Effects of Heating on the Binding of Rare Earth Elements to Humic Acids
Abstract
1. Introduction
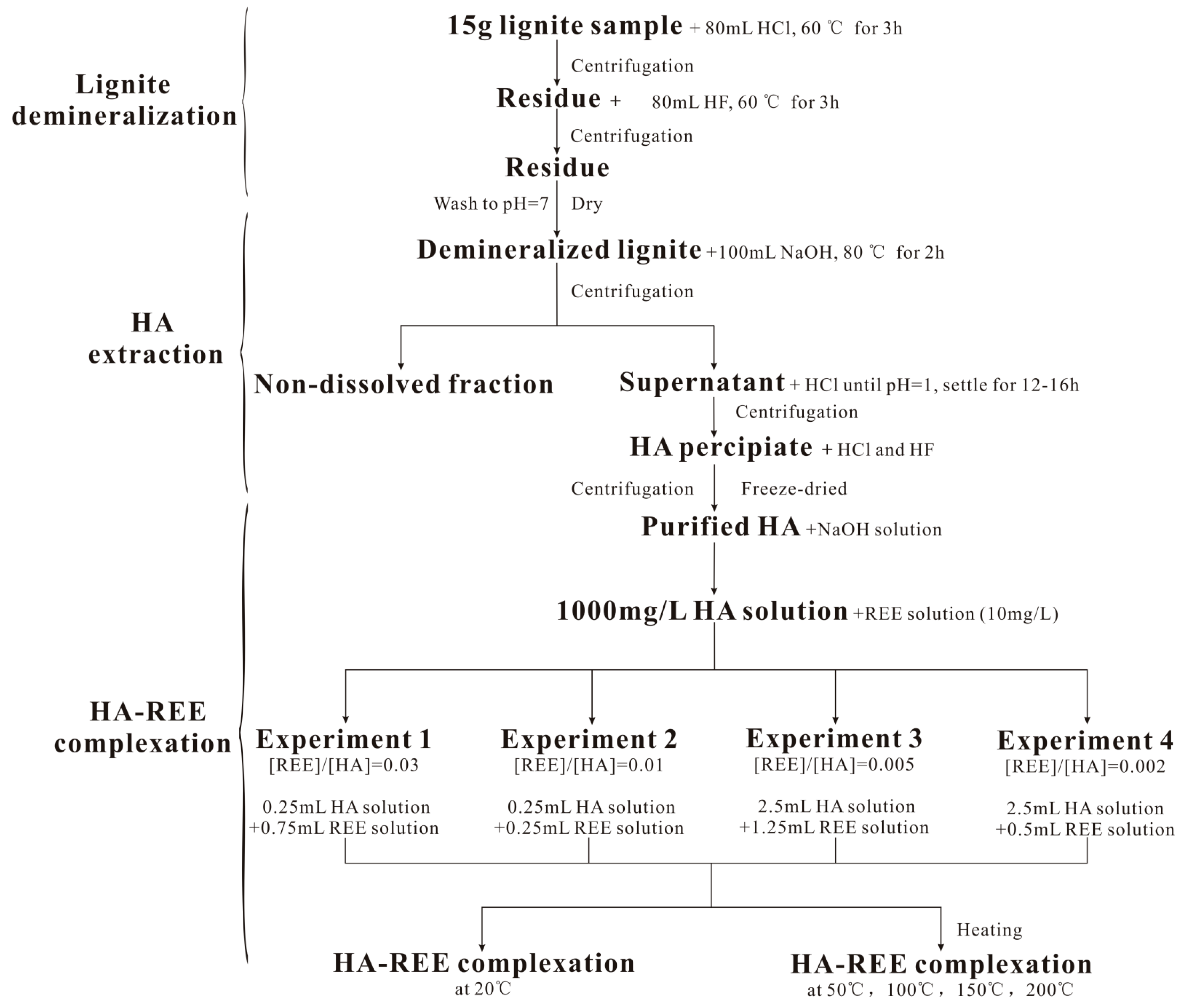
2. Materials and Methods
2.1. Lignite Samples
2.2. Humic Acid Extraction
2.3. Complexation Experiment
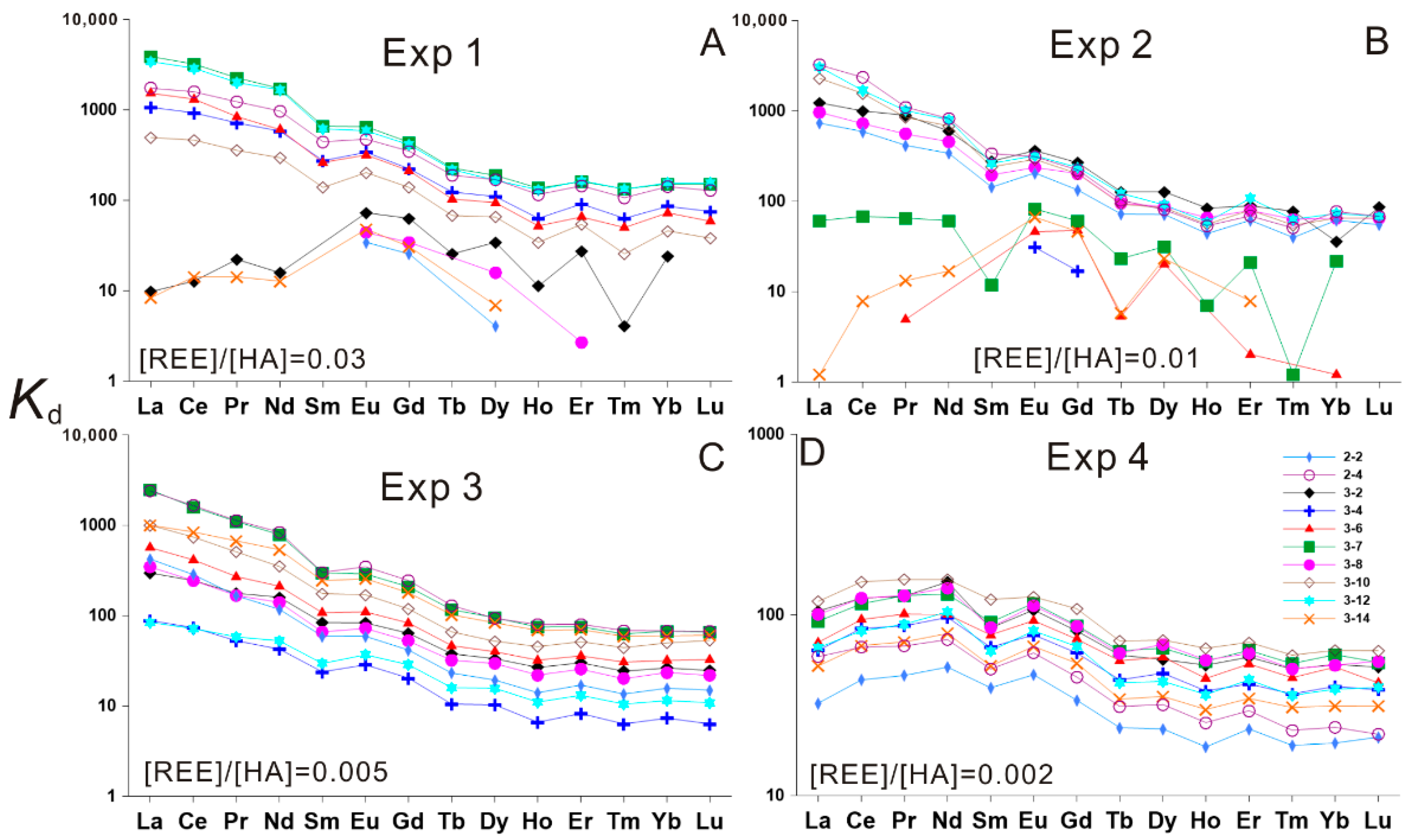
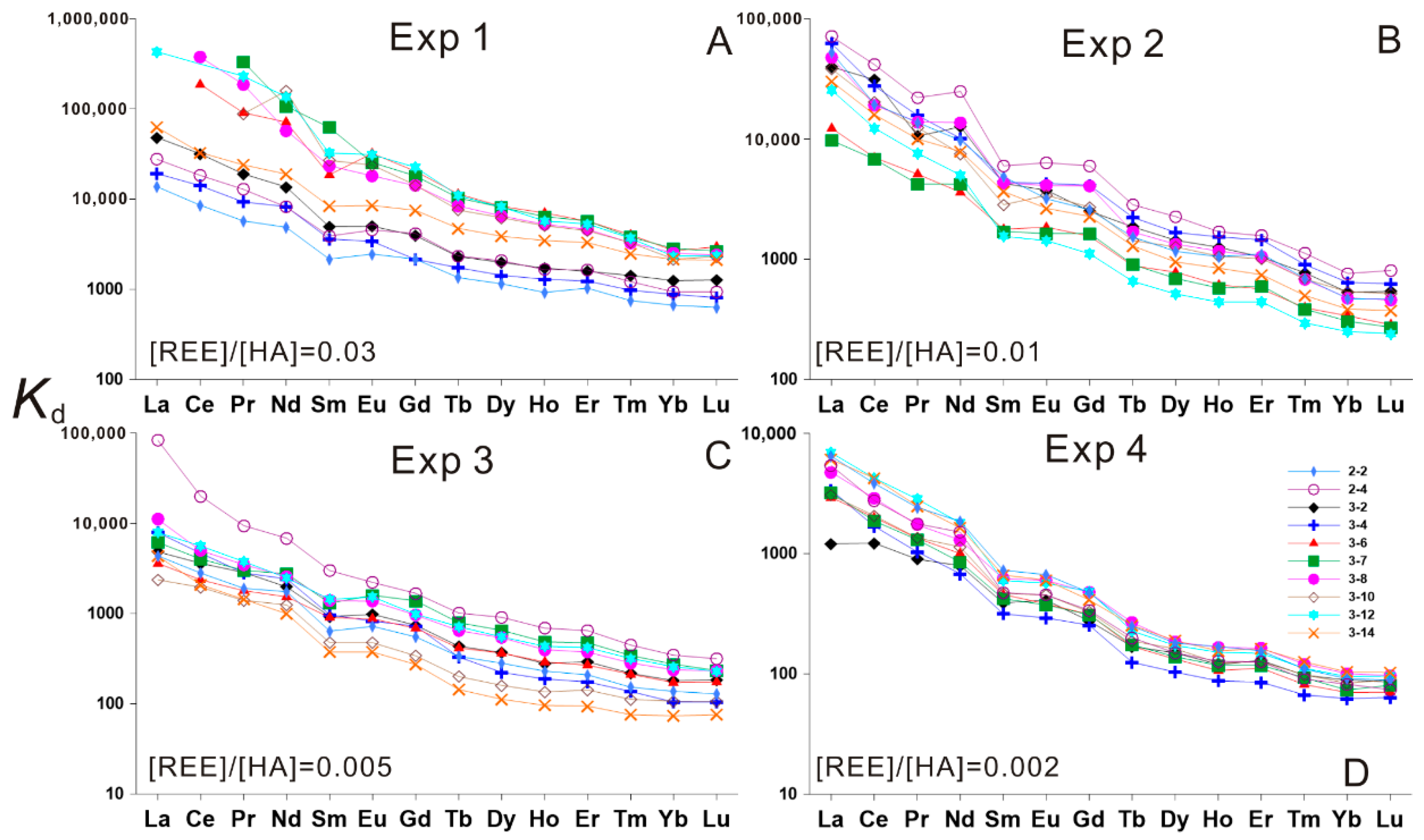
3. Results
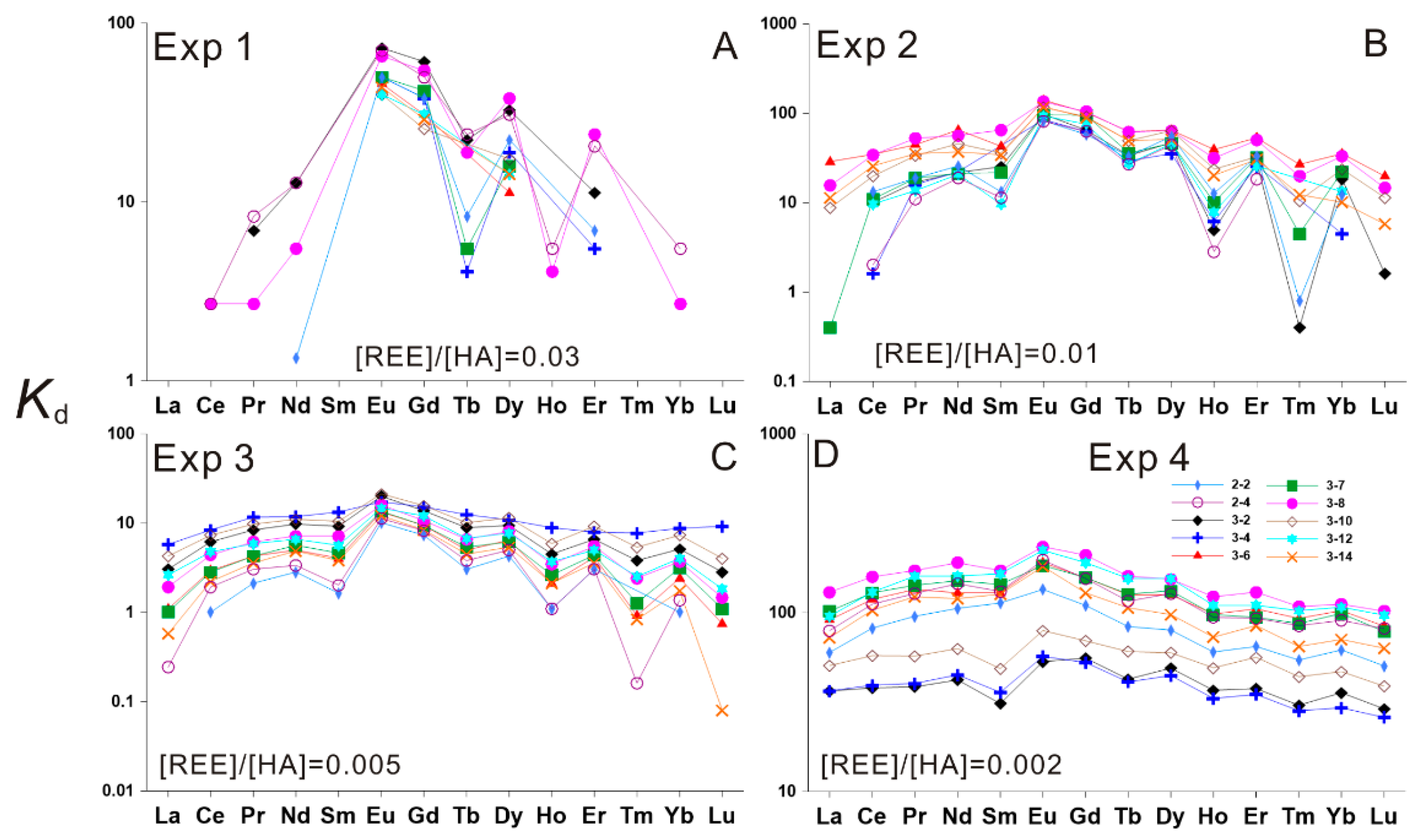
3.1. HA–REE Complexation Characteristics at Room Temperature (about 20 °C)
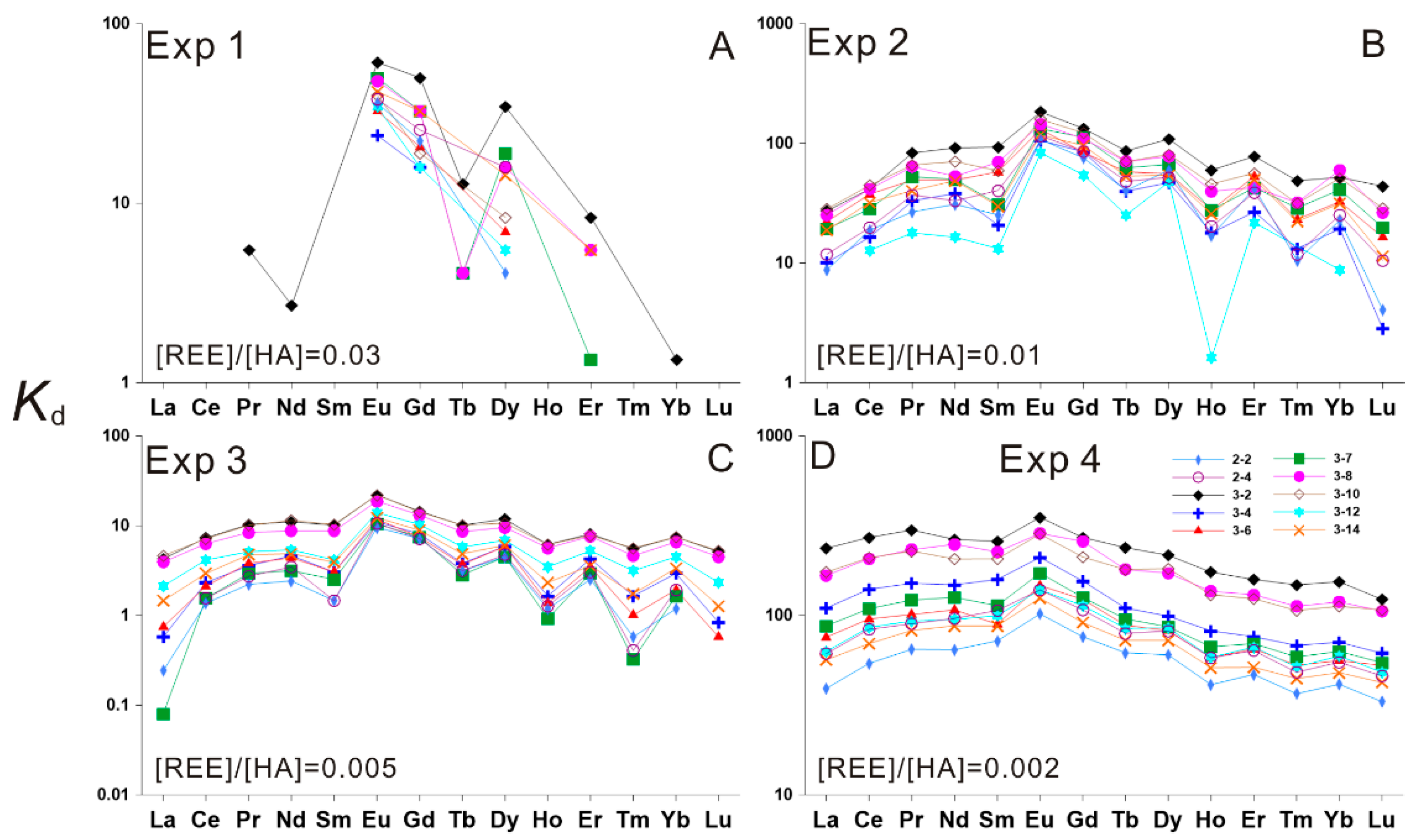
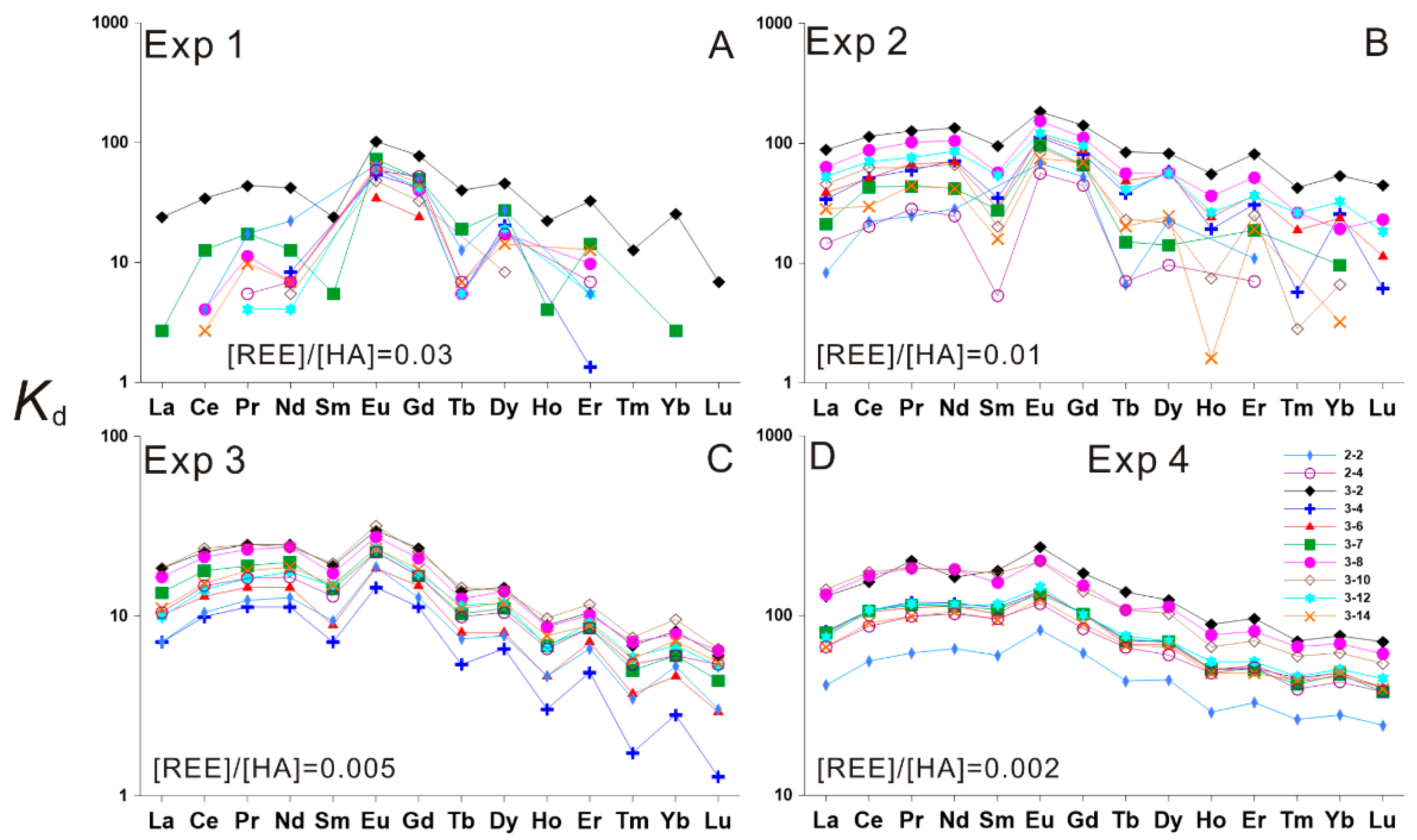
3.2. HA–REE Complexation Characteristics during Heating
3.2.1. HA–REE Complexation Characteristics at 50 and 100 °C
3.2.2. HA–REE Complexation Characteristics at 150 and 200 °C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schatzel, S.J.; Stewart, B.W. Rare earth element sources and modification in the Lower Kittanning coal bed, Pennsylvania: Implications for the origin of coal mineral matter and rare earth element exposure in underground mines. Int. J. Coal Geol. 2003, 54, 223–251. [Google Scholar] [CrossRef]
- Pourret, O.; Martinez, R.E. Modeling lanthanide series binding sites on humic acid. J. Colloid Interface Sci. 2009, 330, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Marsac, R.; Davranche, M.; Gruau, G.; Bouhnik-Le Coz, M.; Dia, A. An improved description of the interactions between rare earth elements and humic acids by modeling: PHREEQC-Model VI coupling. Geochim. Cosmochim. Acta 2011, 75, 5625–5637. [Google Scholar] [CrossRef]
- Marsac, R.; Davranche, M.; Gruau, G.; Dia, A.; Pédrot, M.; Le Coz-Bouhnik, M.; Briant, N. Effects of Fe competition on REE binding to humic acid: Origin of REE pattern variability in organic waters. Chem. Geol. 2013, 342, 119–127. [Google Scholar] [CrossRef]
- Stern, J.C.; Sonke, J.E.; Salters, V.J.M. A capillary electrophoresis-ICP-MS study of rare earth element complexation by humic acids. Chem. Geol. 2007, 246, 170–180. [Google Scholar] [CrossRef]
- Stolpe, B.; Guo, L.; Shiller, A.M. Binding and transport of rare earth elements by organic and iron-rich nanocolloids in alaskan rivers, as revealed by field-flow fractionation and ICP-MS. Geochim. Cosmochim. Acta 2013, 106, 446–462. [Google Scholar] [CrossRef]
- Marsac, R.; Catrouillet, C.; Davranche, M.; Bouhnik-Le Coz, M.; Briant, N.; Janot, N.; Otero-Fariña, A.; Groenenberg, J.E.; Pédrot, M.; Dia, A. Modeling rare earth elements binding to humic acids with model VII. Chem. Geol. 2021, 567, 120099. [Google Scholar] [CrossRef]
- Khan, K.F.; Dar, S.A.; Khan, S.A. Rare earth element (REE) geochemistry of phosphorites of the Sonrai area of Paleoproterozoic Bijawar basin, Uttar Pradesh, India. J. Rare Earths 2012, 30, 507–514. [Google Scholar] [CrossRef]
- Davranche, M.; Pourret, O.; Gruau, G.; Dia, A.; Jin, D.; Gaertner, D. Competitive binding of REE to humic acid and manganese oxide: Impact of reaction kinetics on development of cerium anomaly and REE adsorption. Chem. Geol. 2008, 247, 154–170. [Google Scholar] [CrossRef]
- Madhavaraju, J.; Löser, H.; Lee, Y.I.L.; Santacruz, R.L.; Pi-Puig, T. Geochemistry of Lower Cretaceous limestones of the Alisitos Formation, Baja California, México: Implications for REE source and paleo-redox conditions. J. S. Am. Earth Sci. 2016, 66, 149–165. [Google Scholar] [CrossRef]
- Chen, Y.; Fabbricino, M.; Benedetti, M.F.; Korshin, G.V. Spectroscopic in situ examination of interactions of rare earth ions with humic substances. Water Res. 2015, 68, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Puebla, R.A.; Aroca, R.F.; Valenzuela-Calahorro, C.; Garrido, J.J. Retention of cobalt on a humin derived from brown coal. J. Hazard. Mater. 2006, 135, 122–128. [Google Scholar] [CrossRef]
- Ghabbour, E.A.; Scheinost, A.C.; Davies, G. XAFS studies of cobalt(II) binding by solid peat and soil-derived humic acids and plant-derived humic acid-like substances. Chemosphere 2007, 67, 285–291. [Google Scholar] [CrossRef]
- Bu, G.J.; He, X.S.; Li, T.T.; Wang, Z.X. Insight into indicators related to the humification and distribution of humic substances in Sphagnum and peat at different depths in the Qi Zimei Mountains. Ecol. Indic. 2019, 98, 430–441. [Google Scholar] [CrossRef]
- Katsumi, N.; Yonebayashi, K.; Okazaki, M. Effects of heating on composition, degree of darkness, and stacking nanostructure of soil humic acids. Sci. Total Environ. 2016, 541, 23–32. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, A.; Bermejo-Barrera, A.; Bermejo-Barrera, P. New trends involving the use of ultrasound energy for the extraction of humic substances from marine sediments. Anal. Chim. Acta 2004, 524, 97–107. [Google Scholar] [CrossRef]
- Pourret, O.; Davranche, M.; Gruau, G.; Dia, A. Rare earth elements complexation with humic acid. Chem. Geol. 2007, 243, 128–141. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Takahashi, Y.; Shimizu, H. Interpretation of REE patterns in natural water based on the stability constants. Geochim. Cosmochim. Acta 2006, 70, A717. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Takahashi, Y.; Shimizu, H. Systematic change in relative stabilities of REE-humic complexes at various metal loading levels. Geochem. J. 2010, 44, 39–63. [Google Scholar] [CrossRef]
- Jerzykiewicz, M. Humic and hymatomelanic acids interaction with lanthanide ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 127–131. [Google Scholar] [CrossRef]
- Marsac, R.; Davranche, M.; Gruau, G.; Dia, A. Metal loading effect on rare earth element binding to humic acid: Experimental and modelling evidence. Geochim. Cosmochim. Acta 2010, 74, 1749–1761. [Google Scholar] [CrossRef]
- Marsac, R.; Davranche, M.; Gruau, G.; Dia, A.; Bouhnik-Le Coz, M. Aluminium competitive effect on rare earth elements binding to humic acid. Geochim. Cosmochim. Acta 2012, 89, 1–9. [Google Scholar] [CrossRef]
- Marsac, R.; Davranche, M.; Morin, G.; Takahashi, Y.; Gruau, G.; Briant, N.; Dia, A. Effect of loading on the nature of the REE-humate complexes as determined by Yb3+and Sm3+LIII-edge EXAFS analysis. Chem. Geol. 2015, 396, 218–227. [Google Scholar] [CrossRef]
- Pourret, O.; Davranche, M.; Gruau, G.; Dia, A. Competition between humic acid and carbonates for rare earth elements complexation. J. Colloid Interface Sci. 2007, 305, 25–31. [Google Scholar] [CrossRef]
- Pourret, O.; Houben, D. Characterization of metal binding sites onto biochar using rare earth elements as a fingerprint. Heliyon 2018, 4, e00543. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.C.; Foustoukos, D.I.; Sonke, J.E.; Salters, V.J.M. Humic acid complexation of Th, Hf and Zr in ligand competition experiments: Metal loading and pH effects. Chem. Geol. 2014, 363, 241–249. [Google Scholar] [CrossRef]
- Sonke, J.E.; Salters, V.J.M. Lanthanide-humic substances complexation. I. Experimental evidence for a lanthanide contraction effect. Geochim. Cosmochim. Acta 2006, 70, 1495–1506. [Google Scholar] [CrossRef]
- Sonke, J.E. Lanthanide—Humic substances complexation. II. Calibration of humic ion-binding model V. Environ. Sci. Technol. 2006, 40, 7481–7487. [Google Scholar] [CrossRef]
- Pédrot, M.; Dia, A.; Davranche, M. Dynamic structure of humic substances: Rare earth elements as a fingerprint. J. Colloid Interface Sci. 2010, 345, 206–213. [Google Scholar] [CrossRef]
- Marsac, R.; Banik, N.L.; Lützenkirchen, J.; Catrouillet, C.; Marquardt, C.M.; Johannesson, K.H. Modeling metal ion-humic substances complexation in highly saline conditions. Appl Geochemistry 2017, 79, 52–64. [Google Scholar] [CrossRef]
- Catrouillet, C.; Guenet, H.; Pierson-Wickmann, A.C.; Dia, A.; Lecoz, M.B.; Deville, S.; Lenne, Q.; Suko, Y.; Davranche, M. Rare earth elements as tracers of active colloidal organic matter composition. Environ. Chem. 2020, 17, 133–139. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamamoto, M.; Yamamoto, Y.; Tanaka, K. EXAFS study on the cause of enrichment of heavy REEs on bacterial cell surfaces. Geochim. Cosmochim. Acta 2010, 74, 5443–5462. [Google Scholar] [CrossRef]
- Tang, J.; Johannesson, K.H. Speciation of rare earth elements in natural terrestrial waters: Assessing the role of dissolved organic matter from the modeling approach. Geochim. Cosmochim. Acta 2003, 67, 2321–2339. [Google Scholar] [CrossRef]
- Tang, J.; Johannesson, K.H. Ligand extraction of rare earth elements from aquifer sediments: Implications for rare earth element complexation with organic matter in natural waters. Geochim. Cosmochim. Acta 2010, 74, 6690–6705. [Google Scholar] [CrossRef]
- Eltantawy, I.M. The Effect of Heating on Humic Acid Structure and Electronic Spin Resonance Signal. Soil Sci. Soc. Am. J. 1980, 44, 512–514. [Google Scholar] [CrossRef]
- Francioso, O.; Montecchio, D.; Gioacchini, P.; Ciavatta, C. Thermal analysis (TG-DTA) and isotopic characterization ( 13C-15N) of humic acids from different origins. Appl. Geochem. 2005, 20, 537–544. [Google Scholar] [CrossRef]
- Skhonde, M.P.; Herod, A.A.; van der Walt, T.J.; Tsatsi, W.L.; Mokoena, K. The effect of thermal treatment on the compositional structure of humic acids extracted from South African bituminous coal. Int. J. Miner. Process. 2006, 81, 51–57. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Li, J. Variation in humic and fulvic acids during thermal sludge treatment assessed by size fractionation, elementary analysis, and spectroscopic methods. Front. Environ. Sci. Eng. 2014, 8, 854–862. [Google Scholar] [CrossRef]
- Prado, A.G.S.; Torres, J.D.; Martins, P.C.; Pertusatti, J.; Bolzon, L.B.; Faria, E.A. Studies on copper(II)- and zinc(II)-mixed ligand complexes of humic acid. J. Hazard. Mater. 2006, 136, 585–588. [Google Scholar] [CrossRef]
- Fekete, J.; Sajgó, C.; Kramarics, Á.; Eke, Z.; Kovács, K.; Kárpáti, Z. Aquathermolysis of humic and fulvic acids: Simulation of organic matter maturation in hot thermal waters. Org. Geochem. 2012, 53, 109–118. [Google Scholar] [CrossRef]
- Schwieger, A.C.; Gebauer, K.; Ohle, A.; Beckmann, M. Determination of mercury binding forms in humic substances of lignite. Fuel 2020, 274, 117800. [Google Scholar] [CrossRef]
- Nakada, R.; Waseda, A.; Okumura, F.; Takahashi, Y. Impact of the decarboxylation reaction on rare earth elements binding to organic matter: From humic substances to crude oil. Chem. Geol. 2016, 420, 231–239. [Google Scholar] [CrossRef]
- Cornu, J.Y.; Schneider, A.; Jezequel, K.; Denaix, L. Modelling the complexation of Cd in soil solution at different temperatures using the UV-absorbance of dissolved organic matter. Geoderma 2011, 162, 65–70. [Google Scholar] [CrossRef]
| Isotope | Detection Limit | |
|---|---|---|
| La | 139 | 0.002 |
| Ce | 140 | 0.004 |
| Pr | 141 | 0.001 |
| Nd | 146 | 0.004 |
| Sm | 147 | 0.001 |
| Eu | 151 | 0.001 |
| Gd | 157 | 0.001 |
| Tb | 159 | 0.001 |
| Dy | 163 | 0.001 |
| Ho | 165 | 0.001 |
| Er | 166 | 0.001 |
| Tm | 169 | 0.001 |
| Yb | 172 | 0.001 |
| Lu | 175 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Deng, F.; Cheng, H.; Ning, S.; Li, B.; Pan, S.; Yin, X. Effects of Heating on the Binding of Rare Earth Elements to Humic Acids. Energies 2022, 15, 7362. https://doi.org/10.3390/en15197362
Wang X, Deng F, Cheng H, Ning S, Li B, Pan S, Yin X. Effects of Heating on the Binding of Rare Earth Elements to Humic Acids. Energies. 2022; 15(19):7362. https://doi.org/10.3390/en15197362
Chicago/Turabian StyleWang, Xiaomei, Fan Deng, Haijian Cheng, Shuzheng Ning, Baoqing Li, Sidong Pan, and Xuebo Yin. 2022. "Effects of Heating on the Binding of Rare Earth Elements to Humic Acids" Energies 15, no. 19: 7362. https://doi.org/10.3390/en15197362
APA StyleWang, X., Deng, F., Cheng, H., Ning, S., Li, B., Pan, S., & Yin, X. (2022). Effects of Heating on the Binding of Rare Earth Elements to Humic Acids. Energies, 15(19), 7362. https://doi.org/10.3390/en15197362







