Use of Reduced Graphene Oxide to Modify Melamine and Polyurethane for the Removal of Organic and Oil Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Melamine–rGO and Polyurethane–rGO Foam Composites
2.2.1. Synthesis of rGO Precursors
2.2.2. Preparation of Composites
2.3. Characterization of Composites
2.4. Sorption Experiment Procedure
3. Results and Discussion
3.1. Synthesis of the rGO
3.2. Comparison of Obtained rGO–Foam Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, P.; Zou, C.; Zhong, H. The study of highly oil absorption polyurethane foam material and its application in the emergency disposal of hazardous chemicals. Adv. Mater. Res. 2012, 518–523, 847–853. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Pinto, J.; Fragouli, D.; Athanassiou, A. Facile oil removal from water-in-oil stable emulsions using PU foams. Materials 2018, 11, 2382. [Google Scholar] [CrossRef] [PubMed]
- Anju, M.; Renuka, N.K. Magnetically actuated graphene coated polyurethane foam as potential sorbent for oils and organics. Arab. J. Chem. 2020, 13, 1752–1762. [Google Scholar] [CrossRef]
- Lin, J.; Shang, Y.; Ding, B.; Yang, J.; Yu, J.; Al-Deyab, S.S. Nanoporous polystyrene fibers for oil spill cleanup. Mar. Pollut. Bull. 2012, 64, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, X.; Jiao, Y.; Shi, J. Fabrication of a superhydrophobic, fire-resistant, and mechanical robust sponge upon polyphenol chemistry for efficiently absorbing oils/organic solvents. Ind. Eng. Chem. Res. 2015, 54, 1842–1848. [Google Scholar] [CrossRef]
- Chen, X.; Weibel, J.A.; Garimella, S.V. Continuous Oil-Water Separation Using Polydimethylsiloxane-Functionalized Melamine Sponge. Ind. Eng. Chem. Res. 2016, 55, 3596–3602. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, D.; An, W.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A Robust and Cost-Effective Superhydrophobic Graphene Foam for Efficient Oil and Organic Solvent Recovery. Small 2015, 11, 5222–5229. [Google Scholar] [CrossRef]
- Si, H.; Liu, Q.; Fan, Z.; Wang, B.; Tong, Q.; Lin, M. A Durable Magnetic Superhydrophobic Melamine Sponge: For Solving Complex Marine Oil Spills. Nanomaterials 2022, 12, 2488. [Google Scholar] [CrossRef]
- Shui, Y.; Xian, Y.; Chen, L.; Li, M.; Yao, Y.; Zhang, Q. High oil absorbable superhydrophobic melamine sponges and evaluation in oil spill clean-ups. J. Appl. Polym. Sci. 2020, 137, 49306. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Tai, N.H.; Lee, S.B.; Kuo, W.S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 7908–7912. [Google Scholar] [CrossRef]
- Gupta, S.; He, W.-D.; Tai, N.H. A comparative study on superhydrophobic sponges and their application as fluid channel for continuous separation of oils and organic solvents from water. Compos. Part B Eng. 2016, 101, 99–106. [Google Scholar] [CrossRef]
- Dashairya, L.; Sahu, A.; Saha, P. Stearic acid treated polypyrrole-encapsulated melamine formaldehyde superhydrophobic sponge for oil recovery. Adv. Compos. Hybrid Mater. 2019, 2, 70–82. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Toyoda, M.; Inagaki, M. Sorption and recovery of heavy oils by using exfoliated graphite. Spill Sci. Technol. Bull. 2003, 8, 467–474. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, T.; Kose, H.S.; Karanfil, T. Adsorption of Aromatic Compounds by Carbonaceous Adsorbents: A Comparative Study on Granular Activated Carbon, Activated Carbon Fiber and Carbon Nanotubes. Environ. Sci. Technol. 2010, 12, 6377–6383. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Guan, Q.F.; Chen, L.F.; Zhu, Z.; Zhang, W.J.; Yu, S.H. Macroscopic-scale template synthesis of robust carbonaceous nanofiber hydrogels and aerogels and their applications. Angew. Chemie Int. Ed. 2012, 51, 5101–5105. [Google Scholar] [CrossRef]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon nanotube sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; El Kamash, A.M.; Ali, H.F.; Hung, Y.-T. Overview on Recent Trends and Developments in Radioactive Liquid Waste Treatment Part 1: Sorption/Ion Exchange Technique. Int. J. Environ. Eng. Sci. 2011, 2, 1–16. [Google Scholar]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef]
- Paşka, O.M.; Pacurariu, C.; Muntean, S.G. Kinetic and thermodynamic studies on methylene blue biosorption using corn-husk. RSC Adv. 2014, 4, 62621–62630. [Google Scholar] [CrossRef]
- Hou, X.X.; Deng, Q.F.; Ren, T.Z.; Yuan, Z.Y. Adsorption of Cu2+ and methyl orange from aqueous solutions by activated carbons of corncob-derived char wastes. Environ. Sci. Pollut. Res. 2013, 20, 8521–8534. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Singh, E.; Chen, Z.; Houshmand, F.; Ren, W.; Peles, Y.; Cheng, H.M.; Koratkar, N. Superhydrophobic graphene foams. Small 2013, 9, 75–80. [Google Scholar] [CrossRef]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced graphene oxide molecular sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-Doped Graphene and Its Application in Electrochemical Biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Ren, T.Z.; Liu, Y.P.; Yuan, Z.Y. In situ simultaneous reduction-doping route to synthesize hematite/N-doped graphene nanohybrids with excellent photoactivity. RSC Adv. 2014, 4, 31754–31758. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Wang, J.; Ren, Y.; Xuan, C.; Liu, C.; Shen, C. Superhydrophobic and superoleophilic porous reduced graphene oxide/polycarbonate monoliths for high-efficiency oil/water separation. J. Hazard. Mater. 2018, 344, 849–856. [Google Scholar] [CrossRef]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Liu, F.; Chung, S.; Oh, G.; Seo, T.S. Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. ACS Appl. Mater. Interfaces 2012, 4, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, Z.; Liang, W.; Yang, B.; Qin, X.; Zhao, X.; Pei, C.; La, P.; Li, A. Reduced graphene oxide-coated cottons for selective absorption of organic solvents and oils from water. RSC Adv. 2014, 4, 30587–30591. [Google Scholar] [CrossRef]
- Sun, H.; Cao, L.; Lu, L. Magnetite/reduced graphene oxide nanocomposites: One step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, S.; Wan, J.; Tang, H.; Chang, L.; He, L.; Zhao, H.; Gao, Y.; Tang, Z. Three-dimensional graphene/metal oxide nanoparticle hybrids for high-performance capacitive deionization of saline water. Adv. Mater. 2013, 25, 6270–6276. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Liu, H. Oil Adsorption and Reuse Performance of Multi-Walled Carbon Nanotubes Taoener. Procedia Eng. 2015, 102, 1896–1902. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, W.; Cheng, H.M. Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J. Mater. Chem. 2012, 22, 20197–20202. [Google Scholar] [CrossRef]
- Wu, R.; Yu, B.; Liu, X.; Li, H.; Wang, W.; Chen, L.; Bai, Y.; Ming, Z.; Yang, S.T. One-pot hydrothermal preparation of graphene sponge for the removal of oils and organic solvents. Appl. Surf. Sci. 2016, 362, 56–62. [Google Scholar] [CrossRef]
- Hong, J.Y.; Sohn, E.H.; Park, S.; Park, H.S. Highly-efficient and recyclable oil absorbing performance of functionalized graphene aerogel. Chem. Eng. J. 2015, 269, 229–235. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Robinson, J.T.; Li, X.; Dai, H. Solvothermal Reduction of Chemically Exfoliated Graphene Sheets. J. Am. Chem. Soc. 2009, 131, 9910–9911. [Google Scholar] [CrossRef]
- Feng, Y.; Yao, J. Design of Melamine Sponge-Based Three-Dimensional Porous Materials toward Applications. Ind. Eng. Chem. Res. 2018, 57, 7322–7330. [Google Scholar] [CrossRef]
- Oribayo, O.; Feng, X.; Rempel, G.L.; Pan, Q. Modification of formaldehyde-melamine-sodium bisulfite copolymer foam and its application as effective sorbents for clean up of oil spills. Chem. Eng. Sci. 2017, 160, 384–395. [Google Scholar] [CrossRef]
- Chen, J.; You, H.; Xu, L.; Li, T.; Jiang, X.; Li, C.M. Facile synthesis of a two-tier hierarchical structured superhydrophobic-superoleophilic melamine sponge for rapid and efficient oil/water separation. J. Colloid Interface Sci. 2017, 506, 659–668. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Zhang, R.; Liu, S.; Zhou, Y. A superhydrophobic and elastic melamine sponge for oil/water separation. New J. Chem. 2019, 43, 6343–6349. [Google Scholar] [CrossRef]
- Wu, D.; Fang, L.; Qin, Y.; Wu, W.; Mao, C.; Zhu, H. Oil sorbents with high sorption capacity, oil/water selectivity and reusability for oil spill cleanup. Mar. Pollut. Bull. 2014, 84, 263–267. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Dideikin, A.T.; Kirilenko, D.A.; Baidakova, M.V.; Shnitov, V.V.; Roth, F.; Konyakhin, S.V.; Besedina, N.A.; Pavlov, S.I.; Kuricyn, R.A.; et al. Facile reduction of graphene oxide suspensions and films using glass wafers. Sci. Rep. 2018, 8, 14154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Bardhan, N.M.; Tongay, S.; Wu, J.; Belcher, A.M.; Grossman, J.C. Scalable enhancement of graphene oxide properties by thermally driven phase transformation. Nat. Chem. 2014, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.A.; Xian, L.; Chou, M.Y. Structural and electronic properties of oxidized graphene. Phys. Rev. Lett. 2009, 103, 086802. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing sugar: New functional molecules for the green synthesis of graphene nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yan, Z. Adsorption Mechanism of Oil by Resilient Graphene Aerogels from Oil-Water Emulsion. Langmuir 2018, 34, 1890–1898. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.H.; Altalhi, T.; Losic, D. Outstanding adsorption performance of graphene-carbon nanotube aerogels for continuous oil removal. Carbon N. Y. 2014, 80, 523–533. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Yang, F. Covalent assembly of 3D graphene/polypyrrole foams for oil spill cleanup. J. Mater. Chem. A 2013, 1, 3446–3453. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Z.; Li, P.; Yang, G.; Zheng, Q.; Yang, J. Ammonia solution strengthened three-dimensional macro-porous graphene aerogel. Nanoscale 2013, 5, 5462–5467. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Wu, X.; Qian, R.; Jiang, P. Mechanically flexible and multifunctional polymer-based graphene foams for elastic conductors and oil-water separators. Adv. Mater. 2013, 25, 5658–5662. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Chen, C.; Kong, W.; Duan, H.M.; Zhang, J. Theoretical simulation of reduction mechanism of graphene oxide in sodium hydroxide solution. Phys. Chem. Chem. Phys. 2014, 16, 12858–12864. [Google Scholar] [CrossRef] [PubMed]

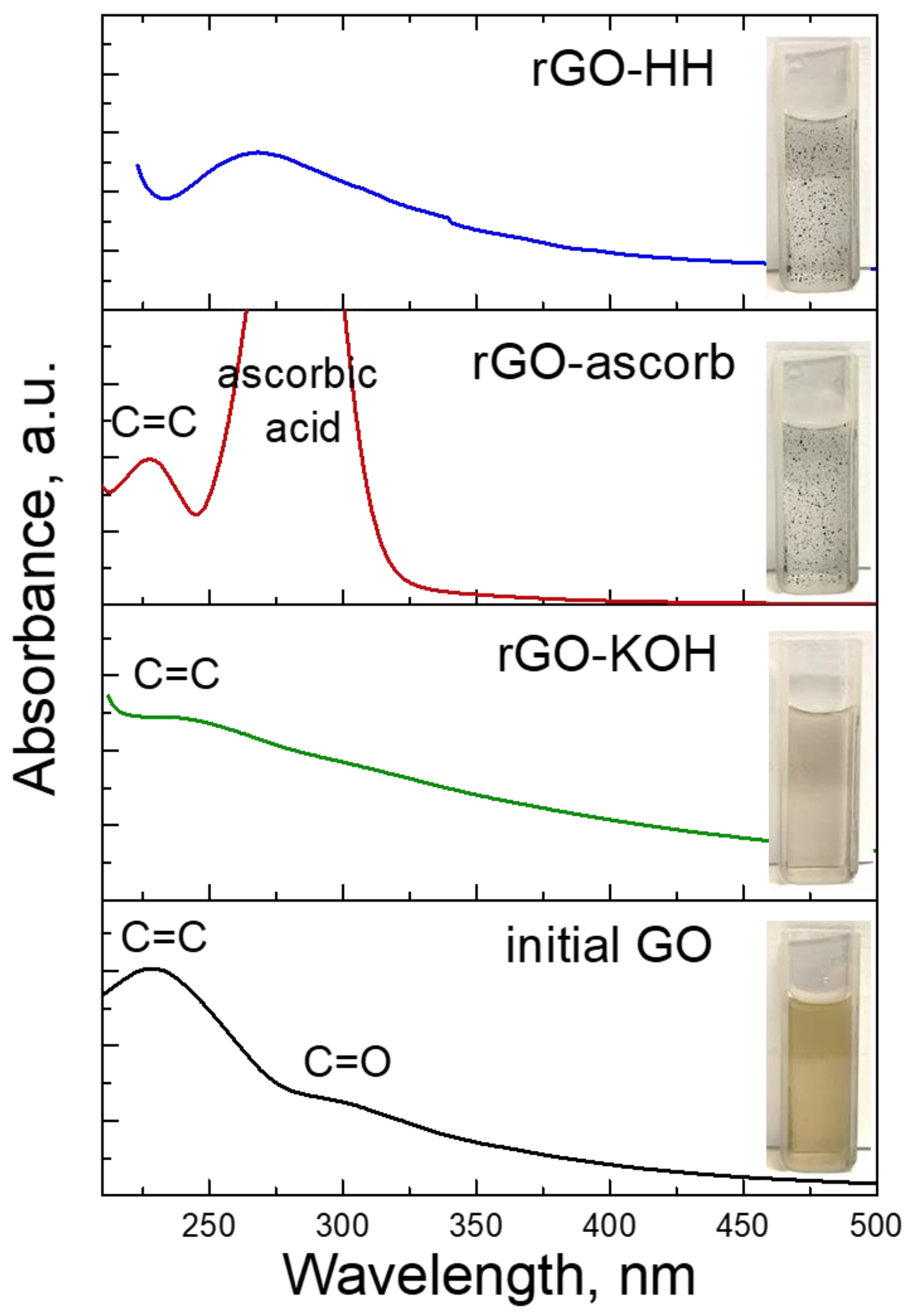



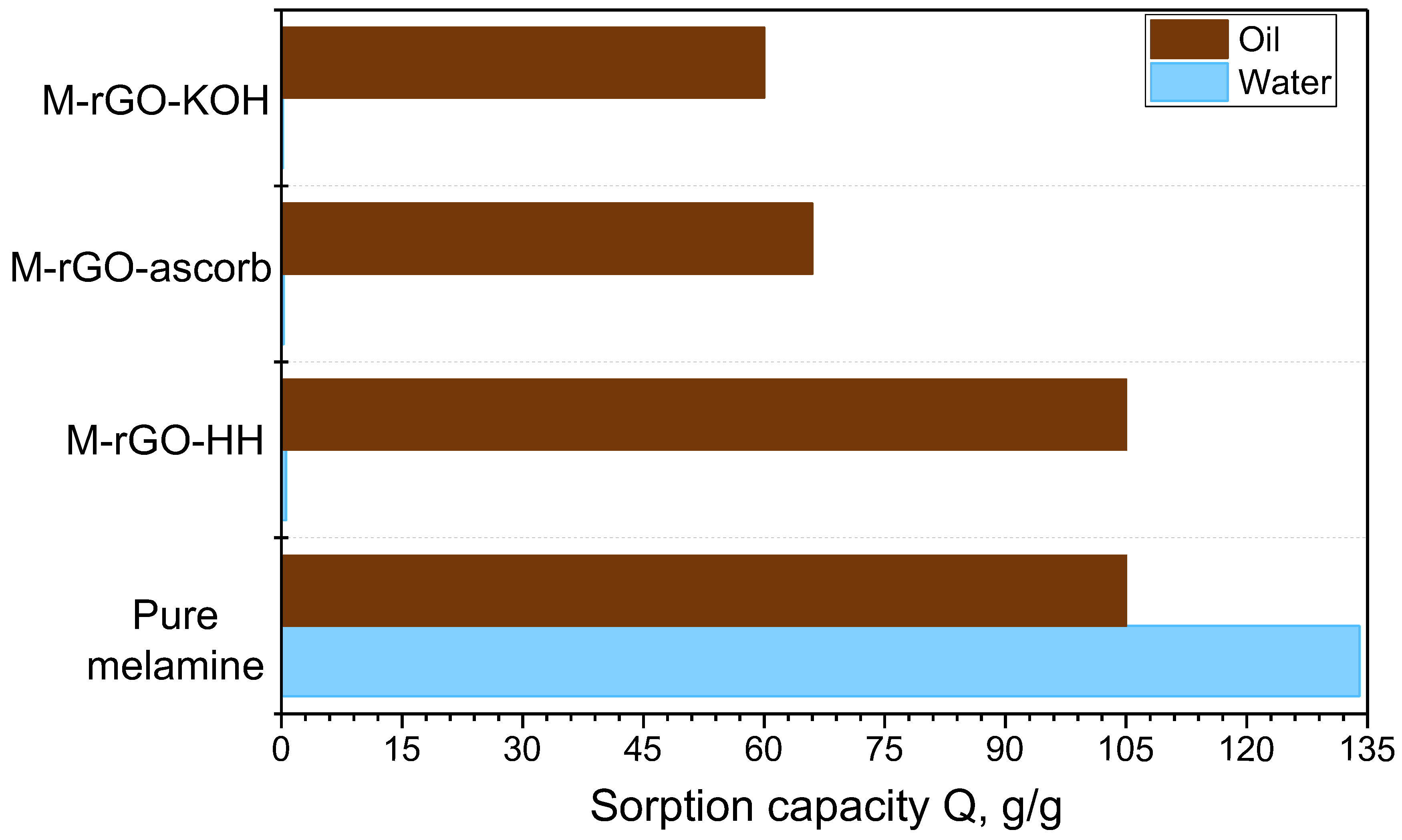
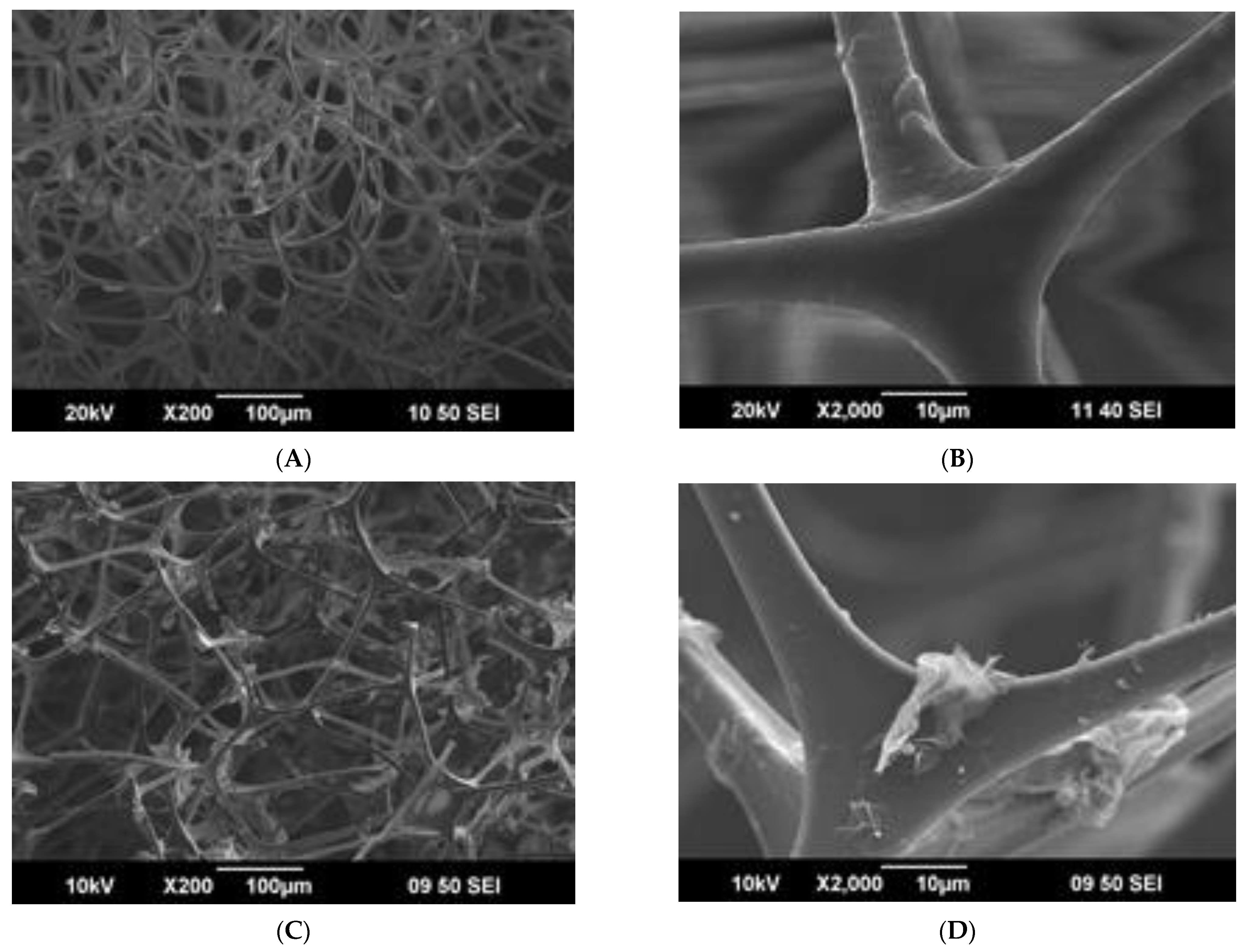
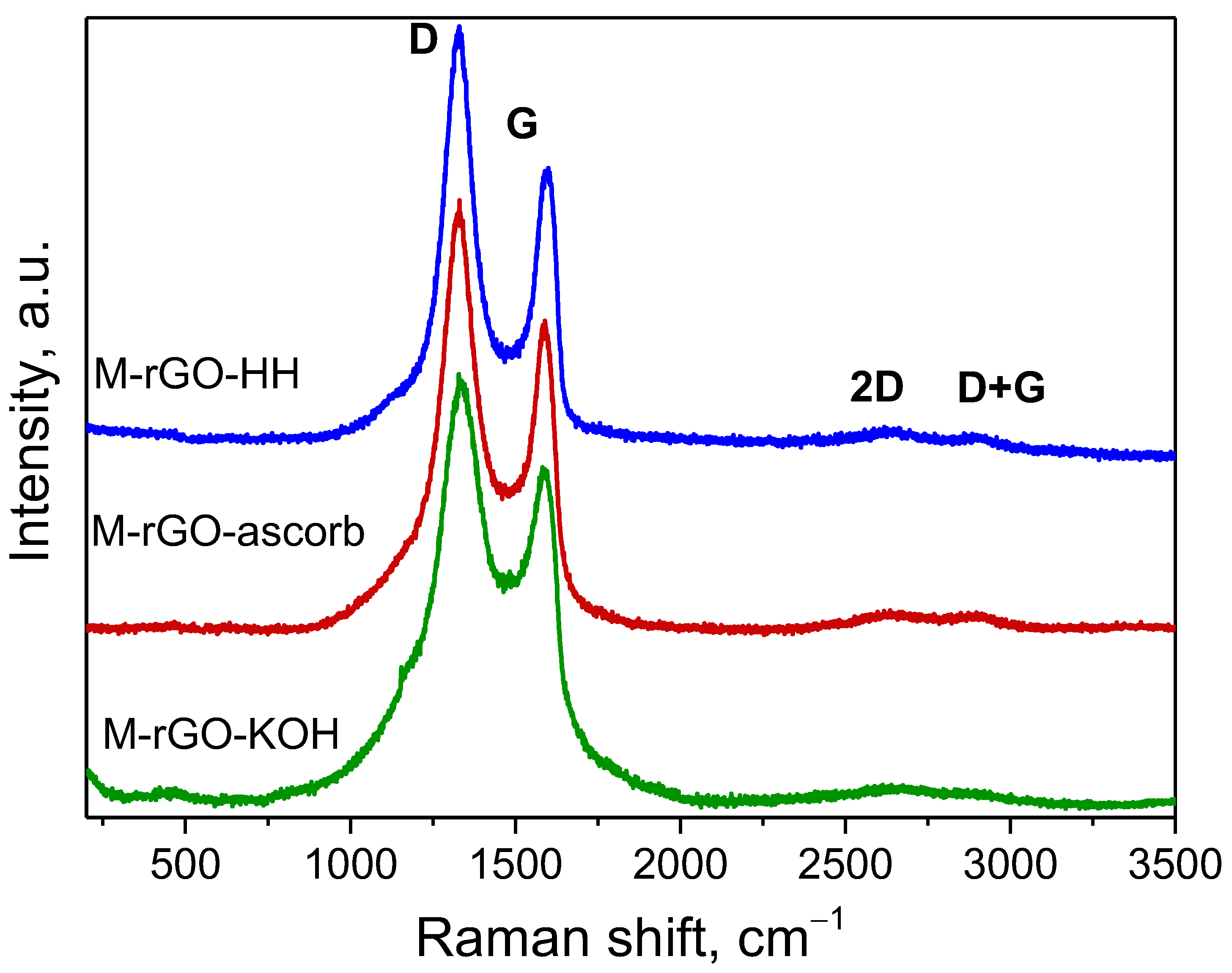

| Sample | Gravimetric Sorption Capacity, Qm, g/g | Selectivity | |||
|---|---|---|---|---|---|
| Qoil/Qwater | |||||
| Oil | Diesel Fuel | Gasoline | Water | ||
| Pure Melamine | 105 | 98 | 80 | 134 | 0.8 |
| M–rGO–KOH | 60 | 69 | 55 | 0.2 | 300 |
| M–rGO–ascorb | 66 | 87 | 60 | 0.3 | 220 |
| M–rGO–HH | 105 | 98 | 80 | 0.6 | 175 |
| Pure | 2 | 47 | 28 | 4 | 0.5 |
| Polyurethane | |||||
| PF–rGO–KOH | 40 | 38 | 22 | 0.6 | 66.7 |
| PF–rGO–ascorb | 49 | 45 | 26 | 0.7 | 70 |
| PF–rGO–HH | 51 | 46 | 30 | 2 | 25.5 |
| Sample | Position, cm−1 | ID/IG | La, nm | |||
|---|---|---|---|---|---|---|
| D-Mode | G-Mode | 2D-Mode | D+G-Mode | |||
| M–rGO–KOH | 1329 | 1582 | 2617 | 2908 | 1.17 | 32.9 |
| M–rGO–ascorb | 1331 | 1587 | 2604 | 2882 | 1.34 | 28.8 |
| M–rGO–HH | 1330 | 1591 | 2642 | 2890 | 1.48 | 26.0 |
| Sample | Fraction, at % | ||||
|---|---|---|---|---|---|
| C–C (sp2) | C–C (sp3) | C−O | N=C(−N)−N, O=C−O, O=C−N | Carbonate (CO32−) | |
| Pure Melamine | – | 46 | 16 | 5.3 | 4.0 |
| M–rGO–KOH | 50 | 11 | 6.9 | 8.3 | 0.2 |
| M–rGO–ascorb | 46 | 14 | 10 | 6.3 | 1.6 |
| M–rGO–HH | 46 | 13 | 4.4 | 10 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhiia, T.; Romanchuk, A.Y.; Maslakov, K.I.; Averin, A.A.; Kalmykov, S.N. Use of Reduced Graphene Oxide to Modify Melamine and Polyurethane for the Removal of Organic and Oil Wastes. Energies 2022, 15, 7371. https://doi.org/10.3390/en15197371
Bakhiia T, Romanchuk AY, Maslakov KI, Averin AA, Kalmykov SN. Use of Reduced Graphene Oxide to Modify Melamine and Polyurethane for the Removal of Organic and Oil Wastes. Energies. 2022; 15(19):7371. https://doi.org/10.3390/en15197371
Chicago/Turabian StyleBakhiia, Tamuna, Anna Yu. Romanchuk, Konstantin I. Maslakov, Alexey A. Averin, and Stepan N. Kalmykov. 2022. "Use of Reduced Graphene Oxide to Modify Melamine and Polyurethane for the Removal of Organic and Oil Wastes" Energies 15, no. 19: 7371. https://doi.org/10.3390/en15197371
APA StyleBakhiia, T., Romanchuk, A. Y., Maslakov, K. I., Averin, A. A., & Kalmykov, S. N. (2022). Use of Reduced Graphene Oxide to Modify Melamine and Polyurethane for the Removal of Organic and Oil Wastes. Energies, 15(19), 7371. https://doi.org/10.3390/en15197371






