Abstract
Ricinus communis is a species distributed worldwide. Its seeds are used to produce castor oil, which can be used for the production of biofuels; yield improvement can be achieved with elicitors that are substances of biological origin that can induce increased productivity of primary and secondary metabolism, when applied to plants. Salicylic acid (SA) is a natural constituent of plants, and applied exogenously acts as an elicitor. The aim of this work is to evaluate the oil content of castor bean plants elicitated with 900, 600, 300, and 100 µM of salicylic acid and its emissions derived from biodiesel made with the oil in blends (0, 10 and 20%) with commercial fuel in a 296 cc diesel cycle engine; elicitation was foliar sprayed. The oil content increased 39% when 900 µM SA was applied compared to control, and the evaluation of emissions showed the maximum reduction with 20% of Ricinus communis biodiesel (RCB) in all different RPM rates. Otherwise, the use of SA could be a method to increase oil content in castor plants as there is no difference in emission reduction derived from the SA application compared to control.
1. Introduction
Castor oil plant (Ricinus communis), a member of the Euphorbiaceae family, is a native of tropical Africa and is being cultivated around the world on tropical weather [1]. Castor oil is obtained from this plant and generates one of the highest yields in weight, with around 50% of to the weight of seed; this oil is constituted of fatty acids: ricinoleic acid 82%, 6% linoleic acid, 5% oleic acid, 3% stearic acid, 2% palmitic acid, and 2% linolenic acid [2]. Castor oil is viscous, pale yellow, non-volatile, and used as a purgative. This oil is more versatile than other vegetable oils, due to its unique structure; it is widely used as a raw material to produce various chemical products such as paints, coatings, inks, lubricants, and a wide variety of other products [3]. In recent years, it has been used to produce biofuels such as bioturbosine and biodiesel [4].
Castor oil is the only source oil of hydroxy fatty acid (12-hydroxyoleic acid, 18:1 OH), used for the production of different products [5], and in comparison to other oils, its application in the biofuel industry does not create and alimentary crisis and does not elevate critical environmental preoccupations [6,7,8,9,10]. Due to the high industrial use and cost of approximately USD 2500 per ton [11], it is very important to have higher yields than what is reported today, which is 36–50% [12]. To increase the yield, different techniques can be carried out; one of them is elicitation, which can improve the immune system and also the protection of plants, productivity, and the quality after the application of this [13]. Elicitors are substances that can induce increased productivity, defensive responses, and activate the biochemical system, among other reported activities, when applied to plant tissues or cells [14]. In addition, its application in some crops helps to increase the production of primary and secondary metabolites [15]. There are several substances that can be used as elicitors—for example, salicylic acid, jasmonic acid, hydrogen peroxide, among others [16].
Salicylic acid (SA) is a natural compound [17]. It can act as a regulator on the balance of plant cells, induce morphological and physiological responses, and participate in the activity of catalase and other enzymes responsible for controlling oxidation in mitochondria [2]. In addition, it is considered as a bioregulator of growth and its application in plants favors the increase in foliar biomass, fruits, and roots. It has also been documented that SA induces biotic and abiotic responses and enhances defense in plants affected by pathogens [18]. It has been found that the application of SA in Jatropha curcas (Euphorbiaceae), the same family as castor oil plants, increases the production of secondary metabolite by cell culture [19]. In addition, elicitation with SA has shown interesting results in Ricinus communis plants with other specialized metabolites such as the alkaloid ricinine [20].
Gorni and Pacheco [21] also showed an increase in oil content in 58% with the application of 0.50 mM SA to Achillea millefolium. The same was observed by Estaji and Niknam [22], who showed an increased seed oil content of 7% when Silybum marianum was elicitated with SA (1 Mm). In addition, Momeni et al. [23] observed that the oil content of Thymbra spicata increased 48% with the foliar application of SA at 5.0 mM. On the other hand, there are no studies of elicitation of ricinoleic acid, but, with the realization of this study, it is observed that the application of SA at the concentration proved that there was no beneficial effect.
It is known that the CO and CO2 emission levels reduce with the usage of Ricinus communis biodiesel due to two principal factors, the quantity of oxygen in RCB that completes combustion and the lower carbon content compared to diesel fuel [24]. The objective of the present work was to evaluate the oil content of Ricinus communis plants treated with different concentrations of salicylic acid, and also its emissions in a diesel cycle engine derived from the use of blends with conventional diesel.
2. Materials and Methods
2.1. Experimental Conditions
The castor oil plant was cultivated from July of 2018 to February of 2019 in a greenhouse of the Amazcala Campus from the Faculty of Engineering of the Autonomous University of Querétaro in the Municipally of El Marques, of the state of Querétaro, Mexico located at the coordinates: 20°42′18.576″ N latitude, 100°15′57.24″ W longitude. Experiment weather conditions were 27.8 ± 4.7 °C, a photoperiod of 14 h/10 h, and 43% of relative humidity.
2.2. Plant Materials
The model plant selected in this study was castor bean (Ricinus communis). A Guanajuatoil variety was used, and the seed was provided by National Institute of Forestry, Agricultural and Livestock Research (INIFAP) Celaya experimental field. The experiment was carried out with a total of 300 plants, divided into 4 replicates and 5 treatments with 15 experimental units each. Guanajuatoil is a variety of free pollination developed for rainfed conditions, which originated from 56 collections made and evaluated in yield trials in the state of Guanajuato.
Sowing was carried out on 24 July 2019. Seeds were sown, at 3 cm each, with a distance between each plant of 70 cm, and 100 cm between groves. The greenhouse had 10 lines, where 30 seeds were placed per line. Simultaneously in a seed bed, 105 plants were planted, this to be able to transplant the plants that did not germinate to the greenhouse. Of the plants that did not germinate, the sprout seedlings were transferred to the greenhouse when they presented 3 to 4 true leaves, as well as the plants where there were two emergencies in the greenhouse.
Weeding activities and cleaning of the greenhouse area were carried out every 2 days to prevent the proliferation of weeds.
The fertilization dosage was 60-40-00 NPK (Nitrogen, Phosphorus, Potassium), giving an application of half of the N and the total of P the day sowed. Forty-five days later, a second application with the rest of N was given [25].
2.3. Elicitation Process and Harvest
After sowing, there was an acclimatization period of 15 days. Subsequently, morphological measurements were made every 7 days until day 42. Finally, the elicitation was carried out on day 60. Elicitation was carried out on 21 September 2019 with SA, by foliar application using a fumigation tank in which the different salicylic acid solutions to be evaluated were placed independently: 100, 300, 600, and 900 µM, and by drench applying 50 mL of the same solutions with a 50 mL beaker simultaneously. Control was treated with distilled water.
Harvest took place on 18 December 2019. The fruits were collected in bags perfectly identified with the type of treatment received. Later, they were taken to the Laboratory of Natural Compounds and Insecticide of the Faculty of Chemistry of the Autonomous University of Queretaro. Finally, the fruits were dried in the sun for two weeks to be able to extract the seeds more easily. The seeds were stored at room temperature in perfectly identified paper bags, protected from humidity and in the dark to avoid the proliferation of microorganisms.
2.4. Plant Measurements
Morphological measurements were made of the plants. Two measurements of the plants were taken: the diameter at ground level and the height measured from ground level to the tip. These measurements were carried out every 7 days for 42 days to observe homogeneity before elicitation. The stem diameter and height of the plants were measured with a measuring tape (Trupper H-1766, Jilotepec, State of Mexico, Mexico) before applying the elicitor salicylic acid at day 60.
2.5. Soxhlet Extraction
For the oil extraction, Soxhlet methodology was followed. Samples of 30 g of seed were weighed and inserted into a Soxhlet extractor connected to a 500 mL flask containing 250 mL of n-hexane (Sigma-Aldrich, Toluca, State of Mexico, Mexico). Extraction was conducted at boiling temperature. When the extraction finished, an ice bath was placed so the sample temperature does not increase significantly. Then, the samples were centrifuged at 110 RPM and separated into layers to remove the solvent with a rotary evaporator IKA RV 10 basic at 55 °C and 110 RPM (Staufen im Breisgau, BW, Germany). After the extraction process, the castor oil was weighed to determine the oil content.
Oil Extraction and Purification for Biodiesel Preparation
Oil was obtained from the seeds by mechanical pressing at 95 °C in an extractor press Zagaon Tech (Queretaro, Mexico), 5 kg h−1. The oil was filtered in batches in a Kitasato flask (1 L) and Büschner funnel (500 mL), with a vacuum pump at 500 mm HG to separate the solids resulting from the endosperm of the seed and the oil.
2.6. Oil Characterization
BSTFA (N, O-bis [trimethylsilyl] trifluoroacetamide) (Sigma-Aldrich, Toluca, State of Mexico, Mexico) was used as a derivatizing agent, added to the castor oil sample and stirred for 2 min at room temperature. Subsequently, 200 μL were injected for the analysis. GC-MS chromatograph, Agilent 7890A series (Wilmington, DE, USA) and an Agilent simple quadrupole MS detector (model 5975C) were used. The equipment used consisted of a GC with an electron energy set at 70 eV and a mass range of 50–700 m/z, an HP-5MS capillary column (30 m × 0.25 mm internal diameter × 0.25 mm), and the splits/splitless injector (2 mm diameter). The temperature of the injector was 250 °C. GC was used in splitless mode with a time of 2.5 min without splitless. The initial oven temperature was 100 °C for 1 min and rose to 220 °C at 6 °C/min, held for 1.23 min, raised to 290 °C at 10 °C/min, then raised to 310 °C at 40 °C/min, and held for 7.5 min. The carrier gas (Helium) flow rate was maintained at 1 mL/min. GC-MS control and data processing were performed with Chem-Station software (Agilent Technologies, Santa Clara, CA, USA).
2.7. Biodiesel Production
Batch transesterification was carried out in 600 mL beaker, at a constant temperature and stirring of 55 °C and 250 rpm, respectively, a 6:1 methanol/oil molar ratio was used and 1% by weight of oil was used for the catalyst. A solution of previously crushed NaOH (Sigma-Aldrich, Toluca, State of Mexico, Mexico) and CH3OH (MERCK KGaA, Darmstadt, Germany) was prepared (sodium methoxide). The oil was brought to an approximate temperature of 55 °C in a heating dish with stirring and sodium methoxide was added; forty minutes were taken for the batches. The contents of the reaction were placed in separatory funnels of 1l and allowed to stand for 12 h; the glycerol and the methyl esters were separated. Washes were carried out with distilled water in a 1:1 (v/v) ratio, in triplicate for each batch of biodiesel with stirring at room temperature, preventing emulsification. The water was separated by separation funnel. Finally, the washed biodiesel was subjected to drying in heating dish at 110 °C for 10 min to evaporate remaining water.
Biodiesel yield was calculated by the relation between purified biodiesel and oil used for transesterification for all the experiments.
2.8. Emission Tests
RCB was used in a concentration of 0, 10, and 20% (B0, B10, and B20) in mixtures with conventional diesel fuel (PEMEX) in a diesel cycle engine (Mpower 178 FD, 6 HP/3600 rpm 296 cm3); CO and CO2 were determined by electrochemical and non-dispersive infrared sensors, respectively, while opacity was determined by the extinction coefficient method; additionally, temperature was determined by a type J thermocouple. The CO and CO2 emissions were measured from the beginning of the experiment with the sensor directly pointed at the engine exhaust tip at a safe distance of 1.5 m, preventing the temperature rise from degrading the sensor housing, the variables in this experiment were biodiesel content (B0, B10 and B20), Engine RPM (1750, 2850 and 3500) SA content was present in sowing for seed harvest, oil preparation, and biodiesel obtention (0, 100, 300, 600, and 900 µM). For opacity and temperature, tests were carried out the same way as CO and CO2 tests; in particular, the opacity probe and the thermocouple were disposed directly at the exhaust tip, to ensure the conditions of operation of both instruments.
Engine was preheated with B0 for 20 min, and residual fuel was removed from the deposit for first run, mixtures were tested randomly according to the experimental design, and, assuring the removal of residual fuel by the end of the measuring of the variables, to assure the reliability of the data, the engine was heated for 5 min with each mixture. A constant load of 100 N was present in the engine crank for all experiments; relative humidity and temperature were not controlled or recorded.
2.9. Ricinus communis Biodiesel Physicochemical Properties
For each experiment with SA, RCB was prepared, and its physicochemical properties were determined by triplicate including Density (by weight/volume relation), viscosity (by digital viscometer), Ash content (by ASTM D482 methodology), Calorimetry (by differential sweep calorimetry), Inflammability point (by thermos-gravimetric analysis), and pH (by pH meter).
2.10. Statistical Analysis for Oil Content and Emissions
The oil content was performed with three replications; in addition, the ricinoleic acid content, ANOVA (p = 0.05), and Tuckey’s multiple comparison (0.05%) tests were carried out for the statistical significance analysis using SYSTAT software 9.0. For emission tests, two replications were made in a completely randomized factorial, using RPM, biodiesel proportion, SA content, CO, CO2, opacity, and emissions temperature with ANOVA (p = 0.05) tests were carried out using R project for statistical analysis ver. 4.2.
3. Results and Discussion
3.1. Plant Morphology Measurement and Seed Weight
The stem diameter and height of the plants were measured after acclimatization of 15 days, every 7 days to observe if there was homogeneity in the growth. In addition, the weight of 1000 seeds was measured after harvest (Figure 1). The range of the stem diameter was 4.09 cm to 4.89 cm at the first measurement, while the last measurement at day 42 ranged from 8.7 cm to 9.2 cm. The height range of plants for the first measurement on day 7 was from 51.1 cm to 58.3 cm, and the range after day 42 was from 195.7 cm to 216.3 cm. The weight of the bunches was calculated per hectare having a theorical density of 14,300 plants, the weight ranged from 4283 kg ha−1 to 5887 kg ha−1, and the weight of 1000 seeds ranged from 357.4 to 511.1 (Table 1). The total weight of bunches means that there was homogeneity in the morphological characteristics of the plants as compared with the weight of 1000 seeds where a statistical difference was found, the plants treated with 900 µM of SA having a higher weight. This study showed homogeneity in the growth of all plants. Expected results were obtained, which was a homogeneous growth of the crop. Studies carried out by other authors show similar results in the total weight of the bunches (kg ha−1) and weight of 1000 seeds. In addition, in Table 1, the total weight of the bunches and weight of 1000 seeds showed a hormesis curve with the peak at 300 µM of SA, which had a weight of 4283 kg ha−1 and 357.4 g, respectively.

Figure 1.
Bunches, seeds, and weight of 1000 seeds of Ricinus communis plants.

Table 1.
Weight of bunches and 1000 seeds of Ricinus communis plants treated with different concentrations of SA.
3.2. Oil Content and Characterization of Ricinus communis Seeds
There was an increase in the oil content due to the elicitation process with different concentrations of SA. Ricinus communis plants with no elicitation have 36.2% of oil content and a concentration of 900 µM of 50.2%. This corresponds to an increase of 38% compared to the plants without elicitation. On the other hand, ricinoleic acid content decreases 4%; plants without elicitation have 74.7% compared to those elicitated with 900 µM having 71.7%. Likewise, an average composition of the following fatty acids was obtained: Ricinoleic acid 73.4%, Linolenic acid 12.4%, Oleic acid 6.5%, Stearic acid 3.0%, Linoleic acid 2.5%, and Palmitic acid 2.09% (Table 2).

Table 2.
Fatty acid and oil content of Ricinus communis plants treated with salicylic acid at different concentrations.
Since the Guanajuatoil variety is an improved species to produce in the open field, when it is produced in a greenhouse, a stress is generated. In the same way as when SA is applied, an eustresic effect is generated, which could affect the production of ricinoleic acid. Akbari et al. [26] reported a decrease of stearic acid on Carthamus tinctorius when applying drought stress. Another study by Shaki et al. [27] showed a decrease of oleic and palmitic acid under salt-stress and application of SA in Carthamus tinctorius. On the other hand, Zhou et al. [28] showed that, when heat stress is applied to Oilseed monkfish (Brassica napus L.), the concentration of different fatty acids decreased.
Many investigations report the promoting influence of SA on crops; some examples are: higher yield of common Phaseolus vulgaris [29], Allium cepa [30], Fragaria vesca [31], and SA in 2 mM resulted in better F. vesca performance than did other rates of these compounds compared to control [32].
Investigation of essential oil content result showed that application of 8 mM SA had the most essential oil content at full irrigation, water stress at stemming, and 50% flowering stages in Fennel [33]. On the other hand, interaction of water stress and SA on essential oil yield showed that application of 3 mM SA had the most essential oil yield under full irrigation SA plays a very important part in the regulation of some physiological processes in plants such as effects on growth and development, ion uptake, and transport and membrane permeability [34]. Miura and Tada [35] reported that the effects of SA on the physiological processes of plants depend on its concentration, type of plant, the stage of plant growth, and environmental conditions. Generally, low concentrations of SA may enhance the antioxidant capacity and tolerance to abiotic stresses, but high concentrations of SA may cause cell death or susceptibility to abiotic stresses [33,36].
The influence of elicitation with salicylic acid and chitosan significantly increased the content of secondary metabolites of the safflower callus to counteract the adverse effects of salinity stress [37]. Trees that received the application of salicylic acid at 400 ppm showed an increase in yield (32.7%) compared to plants that did not receive the application of any product. Similar results were obtained by Khodary [38], who found that application of SA increased fruit weight and fruit number on barley. The role of salicylic acid was reported by Raskin et al. [39], who stated that SA acted as an endogenous raise regulator of flowering and florigenic purposes.
On the other hand, it was observed that the composition of fatty acid of the Guanajuatoil variety as compared to other varieties differed in the concentration of Ricinoleic acid by 18% less and Linolenic acid by 12% more. Geethanjali et al. [40] reported the following composition of fatty acid in castor oil: Ricinoleic 89.6 %, Oleic 3.2%, Linoleic 3.6%, Stearic 1.9%, and Palmitic 1.7%. Another study by Román-Figueroa et al. [41] also shows a similar composition of fatty acid in castor oil: Ricinoleic 89.6%, Linolenic 0.5%, Oleic 2.7%, Linoleic 4.9%, Stearic 1.2%, and Palmitic 1.1%. In the same way, Huang et al. [42] found similar concentrations of fatty acid: Ricinoleic 88.6%, Linoleic 4.7%, Oleic 3.5%, Linolenic 0.6%, Stearic 1.1%, and Palmitic 1.1%. On the contrary, other studies found similar concentrations of ricinoleic acid. Jachmanian et al. [43] showed 72% ricinoleic acid in the Nordestina variety. Rivera-Brenes and Hernández-López [44] reported a similar composition in the ColBio HR 268 variety with 69% ricinoleic acid.
3.3. Emission Tests
In general, it is observed that there is a downward trend in emissions, opacity, and temperature with the increase in RCB content present in the mixture despite the application of SA in the sowing of RC.
Figure 2 and Figure 3 show the results of CO and CO2 emissions in ppm, having the maximum CO reduction (58.8%) with B20 @ 2850 RPM and CO2 reduction (45.4%) with B20 @ 1750 RPM for T3 and T5, respectively, compared to the maximum emissions recorded during the B0 experiments at the same RPM rate.
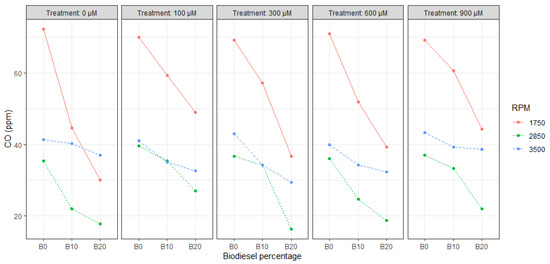
Figure 2.
Correlation plot between CO, SA content, RPM, and biodiesel percentage.
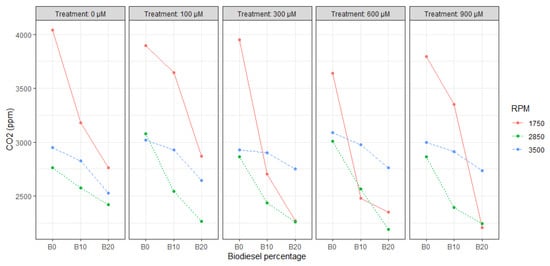
Figure 3.
Correlation plot between CO2, SA content, RPM, and biodiesel percentage.
For the opacity test, it can be shown (Figure 4) that there is a downward trend with the increase in RCB content present in the mixture despite the application of SA in the sowing of RC.
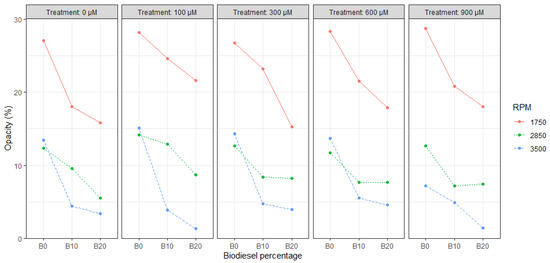
Figure 4.
Correlation plot between opacity, SA content, RPM, and biodiesel percentage.
For the opacity test, it can be shown (Figure 4) that the maximum reduction of 90% was present in B20 @ 3500 RPM for T2 compared to the maximum opacity recorded during the B0 experiments at the same RPM rate.
For temperature tests, the maximum reduction (28%) can be shown in B10 @ 3500 RPM with T2 compared to the maximum opacity recorded during the B0 experiments at the same RPM rate (Figure 5).
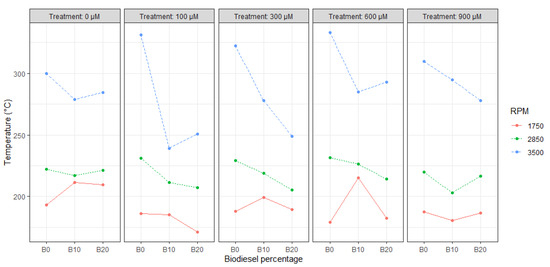
Figure 5.
Correlation plot between temperature, SA content, RPM, and biodiesel percentage.
3.4. Biodiesel of Ricinus communis Elicitated by Salicilyc Acid Effect on Emissions
There was a decrease of CO and CO2 emissions from 1750 to 2850 RPM due to the increase of biodiesel, which can be explained by RC biodiesel composition itself (by the presence of oxygen and because less carbon is present) [24,45] for every SA RCB. From 2850 to 3500 RPM, an augmentation of emissions was present for all experiments. These results are explained because there is more fuel consumption in a small amount of time due to a high RPM rate and more emissions being produced [24,45,46,47]. Otherwise, for 3500 RPM, a downward trend in emissions with B10 and B20 is shown, demonstrating that RC biodiesel helps to mitigate CO and CO2 emissions.
SA seems to not be of statistical significance in ANOVA tests contributing to the decrease in emissions. For opacity, a decrease is shown due to the RPM rate and the biodiesel quantity. This is explained because biodiesel contributes to completing the combustion of fuel due to the presence of oxygen explained before, and SA does not seem to affect the level of opacity. Finally, the temperature of emissions seems to be affected principally by the RPM rate because, with the increase of RPM, there is less time to allow the engine exhaust tip to cool and, because fuel burns faster and in more quantity, there seems to be a downward trend in temperature in relation to the biodiesel, which can be explained because, with the addition, the mixture can liberate less energy compared to B0 as less carbon is present [48]. SA does not seem to be statistically significant in the contribution of emissions and temperature and can only be attributed to RCB, as it reduces flame temperatures [47].
3.5. Physicochemical Properties of Ricinus communis Biodiesel
Means of replicates of physicochemical properties of RC biodiesel with standard deviation are listed in the next table (Table 3), and it can be shown that the treatments were not significant between each.

Table 3.
Physicochemical properties of Ricinus communis biodiesel.
Biodiesel transesterification yield was determined by total weight of oil and biodiesel used for experiments (Table 4).

Table 4.
Oil transesterification yield.
4. Conclusions
In this study, it was found that the application of SA in Ricinus communis plants was an effective method to improve oil content at a concentration of 900 µM. On the other hand, it was observed that the application of SA at different concentrations (100, 300, 600, and 900 µM) might not be a favorable method to increase ricinoleic acid concentration. Due to the importance of castor oil in many industrial applications, elicitation with SA at 900 µM by drench and spray may be an adequate strategy to increase its yield in the seeds of the Ricinus communis plant.
For emission tests, it was found that SA elicitation does not affect the opacity, levels of CO, CO2, and the temperature measures in B0, B10, and B20 concentrations with biodiesel prepared from oil obtained by this method in mixtures with conventional diesel in a diesel cycle engine with no modifications. However, it can be concluded that the addition of RC biodiesel in mixtures with conventional diesel helps to reduce the emissions of CO and CO2 as it helps with improving the combustion, and this can be reaffirmed with the measures of opacity.
Author Contributions
L.A.G.-C., M.A.R.-L. and C.E.Z.-G. conceived the experiments and wrote parts of the manuscript; J.C.-G. conducted the experiments; D.L.Q.-M. and L.A.M.-H. collected the data; F.J.D.M.-F. performed the plant measurements; J.A.R.-M. performed the statistical analysis; J.A.R.-M. designed the experiment; A.F.-M. performed the chemical analyses; L.A.G.-C., conducted the oil extraction, biodiesel preparation, and tests and article writing; C.E.Z.-G., reviewed and edited the manuscript; and M.A.R.-L. and A.A.F.-P. performed the statistical analysis and wrote part of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the National Council of Science and Technology, México (CONACYT) for Grant Nos. 714272 and 741646. In addition, the authors express their gratitude to Miguel Hernández Martínez of the National Institute of Forestry, Agricultural, and Livestock Research (INIFAP), who provided castor seed for this investigation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mondal, B.; Das, S.K. Comparative evaluation of mahua (Bassia latifolia) oil cake and castor bean (Ricinus communis) seed as fish toxicants for tilapia (Oreochromis mossambicus) and panchax (Aplocheilus panchax) with residual toxicity assessment on Labeo bata. Aquac. Res. 2019, 50, 2341–2349. [Google Scholar] [CrossRef]
- Singh, P.K.; Gautam, S. Role of salicylic acid on physiological and biochemical mechanism of salinity stress tolerance in plants. Acta Physiol. Plant. 2013, 35, 2345–2353. [Google Scholar] [CrossRef]
- Nekhavhambe, E.; Mukaya, H.E.; Nkazi, D.B. Development of castor oil–based polymers: A review. J. Adv. Manuf. Process. 2019, 1, e10030. [Google Scholar] [CrossRef]
- Baskar, G.; Aberna Ebenezer Selvakumari, I.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef]
- Chen, G.Q.; Johnson, K.; Morales, E.; Ibáñez, A.M.; Lin, J.-T. A high-Oil castor cultivar developed through recurrent selection. Ind. Crop. Prod. 2018, 111, 8–10. [Google Scholar] [CrossRef]
- Canoira, L.; Galeán, J.G.; Alcántara, R.; Lapuerta, M.; García-Contreras, R. Fatty acid methyl esters (FAMEs) from castor oil: Production process assessment and synergistic effects in its properties. Renew. Energy 2010, 35, 208–217. [Google Scholar] [CrossRef]
- Conceição, M.M.; Candeia, R.A.; Silva, F.C.; Bezerra, A.F.; Fernandes, V.J.; Souza, A.G. Thermoanalytical characterization of castor oil biodiesel. Renew. Sustain. Energy Rev. 2007, 11, 964–975. [Google Scholar] [CrossRef]
- Maleki, E.; Aroua, M.K.; Sulaiman, N.M.N. Castor oil—A more suitable feedstock for enzymatic production of methyl esters. Fuel Process. Technol. 2013, 112, 129–132. [Google Scholar] [CrossRef]
- Mukesh, D.; Iyer, R.S.; Wagh, J.S.; Mokashi, A.A.; Banerji, A.A.; Newadkar, R.V.; Bevinakatti, H.S. Lipase catalysed transesterification of castor oil. Biotechnol. Lett. 1993, 15, 251–256. [Google Scholar] [CrossRef]
- Panwar, N.; Shrirame, H.Y.; Rathore, N.; Jindal, S.; Kurchania, A. Performance evaluation of a diesel engine fueled with methyl ester of castor seed oil. Appl. Therm. Eng. 2010, 30, 245–249. [Google Scholar] [CrossRef]
- Dimian, A.C.; Iancu, P.; Plesu, V.; Bonet-Ruiz, A.-E.; Bonet-Ruiz, J. Castor oil biorefinery: Conceptual process design, simulation and economic analysis. Chem. Eng. Res. Des. 2019, 141, 198–219. [Google Scholar] [CrossRef]
- Leal, J.F.V.; Rios, I.H.; Méndez-Gallegos, S.D.J.; Ventura-Ramos, E.J.; Cuellar-Núñez, M.L.; Mosquera-Artamonov, J.D. Relación entre la composición química de la semilla y la calidad de aceite de doce accesiones de Ricinus communis L. Rev. Mex. De Cienc. Agrícolas 2017, 8, 1343–1356. [Google Scholar] [CrossRef][Green Version]
- Luciano, A.-J.; Irineo, T.-P.; Virginia, O.-V.R.; Feregrino-Perez, A.A.; Hernandez, A.C.; Gerardo, G.-G.R. Integrating Plant Nutrients and Elicitors for Production of Secondary Metabolites, Sustainable Crop Production and Human Health: A Review. Int. J. Agric. Biol. 2017, 19, 391–402. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Chitosan-induced antiviral activity and innate immunity in plants. Environ. Sci. Pollut. Res. 2015, 22, 2935–2944. [Google Scholar] [CrossRef]
- Garcia-Mier, L.; Jimenez-Garcia, S.N.; Guevara-González, R.G.; Feregrino-Perez, A.A.; Contreras-Medina, L.M.; Torres-Pacheco, I. Elicitor Mixtures Significantly Increase Bioactive Compounds, Antioxidant Activity, and Quality Parameters in Sweet Bell Pepper. J. Chem. 2015, 2015, 269296. [Google Scholar] [CrossRef]
- Jimenez-Garcia, S.N.; Vazquez-Cruz, M.A.; Guevara-González, R.G.; Torres-Pacheco, I.; Cruz-Hernandez, A.; Feregrino-Perez, A.A. Current Approaches for Enhanced Expression of Secondary Metabolites as Bioactive Compounds in Plants for Agronomic and Human Health Purposes. Pol. J. Food Nutr. Sci. 2013, 63, 67–78. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef]
- Tiwari, P.; Indoliya, Y.; Chauhan, A.S.; Singh, P.; Singh, P.K.; Singh, P.C.; Srivastava, S.; Pande, V.; Chakrabarty, D. Auxin-salicylic acid cross-talk ameliorates OsMYB–R1 mediated defense towards heavy metal, drought and fungal stress. J. Hazard. Mater. 2020, 399, 122811. [Google Scholar] [CrossRef]
- Mahalakshmi, R.; Eganathan, P.; Parida, A.; Parida, A. Salicylic acid elicitation on production of secondary metabolite by cell cultures of Jatropha Curcas L. Int. J. Pharm. Pharm. Sci. 2013, 5, 655–659. [Google Scholar]
- Zavala-Gómez, C.E.; Rodríguez-Deleón, E.; Bah, M.M.; Feregrino-Pérez, A.A.; Campos-Guillén, J.; Amaro-Reyes, A.; Rodríguez-Morales, J.A.; García-Trejo, J.F.; Flores-Macias, A.; Figueroa-Brito, R.; et al. Effect of Salicylic Acid in the Yield of Ricinine in Ricinus communis under Greenhouse Condition. Plants 2021, 10, 1902. [Google Scholar] [CrossRef]
- Gorni, P.H.; Pacheco, A.C. Growth promotion and elicitor activity of salicylic acid in Achillea millefolium L. Afr. J. Biotechnol. 2016, 15, 657–665. [Google Scholar] [CrossRef]
- Estaji, A.; Niknam, F. Foliar salicylic acid spraying effect’ on growth, seed oil content, and physiology of drought-stressed Silybum marianum L. plant. Agric. Water Manag. 2020, 234, 106116. [Google Scholar] [CrossRef]
- Momeni, M.; Pirbalouti, A.G.; Mousavi, A.; Badi, H.N. Effect of Foliar Applications of Salicylic Acid and Chitosan on the Essential Oil of Thymbra spicata L. under Different Soil Moisture Conditions. J. Essent. Oil Bear. Plants 2020, 23, 1142–1153. [Google Scholar] [CrossRef]
- Arunkumar, M.; Kannan, M.; Murali, G. Experimental studies on engine performance and emission characteristics using castor biodiesel as fuel in CI engine. Renew. Energy 2019, 131, 737–744. [Google Scholar] [CrossRef]
- Salvador, M.H.; Guadalupe, C.H.M.; Miguel, H.M.; Tomás, M.C. Guanajuatoil Variedad de Higuerilla Para la Extracción de Aceite Industrial Para Guanajuato. Guanajuato México, August 2018. Available online: https://es.scribd.com/document/447504555/4774-Produccion-de-semilla-de-higuerilla-Ricinus-communis-L-en-Guanajuato (accessed on 24 August 2022).
- Akbari, G.A.; Heshmati, S.; Soltani, E.; Dehaghi, M.A. Influence of Seed Priming on Seed Yield, Oil Content and Fatty Acid Composition of Safflower (Carthamus tinctorius L.) Grown Under Water Deficit. Int. J. Plant Prod. 2019, 14, 245–258. [Google Scholar] [CrossRef]
- Shaki, F.; Maboud, H.E.; Niknam, V. Effects of salicylic acid on hormonal cross talk, fatty acids profile, and ions homeostasis from salt-stressed safflower. J. Plant Interact. 2019, 14, 340–346. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, T.; Chen, X.; Li, Z.; Wu, D.; Hua, S.; Jiang, L. Effect of high night temperature on storage lipids and transcriptome changes in developing seeds of oilseed rape. J. Exp. Bot. 2018, 69, 1721–1733. [Google Scholar] [CrossRef]
- El-Shraiy, M.; Hegazi, A.M. Effect of acetylsalicylic acid, indole-3- bytric acid and gibberellic acid on plant growth and yield of pea (Pisum sativum L.). Aust. J. Basic Appl. Sci. 2009, 3, 3514–3523. [Google Scholar]
- Amin, A.; Rashad, E.M.; El-Abagy, H.M. Physiological Effect of Indole-3-Butyric Acid and Salicylic Acid on Growth, Yield and Chemical Constituents of Onion Plants. J. Appl. Sci. Res. 2007, 3, 1554–1563. [Google Scholar]
- Karlidag, H.; Yildirim, E.; Turan, M. Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci. Agric. 2009, 66, 180–187. [Google Scholar] [CrossRef]
- Aghaeifard, F.; Babalar, M.; Fallahi, E.; Ahmadi, A. Influence of humic acid and salicylic acid on yield, fruit quality, and leaf mineral elements of strawberry (Fragaria × Ananassa duch.) cv. Camarosa. J. Plant Nutr. 2016, 39, 1821–1829. [Google Scholar] [CrossRef]
- Ghilavizadeh, A.; Masouleh, E.H.; Zakerin, H.R.; Valadabadi, S.A.R.; Sayfzadeh, S.; Yousefi, M. Influence of Salicylic Acid on Growth, Yield and Macro-elements Absorption of Fennel (Foeniculum vulgare Mill.) under Water Stress. J. Med. Plants By-Prod. 2019, 8, 67–75. [Google Scholar] [CrossRef]
- Simaei, M.; Khavari-Nejad, R.A.; Bernard, F. Exogenous Application of Salicylic Acid and Nitric Oxide on the Ionic Contents and Enzymatic Activities in NaCl-Stressed Soybean Plants. Am. J. Plant Sci. 2012, 03, 1495–1503. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- Hara, M.; Furukawa, J.; Sato, A.; Mizoguchi, T.; Miura, K. Abiotic Stress and Role of Salicylic Acid in Plants. In Abiotic Stress Responses in Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 235–251. [Google Scholar] [CrossRef]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tissue Organ Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Khodary, S.E.A. Effect of Salicylic Acid on the Growth, Photosynthesis and Carbohydrate Metabolism in Salt Stressed Maize Plants. Int. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Raskin, I.; Ehmann, A.; Melander, W.R.; Meeuse, B.J.D. Salicylic Acid: A Natural Inducer of Heat Production in Arum Lilies. Science 1987, 237, 1601–1602. [Google Scholar] [CrossRef]
- Geethanjali, G.; Padmaja, K.V.; Prasad, R.B.N. Synthesis, Characterization, and Evaluation of Castor Oil-Based Acylated Derivatives as Potential Lubricant Base Stocks. Ind. Eng. Chem. Res. 2016, 55, 9109–9117. [Google Scholar] [CrossRef]
- Román-Figueroa, C.; Cea, M.; Paneque, M.; González, M.E. Oil Content and Fatty Acid Composition in Castor Bean Naturalized Accessions under Mediterranean Conditions in Chile. Agronomy 2020, 10, 1145. [Google Scholar] [CrossRef]
- Huang, F.; Bao, C.; Peng, M.; Zhu, G.; He, Z.; Chen, X.; Luo, R.; Zhao, Y. Chromatographic analysis of fatty acid composition in differently sized seeds of castor accessions. Biotechnol. Biotechnol. Equip. 2015, 29, 892–900. [Google Scholar] [CrossRef]
- Jachmanian, I.; Villamil, J.; Villamil, J.J. El Cultivo De Tártago (Ricinus communis L.) En El Uruguay: Información Preliminar,” Uruguay. 2009. Available online: http://www.inia.org.uy (accessed on 24 August 2022).
- Rivera-Brenes, P.A.; Hernández-López, J. Evaluación del rendimiento y calidad del aceite de siete variedades de Ricinus communis. Agron. Mesoam. 2015, 27, 183–190. [Google Scholar] [CrossRef][Green Version]
- Özcanli, M.; Serin, H.; Saribiyik, O.Y.; Aydin, K.; Serin, S. Performance and Emission Studies of Castor Bean (Ricinus communis) Oil Biodiesel and Its Blends with Diesel Fuel. Energy Sources Part A Recover. Util. Environ. Eff. 2012, 34, 1808–1814. [Google Scholar] [CrossRef]
- Azad, K.; Rasul, M. Performance and combustion analysis of diesel engine fueled with grape seed and waste cooking biodiesel. Energy Procedia 2019, 160, 340–347. [Google Scholar] [CrossRef]
- Bueno, A.V.; Pereira, M.P.B.; Pontes, J.V.D.O.; de Luna, F.M.T.; Cavalcante, C.L. Performance and emissions characteristics of castor oil biodiesel fuel blends. Appl. Therm. Eng. 2017, 125, 559–566. [Google Scholar] [CrossRef]
- Arunkumar, M.; Mohanavel, V.; Afzal, A.; Sathish, T.; Ravichandran, M.; Khan, S.; Abdullah, N.; Bin Azami, M.; Asif, M. A Study on Performance and Emission Characteristics of Diesel Engine Using Ricinus communis (Castor Oil) Ethyl Esters. Energies 2021, 14, 4320. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).