Biohydrogen—A Green Fuel for Sustainable Energy Solutions
Abstract
1. Introduction
1.1. Global Energy Scenario and Global Warming
1.2. Sustainable Energy Economy
“Sustainable energy is defined as energy providing affordable, accessible and reliable energy services that meet the economic, social and environmental needs within the overall developmental context of the society for which the services are intended while recognising equitable distribution in meeting those needs.”
1.3. Sustainable Hydrogen Economy
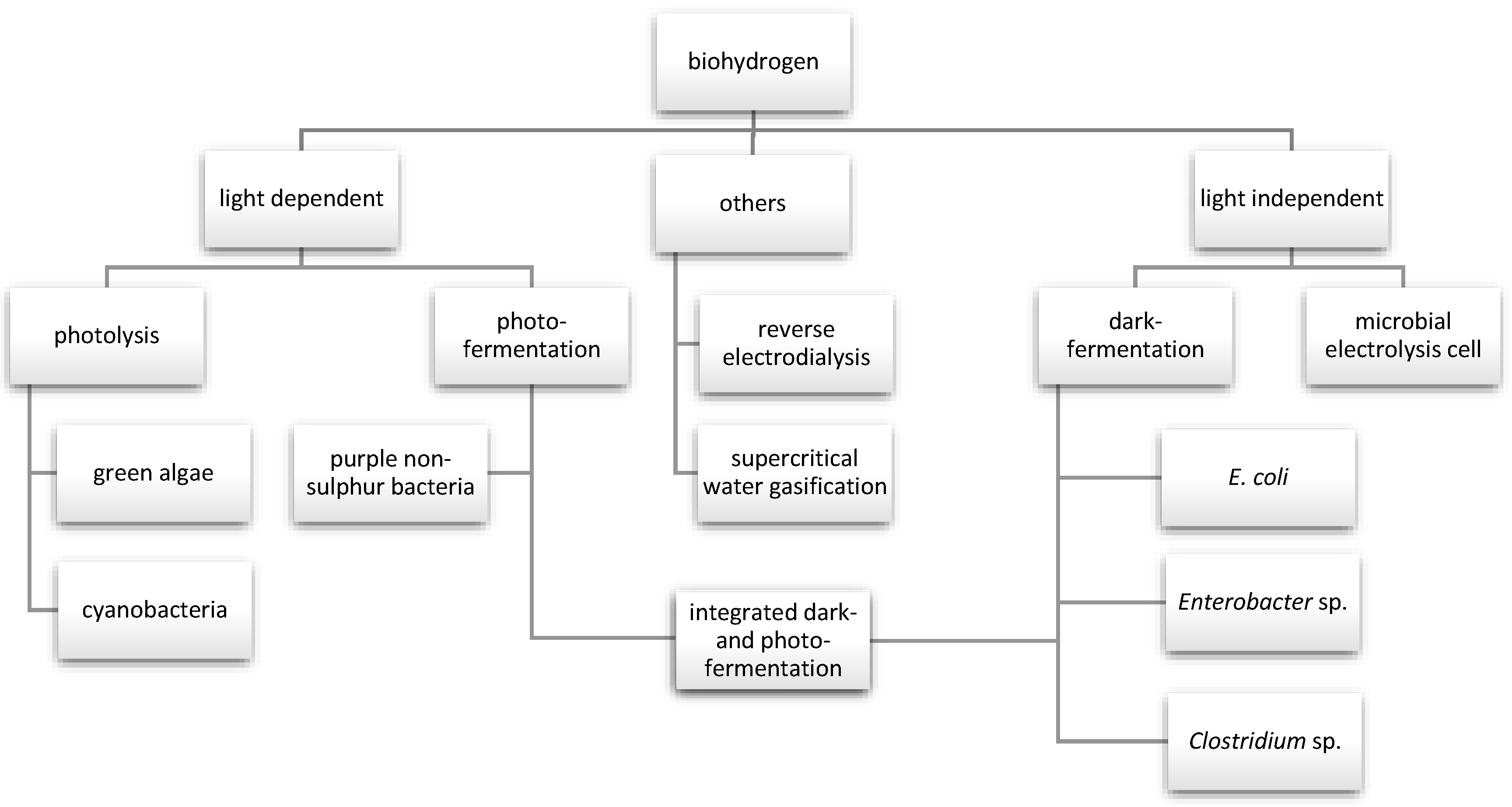
2. Biological Routes of Hydrogen Production
2.1. Biophotolysis
2.2. Photofermentation Process
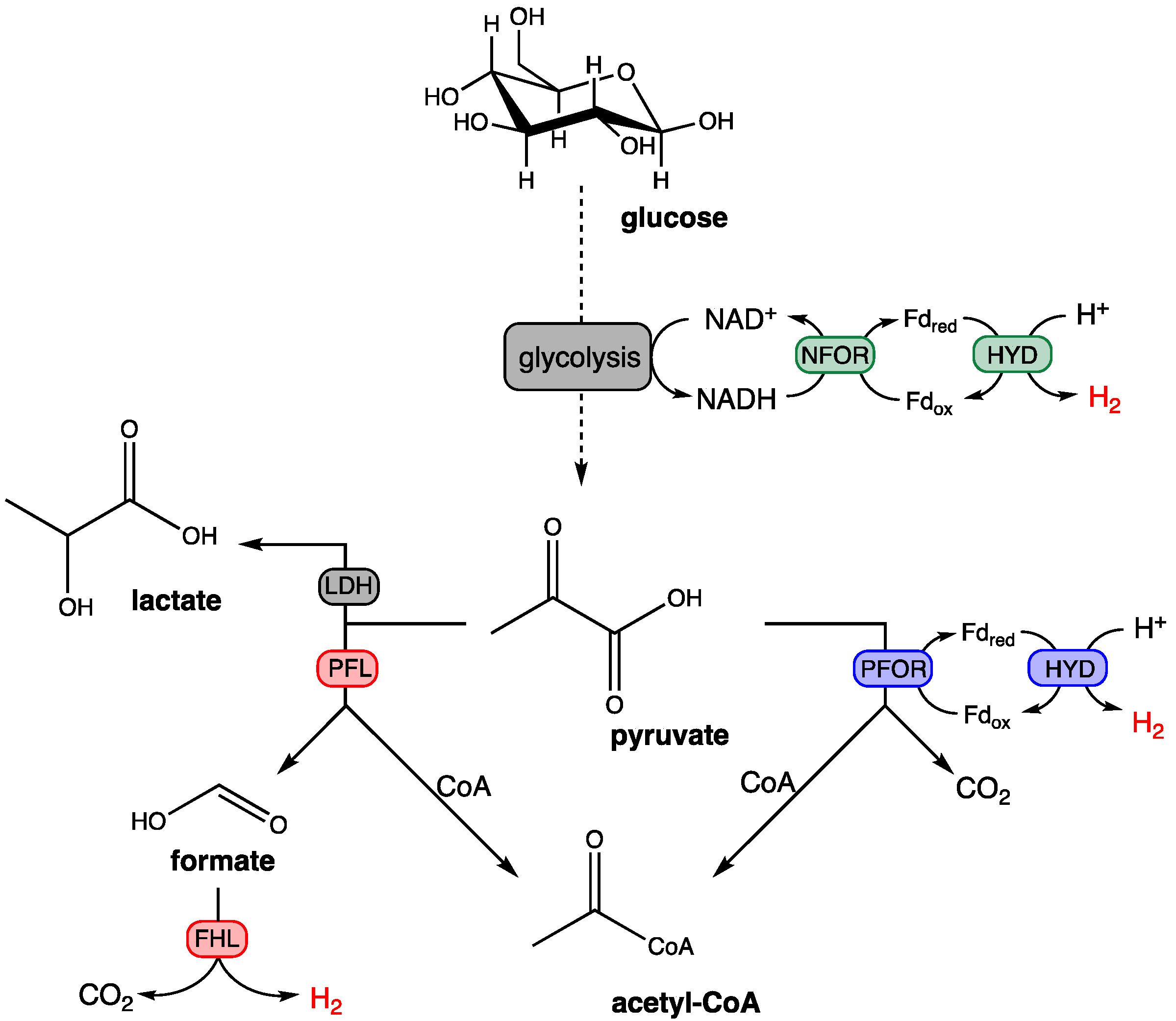
2.3. Dark Fermentation Process
2.4. Microbial Electrolysis Cell
3. Feedstock for Biohydrogen Production
4. Diversity of Biohydrogen-Producing Bacteria
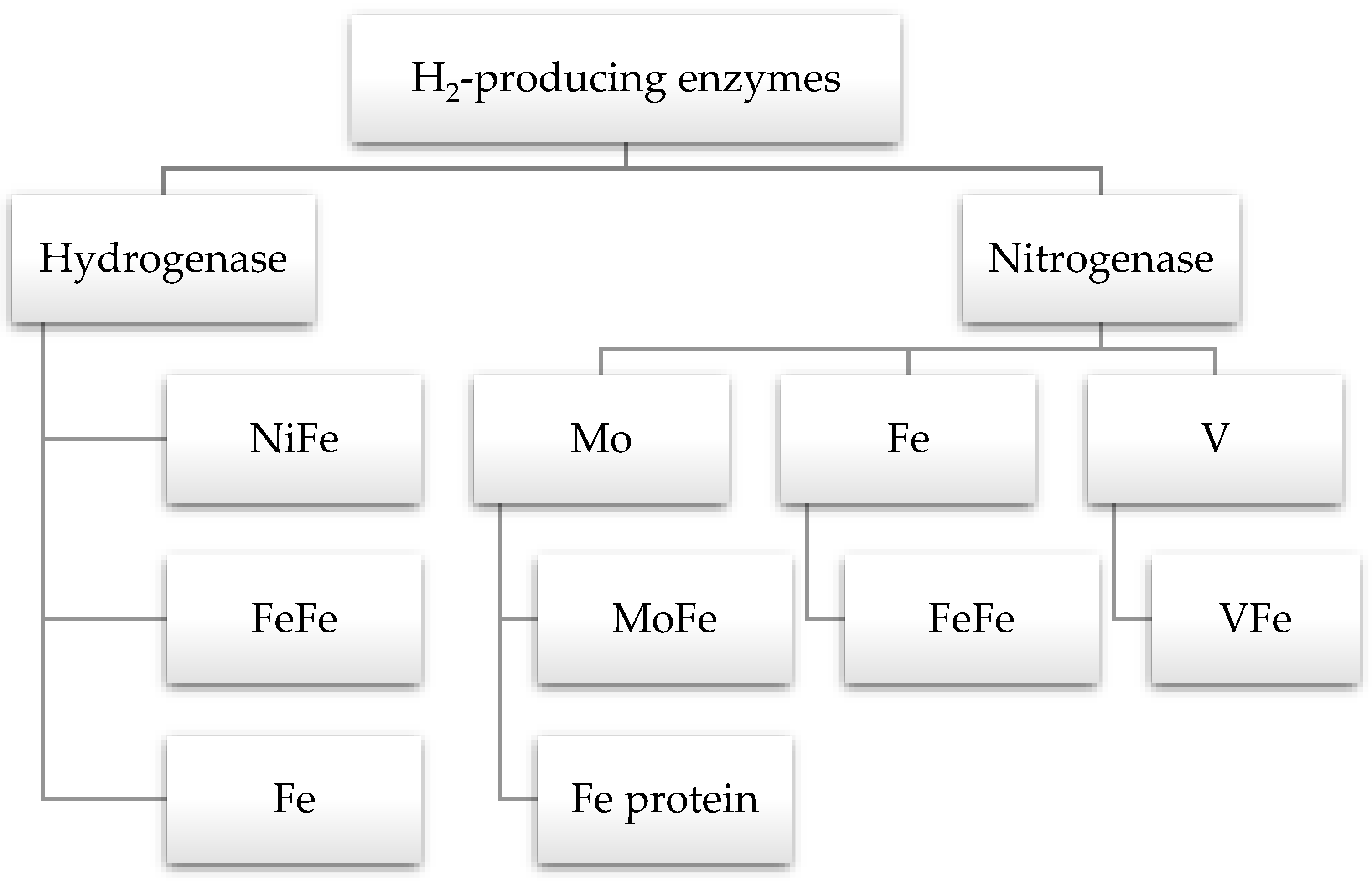
5. Enzymes
6. Factors Affecting the Production of Hydrogen
6.1. Pretreatment Methods
6.2. Effect of Substrate Concentration
6.3. Effect of Initial pH
6.4. Effect of Operational Temperature
6.5. Effect of Nutrients
6.6. Effect of Light Intensity
6.7. Effect of Metal Ions
7. Nanotechnology and Biohydrogen
Enhancement of Biohydrogen by Metallic Nanoparticles
8. Future Perspective of H2 Production
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- U.S. Energy Information Administration. International Energy Outlook. Washington, DC 20585: 2021. Available online: https://www.eia.gov/ieo (accessed on 20 September 2022).
- Bayro-Kaiser, V.; Nelson, N. Microalgal hydrogen production: Prospects of an essential technology for a clean and sustainable energy economy. Photosynth. Res. 2017, 133, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.; von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.P.; Martin, M.V.; Heald, C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang. 2014, 4, 817. [Google Scholar] [CrossRef]
- Schaeffer, M.; Hare, W.; Rahmstorf, S.; Vermeer, M. Long-term sea-level rise implied by 1.5 c and 2 c warming levels. Nat. Clim. Chang. 2012, 2, 867. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef]
- Franks, S.J.; Weber, J.J.; Aitken, S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2014, 7, 123–139. [Google Scholar] [CrossRef]
- Moritz, C.; Agudo, R. The future of species under climate change: Resilience or decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef]
- Vidadili, N.; Suleymanov, E.; Bulut, C.; Mahmudlu, C. Transition to renewable energy and sustainable energy development in azerbaijan. Renew. Sustain. Energy Rev. 2017, 80, 1153–1161. [Google Scholar] [CrossRef]
- Ozturk, M.; Yuksel, Y.E. Energy structure of turkey for sustainable development. Renew. Sustain. Energy Rev. 2016, 53, 1259–1272. [Google Scholar] [CrossRef]
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, M.; Poudyal, R.; Tiwari, I.; Voloshin, R.; Zharmukhamedov, S.; Nam, H.; Zayadan, B.; Bruce, B.; Hou, H.; Allakhverdiev, S. Biofuel production: Challenges and opportunities. Int. J. Hydrog. Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Veziroglu, T.N.; Allakhverdiev, S.I. Biofuel production from plant and algal biomass. Int. J. Hydrog. Energy 2016, 41, 17257–17273. [Google Scholar] [CrossRef]
- Mitra, R.; Balachandar, G.; Singh, V.; Sinha, P.; Das, D. Improvement in energy recovery by dark fermentative biohydrogen followed by biobutanol production process using obligate anaerobes. Int. J. Hydrog. Energy 2017, 42, 4880–4892. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production–a review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef]
- Oey, M.; Sawyer, A.L.; Ross, I.L.; Hankamer, B. Challenges and opportunities for hydrogen production from microalgae. Plant Biotechnol. J. 2016, 14, 1487–1499. [Google Scholar] [CrossRef]
- Bundhoo, M.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrog. Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Memon, S.; Rahman, Z.U.; Lee, K.; Han, J.-I. Current status, barriers and developments in biohydrogen production by microalgae. Renew. Sustain. Energy Rev. 2013, 22, 571–579. [Google Scholar] [CrossRef]
- Demirbas, A. Hydrogen-rich gas from fruit shells via supercritical water extraction. Int. J. Hydrog. Energy 2004, 29, 1237–1243. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A critical review on catalyst design for aqueous phase reforming. Int. J. Hydrog. Energy 2022, 47, 151–180. [Google Scholar] [CrossRef]
- Tak, S.S.; Shetye, O.; Muley, O.; Jaiswal, H.; Malik, S.N. Emerging technologies for hydrogen production from wastewater. Int. J. Hydrog. Energy 2022. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Galletti, C.; Rizzo, A.M.; Chiaramonti, D.; Pirone, R.; Bensaid, S. Aqueous phase reforming of lignin-rich hydrothermal liquefaction by-products: A study on catalyst deactivation. Catal. Today 2021, 365, 206–213. [Google Scholar] [CrossRef]
- Rahman, S.; Masdar, M.; Rosli, M.; Majlan, E.; Husaini, T.; Kamarudin, S.; Daud, W. Overview biohydrogen technologies and application in fuel cell technology. Renew. Sustain. Energy Rev. 2016, 66, 137–162. [Google Scholar] [CrossRef]
- Torzillo, G.; Scoma, A.; Faraloni, C.; Giannelli, L. Advances in the biotechnology of hydrogen production with the microalga chlamydomonas reinhardtii. Crit. Rev. Biotechnol. 2015, 35, 485–496. [Google Scholar] [CrossRef]
- Rumpel, S.; Siebel, J.F.; Farès, C.; Duan, J.; Reijerse, E.; Happe, T.; Lubitz, W.; Winkler, M. Enhancing hydrogen production of microalgae by redirecting electrons from photosystem i to hydrogenase. Energy Environ. Sci. 2014, 7, 3296–3301. [Google Scholar] [CrossRef]
- Liu, H.; Hu, H.; Chignell, J.; Fan, Y. Microbial electrolysis: Novel technology for hydrogen production from biomass. Biofuels 2010, 1, 129–142. [Google Scholar] [CrossRef]
- Lakshmidevi, R.; Muthukumar, K. Enzymatic saccharification and fermentation of paper and pulp industry effluent for biohydrogen production. Int. J. Hydrog. Energy 2010, 35, 3389–3400. [Google Scholar] [CrossRef]
- Silva, J.S.; Mendes, J.S.; Correia, J.A.C.; Rocha, M.V.P.; Micoli, L. Cashew apple bagasse as new feedstock for the hydrogen production using dark fermentation process. J. Biotechnol. 2018, 286, 71–78. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative hydrogen production using pretreated microalgal biomass as feedstock. Microb. Cell Factories 2018, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Arya, S.K. Hydrogen from algal biomass: A review of production process. Biotechnol. Rep. 2017, 15, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. A review on biohydrogen production through photo fermentation of lignocellulosic biomass. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Arimi, M.M.; Knodel, J.; Kiprop, A.; Namango, S.S.; Zhang, Y.; Geißen, S.-U. Strategies for improvement of biohydrogen production from organic-rich wastewater: A review. Biomass Bioenergy 2015, 75, 101–118. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, B.-N.; Kim, Y.-H.; Min, J. Whole-genome sequence of purple non-sulfur bacteria, rhodobacter sphaeroides strain mbtlj-8 with improved co2 reduction capacity. J. Biotechnol. 2018, 288, 9–14. [Google Scholar] [CrossRef]
- Seifert, K.; Waligorska, M.; Laniecki, M. Brewery wastewaters in photobiological hydrogen generation in presence of rhodobacter sphaeroides O.U. 001. Int. J. Hydrog. Energy 2010, 35, 4085–4091. [Google Scholar] [CrossRef]
- Han, H.; Liu, B.; Yang, H.; Shen, J. Effect of carbon sources on the photobiological production of hydrogen using rhodobacter sphaeroides rv. Int. J. Hydrog. Energy 2012, 37, 12167–12174. [Google Scholar] [CrossRef]
- Basak, N.; Das, D. Photofermentative hydrogen production using purple non-sulfur bacteria rhodobacter sphaeroides ou 001 in an annular photobioreactor: A case study. Biomass Bioenergy 2009, 33, 911–919. [Google Scholar] [CrossRef]
- Akroum-Amrouche, D.; Abdi, N.; Lounici, H.; Mameri, N. Effect of physico-chemical parameters on biohydrogen production and growth characteristics by batch culture of rhodobacter sphaeroides cip 60.6. Appl. Energy 2011, 88, 2130–2135. [Google Scholar] [CrossRef]
- Yang, H.; Shao, P.; Lu, T.; Shen, J.; Wang, D.; Xu, Z.; Yuan, X. Continuous bio-hydrogen production from citric acid wastewater via facultative anaerobic bacteria. Int. J. Hydrog. Energy 2006, 31, 1306–1313. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P.; Asthana, R.K.; Singh, S. Biohydrogen production from sugarcane bagasse by integrating dark- and photo fermentation. Bioresour. Technol. 2014, 152, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Enhancing photo fermentative hydrogen production using ethanol rich dark fermentation effluents. Int. J. Hydrog. Energy 2022, 47, 117–126. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Pugazhendhi, A.; Thi, N.B.D.; Zhen, G.; Chandrasekhar, K.; Kadier, A. A comprehensive overview on light independent fermentative hydrogen production from wastewater feedstock and possible integrative options. Energy Convers. Manag. 2017, 141, 390–402. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Fermentative hydrogen production: Principles, progress, and prognosis. Int. J. Hydrog. Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Singh, R.; White, D.; Demirel, Y.; Kelly, R.; Noll, K.; Blum, P. Uncoupling fermentative synthesis of molecular hydrogen from biomass formation in thermotoga maritima. Appl. Environ. Microbiol. 2018, 84, e00998-18. [Google Scholar] [CrossRef]
- Ergal, İ.; Gräf, O.; Hasibar, B.; Steiner, M.; Vukotić, S.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.M.R. Biohydrogen production beyond the thauer limit by precision design of artificial microbial consortia. Commun. Biol. 2020, 3, 443. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-economic and environmental impact assessment of hydrogen production processes using bio-waste as renewable energy resource. Renew. Sustain. Energy Rev. 2022, 156, 111991. [Google Scholar] [CrossRef]
- Guo, W.-Q.; Ren, N.-Q.; Wang, X.-J.; Xiang, W.-S.; Meng, Z.-H.; Ding, J.; Qu, Y.-Y.; Zhang, L.-S. Biohydrogen production from ethanol-type fermentation of molasses in an expanded granular sludge bed (egsb) reactor. Int. J. Hydrog. Energy 2008, 33, 4981–4988. [Google Scholar] [CrossRef]
- Skonieczny, M.T.; Yargeau, V. Biohydrogen production from wastewater by clostridium beijerinckii: Effect of ph and substrate concentration. Int. J. Hydrog. Energy 2009, 34, 3288–3294. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the biological hydrogen production pathway of dark fermentation: Insight into the impact of strain improvement. Microb. Cell Factories 2022, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Ramprakash, B.; Lindblad, P.; Eaton-Rye, J.J.; Incharoensakdi, A. Current strategies and future perspectives in biological hydrogen production: A review. Renew. Sustain. Energy Rev. 2022, 168, 112773. [Google Scholar] [CrossRef]
- Pontrelli, S.; Chiu, T.-Y.; Lan, E.I.; Chen, F.Y.H.; Chang, P.; Liao, J.C. Escherichia coli as a host for metabolic engineering. Metab. Eng. 2018, 50, 16–46. [Google Scholar] [CrossRef] [PubMed]
- Modestra, J.A.; Babu, M.L.; Mohan, S.V. Electro-fermentation of real-field acidogenic spent wash effluents for additional biohydrogen production with simultaneous treatment in a microbial electrolysis cell. Sep. Purif. Technol. 2015, 150, 308–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Microbial electrochemical systems and technologies: It is time to report the capital costs. Environ. Sci. Technol. 2016, 50, 5432–5433. [Google Scholar] [CrossRef]
- Butti, S.K.; Velvizhi, G.; Sulonen, M.L.K.; Haavisto, J.M.; Koroglu, E.O.; Cetinkaya, A.Y.; Singh, S.; Arya, D.; Modestra, J.A.; Krishna, K.V.; et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: Maneuvering towards upscaling. Renew. Sustain. Energy Rev. 2016, 53, 462–476. [Google Scholar] [CrossRef]
- Rivera, I.; Buitrón, G.; Bakonyi, P.; Nemestóthy, N.; Bélafi-Bakó, K. Hydrogen production in a microbial electrolysis cell fed with a dark fermentation effluent. J. Appl. Electrochem. 2015, 45, 1223–1229. [Google Scholar] [CrossRef]
- Kadier, A.; Jain, P.; Lai, B.; Kalil, M.S.; Kondaveeti, S.; Alabbosh, K.F.S.; Abu-Reesh, I.M.; Mohanakrishna, G. Biorefinery perspectives of microbial electrolysis cells (mecs) for hydrogen and valuable chemicals production through wastewater treatment. Biofuel Res. J. 2020, 7, 1128–1142. [Google Scholar] [CrossRef]
- Kadier, A.; Singh, R.; Song, D.; Ghanbari, F.; Zaidi, N.S.; Aryanti, P.T.P.; Jadhav, D.A.; Islam, M.A.; Kalil, M.S.; Nabgan, W.; et al. A novel pico-hydro power (php)-microbial electrolysis cell (mec) coupled system for sustainable hydrogen production during palm oil mill effluent (pome) wastewater treatment. Int. J. Hydrog. Energy 2022. [Google Scholar] [CrossRef]
- Lee, H.-S.; Xin, W.; Katakojwala, R.; Mohan, S.V.; Tabish, N.M.D. Microbial electrolysis cells for the production of biohydrogen in dark fermentation—A review. Bioresour. Technol. 2022, 363, 127934. [Google Scholar] [CrossRef]
- Li, W.; Cheng, C.; Cao, G.; Ren, N. Enhanced biohydrogen production from sugarcane molasses by adding ginkgo biloba leaves. Bioresour. Technol. 2020, 298, 122523. [Google Scholar] [CrossRef] [PubMed]
- Oceguera-Contreras, E.; Aguilar-Juarez, O.; Oseguera-Galindo, D.; Macías-Barragán, J.; Ortiz-Torres, G.; Pita-López, M.L.; Domínguez, J.; Titov, I.; Kamen, A. Establishment of the upstream processing for renewable production of hydrogen using vermicomposting-tea and molasses as substrate. Waste Manag. 2022, 139, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Fuess, L.T.; Soares, L.A.; Damianovic, M.H.R.Z. Thermophilic biohydrogen production from sugarcane molasses under low ph: Metabolic and microbial aspects. Int. J. Hydrog. Energy 2020, 45, 4182–4192. [Google Scholar] [CrossRef]
- Kars, G.; Alparslan, Ü. Valorization of sugar beet molasses for the production of biohydrogen and 5-aminolevulinic acid by rhodobacter sphaeroides o.U.001 in a biorefinery concept. Int. J. Hydrog. Energy 2013, 38, 14488–14494. [Google Scholar] [CrossRef]
- Sagir, E.; Ozgur, E.; Gunduz, U.; Eroglu, I.; Yucel, M. Single-stage photofermentative biohydrogen production from sugar beet molasses by different purple non-sulfur bacteria. Bioprocess Biosyst. Eng. 2017, 40, 1589–1601. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Z.-T.; Zhang, X. Biohydrogen production from starch wastewater and application in fuel cell. Int. J. Hydrog. Energy 2010, 35, 2949–2952. [Google Scholar] [CrossRef]
- Cota-Navarro, C.B.; Carrillo-Reyes, J.; Davila-Vazquez, G.; Alatriste-Mondragón, F.; Razo-Flores, E. Continuous hydrogen and methane production in a two-stage cheese whey fermentation system. Water Sci. Technol. 2011, 64, 367–374. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Lee, M.-Y.; Shin, H.-S. Two-stage uasb reactor converting coffee drink manufacturing wastewater to hydrogen and methane. Int. J. Hydrog. Energy 2012, 37, 7473–7481. [Google Scholar] [CrossRef]
- Bala-Amutha, K.; Murugesan, A.G. Biohydrogen production using corn stalk employing bacillus licheniformis msu agm 2 strain. Renew. Energy 2013, 50, 621–627. [Google Scholar] [CrossRef]
- Chookaew, T.; O-Thong, S.; Prasertsan, P. Fermentative production of hydrogen and soluble metabolites from crude glycerol of biodiesel plant by the newly isolated thermotolerant klebsiella pneumoniae tr17. Int. J. Hydrog. Energy 2012, 37, 13314–13322. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Li, C.; Zhang, T. Acidophilic biohydrogen production from rice slurry. Int. J. Hydrog. Energy 2006, 31, 683–692. [Google Scholar] [CrossRef]
- Anam, K.; Habibi, M.S.; Harwati, T.U.; Susilaningsih, D. Photofermentative hydrogen production using rhodobium marinum from bagasse and soy sauce wastewater. Int. J. Hydrog. Energy 2012, 37, 15436–15442. [Google Scholar] [CrossRef]
- Adessi, A.; McKinlay, J.B.; Harwood, C.S.; de Philippis, R. A rhodopseudomonas palustris nifa* mutant produces h2 from nh4+-containing vegetable wastes. Int. J. Hydrog. Energy 2012, 37, 15893–15900. [Google Scholar] [CrossRef]
- Alam, M.Z.; Jamal, P.; Nadzir, M.M. Bioconversion of palm oil mill effluent for citric acid production: Statistical optimization of fermentation media and time by central composite design. World J. Microbiol. Biotechnol. 2008, 24, 1177–1185. [Google Scholar] [CrossRef]
- Akil, K.; Jayanthi, S. The biohydrogen potential of distillery wastewater by dark fermentation in an anaerobic sequencing batch reactor. Int. J. Green Energy 2014, 11, 28–39. [Google Scholar] [CrossRef]
- Kars, G.; Ceylan, A. Biohydrogen and 5-aminolevulinic acid production from waste barley by rhodobacter sphaeroides o.U.001 in a biorefinery concept. Int. J. Hydrog. Energy 2013, 38, 5573–5579. [Google Scholar] [CrossRef]
- Pan, C.; Fan, Y.; Hou, H. Fermentative production of hydrogen from wheat bran by mixed anaerobic cultures. Ind. Eng. Chem. Res. 2008, 47, 5812–5818. [Google Scholar] [CrossRef]
- Han, W.; Wang, X.; Ye, L.; Huang, J.; Tang, J.; Li, Y.; Ren, N. Fermentative hydrogen production using wheat flour hydrolysate by mixed culture. Int. J. Hydrog. Energy 2015, 40, 4474–4480. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F.; Oztekin, R.; Argun, H. Bio-hydrogen production from acid hydrolyzed wheat starch by photo fermentation using different rhodobacter sp. Int. J. Hydrog. Energy 2009, 34, 2201–2207. [Google Scholar] [CrossRef]
- Kargi, F.; Eren, N.S.; Ozmihci, S. Bio-hydrogen production from cheese whey powder (cwp) solution: Comparison of thermophilic and mesophilic dark fermentations. Int. J. Hydrog. Energy 2012, 37, 8338–8342. [Google Scholar] [CrossRef]
- Pattanamanee, W.; Choorit, W.; Deesan, C.; Sirisansaneeyakul, S.; Chisti, Y. Photofermentive production of biohydrogen from oil palm waste hydrolysate. Int. J. Hydrog. Energy 2012, 37, 4077–4087. [Google Scholar] [CrossRef]
- Hu, B.-B.; Li, M.-Y.; Wang, Y.-T.; Zhu, M.-J. High-yield biohydrogen production from non-detoxified sugarcane bagasse: Fermentation strategy and mechanism. Chem. Eng. J. 2018, 335, 979–987. [Google Scholar] [CrossRef]
- Sangyoka, S.; Reungsang, A.; Lin, C.-Y. Optimization of biohydrogen production from sugarcane bagasse by mixed cultures using a statistical method. Sustain. Environ. Res. 2016, 26, 235–242. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Ge, X.; Wang, Y.; Zhou, X.; You, X.; Liu, H.; Zhang, Q. Bio-hydrogen production from apple waste by photosynthetic bacteria hau-m1. Int. J. Hydrog. Energy 2016, 41, 13399–13407. [Google Scholar] [CrossRef]
- Akinbomi, J.; Taherzadeh, M.J. Evaluation of fermentative hydrogen production from single and mixed fruit wastes. Energies 2015, 8, 4253–4272. [Google Scholar] [CrossRef]
- Argun, H.; Dao, S. Bio-hydrogen production from waste peach pulp by dark fermentation: Effect of inoculum addition. Int. J. Hydrog. Energy 2017, 42, 2569–2574. [Google Scholar] [CrossRef]
- Shi, X.-Y.; Jin, D.-W.; Sun, Q.-Y.; Li, W.-W. Optimization of conditions for hydrogen production from brewery wastewater by anaerobic sludge using desirability function approach. Renew. Energy 2010, 35, 1493–1498. [Google Scholar] [CrossRef]
- Cappelletti, M.; Bucchi, G.; Mendes, J.D.; Alberini, A.; Fedi, S.; Bertin, L.; Frascari, D. Biohydrogen production from glucose, molasses and cheese whey by suspended and attached cells of four hyperthermophilic thermotoga strains. J. Chem. Technol. Biotechnol. 2012, 87, 1291–1301. [Google Scholar] [CrossRef]
- Han, W.; Wang, B.; Zhou, Y.; Wang, D.-X.; Wang, Y.; Yue, L.-R.; Li, Y.-F.; Ren, N.-Q. Fermentative hydrogen production from molasses wastewater in a continuous mixed immobilized sludge reactor. Bioresour. Technol. 2012, 110, 219–223. [Google Scholar] [CrossRef]
- Kothandapani, S.; Preetha, B. Effect of initial ph on biohydrogen production using distillery wastewater by batch process. J. Chem. Pharm. Sci. 2015, 8, 148–151. [Google Scholar]
- Moreno-Dávila, I.M.M.; Ríos-González, L.J.; Garza-García, Y.; Garza, J.A.R.-D.L.; Rodríguez-Martínez, J. Biohydrogen production from diary processing wastewater by anaerobic biofilm reactors. Afr. J. Biotechnol. 2011, 10, 5320. [Google Scholar]
- Zhu, H.; Ueda, S.; Asada, Y.; Miyake, J. Hydrogen production as a novel process of wastewater treatment—Studies on tofu wastewater with entrapped r. Sphaeroides and mutagenesis. Int. J. Hydrog. Energy 2002, 27, 1349–1357. [Google Scholar] [CrossRef]
- Zheng, G.H.; Wang, L.; Kang, Z.H. Feasibility of biohydrogen production from tofu wastewater with glutamine auxotrophic mutant of rhodobacter sphaeroides. Renew. Energy 2010, 35, 2910–2913. [Google Scholar] [CrossRef]
- Lay, C.-H.; Sen, B.; Huang, S.-C.; Chen, C.-C.; Lin, C.-Y. Sustainable bioenergy production from tofu-processing wastewater by anaerobic hydrogen fermentation for onsite energy recovery. Renew. Energy 2013, 58, 60–67. [Google Scholar] [CrossRef]
- Jamil, Z.; Annuar, M.S.M.; Ibrahim, S.; Vikineswary, S. Optimization of phototrophic hydrogen production by rhodopseudomonas palustris pbum001 via statistical experimental design. Int. J. Hydrog. Energy 2009, 34, 7502–7512. [Google Scholar] [CrossRef]
- Garritano, A.D.N.; de Sá, L.R.V.; Aguieiras, É.C.G.; Freire, D.M.G.; Ferreira-Leitão, V.S. Efficient biohydrogen production via dark fermentation from hydrolized palm oil mill effluent by non-commercial enzyme preparation. Int. J. Hydrog. Energy 2017, 42, 29166–29174. [Google Scholar] [CrossRef]
- Park, J.-I.; Lee, J.; Sim, S.J.; Lee, J.-H. Production of hydrogen from marine macro-algae biomass using anaerobic sewage sludge microflora. Biotechnol. Bioprocess Eng. 2009, 14, 307. [Google Scholar] [CrossRef]
- Nguyen, T.-A.D.; Kim, K.-R.; Nguyen, M.-T.; Kim, M.S.; Kim, D.; Sim, S.J. Enhancement of fermentative hydrogen production from green algal biomass of thermotoga neapolitana by various pretreatment methods. Int. J. Hydrog. Energy 2010, 35, 13035–13040. [Google Scholar] [CrossRef]
- Pattra, S.; Lay, C.-H.; Lin, C.-Y.; O-Thong, S.; Reungsang, A. Performance and population analysis of hydrogen production from sugarcane juice by non-sterile continuous stirred tank reactor augmented with clostridium butyricum. Int. J. Hydrog. Energy 2011, 36, 8697–8703. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, J.; Chen, G.D.; Feng, R.; Yang, J.; Zhao, Y.F.; Wei, Q.; Du, B.; Zhang, Y.F. Anaerobic biohydrogen production by the mixed culture with mesoporous Fe3O4 nanoparticles activation. Presented at Advanced Materials Research. Trans. Tech. Publ. 2011, 306, 1528–1531. [Google Scholar]
- Jayasinghearachchi, H.S.; Singh, S.; Sarma, P.M.; Aginihotri, A.; Lal, B. Fermentative hydrogen production by new marine clostridium amygdalinum strain c9 isolated from offshore crude oil pipeline. Int. J. Hydrog. Energy 2010, 35, 6665–6673. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Liu, J.; Zhang, X.; Li, F. Pretreatment enhanced structural disruption, enzymatic hydrolysis, fermentative hydrogen production from rice straw. Int. J. Hydrog. Energy 2022, 47, 11778–11786. [Google Scholar] [CrossRef]
- Zhang, H.; Bruns, M.A.; Logan, B.E. Biological hydrogen production by clostridium acetobutylicum in an unsaturated flow reactor. Water Res. 2006, 40, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.-L.; Rahim, R.A.; Shirai, Y.; Hassan, M.A. Biohydrogen production by clostridium butyricum eb6 from palm oil mill effluent. Int. J. Hydrog. Energy 2009, 34, 764–771. [Google Scholar] [CrossRef]
- Rao, R.; Basak, N. Optimization and modelling of dark fermentative hydrogen production from cheese whey by enterobacter aerogenes 2822. Int. J. Hydrog. Energy 2021, 46, 1777–1800. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef]
- Singh, V.; Das, D. Chapter 3—Potential of hydrogen production from biomass. In Science and Engineering of Hydrogen-Based Energy Technologies; de Miranda, P.E.V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 123–164. [Google Scholar]
- Massanet-Nicolau, J.; Dinsdale, R.; Guwy, A. Hydrogen production from sewage sludge using mixed microflora inoculum: Effect of ph and enzymatic pretreatment. Bioresour. Technol. 2008, 99, 6325–6331. [Google Scholar] [CrossRef]
- Xu, T.; Chen, D.; Hu, X. Hydrogen-activating models of hydrogenases. Coord. Chem. Rev. 2015, 303, 32–41. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, M.-S. Hydrogenases for biological hydrogen production. Bioresour. Technol. 2011, 102, 8423–8431. [Google Scholar] [CrossRef]
- Ghosh, S.; Dairkee, U.K.; Chowdhury, R.; Bhattacharya, P. Hydrogen from food processing wastes via photofermentation using purple non-sulfur bacteria (pnsb)–a review. Energy Convers. Manag. 2017, 141, 299–314. [Google Scholar] [CrossRef]
- Jang, S.; Kim, D.-H.; Yun, Y.-M.; Lee, M.-K.; Moon, C.; Kang, W.-S.; Kwak, S.-S.; Kim, M.-S. Hydrogen fermentation of food waste by alkali-shock pretreatment: Microbial community analysis and limitation of continuous operation. Bioresour. Technol. 2015, 186, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jarunglumlert, T.; Prommuak, C.; Putmai, N.; Pavasant, P. Scaling-up bio-hydrogen production from food waste: Feasibilities and challenges. Int. J. Hydrog. Energy 2018, 43, 634–648. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int. J. Hydrog. Energy 2014, 39, 7721–7729. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G. Comparative study of the effect of ultrasonication on the anaerobic biodegradability of food waste in single and two-stage systems. Bioresour. Technol. 2011, 102, 6449–6457. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Dhar, B.R.; Nakhla, G. Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int. J. Hydrog. Energy 2011, 36, 11379–11387. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Nakhla, G. Viability of ultrasonication of food waste for hydrogen production. Int. J. Hydrog. Energy 2012, 37, 2960–2964. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Hydrogen fermentation of food waste without inoculum addition. Enzym. Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- Kothari, R.; Kumar, V.; Pathak, V.V.; Ahmad, S.; Aoyi, O.; Tyagi, V. A critical review on factors influencing fermentative hydrogen production. Front. Biosci. 2017, 22, 1195–1220. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, M.-S.; Lee, J.K. Hydrogen evolution under photoheterotrophic and dark fermentative conditions by recombinant rhodobacter sphaeroides containing the genes for fermentative pyruvate metabolism of rhodospirillum rubrum. Int. J. Hydrog. Energy 2008, 33, 5131–5136. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, D.-H.; Kim, S.-H.; Yoon, J.-J.; Park, H.-D. Effect of substrate concentration on the competition between clostridium and lactobacillus during biohydrogen production. Int. J. Hydrog. Energy 2017, 43, 11460–11469. [Google Scholar] [CrossRef]
- Dong-Jie, N.; Jing-Yuan, W.; Bao-Ying, W.; You-Cai, Z. Effect of mo-containing additives on biohydrogen fermentation from cassava’s stillage. Int. J. Hydrog. Energy 2011, 36, 5289–5295. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.-S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Maneeruttanarungroj, C.; Phunpruch, S. Effect of ph on biohydrogen production in green alga tetraspora sp. Cu2551. Energy Procedia 2017, 138, 1085–1092. [Google Scholar] [CrossRef]
- Wang, B.-N.; Yang, C.-F.; Lee, C.-M. The factors influencing direct photohydrogen production and anaerobic fermentation hydrogen production combination bioreactors. Int. J. Hydrog. Energy 2011, 36, 14069–14077. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrere, H.; Steyer, J.-P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrog. Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Sinha, P.; Pandey, A. An evaluative report and challenges for fermentative biohydrogen production. Int. J. Hydrog. Energy 2011, 36, 7460–7478. [Google Scholar] [CrossRef]
- Saady, N.M.C. Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: Unresolved challenge. Int. J. Hydrog. Energy 2013, 38, 13172–13191. [Google Scholar] [CrossRef]
- Pawar, S.S.; van Niel, E.W. Thermophilic biohydrogen production: How far are we? Appl. Microbiol. Biotechnol. 2013, 97, 7999–8009. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Choi, J.-A.; Oh, Y.-K.; Abou-Shanab, R.A.; Song, H.; Min, B.; Cho, Y.; Na, J.-G.; Koo, J.; Jeon, B.-H. Hydrogen production from sulfate-and ferrous-enriched wastewater. Int. J. Hydrog. Energy 2011, 36, 13984–13990. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Nakhla, G. Influence of iron on sulfide inhibition in dark biohydrogen fermentation. Bioresour. Technol. 2012, 126, 123–130. [Google Scholar] [CrossRef]
- Ahmadi-Pirlou, M.; Ebrahimi-Nik, M.; Khojastehpour, M.; Ebrahimi, S.H. Mesophilic co-digestion of municipal solid waste and sewage sludge: Effect of mixing ratio, total solids, and alkaline pretreatment. Int. Biodeterior. Biodegrad. 2017, 125, 97–104. [Google Scholar] [CrossRef]
- del Pilar Anzola-Rojas, M.; da Fonseca, S.G.; da Silva, C.C.; de Oliveira, V.M.; Zaiat, M. The use of the carbon/nitrogen ratio and specific organic loading rate as tools for improving biohydrogen production in fixed-bed reactors. Biotechnol. Rep. 2015, 5, 46–54. [Google Scholar] [CrossRef]
- Salerno, M.B.; Park, W.; Zuo, Y.; Logan, B.E. Inhibition of biohydrogen production by ammonia. Water Res. 2006, 40, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Elbeshbishy, E.; Nakhla, G. Optimization of biological hydrogen production for anaerobic co-digestion of food waste and wastewater biosolids. Bioresour. Technol. 2013, 130, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lay, C. Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int. J. Hydrog. Energy 2004, 29, 41–45. [Google Scholar] [CrossRef]
- Assawamongkholsiri, T.; Reungsang, A. Photo fermentational hydrogen production of rhodobacter sp. Kku-ps1 isolated from an uasb reactor. Electron. J. Biotechnol. 2015, 18, 221–230. [Google Scholar] [CrossRef]
- Kim, M.-S.; Baek, J.-S.; Lee, J.K. Comparison of h2 accumulation by rhodobacter sphaeroides kd131 and its uptake hydrogenase and phb synthase deficient mutant. Int. J. Hydrog. Energy 2006, 31, 121–127. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371. [Google Scholar] [CrossRef]
- Le, D.T.H.; Nitisoravut, R. Modified hydrotalcites for enhancement of biohydrogen production. Int. J. Hydrog. Energy 2015, 40, 12169–12176. [Google Scholar] [CrossRef]
- Junghare, M.; Subudhi, S.; Lal, B. Improvement of hydrogen production under decreased partial pressure by newly isolated alkaline tolerant anaerobe, clostridium butyricum tm-9a: Optimization of process parameters. Int. J. Hydrog. Energy 2012, 37, 3160–3168. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, Y. The influence of sodium on biohydrogen production from food waste by anaerobic fermentation. J. Mater. Cycles Waste Manag. 2009, 11, 244–250. [Google Scholar] [CrossRef]
- Yu, H.-Q.; Tay, J.-H.; Fang, H.H. The roles of calcium in sludge granulation during uasb reactor start-up. Water Res. 2001, 35, 1052–1060. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Christophe, G.; Fontanille, P.; Larroche, C. Biological upgrading of volatile fatty acids, key intermediates for the valorization of biowaste through dark anaerobic fermentation. Bioresour. Technol. 2013, 145, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Elreedy, A.; Ibrahim, E.; Hassan, N.; El-Dissouky, A.; Fujii, M.; Yoshimura, C.; Tawfik, A. Nickel-graphene nanocomposite as a novel supplement for enhancement of biohydrogen production from industrial wastewater containing mono-ethylene glycol. Energy Convers. Manag. 2017, 140, 133–144. [Google Scholar] [CrossRef]
- Patel, S.K.; Choi, S.H.; Kang, Y.C.; Lee, J.-K. Large-scale aerosol-assisted synthesis of biofriendly fe 2 o 3 yolk–shell particles: A promising support for enzyme immobilization. Nanoscale 2016, 8, 6728–6738. [Google Scholar] [CrossRef]
- Otari, S.; Pawar, S.; Patel, S.K.; Singh, R.K.; Kim, S.-Y.; Lee, J.H.; Zhang, L.; Lee, J.-K. Canna edulis leaf extract-mediated preparation of stabilized silver nanoparticles: Characterization, antimicrobial activity, and toxicity studies. J. Microbiol. Biotechnol. 2017, 27, 731–738. [Google Scholar] [CrossRef]
- Beckers, L.; Hiligsmann, S.; Lambert, S.D.; Heinrichs, B.; Thonart, P. Improving effect of metal and oxide nanoparticles encapsulated in porous silica on fermentative biohydrogen production by clostridium butyricum. Bioresour. Technol. 2013, 133, 109–117. [Google Scholar] [CrossRef]
- Mohanraj, S.; Anbalagan, K.; Kodhaiyolii, S.; Pugalenthi, V. Comparative evaluation of fermentative hydrogen production using enterobacter cloacae and mixed culture: Effect of Pd(ii) ion and phytogenic palladium nanoparticles. J. Biotechnol. 2014, 192, 87–95. [Google Scholar] [CrossRef]
- Mohanraj, S.; Anbalagan, K.; Rajaguru, P.; Pugalenthi, V. Effects of phytogenic copper nanoparticles on fermentative hydrogen production by enterobacter cloacae and clostridium acetobutylicum. Int. J. Hydrog. Energy 2016, 41, 10639–10645. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Influence of nickel and hematite nanoparticle powder on the production of biohydrogen from complex distillery wastewater in batch fermentation. Int. J. Hydrog. Energy 2015, 40, 10734–10743. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J. Enhancement effect of gold nanoparticles on biohydrogen production from artificial wastewater. Int. J. Hydrog. Energy 2007, 32, 17–23. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Du, B.; Wei, D.; Wei, Q.; Zhao, Y. Enhancement effect of silver nanoparticles on fermentative biohydrogen production using mixed bacteria. Bioresour. Technol. 2013, 142, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The effects of fe0 and ni0 nanoparticles versus fe2+ and ni2+ ions on dark hydrogen fermentation. Int. J. Hydrog. Energy 2016, 41, 167–173. [Google Scholar] [CrossRef]
- Nath, D.; Manhar, A.K.; Gupta, K.; Saikia, D.; Das, S.K.; Mandal, M. Phytosynthesized iron nanoparticles: Effects on fermentative hydrogen production by enterobacter cloacae dh-89. Bull. Mater. Sci. 2015, 38, 1533–1538. [Google Scholar] [CrossRef]
- Mahmood, T. Effect of iron nanoparticles on hyacinth’ s fermentation. Int. J. Sci. 2013, 2, 106–121. [Google Scholar]
- Zhang, L.; Zhang, L.; Li, D. Enhanced dark fermentative hydrogen production by zero-valent iron activated carbon micro-electrolysis. Int. J. Hydrog. Energy 2015, 40, 12201–12208. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Liu, M.; Zhou, J.; Cen, K. Enhanced dark hydrogen fermentation by addition of ferric oxide nanoparticles using enterobacter aerogenes. Bioresour. Technol. 2016, 207, 213–219. [Google Scholar] [CrossRef]
- Nasr, M.; Tawfik, A.; Ookawara, S.; Suzuki, M.; Kumari, S.; Bux, F. Continuous biohydrogen production from starch wastewater via sequential dark-photo fermentation with emphasize on maghemite nanoparticles. J. Ind. Eng. Chem. 2015, 21, 500–506. [Google Scholar] [CrossRef]
- Reddy, K.; Nasr, M.; Kumari, S.; Kumar, S.; Gupta, S.K.; Enitan, A.M.; Bux, F. Biohydrogen production from sugarcane bagasse hydrolysate: Effects of ph, s/x, Fe2+, and magnetite nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 8790–8804. [Google Scholar] [CrossRef]
- Dolly, S.; Pandey, A.; Pandey, B.K.; Gopal, R. Process parameter optimization and enhancement of photo-biohydrogen production by mixed culture of rhodobacter sphaeroides nmbl-02 and escherichia coli nmbl-04 using fe-nanoparticle. Int. J. Hydrog. Energy 2015, 40, 16010–16020. [Google Scholar] [CrossRef]
- Zada, B.; Mahmood, T.; Malik, S.A. Effect of zinc oxide nanoparticles on hyacinth’s fermentation. Int. J. Enhanc. Res. Sci. Technol. Eng. 2014, 3, 78–92. [Google Scholar]
- Pandey, A.; Gupta, K.; Pandey, A. Effect of nanosized tio2 on photofermentation by rhodobacter sphaeroides nmbl-02. Biomass Bioenergy 2015, 72, 273–279. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y. Nano-tio2 enhanced photofermentative hydrogen produced from the dark fermentation liquid of waste activated sludge. Environ. Sci. Technol. 2011, 45, 8589–8595. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, L.; Torzillo, G. Hydrogen production with the microalga chlamydomonas reinhardtii grown in a compact tubular photobioreactor immersed in a scattering light nanoparticle suspension. Int. J. Hydrog. Energy 2012, 37, 16951–16961. [Google Scholar] [CrossRef]
- Mullai, P.; Yogeswari, M.K.; Sridevi, K. Optimisation and enhancement of biohydrogen production using nickel nanoparticles—A novel approach. Bioresour. Technol. 2013, 141, 212–219. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Nanoparticles in biological hydrogen production: An overview. Indian J. Microbiol. 2018, 58, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Tahir, A.; Shah, S.A.Q.; Tsuzuki, T.; Nisbet, D.; Chen, J.; Rehman, Y. Effect of phyto-fabricated nanoscale organic-iron complex on photo-fermentative hydrogen production by rhodopseudomonas palustris mp2 and rhodopseudomonas palustris mp4. Biomass Bioenergy 2020, 140, 105667. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, F.; Zheng, H.; Zhang, C.; Wei, F.; Xing, X.-H. Enhanced hydrogen production in a uasb reactor by retaining microbial consortium onto carbon nanotubes (cnts). Int. J. Hydrog. Energy 2012, 37, 10619–10626. [Google Scholar] [CrossRef]


| Type | Feedstock | Fermentation Process | Substrate Conc. | pH | Hydrogen % Yield | Ref. |
|---|---|---|---|---|---|---|
| Solid food waste | Wheat bran and flour | Anaerobic degradation | - | 5.0 | 0.13 L/g | [78] |
| Dark fermentation | 10.7 g/L | 4.5 | 0.24 L/g glucose | [79] | ||
| Photofermentation | 5 g/L | 7.0 | 0.15 L/g glucose | [80] | ||
| Waste barley | Photofermentation | 11 g/L | 7.0 | 0.4 L/L culture | [77] | |
| Cheese whey powder | Dark fermentation | - | - | 0.11 L/g sugar | [81] | |
| Oil palm waste | Photofermentation | - | 7.0 | 0.02 L/L/h | [82] | |
| Sugar cane bagasse | Photofermentation | - | 7.0 | 0.04 L | [73] | |
| 10% w/v | 7.0 | 0.35 L/L | [42,83] | |||
| Dark fermentation | 22.8 g/L | 6.0 | 6.98 L/L | [84] | ||
| - | 6.8 | 0.76 L/L | [42] | |||
| Two-stage anaerobic digestion | - | 6.5–7.0 | 6.2 L/L | |||
| Vegetable and fruit waste | Photofermentation | - | 6.8 | 0.004 L/L/h | [74] | |
| - | 7.1 | 0.11 L/g | [85] | |||
| Dark fermentation | - | 5.0 | 0.51 L/g volatile solid | [86] | ||
| - | 5.9 | 0.12 L/g TOC | [87] | |||
| Food and beverage industry wastewater | Brewing industry wastewater | Anaerobic dark fermentation | - | 5.95 | 0.15 L /g COD | [88] |
| Photofermentation | 10% v/v | 7.2 | 2.24 L/L medium | [37] | ||
| Sugarcane and sugar beet molasses | Photofermentation | 28 g/L | 7.0 | 1.01 L/L culture | [65] | |
| 10 mM | 7.5 | 1.24 L/g sucrose | [66] | |||
| Dark fermentation | - | - | 0.37 L/g glucose | [89] | ||
| - | 5.5 | 0.02 L/g glucose | [90] | |||
| Distillery wastewater | Dark fermentation | - | 5.8 | 0.04 L/g COD | [76] | |
| Photofermentation | - | 6.5 | - | [91] | ||
| Soy sauce wastewater | Photofermentation | - | 7.0 | 0.2 L | [73] | |
| Dairy industry wastewater | Dark fermentation | 21.1 g/L | 7.0 | 0.29 L/g COD | [92] | |
| Tofu wastewater | Photofermentation | - | 7.0 | 2.2 L/L | [93] | |
| - | 7.2 | - | [94] | |||
| Dark fermentation | 20 g COD/L | 5.5 | 0.11 L/g COD | [95] | ||
| Palm oil mill wastewater | Photofermentation | - | 6.0 | 0.66 L/L POME | [96] | |
| Dark fermentation | 48 g/L | 4.5 | 0.06 L/g | [97] | ||
| Algal biomass | Dark fermentation | 50 g/L | 7.5 | 0.07 L/L/h | [98] | |
| 5.0 g/L | 7.4 | 0.31 L/g glucose | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwal, F.; Torriero, A.A.J. Biohydrogen—A Green Fuel for Sustainable Energy Solutions. Energies 2022, 15, 7783. https://doi.org/10.3390/en15207783
Kanwal F, Torriero AAJ. Biohydrogen—A Green Fuel for Sustainable Energy Solutions. Energies. 2022; 15(20):7783. https://doi.org/10.3390/en15207783
Chicago/Turabian StyleKanwal, Fariha, and Angel A. J. Torriero. 2022. "Biohydrogen—A Green Fuel for Sustainable Energy Solutions" Energies 15, no. 20: 7783. https://doi.org/10.3390/en15207783
APA StyleKanwal, F., & Torriero, A. A. J. (2022). Biohydrogen—A Green Fuel for Sustainable Energy Solutions. Energies, 15(20), 7783. https://doi.org/10.3390/en15207783







