Abstract
The use of fossil fuels (methane, oil, etc.) is undergoing an unprecedented crisis now. There is the urgent need to search for alternative energy sources. A wide range of degraded organic materials can be effectively used to provide energy together with environmental protection. Soapstock is a hazardous waste containing a high concentration of toxic organic compounds of man-made origin (fatty acids, surfactants, dyes, etc.). To prevent environmental contamination such substances require an effective treatment approach. The goal of the study was to isolate the adapted-to-fatty-acids methanogenic microbiome and investigate the patterns of sodium acetate and soapstock degradation with simultaneous biomethane synthesis. The effectiveness of the degradation of sodium acetate and soapstock by non-adapted and adapted microbiomes was evaluated by decreasing the concentration of dissolved organic compounds. The effectiveness of the fermentation process was determined by the biogas (mixture of CH4 and CO2) yield. The most effective degradation occurred in the variant with sodium acetate and adapted methanogens and amounted to 77.9%. In other variants, the patterns and the efficiency of purification were similar ranging from 60.6 to 68.0%. The biomethane was mostly synthesized by adapted methanogens on the soapstock and sodium acetate as substrates. Thus, the CH4 yield was 368.4 L/kg of dissolved organic compounds or 127.5 L/kg of soapstock. The results of this study demonstrated the potential of methanogenic microorganisms in the biodegradation of soapstock with simultaneous biogas synthesis. The results can serve as a basis to reduce the reliance on fossil fuels by generating biomethane via the fermentation of toxic organics.
1. Introduction
Currently, the economy of the globe is facing a total energy crisis. The reasons for such a situation are the depletion of fossil fuels, the negative effect on the environment due to their use (greenhouse effect, etc.), and market instability. In this regard, there is an urgent need to find alternative energy sources to provide a gradual replacement of fossil fuels by green energy. Fuel provided as the result of biological processes is considered to be a promising alternative. For example, methanogenic fermentation of organic compounds can provide not only methane production but also environmental protection. Thus, the development and optimization of the fermentation processes is a promising approach to support the economy with alternative energy [1].
Pollution of the environment by organic toxicants is one of the most hazardous environmental problems. Today, natural and synthetic organic compounds predominate over inorganic ones in landfills and industrial wastewater. Accumulated as a result of human activities and imperfect technological processes in chemical enterprises, as well as the absence or ineffectiveness of their detoxification, persistent organic compounds possess hazards to the environment [1,2]. Since they are degraded very slowly, synthetic organic compounds persist in the environment for a long time, disrupting normal functioning flora and fauna. A soapstock is a striking example of an organic contaminant. Soapstock from alkali refining is a rich source of fatty acids, but it also presents a handling, storage, and disposal problem [1]. Substances containing fatty acids are widely used in everyday life as antimicrobial agents, preservatives, stabilizers, etc. [3]. Soapstock is formed both at industrial enterprises [4] and as a result of everyday human activities and the intensive use of detergents, including solid and liquid soaps. Due to the spread of the COVID-19 virus, the intensity of soap use has increased significantly [5]. Due to the high demand for detergents, it is impossible to satisfy the needs only from soaps synthesized from natural ingredients (oils, animal fats, etc.). In hard water, a soap of natural origin loses its detergent effect because insoluble magnesium and calcium salts of higher carboxylic acids are formed. In this regard, the production of synthetic detergents has been widely developed. They have a good cleaning effect and do not lose it in hard water. Long-chain fatty acids with high molecular weight are used for the production of synthetic detergents. The fatty acids necessary for the production of soap are extracted from crude oil [6] or paraffin oxidation [7]. A toilet soap is produced by the neutralization of acids containing from 10 to 16 carbon atoms in the molecule yields. Household soap and soap for technical purposes is produced using long-chain fatty acids containing from 17 to 21 carbon atoms. Thus, organic acids and their salts that are accumulated in the environment are mainly represented by two groups: short-chain (SCFA) [4] and long-chain fatty acids (LCFA) [8]. Short-chain fatty acids (acetic, propionic, and butyric acids) are saturated carboxylic acids containing six or fewer carbon atoms. Usually, they are the component of landfill leachate [8] and the digestate of anaerobic bioreactors [9]. The long-chain fatty acids in most cases accumulate in the environment as the part of household or industrial wastewater containing alkaline salts of fatty acids and soaps [10].
This process causes eutrophication and foaming, and altering of the salinity, turbidity, and pH of the environment [11]. Such uncontrolled pollution of the environment with fatty acids and their salts has a detrimental effect on the functioning of living organisms. It destabilizes the normal function of natural ecosystems and causes mass extinction of microorganisms in soil and water reservoirs. The antibacterial activity of fatty acids is well known in the literature [12]. Fatty acids inhibit the growth of both spore and non-spore microorganisms. Thus, the growth of strains Clostridium botulinum 62A, Clostridium sporogenes PA3679, and Bacillus cereus F4165/75 were inhibited by lauric, linoleic, and linolenic acids. Minimum inhibitory concentrations ranged from 50–150 mg/L for lauric acid, ≥150 mg/L for myristic acid, 30–100 mg/L for linoleic acid, and 10–75 mg/L for linolenic acid depending on the strain [13]. Fatty acids C4–C16 and oleic acid inhibited the growth of E. coli strain k12/154 by its insertion into the medium in the exponential phase of growth at a concentration of 0.1–0.4% [14]. Due to the high level of pollution of the environment with organic contaminants, especially fatty acids, it is of great interest to develop methods of wastewater purification. Current wastewater treatment approaches include physical, chemical, and biological methods to eliminate pollutants [15]. To date, the most common methods of water purification from organic acids are flotation [16], reverse osmosis [17], nanofiltration [18], electrochemical oxidation [19,20], photocatalytic ozonation [21], the ion exchange method [22], and adsorption [23]. The main problem of existing wastewater treatment methods is the obsolescence of treatment plants, the high cost of their operation, and their maintenance [24]. They are not sustainable to meet the ever-growing water sanitation needs due to rapid industrialization and population growth [25]. The chemical-based purification methods are hazardous due to their negative environmental impacts [4]. Biological and especially microbiological remediation approaches are the better alternative due to their cost-effectiveness and high efficiency compared with traditional physicochemical approaches [26]. Microbiological wastewater purification involves the use of aerobic or anaerobic methods, or a combination of them [27]. The advantages of anaerobic purification are total organics removal, less sludge production, a low energy requirement, and the opportunity for biogas production in a huge amount [28]. Given that salts of fatty acids contained in soap in high concentrations are toxic to microorganisms, the urgent problem is to isolate new microbial strains resistant to organic compounds in high concentrations. Soapstock containing fatty acids can be used as a substrate (the only source of carbon and energy) for organic-resistant microorganisms to produce biogas (biohydrogen or biomethane) [2]. Given the wide variety and metabolic pathways, the microorganisms are the optimal biological agents for wastewater purification from the contamination with synthetic organic contaminants (soapstock, salts of short-chain and long-chain fatty acids, etc.) with energy production. They are also able to adapt to extreme environmental conditions and high concentrations of toxic compounds, as well as to switch microbial metabolism to the degradation of hard-to-reach substrates [29].
It is already known that some microorganisms are resistant to certain fatty acids and are able to transform them. However, the patterns of the methanogenic degradation of the soapstock containing a mixture of fatty acids and other synthetic organic compounds (surfactants, dyes, etc.) are still unstudied. The process of methanogenesis is multistage and includes a number of biochemical reactions: polymer hydrolysis, acetogenesis (including hydrogen synthesis), and methanogenesis. Methanogenesis consists of such stages:
- Hydrolysis of polymers [30]:
- 1.1.
- [C6H12O6]n → nC6H12O6;
- 1.2.
- Lipids → RCH2CH2COOH (LCFA) + glycerol
- 1.3.
- Proteins → amino acids
- Acetogenesis:
- 2.1.
- C6H12O6 + 2H2O = 4H2 + 2CH3COOH (acetate) + 2CO2 (–414 mV) [31];
- 2.2.
- RCH2CH2COOH (LCFA) → ß-oxidation pathway → AcetylCoA → CH3COOH [32]
- Methanogenesis [33]:
- 3.1.
- Acetoclastic methanogenesis: CH3COOH = CH4↑ + CO2 (+120 mV);
- 3.2.
- Hydrogenotrophic methanogenesis: 4H2 + CO2 = CH4↑ +2H2O (+170 mV);
- 3.3.
- Hydrogenotrophic methanogenesis: CO2 + 8H+ + 8e− = CH4↑ +2H2O (–240 mV).
Hydrolysis and acetogenesis reactions, in which long-chain fatty acids are converted into acetate, are important for the degradation of soapstock. Methane is immediately synthesized from acetate during the stage of acetoclastic methanogenesis.
In this regard, the goal of the study was to isolate the adapted-to-fatty-acids methanogenic microbiome and investigate the patterns of sodium acetate and soapstock degradation with simultaneous biomethane synthesis.
2. Materials and Methods
2.1. Design of the Experiment
Liquid household soap and sodium acetate were used as the fermentation substrates (the only source of carbon and energy) to create the model stocks, and to study and compare the efficiency of their degradation by methanogenic microbiome. Sodium acetate and household soap were the model substrates and the sources of short-chain and long-chain fatty acids respectively. The efficiency of the fermentation process was evaluated by the level of the decrease in the concentration of dissolved organic compounds (DOC), also counting the total carbon and biomethane yield. Two types of inoculum were used for the degradation. The first one was the methanogenic microbiome of fermented methane tank sludge, which was not adapted to fatty acids in high concentration. The second one was methanogenic microbiome of fermented sludge of methane tank adapted to fatty acids in high concentration.
2.2. Inoculum Preparation
Fermented methane tank sludge sampled at Bortnytska Aeration Station (BAS) was used as inoculum (Kyiv, Ukraine). It is the only sewage treatment plant within Kyiv and a dozen other localities of the region, not only the Left Bank, but also in Vyshhorod, Irpin, Vyshneve, Chabaniv, Kotsiubynskyy, Sofiivska, and Petropavlivska Borshchahivka. Two inoculum variants (adapted and non-adapted to fatty acids in high concentration) were prepared to compare the degradation efficiency of the model wastewater. The first inoculum was a freshly sampled fermented methane tank sludge containing a diversified community of methanogenic microorganisms. These microorganisms were not previously adapted to grow in the presence of high concentrations of fatty acids. The second inoculum was pre-adapted to grow in the presence of fatty acids in high concentration. For this purpose, the fermented sludge of methane tank was cultivated in a toxic leachate obtained after the fermentation of multicomponent food waste. The cultivation was carried out in the anaerobic conditions in hermetically sealed jars for 30 days. The initial concentration of dissolved organic compounds (total carbon) in the leachate was 1071 mg/L [34]. The initial pH and Eh values of the leachate for the methanogens’ adaptation were 6.1 ± 0.3 and −120 ± 24.0 mV, respectively. Methanogens adapted to the leachate with a high concentration of DOC, increased the pH of the medium to 8.5 ± 0.4, decreased the redox potential to −280 ± 55.0 mV, and also synthesized methane. The culture fluid contained metabolically active methanogens after fermentation was centrifuged [35] on a centrifuge EZeeMini D1008 using mode 2680× g. The precipitate containing the concentrate of adapted methanogenic microorganisms was used as the second inoculum variant for the fermentation of the model soapstock and sodium acetate. The inoculum was added to the experimental jars in the amount of 10% of the medium.
2.3. Fermentation Process
The process of fermentation of sodium acetate and the model soapstock was performed in the hermetically sealed jars with a total volume of 0.5 L. Four variants of the experiment were performed to compare the fermentation efficiency of short-chain and long-chain fatty acids with adapted and non-adapted methanogenic microorganisms (Table 1).

Table 1.
Design of the experiment.
Non-adapted (treatment 1,3) and adapted (treatment 2,4) methanogenic microorganisms were used as inoculum. Model soapstock (treatment 1,2, a source of long-chain fatty acids) and sodium acetate (treatment 3,4, a source of short-chain fatty acid) were used as substrates. Substrates were added into the sterile tap water to a final concentration of dissolved organic compounds of 1000 mg/L (Table 1). Mineral salts were also added to the model mixture as a source of essential nutrients, g/L: NH4Cl—5.0; MgSO4—0.5; KH2PO4 —3.0. The treatments were performed using glass jars with a total volume of 0.5 L. Liquid household soap was used as a carbon source consisting of long-chain fatty acid salts (1000 mg/L DOC). Sodium acetate (CH3COONa) was used as the model source of short-chain fatty acids (1000 mg/L DOC). The glass jars were filled with 0.3 L of model mixture (sodium acetate and soapstock) containing mineral salts, substrates, and inoculums (Table 1). The fermentation jars were closed with rubber stoppers with fittings to sample the aliquots of culture fluid and gas and to remove the synthesized gas. Air (21% O2 and 78% N2) was the initial gas phase of the jars. The fermentation was conducted during 72 days at 30 °C. The synthesized gas was removed by a plastic syringe (0.1 L volume) to measure its volume. The effectiveness of sodium acetate and the model soapstock degradation by different types of inoculums was evaluated by the decrease in the concentration of dissolved organic compounds (DOC). The effectiveness of fermentation process was evaluated by the biogas (CH4, CO2) yield. The statistical analysis was carried out using Microsoft Excel and Origin Pro 7 software. All experiments were performed in triplicate. Each value was presented as the mean ± standard deviation (SD).
2.4. Control of the Fermentation Parameters
The metabolic parameters were determined as following [36]: pH, redox potential (Eh, mV), total concentration of dissolved organic compounds (DOC, mg/L), total concentration of NH4+ ions (mg/L), the volume and content of the gas phase, CH4 and CO2 yield.
The pH and Eh were determined using the ionometer EZODO MP-103 with remote electrodes Ezodo and BNC connectors—models PY41 and PO50, respectively. The reliability of the measurements was confirmed by standard buffer solutions [36].
The determination of gas composition was performed by the standard gas chromatography method [37]. The concentration of dissolved organic carbon (DOC) was determined by a permanganate method [38]. The concentration of ammonium ions was determined by photocolorimetric method via the qualitative reaction with Nessler’s reagent [39]. The model stocks with soap and sodium acetate without inoculums were used as a control variant of the experiment.
2.5. Determination of the Effectiveness of the Degradation Process
The evaluation of the efficiency of the degradation of the model soap stock and sodium acetate was determined by the following parameters:
- -
- the model soap stock and acetate degradation time (T, days)—defined as the duration of the process from the moment of the fermentation start until its termination (the termination of gas synthesis, etc.).
- -
- purification efficiency—calculated as the percentage of decrease in the concentration of DOC, %.
- -
- biomethane yield—calculated as the amount of CH4 (L) synthesized from 1 kg of organics counting also the dissolved organic carbon, L CH4/kg DOC.
- -
- carbon dioxide yield —calculated as the amount of CO2 (L) synthesized from 1 kg of organics counting also the dissolved organic carbon, L CO2/kg DOC.
3. Results
3.1. Dynamic of the Model Soapstock and Sodium Acetate Fermentation by Non-Adapted and Adapted Methanogenic Microorganisms
The degradation of sodium acetate and model soapstock occurred during their fermentation. The progress of the fermentation process is shown in Figure 1.

Figure 1.
The methanogenic fermentation (13 days) of sodium acetate (a) and model soapstock (b), as well as sodium acetate crystals (c) and liquid soap (d), which were added to fermentation jars to prepare model stocks.
The basic fermentation parameters of the model soapstock and sodium acetate fermentation were obtained (Figure 2). The initial values of pH (Figure 2a), redox potential (Eh, Figure 2b), concentration of dissolved organic compounds (DOC, Figure 2c), and ammonium ions (Figure 2d) were approximately the same.
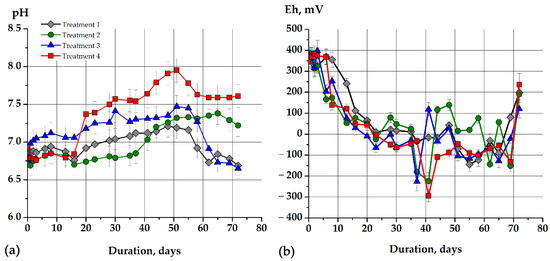
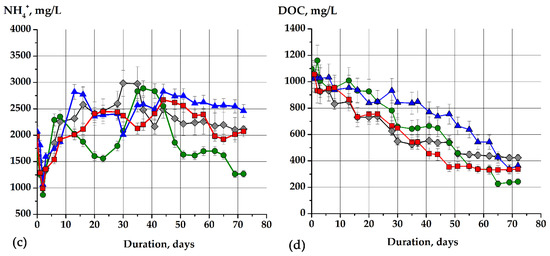
Figure 2.
The basic metabolic parameters of the sodium acetate and model soapstock fermentation process: (a) pH, (b) (Eh), (c) (DOC), (d) NH4+. The figure shows the dynamics of changes in metabolic parameters in different cases with sodium acetate and non-adapted methanogens (gray lines, treatment 1), sodium acetate with adapted methanogens (green lines, treatment 2), model soapstock as a substrate and non-adapted methanogens (blue lines, treatment 3), model soapstock as a substrate and adapted methanogens (red lines, treatment 4).
Thus, the initial pH in all variants of the experiment ranged from 6.72 ± 0.2 to 6.92 ± 0.3 (Figure 2a). In both cases with sodium acetate (treatment 1, treatment 2), the pH increased slowly for the first 20 days. Subsequently (from 28 to 72 days), the pH was stabilized close to the neutral zone from 7.0 ± 0.1 to 7.4 ± 0.1 using both types of inoculum for fermentation (Figure 2a). The pH dynamics of the model soapstock fermentation containing long-chain fatty acids differed from the fermentation of the short-chain fatty acids of sodium acetate. The pH began to rise slowly on the second day of cultivation and increased from 6.8 ± 0.3 (start of fermentation) to 7.1 ± 0.2 (2 days) during the fermentation with adapted methanogens (Figure 2a, treatment 3). The maximum pH value was 7.5 ± 0.2 (51 days). Subsequently, the pH was decreased to slightly acidic values of 6.65 ± 0.2. In contrast to those that were unadapted, adapted methanogens (Figure 2a, treatment 4) raised the pH to a maximum of 7.9 ± 0.4 at 51 days of cultivation. Subsequently, they maintained a pH at a high level of not less than 7.6 ± 0.2 (Figure 2a, treatment 4). The increase in pH from the 20th to the 50th day of cultivation in all variants of the experiment (most in treatment 4, adapted methanogens) can be explained by the accumulation of ammonium ions (Figure 2d) due to the microbial degradation of nitrogen-containing compounds (biomass of microorganisms or stock components (proteins and amino acids) that were introduced into the jars together with the inoculum). Thus, the autoregulation of pH to the slightly alkaline value, which is more optimal for methanogens, was observed.
In all variants of the experiment, the redox potential was gradually decreased to negative values, and were more optimal for methanogens. The maximum decrease in redox potential was observed via the model soapstock fermentation with an adapted methanogenic microbial community (Figure 2b, treatment 4). Thus, Eh decreased from +345 ± 23 mV to −294 ± 29.4 mV. For a long time, Eh remained low and only from 69 to 72 days increased from −132 ± 33.2 mV to +236 ± 53.6 mV, respectively. That indicated the inhibition of the metabolic activity of methanogens (Figure 2b, treatment 4). The Eh did not decrease below −226 ± 45 mV (37 days), but the dynamics and the values of redox potential from 44 to 72 days in both variants were similar during the fermentation of the model soapstock with non-adapted methanogens (Figure 2b, treatments 3, 4). The redox potential had a maximum decreased after 41 days from 389 ± 19.5 mV to −224 ± 41.2 mV, but increased to +116 ± 15.8 mV after 44 days during the fermentation of sodium acetate with adapted methanogens (Figure 2b, treatment 2). Non-adapted methanogens were able to decrease Eh only to −144 mV for 55 days of cultivation, after which it gradually increased to +201 ± 22 mV (Figure 2b, treatment 1). A decrease in the Eh to extremely negative values during the growth of methanogens indicated their high metabolic activity and correlated with an increase in the methane synthesis.
The initial values of ammonium ions’ content ranged from 1539 ± 76.9 mg/L to 2289.3 ± 114.5 mg/L. The concentration of ammonium ions’ NH4+ (Figure 2d) increased till 44 days of cultivation in all variants of the experiment. There was a gradual decrease in the concentration of NH4+ from 44 to 72 days. For example, the NH4+ concentration increased from 1539 ± 76.9 mg/L at the beginning of the fermentation to 2670 ± 133.5 mg/L (44 days) during the soapstock fermentation with adapted methanogens and decreased to 1925 ± 96.2 mg/L on the 65th day. Both salts and inocula (10% of the total model) and biomass of microorganisms were the partial source of ammonium ions. The fluctuations in the concentration of NH4+ in culture medium can be explained by the simultaneous NH4+ consumption by growing microorganisms and the release of cation due to the lysis of microbial biomass. Summing up, the lowest metabolic activity of microorganisms was observed in the variant of sodium acetate fermentation with non-adapted methanogens (Figure 2, treatment 1). Methanogens adapted to high concentrations of organic compounds (Figure 2, treatment 2, treatment 4) increased the pH more intensively and reduced Eh to the optimal values for their growth.
3.2. Dynamic of Sodium Acetate Stock and Model Soapstock Degradation by Methanogenic Microorganisms
The concentration of dissolved organic compounds during the fermentation of sodium acetate and the model soapstock was an important metabolic parameter that characterized the level of its degradation. In addition, the ability to degrade the mixture of short-chain fatty acids (on the example of sodium acetate) and long-chain fatty acids (on the example of soapstock) by non-adapted and adapted to organic compounds in high concentrations methanogens was compared. To create the same conditions for comparing the degradation efficiency, the acetate and liquid soap were loaded to the model stocks to a final concentration of total carbon of 1000 mg/L. It was shown that in all variants of the experiment, a decrease in the concentration of organic compounds throughout the fermentation process took place (Figure 2c). The concentration of dissolved organic compounds decreased 2.5-fold (by 60.6%) from 1075.8 ± 53.8 (start of fermentation) to 423.5 mg/L on the 69th day of fermentation. Furthermore, it was not changed in the variant with sodium acetate and non-adapted methanogens (Figure 2c, treatment 1). Adapted methanogens consumed the sodium acetate as a substrate more efficiently. Thus, there was a 4.5-fold decrease in DOC from 1097.8 ± 102.5 to 242.8 ± 34.1 mg/L with an efficiency of 77.9% (Figure 2c, treatment 2). Non-adapted and adapted methanogens used 64.7% and 68% of total DOC during fermentation of the soapstock, respectively. Thus, the DOC concentration decreased from 1031.8 ± 102.5 mg/L to 363.8 ± 36.4 mg/L (Figure 2c, treatment 3) and from 1053.8 ± 95.6 mg/L to 337.5 ± 33.7 mg/L (Figure 2c, treatment 4), respectively. A decrease in the DOC concentration correlated with an increase in the concentration and yield of CH4 and CO2 (Figure 3). This indicated the presence of the optimal conditions for the growth of methanogens (pH, Eh, temperature, etc.) and effective conversion of substrates (sodium acetate and model soapstock) into biogas.
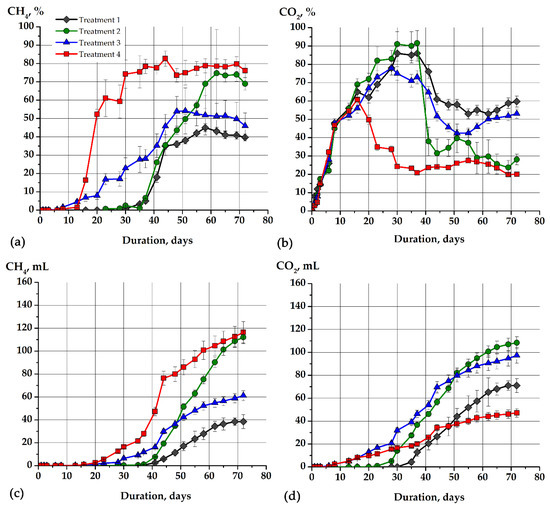
Figure 3.
The production of biogas from sodium acetate and the model soapstock during microbial purification: concentration of CH4 (a), concentration of CO2 (b), cumulative production of CH4 (c), cumulative production of CO2 (d). The figure shows the dynamics of biogas production in different variants with sodium acetate and non-adapted methanogens (gray lines, treatment 1), sodium acetate with adapted methanogens (green lines, treatment 2), model soapstock as a substrate and non-adapted methanogens (blue lines, treatment 3), model soapstock as a substrate and adapted methanogens (red lines, treatment 4).
Thus, the most effective degradation of organic compounds (short-chain and long-chain fatty acids) occurred in the variant with sodium acetate and adapted methanogens and amounted to 77.9%. In other variants, the patterns and efficiency of degradation were similar, regardless of the type of inoculum and ranged from 60.6 to 68%.
3.3. Biogas Production from Sodium Acetate and Model Soapstock by Methanogenic Microorganisms
Simultaneously with microbial degradation of the model soapstock containing synthetic organic compounds via methanogenic fermentation, both adapted and non-adapted methanogens produced biogas (mixture of CH4 and CO2). The maximum concentration of CH4 in all variants of the experiment ranged from 40.8 ± 5.2 (Figure 3, treatment 1) to 82.7 ± 9.8% (Figure 3, treatment 4).
Adapted methanogenic microorganisms synthesized methane more intensively than non-adapted ones. Thus, the concentration of CH4 during model soapstock fermentation by adapted methanogens was 52.3 ± 5.6% on day 20 and 82.7 ± 9.8% on day 44 (Figure 3a, treatment 4). A significantly lower intensity of methane synthesis was observed during the fermentation of soap with non-adapted methanogens (Figure 3a, treatment 3). Thus, on the 20th day the methane concentration was 7.9 ± 2.1%, and on the 51st day it was only 54.1 ± 12.5%. Thus, there was a significant difference in the synthesis of biogas by adapted and non-adapted methanogens. The process of adaptation of the inoculum to the high concentration of organic compounds (especially fatty acids) is described in Section 2.2 (preparation of the inoculum). Adapted methanogens also synthesized methane more intensively during the fermentation of sodium acetate. Thus, the maximum methane concentration was 74.7 ± 23.7% (Figure 3a, treatment 2). The maximum CH4 concentration was only 40.8 ± 5.2% during fermentation of CH3COONa by non-adapted methanogens (Figure 3a, treatment 1).
The concentration of CO2 was also high in all variants of the experiment (Figure 3b). Most of the carbon dioxide was synthesized via fermentation of sodium acetate. For example, the concentration of carbon dioxide was 91.6 ± 6.8% on day 37, and methane was only 6.6 ± 0.3%. The decrease in the concentration of CO2 was correlated with the increase in the concentration of CH4 in all variants of the experiment. During the fermentation of the soapstock, much less carbon dioxide was synthesized. Thus, the maximum CO2 concentration was reached on day 16 and amounted to 60.8 ± 3.1% in the variant with adapted methanogens. However, it decreased to 34.8 ± 3.9% (Figure 3b, treatment 4) on the 23rd day when the methane concentration rose to 61.1 ± 10.5% (Figure 3a, treatment 4). The concentration of CO2 decreased starting from 40 days of cultivation. The concentration of methane increased in this time. Since CO2 can serve as a substrate for methane synthesis, the simultaneous decrease in the CO2 concentration with the increase in the CH4 is quite natural. Thus, the balance shifted from 35–40% CH4/60–65% CO2 (before 40 days) to approximately 60–65% CH4/35–40% CO2.
The efficiency of the fermentation process was also evaluated by the synthesis of the biogas. The total volume of synthesized gas in all variants ranged from 115–256 mL. The gas mixture consisted of CH4 and CO2. The gas yield was calculated as the amount of CH4 (L) synthesized from 1 kg of DOC. Most biomethane was synthesized by adapted methanogens using soap and sodium acetate as substrates. Thus, the CH4 yield was 368.4 L/kg DOC or 127.5 L/kg of soap (treatment 4, Table 1). The biomethane was synthesized by adapted methanogens a little less from sodium acetate—330.9 L/kg DOC or 96.8 L/kg of CH3COONa (treatment 2, Table 1). Much less biogas was synthesized by non-adapted methanogens from both sodium acetate (treatment 1) and soap (treatment 3). Thus, the CH4 yield was 120 L/kg DOC or 35.2 L/kg of CH3COONa (treatment 1) and 197.6 L/kg DOC or 68.3 L/kg of soap (treatment 3) were synthesized.
The least carbon dioxide was formed in the variant of the soap fermentation with adapted methanogens (the most energy-efficient variant in methane production). In this case, the yield of CO2 was 149.3 L/kg DOC or 51.7 L/kg of soap (treatment 4). The highest CO2 yield was in treatment 1–222 L/kg DOC or 64.9 L/kg of CH3COONa.
4. Discussion
The advantages of our study are as follows. We used a diversified microbial community, which contained different physiological groups of microorganisms capable of carrying out all stages of the transformation of organic matter into the final products, CH4 and CO2. We carried out additional adaptation of methanogens to toxic leachate and proved that the studied microbial community is able to quickly adapt to fatty acids in high concentrations. We have shown that methanogens are able not only to synthesize high-energy fuel biomethane by fermenting soap (alkaline salts of fatty acids), but also to degrade fatty acids and soapstock with high efficiency (up to 77.9%). Similar studies are not available in scientific sources. To improve the efficiency of the fermentation process, we did not use additional substrates or enzymes, which is now common in the available literature.
The experimental results show the high efficiency (60.6–77.9%) of soapstock degradation. In addition, adapted methanogens synthesized more biogas from sodium acetate and model soapstock than non-adapted to fatty acid substrate. For example, adapted methanogens synthesized 368.4 L/kg DOC or 127.5 L/kg of soap. Thus, it was experimentally confirmed that the microbial community of fermented methane tank sludge is promising for application in the biotechnologies of bioremediation and biogas synthesis. The main reason for this is the presence of diversified microorganisms of different physiological and taxonomic groups in such communities. Thereby such communities relatively easily adapt to the substrates (fatty acids, oils, etc.) and contribute to their intensive conversion with biogas production [40]. It is a proven fact that the application of municipal waste sludge improves the conversion of fats, oils, and grease. In this case, the adaptation of the microbial community was due to autoselection of Methanosaeta and Methanospirillum, for which the conditions were favorable and the substrate was suitable [40]. Other studies are known where the mechanisms of adaptation of microorganisms to the presence of fatty acids toxic to most bacteria in the bioreactor are established at the molecular level. The metagenomic analysis showed that only the microbes associated with long-chain fatty acids (LCFA) degradation could encode proteins related to “chemotaxis” and “flagellar assembly”, which promoted the ability to move towards the LCFA sources and degrade them [41]. Finally, it was proven that a previously adapted to LCFA inoculum is more efficient in the degradation process of LCFA due to the specialization of the microbial community [41]. In our study, we combined both the application of a diversified microbial community and its pre-adaptation to the similar substrate to increase the efficiency of degradation of sodium acetate (short-chain fatty acid) and model soapstock (source of long-chain fatty acids). In our case, the goal was to purify model stocks from organics (to minimize the concentration of organic compounds) and obtain an energy source—biomethane. Nowadays, household soap consists of synthetic organic compounds, so it is more difficult to degrade it by microorganisms and obtain biogas than from substrates of natural origin. Synthetic soaps and detergents are surfactants and, as such, they have detergent properties. Soap’s chemical properties explain its antimicrobial action. Due to its amphiphilic structure, it is able to interact with the lipid membranes of microorganisms (viruses, bacteria, etc.) and inactivate them [42]. However, it is known that bacteria of Corynebacterium, Pseudomonas, and Bacillus species are able to use soap as the only source of carbon and energy [43]. There are also many other types of microorganisms that can use fatty acids and soap as a substrate. For example, Proteus, Acinetobacter, Enterobacter, Staphylococcus, Bacillus, Pseudomonas, Arthrobacter, Corynebacterium, and Micrococcus [44]. Thus, aerobic processes using aerobic and facultative anaerobic microorganisms are used to transform soap waste. We used methane tank sludge as an inoculum for sodium acetate and soapstock fermentation to degrade them. We have shown that the growth of the non-adapted methanogenic microorganisms in soapstock was not inhibited, as was the growth of the pre-adapted methanogens. This can be explained by the presence of synthetic soaps and other detergents in municipal wastewater in high concentrations [45], which are usually loaded into methane tanks for recycling. Thus, the initial inoculum was also partially adapted and capable of degrading the fatty acids that form the basis of soap. It has been known since the 1930s that methane fermentation has often been used for the conversion of glycerol [46,47] or organic fatty acids (separate components of the soap) into biogas [48]. We have shown that methanogens are able not only to synthesize high-energy fuel biomethane by fermenting soap (alkaline salts of fatty acids), but also to degrade fatty acids and soapstock with high efficiency (up to 77.9%). Similar studies are not available in scientific sources. Further development in this direction will contribute to the development of biotechnologies of contaminated wastewater purification from organic compounds and the development of new alternative methods of biogas production, such as methane fermentation of synthetic organic compounds. The use of fossil fuels (methane, oil, etc.) is undergoing an unprecedented crisis. There is the urgent need to search for alternative energy sources. The degradation of organic compounds by a methanogenic microbiome is the promising approach used for the decomposition of organics in landfills to obtain biomethane [26,27,49]. However, the sources of organics to be degraded are much wider and can be effectively used to provide energy as well as prevent environment pollution [49].
The pretreatment of soapstock with expensive additives, such as enzymes, to increase the efficiency of degradation is now widespread [50,51]. Commercial enzymes [50] or special strains of microorganisms are used for this purpose. For example, Staphylococcus haemolyticus was used to perform enzymatic hydrolysis of lipids [51]. Herein, no additional substances were used to increase the efficiency of fermentation and purification. We used a diversified microbiome containing microorganisms with different types of metabolism, as well as a wide range of enzyme activity, as an inoculum. In addition, we obtained a very high methane yield, which was 368.4 L/kg DOC for soapstock fermentation. In the other study, the methane yield was only 45.4 L/kg-COD after soybean oil refinery wastewater treatment with microbial fuel cells and microbial electrolysis cells at high organic loading (2900 ± 100 mg/L COD) [52]. The efficiency of wastewater degradation in our experiment ranged from 60.6 to 77.9%, which is somewhat lower than using chemical [53] and physical [54] methods (80–99%). However, further research on process optimization will allow the efficiency of soapstock degradation to be increased.
5. Conclusions
The results of this study demonstrated the potential of methanogenic microorganisms in the degradation of sodium acetate and soapstock with the simultaneous biogas synthesis in high amounts. The use of adapted methanogens in the fermentation resulted in the increase in the efficiency of the degradation of the investigated substrates and biomethane production. Since the methanogens fermented and degraded the soapstock with high efficiency, further research of the patterns and mechanisms of industrial synthetic soapstock purification is needed to determine the most effective way of toxic synthetic organic wastewater purification. Further development of this technology is promising for the purification of wastewater and to reduce the reliance on fossil fuels by generating biomethane via the fermentation of toxic soapstock containing long-chain and short-chain fatty acids.
Author Contributions
Conceptualization, V.H., O.T. and G.G.; methodology, A.K. and D.J.; software, O.H. and I.B.; validation, O.S. and O.T.; formal analysis, L.Y. and G.G.; investigation, O.T., V.H., O.H., I.B. and O.S.; resources, O.T. and V.H.; data curation, V.H. and O.T.; writing—original draft preparation, I.B., O.S., O.H., V.H., G.G., L.Y., A.K., D.J. and O.T.; writing—review and editing, I.B., O.S., O.H., V.H., G.G., L.Y., A.K., D.J. and O.T.; visualization, L.Y.; supervision, O.T. and V.H.; project administration, V.H. and O.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded under the fundamental research project of the National Academy of Sciences of Ukraine on the subject #06.30, “Investigation of regularities of sewage treatment from a wide range of toxic organic compounds and metals” and the Kosciuszko Foundation Program for Ukrainian Scientists, grant number 6 April 2022 on the topic “Treatment of solid and liquid organic waste with obtaining of energy and valuable products”.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shah, K.K.R.; Patel, G.B. Biodegradation of Soap Stock: As an Alternative Renewable Energy Resource and Reduce. In Innovations in Environmental Biotechnology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 653–676. [Google Scholar] [CrossRef]
- Postigo, C.; Barceló, D. Synthetic Organic Compounds and Their Transformation Products in Groundwater: Occurrence, Fate and Mitigation. Sci. Total Environ. 2015, 503, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Steber, J.; Berger, H. Biodegradability of Anionic Surfactants. In Biodegradability of Surfactants; Springer: Berlin/Heidelberg, Germany, 1995; pp. 134–182. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Li, Y.; Luo, Y.-L.; Pan, Y.-R.; Liu, J.; Butler, D. Alkaline Environments Benefit Microbial K-Strategists to Efficiently Utilize Protein Substrate and Promote Valorization of Protein Waste into Short-Chain Fatty Acids. Chem. Eng. J. 2021, 404, 127147. [Google Scholar] [CrossRef]
- Chirani, M.R.; Kowsari, E.; Teymourian, T.; Ramakrishna, S. Environmental Impact of Increased Soap Consumption during COVID-19 Pandemic: Biodegradable Soap Production and Sustainable Packaging. Sci. Total Environ. 2021, 796, 149013. [Google Scholar] [CrossRef]
- Bayanmunkh, M.; Bayasgalan, K.; Byambasuren, G.; Batchuluun, K.; Murneren, T. Synthetic Fatty Acid from Crude Oil of Tamsagbulag Petroleum Deposit (Mongolia). Mong. J. Chem. 2021, 22, 1–6. [Google Scholar] [CrossRef]
- Li, S.; Gao, L.; Wang, J.; Rong, G.; Cao, Y. Enhancement of Floatability of Low-Rank Coal Using Oxidized Paraffin Soap. RSC Adv. 2020, 10, 15098–15106. [Google Scholar] [CrossRef]
- Mohammad, A.; Singh, D.N.; Podlasek, A.; Osinski, P.; Koda, E. Leachate Characteristics: Potential Indicators for Monitoring Various Phases of Municipal Solid Waste Decomposition in a Bioreactor Landfill. J. Environ. Manag. 2022, 309, 114683. [Google Scholar] [CrossRef]
- Ofon, U.A.; Ndubuisi-Nnaji, U.U.; Shaibu, S.E.; Fatunla, O.K.; Offiong, N.-A.O. Recycling Anaerobic Digestate Enhances the Co-Digestion Potential of Agro-Industrial Residues: Influence of Different Digestates as Sources of Microbial Inoculum. Environ. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Achaw, O.-W.; Danso-Boateng, E. Soaps and Detergents. In Chemical and Process Industries; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–37. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Khodadoost, F. Effects of Detergents on Natural Ecosystems and Wastewater Treatment Processes: A Review. Environ. Sci. Pollut. Res. 2019, 26, 26439–26448. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An Update of Possible Mechanisms of Action and Implications in the Development of the next-Generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Ababouch, L.; Chaibi, A.; Busta, F.F. Inhibition of Bacterial Spore Growth by Fatty Acids and Their Sodium Salts. J. Food Prot. 1992, 55, 980–984. [Google Scholar] [CrossRef]
- Fay, J.; Farias, R. The Inhibitory Action of Fatty Acids on the Growth of Escherichia coli. Microbiology 1975, 91, 233–240. [Google Scholar] [CrossRef]
- Demirbas, A.; Edris, G.; Alalayah, W.M. Sludge Production from Municipal Wastewater Treatment in Sewage Treatment Plant. Energy Sources Part Recovery Util. Environ. Eff. 2017, 39, 999–1006. [Google Scholar] [CrossRef]
- Peng, W.; Chang, L.; Li, P.; Han, G.; Huang, Y.; Cao, Y. An Overview on the Surfactants Used in Ion Flotation. J. Mol. Liq. 2019, 286, 110955. [Google Scholar] [CrossRef]
- Bartels, C.R.; Wilf, M.; Andes, K.; Iong, J. Design Considerations for Wastewater Treatment by Reverse Osmosis. Water Sci. Technol. 2005, 51, 473–482. [Google Scholar] [CrossRef]
- Bóna, Á.; Bakonyi, P.; Galambos, I.; Bélafi-Bakó, K.; Nemestóthy, N. Separation of Volatile Fatty Acids from Model Anaerobic Effluents Using Various Membrane Technologies. Membranes 2020, 10, 252. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical Oxidation of Organic Pollutants for Wastewater Treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Cao, H. Organic Pollutants Removal in Wastewater by Heterogeneous Photocatalytic Ozonation. Chemosphere 2015, 121, 1–17. [Google Scholar] [CrossRef]
- Kansara, N.; Bhati, L.; Narang, M.; Vaishnavi, R. Wastewater Treatment by Ion Exchange Method: A Review of Past and Recent Researches. Environ. Sci. Indian J. 2016, 12, 143–150. [Google Scholar]
- Reyhanitash, E.; Kersten, S.R.; Schuur, B. Recovery of Volatile Fatty Acids from Fermented Wastewater by Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 9176–9184. [Google Scholar] [CrossRef]
- Singh, P.; Sharda, S.; Cauhan, S.S. Domestic Waste Water Treatment by Fly and Wood Ash along with Additive Materials. Int. J. Civ. Eng. Technol. 2016, 7, 67–75. Available online: http://www.iaeme.com/IJCIET/index.asp (accessed on 5 May 2022).
- Gude, V.G. Wastewater Treatment in Microbial Fuel Cells—An Overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Maier, R.M.; Gentry, T.J. Microorganisms and Organic Pollutants. In Environmental Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 377–413. [Google Scholar] [CrossRef]
- Anijiofor, S.C.; Jamil, N.A.M.; Jabbar, S.; Sakyat, S.; Gomes, C. Aerobic and Anaerobic Sewage Biodegradable Processes: The Gap Analysis. Int. J. Res. Environ. Sci. 2017, 3, 9–19. [Google Scholar] [CrossRef]
- Kong, Z.; Li, L.; Xue, Y.; Yang, M.; Li, Y.-Y. Challenges and Prospects for the Anaerobic Treatment of Chemical-Industrial Organic Wastewater: A Review. J. Clean. Prod. 2019, 231, 913–927. [Google Scholar] [CrossRef]
- Chávez, A.; Gimeno, O.; Rey, A.; Pliego, G.; Oropesa, A.; Álvarez, P.; Beltrán, F. Treatment of Highly Polluted Industrial Wastewater by Means of Sequential Aerobic Biological Oxidation-Ozone Based AOPs. Chem. Eng. J. 2019, 361, 89–98. [Google Scholar] [CrossRef]
- Pereira, M.A. Anaerobic Biodegradation of Long Chain Fatty Acids Biomethanisation of Biomass-Associated LCFA as a Challenge for the Anaerobic Treatment of Effluents with High Lipid/LCFA Content. 2003. Available online: https://hdl.handle.net/1822/4650 (accessed on 28 June 2022).
- De Amorim, E.L.C.; Barros, A.R.; Damianovic, M.H.R.Z.; Silva, L.D. Anaerobic fluidized bed reactor with expanded clay as support for hydrogen production through dark fermentation of glucose. Int. J. Hydrog. Energy 2009, 34, 783–790. [Google Scholar] [CrossRef]
- Sousa, D.Z.; Balk, M.; Alves, M.; Schink, B.; McInerney, M.J.; Smidt, H.; Plugge, C.M.; Stams, A.J. Degradation of Long-Chain Fatty Acids by Sulfate-Reducing and Methanogenic Communities. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Thauer, R.K. Biochemistry of methanogenesis: A tribute to Marjory Stephenson: 1998 Marjory Stephenson prize lecture. Microbiology 1998, 144, 2377–2406. [Google Scholar] [CrossRef]
- Hovorukha, V.; Havryliuk, O.; Bida, I.; Danko, Y.P.; Shabliy, O.; Gladka, G.; Yastremska, L.; Tashyrev, O. Two-stage degradation of solid organic waste and liquid filtrate. Biotechnol. Acta 2021, 14, 70–79. [Google Scholar] [CrossRef]
- Gésan-Guiziou, G. Removal of Bacteria, Spores and Somatic Cells from Milk by Centrifugation and Microfiltration Techniques. In Improving the Safety and Quality of Milk; Elsevier: Amsterdam, The Netherlands, 2010; pp. 349–372. [Google Scholar] [CrossRef]
- Havryliuk, O.; Hovorukha, V.; Savitsky, O.; Trilis, V.; Kalinichenko, A.; Dołhańczuk-Śródka, A.; Janecki, D.; Tashyrev, O. Anaerobic Degradation of Environmentally Hazardous Aquatic Plant Pistia stratiotes and Soluble Cu(II) Detoxification by Methanogenic Granular Microbial Preparation. Energies 2021, 14, 3849. [Google Scholar] [CrossRef]
- Acree, W.E. Basic Gas Chromatography (McNair, Harold M.; Miller, James M.). J. Chem. Educ. 1998, 75, 1094. [Google Scholar] [CrossRef][Green Version]
- Suslova, O.; Govorukha, V.; Brovarskaya, O.; Matveeva, N.; Tashyreva, H.; Tashyrev, O. Method for Determining Organic Compound Concentration in Biological Systems by Permanganate Redox Titration. Int. J. Bioautom. 2014, 18, 45–52. Available online: http://www.biomed.bas.bg/bioautomation/2014/vol_18.1/files/18.1_05.pd (accessed on 12 June 2022).
- Qiu, X.; Liu, G.-P.; Zhu, Y.-Q. Determination of Water-Soluble Ammonium Ion in Soil by Spectrophotometry. Analyst 1987, 112, 909–911. [Google Scholar] [CrossRef]
- Ziels, R.M.; Karlsson, A.; Beck, D.A.; Ejlertsson, J.; Yekta, S.S.; Bjorn, A.; Stensel, H.D.; Svensson, B.H. Microbial Community Adaptation Influences Long-Chain Fatty Acid Conversion during Anaerobic Codigestion of Fats, Oils, and Grease with Municipal Sludge. Water Res. 2016, 103, 372–382. [Google Scholar] [CrossRef]
- Kougias, P.G.; Treu, L.; Campanaro, S.; Zhu, X.; Angelidaki, I. Dynamic Functional Characterization and Phylogenetic Changes Due to Long Chain Fatty Acids Pulses in Biogas Reactors. Sci. Rep. 2016, 6, 28810. [Google Scholar] [CrossRef]
- Coiffard, L.; Couteau, C. Soap and Syndets: Differences and Analogies, Sources of Great Confusion. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11432–11439. [Google Scholar] [CrossRef]
- Nwinyi, O.C.; Umane, P.O. Degradation Potentials of Tropical Soil Bacteria on Detergents. IOP Publ. 2021, 665, 012071. [Google Scholar] [CrossRef]
- Osadebe, A.U.; Onyiliogwu, C.A.; Okpokwasili, G.C. Biodegradation of Anionic Surfactants from Oil Field Detergents in Aquatic Systems. Univers. J. Microbiol. 2018, 6, 7–14. [Google Scholar] [CrossRef]
- Ojo, O.A.; Oso, B.A. Biodegradation of Synthetic Detergents in Wastewater. Afr. J. Biotechnol. 2009, 8, 1090–1109. Available online: http://www.academicjournals.org/AJB (accessed on 10 May 2022).
- Szymanowska-Powałowska, D.; Kubiak, P.; Lewicki, A. The Methane Fermentation Medium as an Attractive Source of Bacteria from Genus Clostridium Capable of Converting Glycerol into 1, 3-Propylene Glycol. Biotechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2012, 93, 68–77. [Google Scholar] [CrossRef]
- Thayer, L.A. Bacterial Genesis of Hydrocarbons from Fatty Acids. AAPG Bull. 1931, 15, 441–453. [Google Scholar] [CrossRef]
- Tarvin, D.; Buswell, A. The Methane Fermentation of Organic Acids and Carbohydrates. J. Am. Chem. Soc. 1934, 56, 1751–1755. [Google Scholar] [CrossRef]
- Pagliano, G.; Ventorino, V.; Panico, A.; Pepe, O. Integrated Systems for Biopolymers and Bioenergy Production from Organic Waste and By-Products: A Review of Microbial Processes. Biotechnol. Biofuels 2017, 10, 113. [Google Scholar] [CrossRef]
- Borowski, S.; Cieciura-Włoch, W. Enzymatic Pretreatment of Byproducts from Soapstock Splitting and Glycerol Processing for Improvement of Biogas Production. Molecules 2021, 26, 6782. [Google Scholar] [CrossRef]
- Cherif, S.; Aloui, F.; Carrière, F.; Sayadi, S. Lipase Pre-Hydrolysis Enhance Anaerobic Biodigestion of Soap Stock from An Oil Refining Industry. J. Oleo Sci. 2014, 63, 109–114. [Google Scholar] [CrossRef]
- Yu, N.; Xing, D.; Li, W.; Yang, Y.; Li, Z.; Li, Y.; Ren, N. Electricity and Methane Production from Soybean Edible Oil Refinery Wastewater Using Microbial Electrochemical Systems. Int. J. Hydrog. Energy 2017, 42, 96–102. [Google Scholar] [CrossRef]
- Thomas, M.; Kozik, V.; Barbusiński, K.; Sochanik, A.; Jampilek, J.; Bąk, A. Potassium Ferrate (VI) as the Multifunctional Agent in the Treatment of Landfill Leachate. Materials 2020, 13, 5017. [Google Scholar] [CrossRef]
- Tałałaj, I.A.; Biedka, P.; Bartkowska, I. Treatment of Landfill Leachates with Biological Pretreatments and Reverse Osmosis. Environ. Chem. Lett. 2019, 17, 1177–1193. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).