel alternative in light service CI engines, i.e., unaltered constant speed direct injection diesel engines often used in stationary applications.
For application in high-speed diesel engines, the two oils need modifications to bring their physicochemical parameters close to average high-speed diesel, which is achieved by their conversion to methyl/ethyl esters.
The physicochemical characteristics of EURO-III and EURO-IV HSD are also presented in Table 4.

Table 4.
Physicochemical characterization of EURO-III and EURO-IV high-speed diesels.
The problem of high viscosity can be answered by blending biodiesel with conventional diesel. Blending biodiesel is a simple process involving mixing conventional diesel with biodiesel in proportion to form a specific blend under appropriate conditions [39]. The letter “B” indicates the percentage of biodiesel in any diesel blend. Various methods exist for blending biodiesel with regular diesel. Most commonly, biodiesel and diesel are mixed in tanks at the manufacturing facility. In the storage tank, there is splash mixing. Metered pump mixing refers to the process wherein a transfer pump draws components from two locations, sets the meter to the desired volume, and mixes the fuels [40].
ASTM has approved B5 biodiesel blends for use in any compression ignition engine designed to run on petroleum diesel. In addition, numerous biodiesel blends are employed, such as B20 (20% biodiesel, 80% petroleum diesel), B5 (5% biodiesel, 95% petroleum diesel), and B2 (5% biodiesel, 95% petroleum diesel) (2 percent biodiesel, 98 percent petroleum diesel) [41]. According to the literature, using biodiesel blends reduces pollutants such as HC (hydrocarbon), CO (carbon monoxide), and PM (particulate matter). However, adding additives or using a cetane number improver can also result in pollutant reduction [42]. According to studies, long-term biodiesel storage and its blends are a difficult problem. Biodiesel is unstable due to the presence of unsaturated fatty acids ester. So, the key concerns are the storage stabilities and oxidation stability of biodiesel and its mixes, which affect biodiesel quality and the numerous components that come into contacts with it, such as vehicle parts and storage containers. The peroxide value, acid value, and viscosity of biodiesel rise with oxidation, but the methyl esters content and iodine content drop. These variations in biodiesel properties may impact its performance and stability [43]. The fuel darkens, silts up, and forms gums using biodiesels and mixtures. This can lead to deposits in the engine’s combustion chamber, injector fouling, and other problems throughout the fuel system, including filter blockage [44].
Therefore, to make biodiesel and its blend a sustainable commercial fuel, it is important to have detailed knowledge of biodiesel oxidation stability.
3. Material and Method
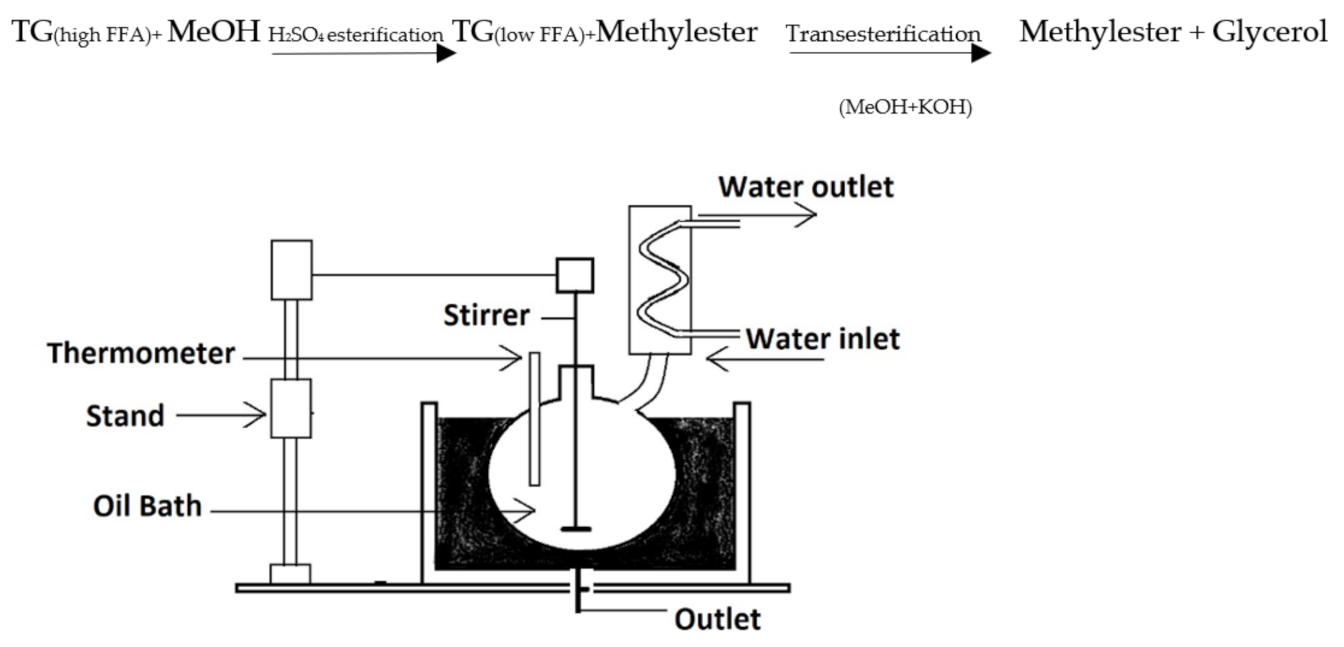
Figure 1 depicts the laboratory setup for optimizing process conditions for methyl ester lab-scale preparation. It has an oil bath, reaction flask with the condenser, and mechanical stirrer with digital rpm control. The glass reactor had a capacity of 1000 mL and had three necks, one for the stirrer and the other for the condenser and reactant intake. The reaction temperature was measured using a thermometer indicator. The batch reactor had a bottom-mounted valve for collecting the finished product. The reaction flask is placed in an oil bath to maintain a consistent temperature. Before beginning the process, the oil sample (500 mL) was preheated to the necessary temperature. The potassium hydroxide–methanol solution was freshly produced to preserve catalytic activity and limit moisture absorption. The methanolic solution was added to the oil in the reaction flask, and the timer was started. This laboratory setup was utilized to optimize major process conditions for the transesterification of the two raw/virgin oils. Due care was made to guarantee that all raw ingredients for chemical reactions were measured and temperature measurements and other safety procedures.
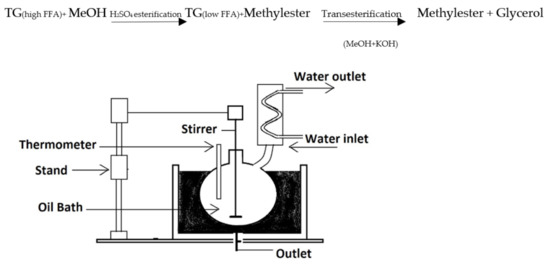
Figure 1.
Schematic diagram of transesterification lab reactor.
In the case of Pongamia, the free fatty acids (FFA) content was between 0.2 to 8%. The high FFA feedstock requires pretreatment to lower its acid value before moving to alkali-catalyzed transesterification [45]. For this purpose, the oil was preheated with methanol and acid catalyst for the esterification of free fatty acid to lower the FFA level, followed by a transesterification process, as shown in the reaction below.
Samples (100 mL) were prepared in beakers by mixing the methyl esters with the diesel fuels to a defined blend level. Four methyl ester blends with each diesel fuel were prepared in 20%, 40%, 60%, and 80% (v/v), respectively. Before testing the physicochemical properties, each sample was heated to about 40 °C and homogenized using a magnetic stirrer. The samples were allowed to attain the ambient temperature before subjecting them to major physicochemical investigations. The samples were also checked for separation of any layer in the beaker to ensure their compatibility prior to proceeding with the tests. The basic objective of this study was to bring the blend viscosity close to the specification range, as the kinematic viscosity of the two methyl esters is higher than the diesel fuels.
German tool Petrotest “PetroOXY(e)-VERSION: 10.08.2011” measured the rancimat period. The oxidation stability of fuel was calculated using ASTM-D 7545-09. The rancimat method measures the time between starting a test cycle and the breaking point (response stoppage time), which is a 10% drop in test vessel pressure as it warms to test temperature. Rancimat time was measured using 5 cc fuel in a sealed chamber. The chamber was inflated with oxygen to 700 kPa and heated to 140 °C. This accelerates oxidation. As fuel oxidizes, it consumes the chamber’s oxygen, causing a pressure reduction. The oxidation stability of the fuel determines the rancimat period. Rancimat time was measured for biodiesel and its blends with commercial diesel fuel with a 2% accuracy.
Kinematic viscosity of biodiesel and its blends were measured at 40 °C and 50% torque by Fungi-lab Expert Series Viscometer, according to ASTM-D 445 method.
The density of diesel samples was measured at 15 C using Anton Paar Density meter DMA-35 Version 3, according to ASTM-D 4052 method
4. Results and Discussion
We have examined the effect of blending conventional diesel with Jatropha and Pongamia biodiesel for which viscosity, density, pour point, flash point, o API, cetane index, aniline point, and rancimat time (oxidation stability) of B-20, B-40, B-60, B-80 blends of Jatropha and Pongamia biodiesel with EURO-III and EURO-IV HSD were analyzed to accesses that the blended fuels are within the specifications range of new generation diesel fuels. The physicochemical properties of blends of Jatropha biodiesel with EURO-III HSD and EURO-IV HSD are presented in Table 5 and Table 6.

Table 5.
Physical and chemical properties of EURO-III HSD and Jatropha curcus methyl ester blends (JB+E-III).

Table 6.
Physical and chemical properties of Euro-IV HSD and Jatropha curcus methyl ester blends (JB+E-IV).
The physicochemical properties of blends of Pongamia biodiesel with EURO-III HSD and EURO–IV HSD are presented in Table 7 and Table 8.

Table 7.
Physical and chemical properties of EURO-III HSD and Pongamia pinnata methyl ester blends (PB+E-III).

Table 8.
Physical and chemical properties of EURO-IV HSD and Pongamia pinnata methyl ester blends (PB+E-IV).
The two esters (biodiesels) are comparable in quality as prescribed by ASTM standard (D-6751). However, comparison with HSD properties shows the two methyl esters to be of higher values with respect to density, kinematics viscosity, and others. This aspect has been examined through blend studies to assess that the blended fuels are within the specifications range of new-generation diesel fuels for their application in the existing engines. The blend compositions have also been further examined with respect to limits to which the blend compositions can be extended beyond which the blend properties overshoot the specification limit. It may be noted that the two esters carry their unsaturation characteristic (presence of olefinic bond) in the blended fuel, due to which their oxidation stability may be lower compared to neat diesel fuels.
Considering the two EURO-III and EURO-IV HSD new generation transport fuel neat (Table 4) it is observed that the fuels are similar in their physicochemical characteristics, but for the concentration of sulfur which is 350 ppm and 50 ppm as the max limit in the two cases, respectively. They differ slightly in pour point because of the increased paraffinicity of EURO-IV fuel, due to which it is of lower density and lower viscosity and is of almost comparable cetane number
Combining research results and Jatropha curcus methyl ester qualities with EURO-III and EURO-IV HSD shows that blending these diesels significantly dilutes the Jatropha curcus methyl esters, notably with respect to viscosity. The ester’s viscosity drops as diesel’s proportion in the blend rises.
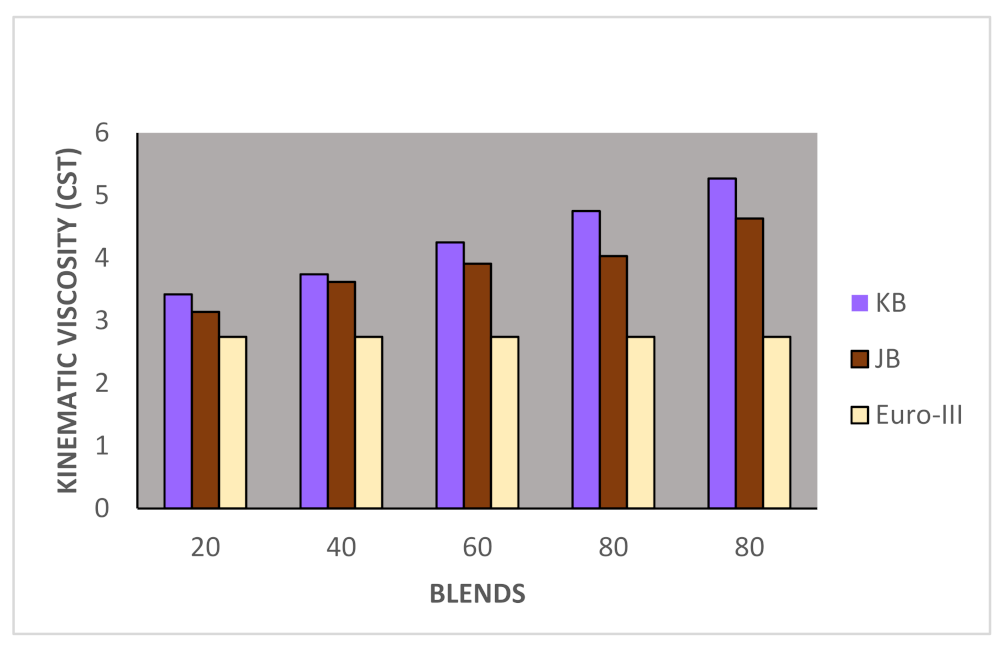
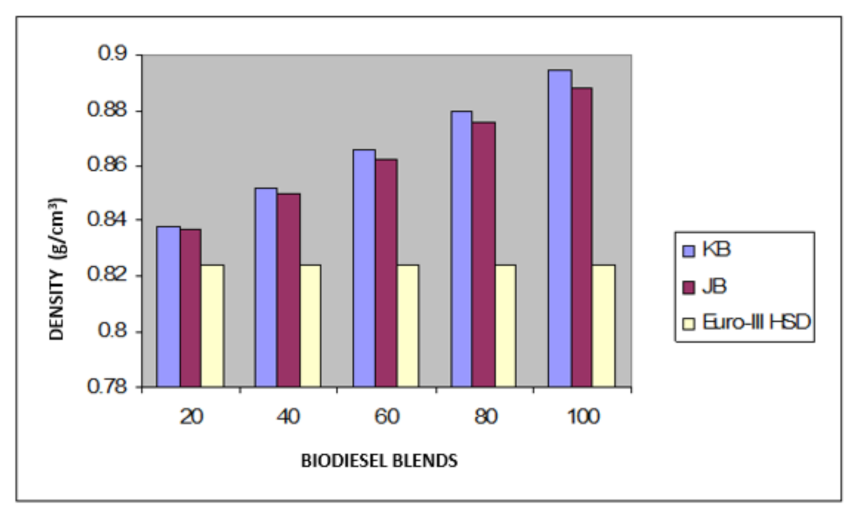
Taking now the results of blending studies between Pongamia pinnata methyl ester with EURO-III and EURO-IV HSD as reported in Table 7 and Table 8, respectively. One observed a similar pattern as in the case of blends with Jatropha curcus methyl esters. There is a considerable viscosity lowering due to the dilution effect of increasing diesel concentration in both cases. This aspect is visible in Figure 2 and Figure 3. It may be noted in this case that blends with fuel beyond 60% diesel go outside the specified viscosity limit of 4.50 cSt at 40 °C. This is in contrast to the observation made earlier with Jatropha curcus methyl ester. The density trends observed with the blends are similar to the ones observed with Jatropha curcus methyl ester blends. All the blends starting with 40% biodiesel concentration overshoot the specification limits of the density, as seen in the case of Jatropha curcus methyl ester. This behavior is also seen in Figure 4 and Figure 5. Other properties, such as the pour point, flash point, and cetane index, are within the specification limit. Unlike Jatropha curcus blend, there is no synergistic effect seen in this case with respect to the cetane index (Figure 6 and Figure 7). This may be due to the very large size of the average carbon number in Pongamia pinnata ester compared to around a maximum of C 25 carbon number in the case of average diesel fuels.
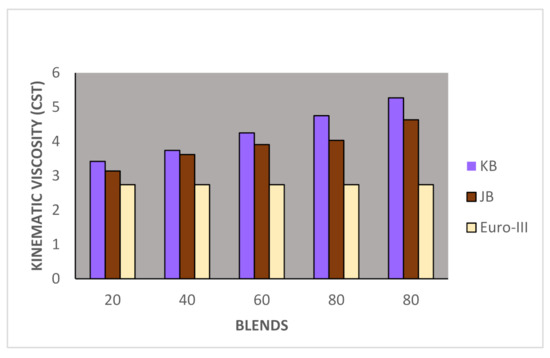
Figure 2.
Variation of kinematics viscosity of Jatropha curcus and Pongamia pinnata methyl esters blends with EURO-III HSD.
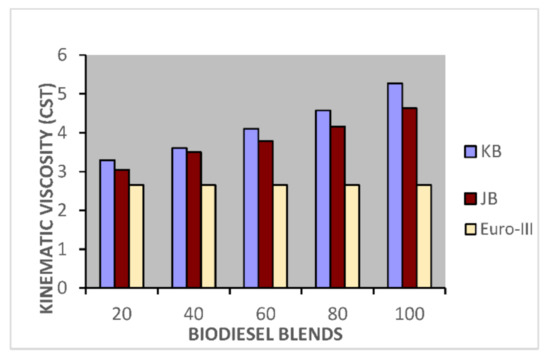
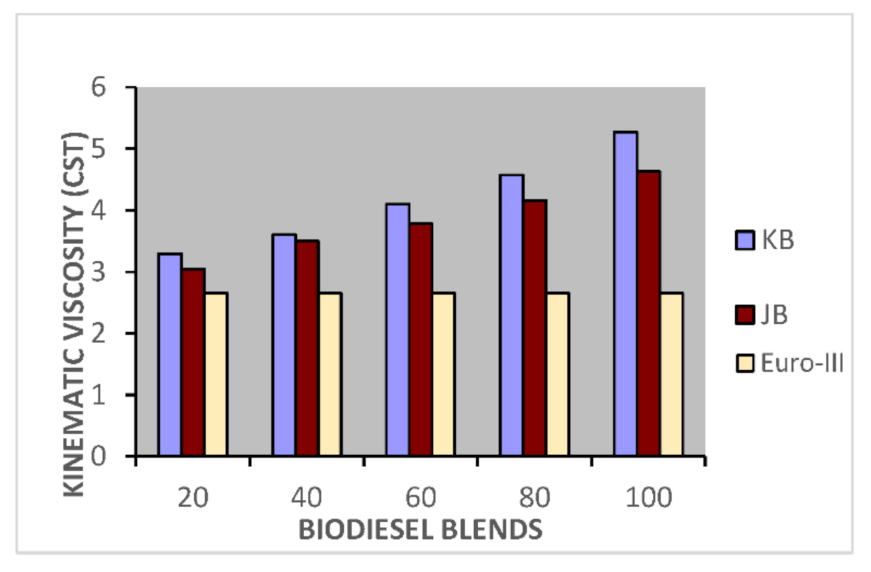
Figure 3.
Variation of kinematics viscosity of Jatropha curcus and Pongamia pinnata methyl esters blends with EURO-IV HSD.
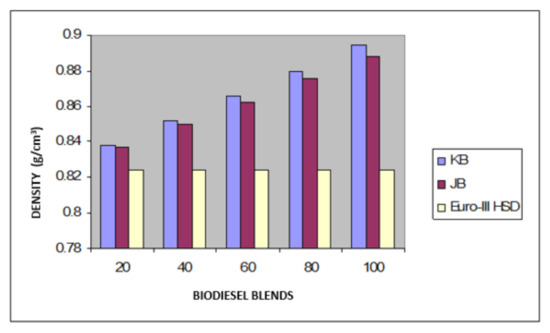
Figure 4.
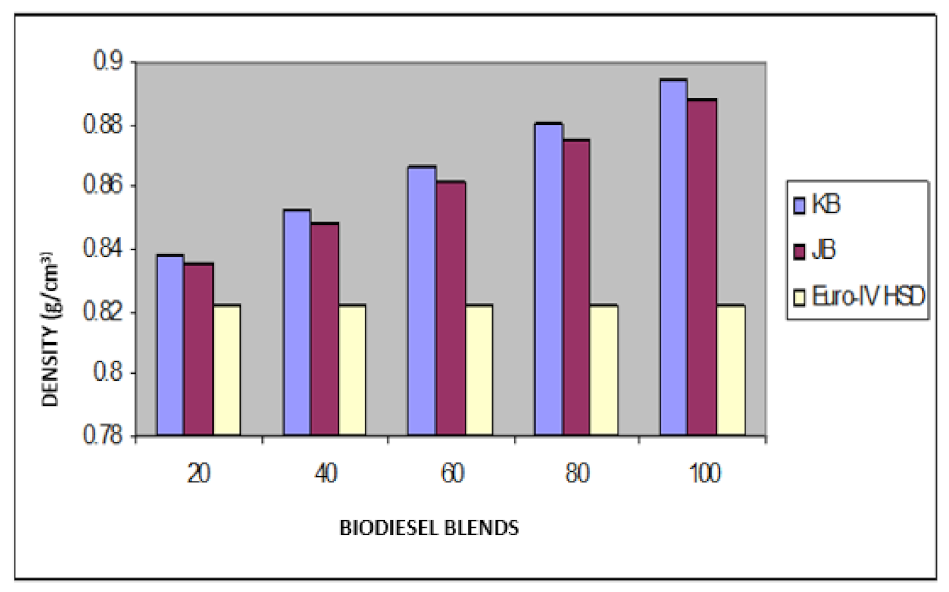
Variation of the density of Jatropha curcus and Pongamia pinnata methyl esters blends with EURO-III HSD.
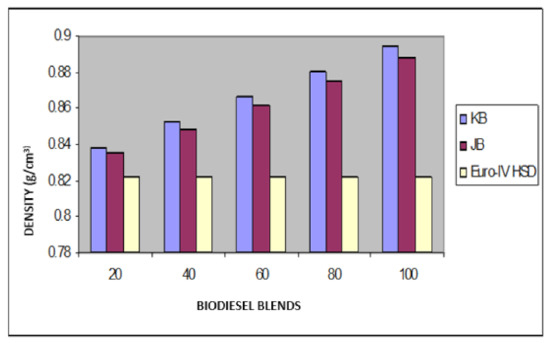
Figure 5.
Variation of density of Jatropha curcus and Pongamia pinnata methyl esters blends with EURO-IV HSD.
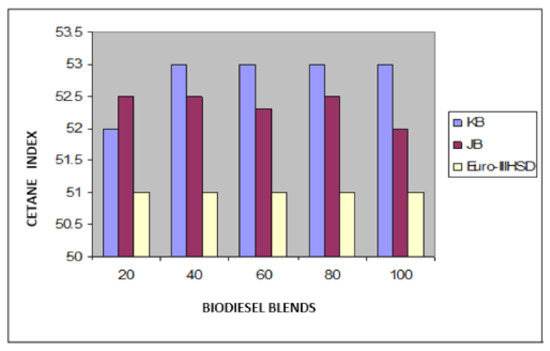
Figure 6.
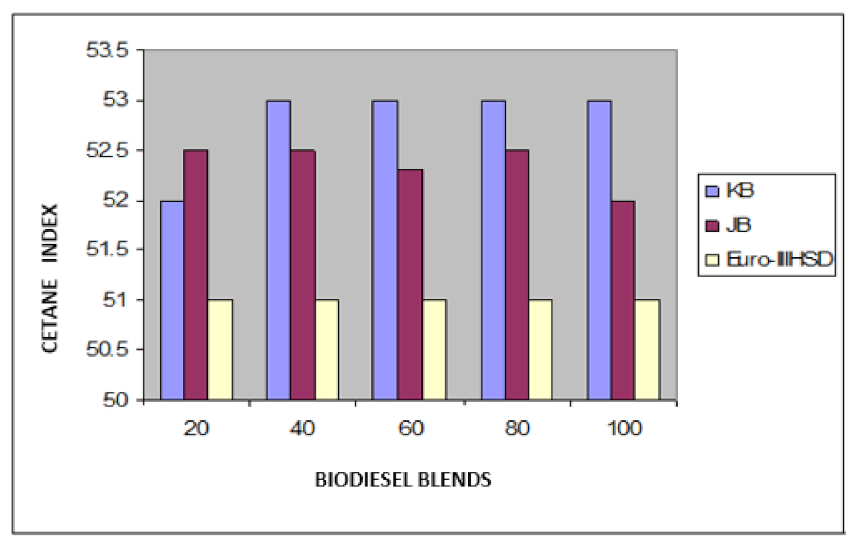
Variation of cetane index of Jatropha curcus and Pongamia pinnata methyl esters blends with EURO-III HSD.
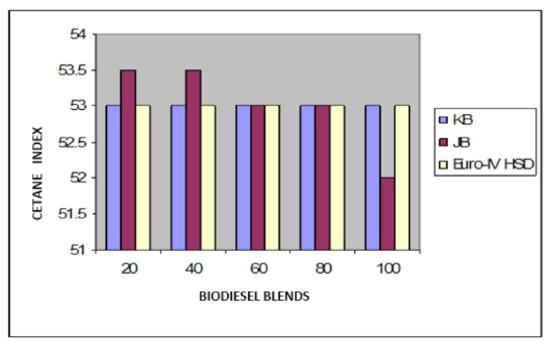
Figure 7.
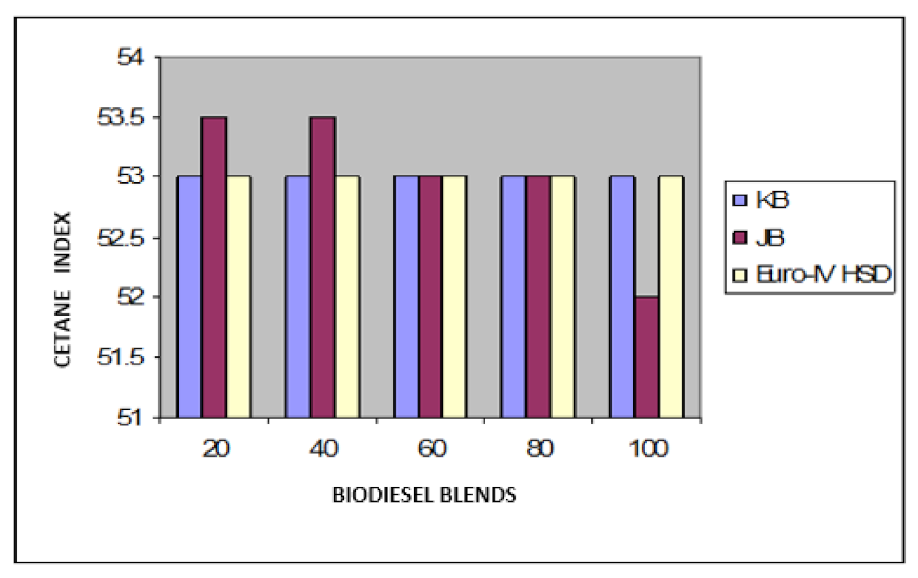
Variation of cetane index of Jatropha curcus and Pongamia pinnata methyl esters blends with EURO-IV HSD.
Jatropha and Pongamia biodiesel are compared with EURO-III and EURO-IV high-speed diesel. The studies interpreted the two methyl esters as having higher values with respect to density, kinematics viscosity, and others. This aspect can be examined through blend studies to assess that the blended fuels are within the specifications range of new generation diesel fuels for their application in the existing engines. Their induction periods indicate the low oxidation stability of clean Jatropha curcus and Pongamia pinnata biodiesels. However, combining biodiesel with commercially available diesel fuels could increase the oxidation stability of both fuels. According to the research, the stability of blends reduces as the biodiesel concentration rises.
The blend compositions have also been further examined with respect to limits to which the blend compositions can be extended beyond which the blend properties overshoot the specification limit. It may be noted that the two esters carry their unsaturation characteristic (presence of olefinic bond) in the blended fuel, due to which their oxidation stability may be lower compared to neat diesel fuels.
Considering the two EURO-III and EURO-IV HSD new generation transport fuel neat, it is observed that the fuels are similar in their physicochemical characteristics, but for the concentration of sulfur which is 350 ppm and 50 ppm as the max limit in the two cases, respectively. They differ slightly in pour point because of the increased paraffinicity of EURO-IV fuel, which is of lower density and viscosity and is of almost comparable cetane number.
The blending research of Jatropha curcus methyl ester with EURO-III and EURO-IV HSD revealed that adding both types of diesel to the Jatropha curcus methyl esters has a significant diluting effect, especially on viscosity. As seen in Figure 2 and Figure 3, the ester’s viscosity reduces as the blend’s diesel percentage increases. Blends containing up to 80 percent biodiesel remain within the viscosity limit. However, adding methyl esters in excess of B-40 concentration results in a density rise in the blends that are outside the maximum 0.8450 g/cm3 at 15 °C requirements. This is also evident in Figure 4 and Figure 5. Other properties of the blends, such as pour point, flash point, and cetane index, are within the prescribed specification limits when compared to the diesel qualities requirement. Figure 6 and Figure 7 show the cetane index of blended fuels containing both diesels from 20 percent onwards. The computed cetane index values from B-20 onwards are higher than those of neat Jatropha curcus methyl ester in both situations. This phenomenon can be explained by the synergistic effect of the two fuel mixes.
The findings of blending trials with Pongamia pinnata methyl ester and EURO-III and EURO-IV HSD revealed a pattern similar to that seen with Jatropha curcus methyl esters. There is a considerable viscosity lowering due to the dilution effect of increasing diesel concentration in both cases. It may be noted in this case that blends with fuel beyond 60% diesel go outside the specified viscosity limit of 4.50 at 40 °C. This is in contrast to the observation made earlier with Jatropha curcus methyl ester. The density trends observed with the blends are similar to the ones observed with Jatropha curcus methyl ester blends. All the blends start with a 40% biodiesel concentration overshoot of the specification limits of the density, as seen in the case of Jatropha curcus methyl ester. This behavior is also seen in Figure 4 and Figure 5. Other properties, such as pour point, flash point, and cetane index, are well within the specification limit, and unlike the Jatropha curcus blend, there is no synergistic effect seen with respect to the cetane index (Figure 6 and Figure 7) in this case. This may be due to the very large size of the average carbon number in Pongamia pinnata ester compared to around the maximum of C 25 carbon number in the case of average diesel fuels.
The literature study on oxidation stability showed that the antioxidant effect is highly dependent on the feedstock used in biodiesel production. When JB is blended with HSD-III, the result is a composition with better oxidation stability. JB-10, TBHQ was determined to be the most efficient antioxidant among all employed antioxidants in this investigation with neat methyl esters and lower mixes [26]. In contrast, BHA was more effective in higher mixes such as JB-20 and JB-40. According to the trial data, the 500 ppm dosages of PY, PrG, and BHT were most effective in plain biodiesel, and its diesel mixes in PB blends with EURO-III HSD. The most stable biodiesel was plain biodiesel with 500 ppm of PY. Except for B40, all other mixes containing 500 ppm PY could be kept for up to 90 days [23]. PY had the best effect in biodiesel stabilization in JB blends with EURO-IV HSD due to its higher number of labile hydrogens, but PrG was found to be highly effective in PB blends with EURO-IV HSD, which could be due to the chemical structure of PB vs JB and the different level of molecular interaction. The study also discovered that the optimal concentration for all antioxidants is 500 ppm and that increasing the concentration to 600 ppm has no impact and raises the fuel cost unnecessarily.
5. Conclusions
The experimental result revealed that the two esters are in the acceptable range as prescribed by ASTM standard (D-6751). However, as compared to HSD properties, the two methyl esters are to be of higher values with respect to density and kinematics viscosity. Blend studies have looked at this part to make sure that the blended fuels are within the range of specifications for new-generation diesel fuels.
In blends of JOME with EURO-III and EURO-IV HSD, the viscosity of the ester reduces with increasing diesel percentage in blends. Blends containing up to 80% biodiesel remain within the viscosity limit. Adding methyl esters beyond B-40 concentration increases blend density beyond the maximum need of 0.8450 g/cm3 at 15 °C. Cetane index values from B-20 forward are higher than the neat JOME. This is due to the synergy between the two fuels. Considering the other properties of the JOME blends, such as pour point, flash point, and cetane index, in relation to diesel properties requirement, their values are within the prescribed specification limits.
KOME blends with EURO-III and EURO-IV HSD showed a similar pattern, except that with both the fuel beyond 60% diesel, the blends go outside the specified limit of viscosity. All blends starting with 40% biodiesel concentration exceed density limitations, as observed in JOME. Other properties such as pour point, flash point, and cetane index are well within the specification limit, and unlike Jatropha curcus blend, there is no synergistic effect seen with respect to the cetane index. This may be attributed to the high average carbon number in KOME compared to diesel fuels (C25).
Author Contributions
Conceptualization, methodology, data curation, formal analysis, investigation, writing—original draft preparation, B.Y.L.; supervision, resources, project administration, funding acquisition, writing—review and editing, W.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
Work is completed under the DST funded project. Project NO-DST/TSG/AF/2011/104, and the National Science and Technology Council, Taiwan, under the grant numbers MOST 109-2221-E-006-040-MY3, MOST 110-2622-E-006-001-CC1, and MOST 110-3116-F-006-003.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Further, the raw data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to the Department of Science and Technology for providing financial support for the experimental study under the sanctioned project DST/TSG/AF/2011/104. I would also like to convey my gratitude to the UPES Management for their unwavering support and encouragement. Mathura Refinery deserves special thanks for giving the fuel samples. The authors also acknowledge the financial support of the National Science and Technology Council, Taiwan, for this research. This research is also supported in part by the Higher Education Sprout Project, Ministry of Education at the Headquarters of the University Advancement at National Cheng Kung University (NCKU).
Conflicts of Interest
The authors declares no conflict of interest.
Nomenclature
| S.NO. | Abbreviation | Full form |
| 1 | HSD | High speed diesel |
| 2 | NOVOD | National oil seeds and vegetable oil development |
| 3 | FFA | Free fatty acid |
| 4 | PAH | Polycyclic aromatics hydrocarbon |
| 5 | ASTM | American Society for Testing and Materials |
| 6 | B | The percentage of biodiesel in any diesel blends |
| 7 | B20 | 20% biodiesel, 80% petroleum diesel |
| 8 | B5 | 5% biodiesel, 95% petroleum diesel |
| 9 | B2 | 2 percent biodiesel, 98 percent petroleum diesel |
| 10 | HC | Hydrocarbon |
| 11 | CO | Carbon monoxide |
| 12 | PM | Particulate matter |
| 13 | FAMEs | Fatty acid Methyl esters |
| 14 | CFPP | Cold filter plugging point |
| 15 | TBHQ | Tert-butylhydroxyquinone |
| 16 | BHT | Butylatedhydroxy toluene |
| 17 | BHA | Butylated hydroxy anisole |
| 18 | DPA | Diphenylamine |
| 19 | PY | Pyrogallol |
| 20 | PG | Propyl gallate |
| 21 | JOME | Jatropha methyl ester |
| 22 | KOME | Pongamia methyl ester |
| 23 | MME | Methyl ester |
References
- Niculescu, R.; Clenci, A.; Iorga-Siman, V. Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines. Energies 2019, 12, 1194. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Xu, J.; Ou, X.; Chang, S.; Wu, M. Techno-Economic Analysis of Bioethanol Production from Lignocellulosic Biomass in China: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. Energies 2015, 8, 4096–4117. [Google Scholar] [CrossRef]
- Caldeira-Pires, A.; Da Luz, S.M.; Palma-Rojas, S.; Rodrigues, T.; Silverio, V.C.; Vilela, F.; Barbosa, P.C.; Alves, A.M. Sustainability of the Biorefinery Industry for Fuel Production. Energies 2013, 6, 329–350. [Google Scholar] [CrossRef]
- (PDF) Biodegradability of Diesel and Biodiesel Blends. Available online: https://www.researchgate.net/publication/27798287_Biodegradability_of_diesel_and_biodiesel_blends (accessed on 9 May 2022).
- Sarada, S.N.; Shailaja, M.; Raju, A.S.R.; Radha, K.K. Optimization of injection pressure for a compression ignition engine with cotton seed oil as an alternate fuel. Int. J. Eng. Sci. Technol. 2011, 2, 142–149. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, L.; Babu, M.; Arora, P.; Singh, V.; Kumar, N.; Varyani, T. Comparative evaluation of performance and emission characteristics of jatropha, karanja and polanga based biodiesel as fuel in a tractor engine. Fuel 2009, 88, 1698–1707. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Silitonga, A.; Masjuki, H.; Ong, H.C.; Sebayang, A.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Liu, J.; Tao, B. Fractionation of fatty acid methyl esters via urea inclusion and its application to improve the low-temperature performance of biodiesel. Biofuel Res. J. 2022, 9, 1617–1629. [Google Scholar] [CrossRef]
- Meira, M.; Santana, P.M.B.; Araújo, A.S.; Silva, C.L.; Filho, J.R.L.; Ferreira, H.T. Oxidative degradation and corrosiveness of biodiesel. Corros. Rev. 2014, 32, 143–161. [Google Scholar] [CrossRef]
- Odziemkowska, M.; Czarnocka, J.; Wawryniuk, K. Study of Stability Changes of Model Fuel Blends. In Improvement Trends for Internal Combustion Engines; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Chandran, D.; Ng, H.; Harrison, L.; Gan, S.; Choo, Y.; Jahis, S. Compatibility of biodiesel fuel with metals and elastomers in fuel delivery system of a diesel engine. J. Oil Palm Res. 2016, 28, 64–73. [Google Scholar] [CrossRef]
- Sharafutdinov, I.; Stratiev, D.; Shishkova, I.; Dinkov, R.; Batchvarov, A.; Petkov, N.; Rudnev, N. Cold flow properties and oxidation stability of blends of near zero sulfur diesel from Ural crude oil and FAME from different origin. Fuel 2012, 96, 556–567. [Google Scholar] [CrossRef]
- Karavalakis, G.; Stournas, S.; Karonis, D. Evaluation of the oxidation stability of diesel/biodiesel blends. Fuel 2010, 89, 2483–2489. [Google Scholar] [CrossRef]
- Lamba, B.Y.; Joshi, G.; Tiwari, A.K.; Rawat, D.S.; Mallick, S. Effect of antioxidants on physico-chemical properties of EURO-III HSD (high speed diesel) and Jatropha biodiesel blends. Energy 2013, 60, 222–229. [Google Scholar] [CrossRef]
- Yang, Z.; Hollebone, B.P.; Wang, Z.; Yang, C.; Landriault, M. Factors affecting oxidation stability of commercially available biodiesel products. Fuel Process. Technol. 2013, 106, 366–375. [Google Scholar] [CrossRef]
- Christensen, E.; McCormick, R.L. Long-term storage stability of biodiesel and biodiesel blends. Fuel Process. Technol. 2014, 128, 339–348. [Google Scholar] [CrossRef]
- Serrano, M.; Bouaid, A.; Martinez, M.; Aracil, J. Oxidation stability of biodiesel from different feedstocks: Influence of commercial additives and purification step. Fuel 2013, 113, 50–58. [Google Scholar] [CrossRef]
- Karavalakis, G.; Hilari, D.; Givalou, L.; Karonis, D.; Stournas, S. Storage stability and ageing effect of biodiesel blends treated with different antioxidants. Energy 2011, 36, 369–374. [Google Scholar] [CrossRef]
- Joshi, G.; Lamba, B.Y.; Rawat, D.S.; Mallick, S.; Murthy, K.S.R. Evaluation of Additive Effects on Oxidation Stability of Jatropha Curcas Biodiesel Blends with Conventional Diesel Sold at Retail Outlets. Ind. Eng. Chem. Res. 2013, 52, 7586–7592. [Google Scholar] [CrossRef]
- Rawat, D.S.; Joshi, G.; Lamba, B.Y.; Tiwari, A.K.; Mallick, S. Impact of additives on storage stability of Karanja (Pongamia Pinnata) biodiesel blends with conventional diesel sold at retail outlets. Fuel 2014, 120, 30–37. [Google Scholar] [CrossRef]
- Rawat, D.S.; Joshi, G.; Lamba, B.Y.; Tiwari, A.K.; Kumar, P. The effect of binary antioxidant proportions on antioxidant synergy and oxidation stability of Jatropha and Karanja biodiesels. Energy 2015, 84, 643–655. [Google Scholar] [CrossRef]
- Lamba, B.Y.; Joshi, G.; Rawat, D.S.; Jain, S.; Kumar, S. Study of oxidation behavior of Jatropha oil methyl esters and Karanja oil methyl esters blends with EURO-IV high speed diesel. Renew. Energy Focus 2018, 27, 59–66. [Google Scholar] [CrossRef]
- Lamba, B.Y.; Jain, S.; Kumar, S. A Review on Jatropha curcas Derived Biodiesel for Economic and Sustainable Development. Int. J. Emerg. Technol. 2020, 11, 1026–1033. Available online: www.researchtrend.net (accessed on 9 May 2022).
- Karavalakis, G.; Stournas, S. Impact of Antioxidant Additives on the Oxidation Stability of Diesel/Biodiesel Blends. Energy Fuels 2010, 24, 3682–3686. [Google Scholar] [CrossRef]
- Reddy, S.N.K.; Wani, M.M. A Comprehensive Review on Effects of Nanoparticles-Antioxidant Additives-Biodiesel Blends on Performance and Emissions of Diesel Engine. Appl. Sci. Eng. Prog. 2020, 13, 285–298. [Google Scholar] [CrossRef]
- Krishnakumar, S.; Khan, T.; Rajashekhar, C.; Soudagar, M.M.; Afzal, A.; Elfasakhany, A. Influence of Graphene Nano Particles and Antioxidants with Waste Cooking Oil Biodiesel and Diesel Blends on Engine Performance and Emissions. Energies 2021, 14, 4306. [Google Scholar] [CrossRef]
- Atabani, A.E.; Mofijur, M.; Masjuki, H.H.; Badruddin, I.A.; Chong, W.T.; Cheng, S.F.; Gouk, S.W. A study of production and characterization of Manketti (Ricinodendron rautonemii) methyl ester and its blends as a potential biodiesel feedstock. Biofuel Res. J. 2014, 4, 139–146. [Google Scholar] [CrossRef]
- EUROPA—Environment—Auto Oil, II. Available online: https://ec.europa.eu/environment/archives/autooil/index.htm (accessed on 23 May 2022).
- Preparation and Characterization of Biodiesel-Diesel Fuels Blends and Experimental Investigations Part II: Preparation of Blends and Experimental Findings|Article Information|J-GLOBAL. Available online: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=201002292865791260 (accessed on 29 April 2022).
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.; Silitonga, A.; Chen, W.-H.; Kusumo, F.; Dharma, S.; Sebayang, A. Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Use of Mahua Oil (Madhuca indica) as a Diesel Fuel Extender. Available online: https://www.researchgate.net/publication/286738970_Use_of_mahua_oil_madhuca_indica_as_a_diesel_fuel_extender (accessed on 9 May 2022).
- Aderibigbe, F.A.; Mustapha, S.I.; Adewoye, T.L.; Mohammed, I.; Gbadegesin, A.B.; Niyi, F.E.; Olowu, O.I.; Soretire, A.G.; Saka, H.B. Qualitative role of heterogeneous catalysts in biodiesel production from Jatropha curcas oil. Biofuel Res. J. 2020, 7, 1159–1169. [Google Scholar] [CrossRef]
- Islam, A.K.M.A.; Chakrabarty, S.; Yaakob, Z.; Ahiduzzaman, M. Koroch (Pongamia pinnata): A Promising Unexploited Resources for the Tropics and Subtropics. In Forest Biomass-From Trees to Energy; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Biodiesel e-Book in English. Available online: http://www.svlele.com/book.htm (accessed on 9 May 2022).
- Preparation and Characterization of Biodiesel-Diesel Fuel Blends and Experimental Investigations. Pt. I. Preparation and Characterization of Blending Components (Journal Article)|ETDEWEB. Available online: https://www.osti.gov/etdeweb/biblio/21246169 (accessed on 29 April 2022).
- Study Of Biodiesel Blends And Emission Characteristics Of Biodiesel. Available online: https://1library.net/document/yro64ljy-study-biodiesel-blends-emission-characteristics-biodiesel.html (accessed on 5 May 2022).
- Alternative Fuels Data Center: Biodiesel Blends. Available online: https://afdc.energy.gov/fuels/biodiesel_blends.html (accessed on 5 May 2022).
- Study of Biodiesel Blends and Emission Characteristics of Biodiesel|Open Access Journals. Available online: https://www.rroij.com/open-access/study-of-biodiesel-blends-and-emission-characteristics-of-biodiesel-.php?aid=46652&msclkid=90bd726bcf7311ec9f7ba04f1dd46e49 (accessed on 9 May 2022).
- Zuleta, E.C.; Baena, L.; Rios, L.A.; Calderon, J.A. The oxidative stability of biodiesel and its impact on the deterioration of metallic and polymeric materials: A review. J. Braz. Chem. Soc. 2012, 23, 2159–2175. [Google Scholar] [CrossRef]
- Yamane, K.; Kawasaki, K.; Sone, K.; Hara, T.; Prakoso, T. Oxidation stability of biodiesel and its effects on diesel combustion and emission characteristics. Int. J. Engine Res. 2007, 8, 307–319. [Google Scholar] [CrossRef]
- Litinas, A.; Geivanidis, S.; Faliakis, A.; Courouclis, Y.; Samaras, Z.; Keder, A.; Krasnoholovets, V.; Gandzha, I.; Zabulonov, Y.; Puhach, O.; et al. Biodiesel production from high FFA feedstocks with a novel chemical multifunctional process intensifier. Biofuel Res. J. 2020, 7, 1170–1177. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).