Synergic Effects of Bed Materials and Catalytic Filter Candle for the Conversion of Tar during Biomass Steam Gasification
Abstract
1. Introduction
- the toluene content lower than 750 mg/Nm3 even if it should be preferably <250 mg/Nm3;
- the naphthalene content lower than 75 mg/Nm3, even if it should be preferably <25 mg/Nm3.
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Apparatus
2.3. Analysis of Products
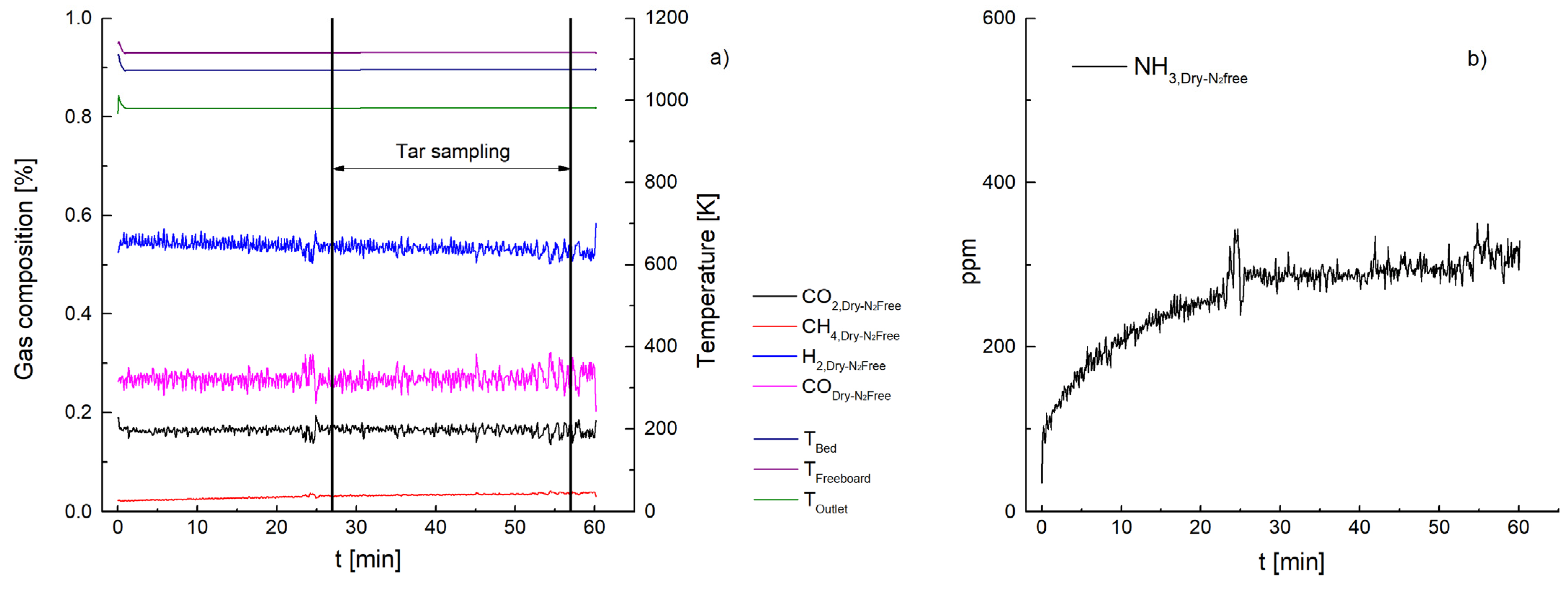
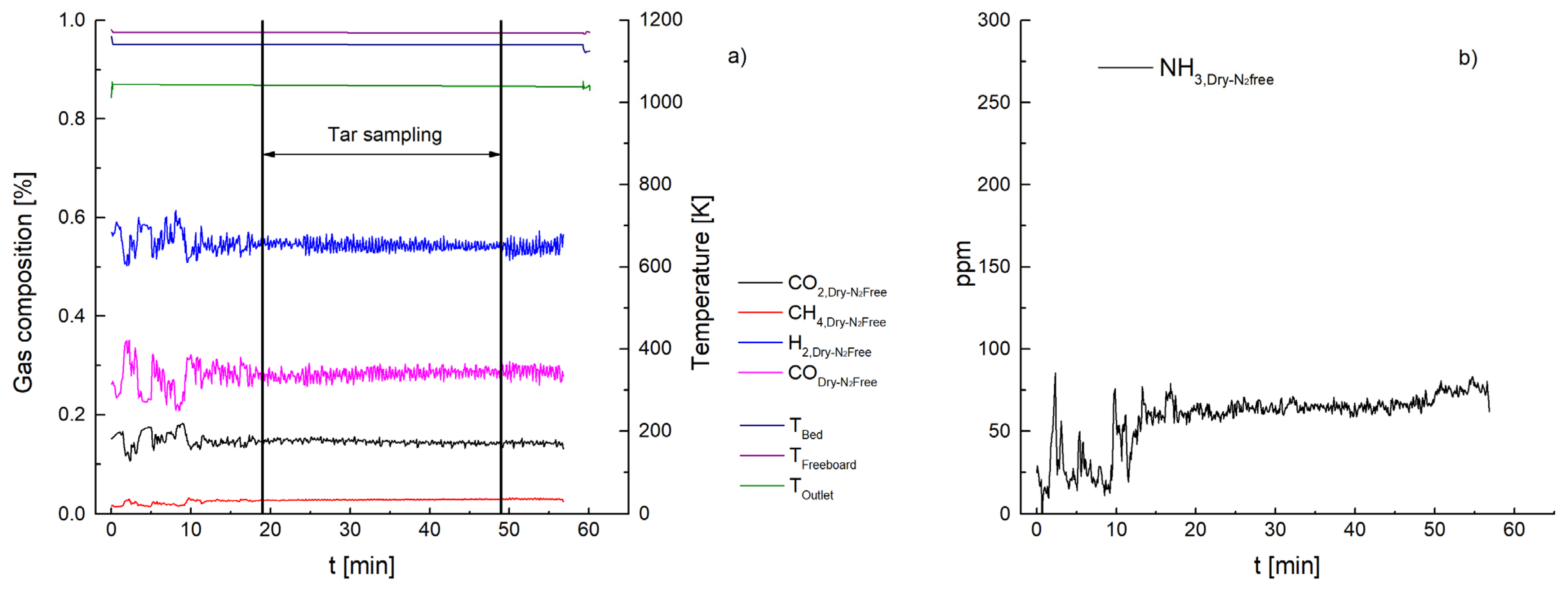
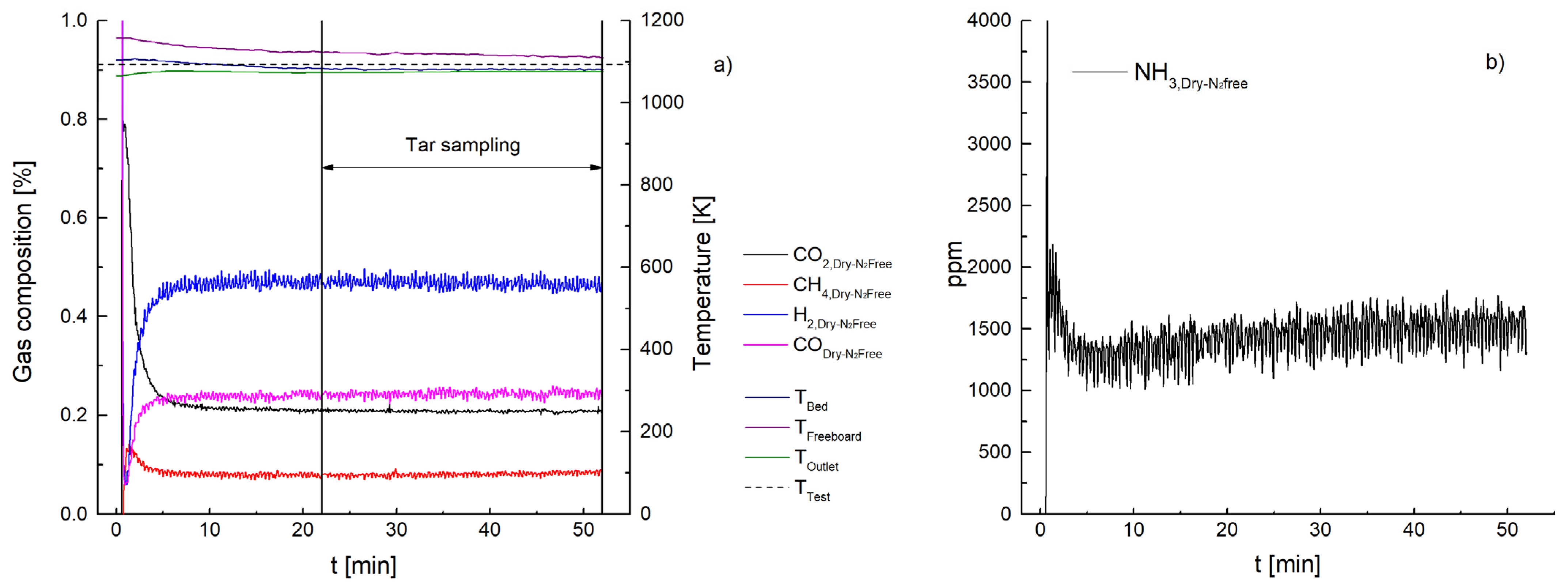
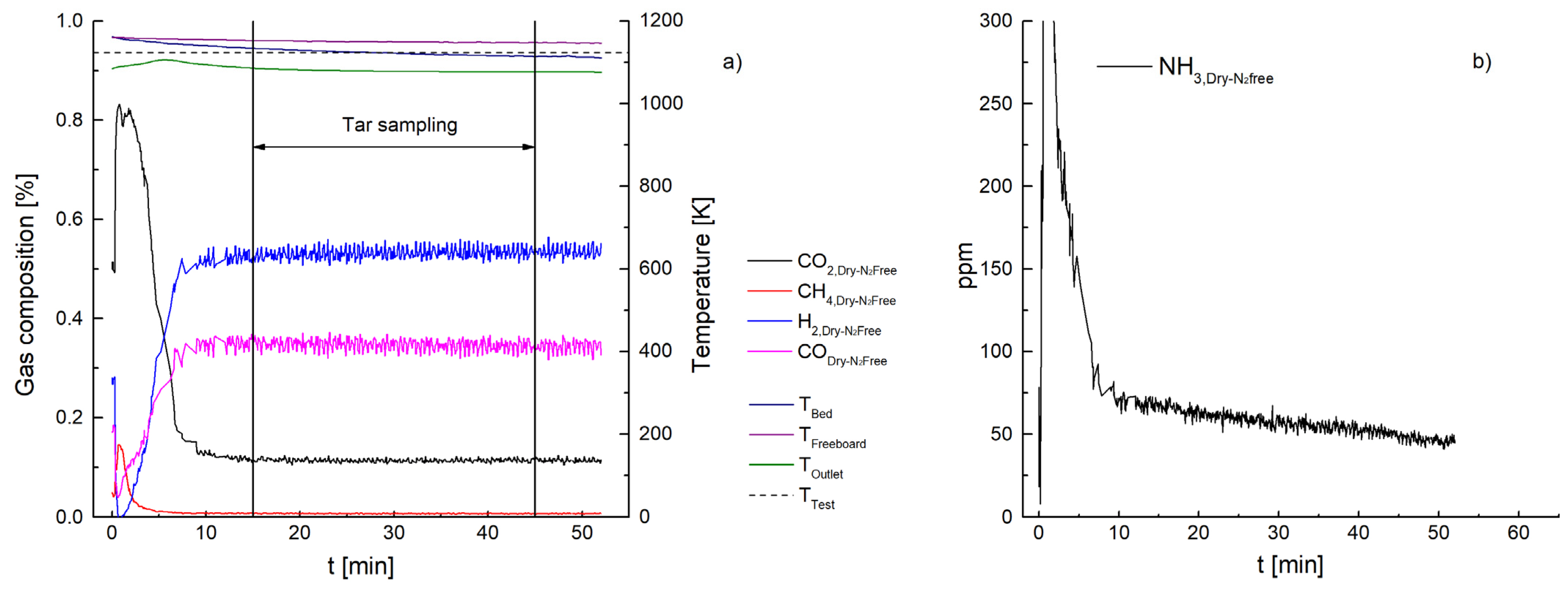
3. Results and Discussion
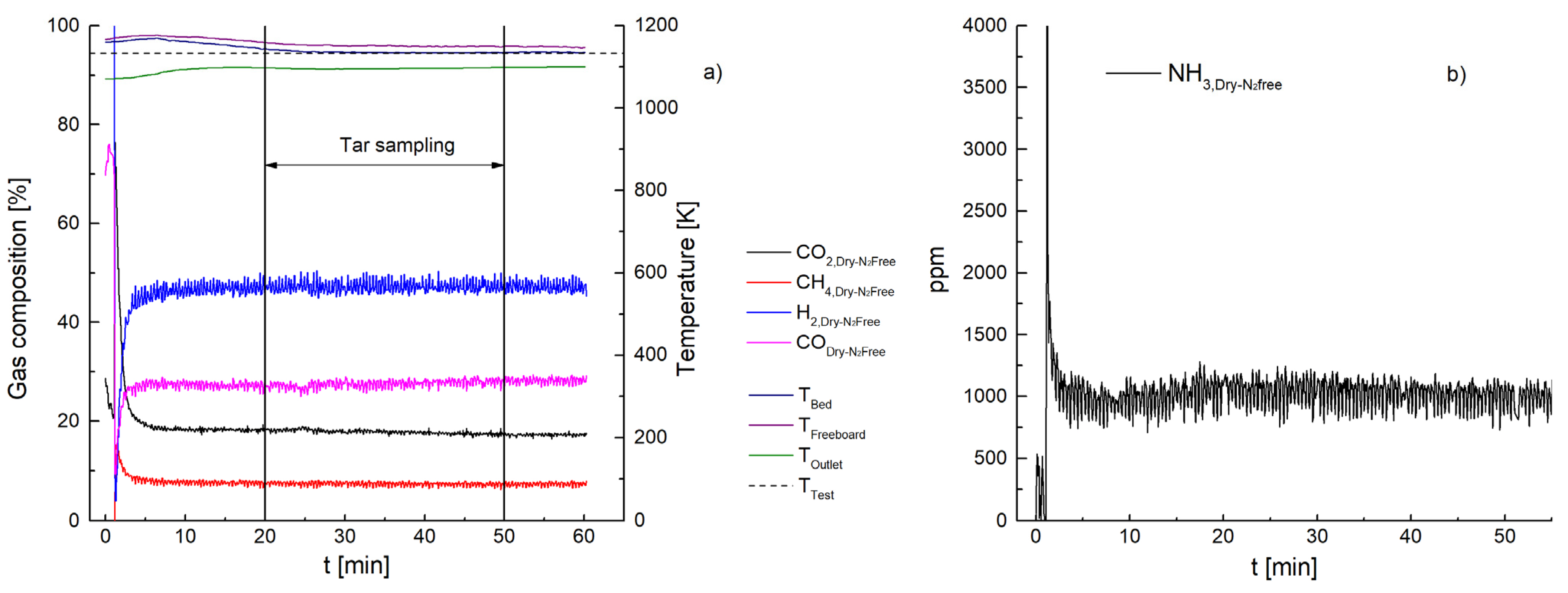
3.1. Ammonia Content
3.2. Tar Content
3.2.1. Ceramic Filter Candle (Test 1–7)
3.2.2. Metallic Filter Candle (Test 8)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- World Health Organization. COP24 Special Report: Health and Climate Change; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- United Nations. Paris Agreement. 21st Conf Parties 2015:3. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 9 August 2021).
- A European Green Deal|European Commission. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 9 August 2021).
- Trattner, A.; Klell, M.; Radner, F. Sustainable Hydrogen Society–Vision, Findings and Development of a Hydrogen Economy Using the Example of Austria. Int. J. Hydrogen Energy 2022, 47, 2059–2079. [Google Scholar] [CrossRef]
- Lin, R.H.; Zhao, Y.Y.; Wu, B.D. Toward a Hydrogen Society: Hydrogen and Smart Grid Integration. Int. J. Hydrogen Energy 2020, 45, 20164–20175. [Google Scholar] [CrossRef]
- Thomson, R.; Kwong, P.; Ahmad, E.; Nigam, K.D.P. Clean Syngas from Small Commercial Biomass Gasifiers; a Review of Gasifier Development, Recent Advances and Performance Evaluation. Int. J. Hydrogen Energy 2020, 45, 21087–21111. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A Review of the Primary Measures for Tar Elimination in Biomass Gasification Processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A Review on Biomass Gasification Syngas Cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Kraussler, M.; Binder, M.; Schindler, P.; Hofbauer, H. Hydrogen Production within a Polygeneration Concept Based on Dual Fluidized Bed Biomass Steam Gasification. Biomass Bioenergy 2018, 111, 320–329. [Google Scholar] [CrossRef]
- Ciferno, J.P.; Marano, J.J. Benchmarking Biomass Gasification Technologies for Fuels, Chemicals and Hydrogen Production; US Department of Energy, National Energy Technology Laboratory: Morgantown, WV, USA, 2002.
- Bocci, E.; Di Carlo, A.; McPhail, S.J.; Gallucci, K.; Foscolo, P.U.; Moneti, M.; Villarini, M.; Carlini, M. Biomass to Fuel Cells State of the Art: A Review of the Most Innovative Technology Solutions. Int. J. Hydrogen Energy 2014, 39, 21876–21895. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; He, V.; Nakajo, A.; Schiffmann, J.; Maréchal, F.; Van Herle, J. Techno-Economic Optimization of an Integrated Biomass Waste Gasifier–Solid Oxide Fuel Cell Plant. Front. Energy Res. 2021, 9, 247. [Google Scholar] [CrossRef]
- Costa, P.; Pinto, F.; Neto André, R.; Marques, P. Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP). Processes 2021, 9, 254. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass Gasification Gas Cleaning for Downstream Applications: A Comparative Critical Review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Larsson, A.; Kuba, M.; Berdugo Vilches, T.; Seemann, M.; Hofbauer, H.; Thunman, H. Steam Gasification of Biomass–Typical Gas Quality and Operational Strategies Derived from Industrial-Scale Plants. Fuel Process. Technol. 2021, 212, 106609. [Google Scholar] [CrossRef]
- Pio, D.T.; Ruivo, L.C.M.; Tarelho, L.A.C.; Frade, J.R.; Kantarelis, E.; Engvall, K. Tar Formation during Eucalyptus Gasification in a Bubbling Fluidized Bed Reactor: Effect of Feedstock and Reactor Bed Composition. Energy Convers. Manag. 2021, 229, 113749. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128130407. [Google Scholar]
- Milne, T.A.; Evans, R.J.; Abatzaglou, N. Biomass Gasifier “Tars”: Their Nature, Formation, and Conversion; U.S. National Renewable Energy Laboratory: Golden, CO, USA, 1998. [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in Biofuel Production from Gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Hasler, P.; Nussbaumer, T. Gas Cleaning for IC Engine Applications from Fixed Bed Biomass Gasification. Biomass Bioenergy 1999, 16, 385–395. [Google Scholar] [CrossRef]
- Abedi, A.; Dalai, A.K. Steam Gasification of Oat Hull Pellets over Ni-Based Catalysts: Syngas Yield and Tar Reduction. Fuel 2019, 254, 115585. [Google Scholar] [CrossRef]
- Cortazar, M.; Alvarez, J.; Lopez, G.; Amutio, M.; Santamaria, L.; Bilbao, J.; Olazar, M. Role of Temperature on Gasification Performance and Tar Composition in a Fountain Enhanced Conical Spouted Bed Reactor. Energy Convers. Manag. 2018, 171, 1589–1597. [Google Scholar] [CrossRef]
- Horvat, A.; Pandey, D.S.; Kwapinska, M.; Mello, B.B.; Gómez-Barea, A.; Fryda, L.E.; Rabou, L.P.L.M.; Kwapinski, W.; Leahy, J.J. Tar Yield and Composition from Poultry Litter Gasification in a Fluidised Bed Reactor: Effects of Equivalence Ratio, Temperature and Limestone Addition. RSC Adv. 2019, 9, 13283–13296. [Google Scholar] [CrossRef]
- Tsekos, C.; del Grosso, M.; de Jong, W. Gasification of Woody Biomass in a Novel Indirectly Heated Bubbling Fluidized Bed Steam Reformer. Fuel Process. Technol. 2021, 224, 107003. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of Literature on Catalysts for Biomass Gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Li, C.; Hirabayashi, D.; Suzuki, K. Development of New Nickel Based Catalyst for Biomass Tar Steam Reforming Producing H2-Rich Syngas. Fuel Process. Technol. 2009, 90, 790–796. [Google Scholar] [CrossRef]
- Pinto, F.; Lopes, H.; André, R.N.; Gulyurtlu, I.; Cabrita, I. Effect of Catalysts in the Quality of Syngas and By-Products Obtained by Co-Gasification of Coal and Wastes. Tars and Nitrogen Compounds Abatement. Fuel 2007, 86, 2052–2063. [Google Scholar] [CrossRef]
- Savuto, E.; Di Carlo, A.; Steele, A.; Heidenreich, S.; Gallucci, K.; Rapagna, S. Syngas Conditioning by Ceramic Filter Candles Filled with Catalyst Pellets and Placed inside the Freeboard of a Fluidized Bed Steam Gasifier. Fuel Process. Technol. 2019, 191, 44–53. [Google Scholar] [CrossRef]
- Dayton, D. Review of the Literature on Catalytic Biomass Tar Destruction: Milestone Completion Report; Report; National Renewable Energy Laboratory (U.S.): Golden, CO, USA, 2002.
- Ma, X.; Zhao, X.; Gu, J.; Shi, J. Co-Gasification of Coal and Biomass Blends Using Dolomite and Olivine as Catalysts. Renew. Energy 2019, 132, 509–514. [Google Scholar] [CrossRef]
- Soomro, A.; Chen, S.; Ma, S.; Xiang, W. Catalytic Activities of Nickel, Dolomite, and Olivine for Tar Removal and H2-Enriched Gas Production in Biomass Gasification Process. Energy Environ. 2018, 29, 839–867. [Google Scholar] [CrossRef]
- Savuto, E.; May, J.; Di Carlo, A.; Gallucci, K.; Di Giuliano, A.; Rapagnà, S. Steam Gasification of Lignite in a Bench-Scale Fluidized-Bed Gasifier Using Olivine as Bed Material. Appl. Sci. 2020, 10, 2931. [Google Scholar] [CrossRef]
- Final Report Summary-UNIFHY (UNIQUE Gasifier for Hydrogen Production)|Report Summary|UNIfHY|FP7|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/299732/reporting/it (accessed on 10 August 2021).
- Kuramoto, K.; Hosokai, S.; Matsuoka, K.; Ishiyama, T.; Kishimoto, H.; Yamaji, K. Degradation Behaviors of SOFC Due to Chemical Interaction between Ni-YSZ Anode and Trace Gaseous Impurities in Coal Syngas. Fuel Process. Technol. 2017, 160, 8–18. [Google Scholar] [CrossRef]
- Ouweltjes, J.P. Report Summarising the Literature Review for Selection of Representative Syngas Compositions Including Organic and Inorganic Contaminants; European Union Funding for Research and Innovation: Brussel, Belgium, 2019. [Google Scholar]
- UNIQUE Gasifier for Hydrogen Production|UNIfHY Project|FP7|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/299732/it (accessed on 10 August 2021).
- Heidenreich, S.; Foscolo, P.U. New Concepts in Biomass Gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Rapagnà, S.; Gallucci, K.; Foscolo, P.U. Olivine, Dolomite and Ceramic Filters in One Vessel to Produce Clean Gas from Biomass. Waste Manag. 2018, 71, 792–800. [Google Scholar] [CrossRef]
- Aravind, P.V.; De Jong, W. Evaluation of High Temperature Gas Cleaning Options for Biomass Gasification Product Gas for Solid Oxide Fuel Cells. Prog. Energy Combust. Sci. 2012, 38, 737–764. [Google Scholar] [CrossRef]
- Xu, C.; Donald, J.; Byambajav, E.; Ohtsuka, Y. Recent Advances in Catalysts for Hot-Gas Removal of Tar and NH3 from Biomass Gasification. Fuel 2010, 89, 1784–1795. [Google Scholar] [CrossRef]
- Franco, C.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. The Study of Reactions Influencing the Biomass Steam Gasification Process. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Simell, P.A.; Hirvensalo, E.K.; Smolander, V.T.; Krause, A.O.I. Steam Reforming of Gasification Gas Tar over Dolomite with Benzene as a Model Compound. Ind. Eng. Chem. Res. 1999, 38, 1250–1257. [Google Scholar] [CrossRef]
- Corella, J.; Caballero, M.A.; Aznar, M.-P.; Brage, C. Two Advanced Models for the Kinetics of the Variation of the Tar Composition in Its Catalytic Elimination in Biomass Gasification. Ind. Eng. Chem. Res. 2003, 42, 3001–3011. [Google Scholar] [CrossRef]


| Proximate Analysis (wt.%) | Ultimate Analysis (wt.% Dry Basis) | ||
|---|---|---|---|
| Moisture | 7.7 | C | 48.90 |
| Ash | 1.1 | H | 6.20 |
| Volatile matter | 71.7 | N | 0.18 |
| Fixed carbon | 19.5 | O | 43.50 |
| Cl | 0.029 | ||
| S | 0.026 | ||
| Test | 1-2-3 | 4-5-6-7 | 8 | ||
|---|---|---|---|---|---|
| Bed material | Olivine | Olivine | Dolomite | Olivine | Dolomite |
| wt.% | 100 | 80.5 | 19.5 | 80 | 20 |
| Sauter mean diameter [μm] | 235 | 250.1 | 330.6 | 250.4 | 339.9 |
| Particle density [kg/m3] | 3000 | 3000 | 1650 | 3000 | 1650 |
| Test | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Temperature [K] | 1032 | 1085 | 1130 | 1081 | 1136 | 1093 | 1137 | 1123 |
| Steam/Biomassdry | 0.50 | 0.51 | 0.51 | 0.53 | 0.52 | 0.51 | 0.50 | 0.58 |
| Filter Candle | Ceramic filter with nickel-based catalyst | Ceramic filter without nickel-based catalyst | Ceramic filter with nickel-based catalyst | Metallic filter with nickel-based catalyst | ||||
| Catalyst [g] | 455 | - | 463 | 1027 | ||||
| Biomass [g/min] | 11.28 | 10.84 | 10.84 | 11.10 | ||||
| Test | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Bed material | Olivine | Olivine—Dolomite | ||||||
| Gas yield [Nm3/kgBiomass] | 1.4 | 1.4 | 1.4 | 1.4 | 1.6 | 1.8 | 1.9 | 1.9 |
| Char residue [gChar/kgBiomass] | 78.1 | 101.7 | 67.1 | 80.6 | 60.4 | 55.9 | ||
| Tar [mg/Nm3] | 7116.0 | 3730.7 | 678.2 | 6217.3 | 3252.3 | 798.0 | 432.8 | 225.2 |
| H2O conversion [%] | 37.8 | 43.7 | 54.7 | 38.5 | 42.7 | 50.0 | 54.8 | 58.2 |
| GHSV [h−1] | 4153 | 4102 | 3922 | ------ | ------ | 4168 | 4227 | 2324 |
| H2 [%voldryN2free] | 53.4 | 53.5 | 54.2 | 46.3 | 46.8 | 55.3 | 55.1 | 53.3 |
| CO [%voldryN2free] | 25.7 | 27.0 | 28.4 | 23.6 | 26.9 | 28.8 | 31.4 | 34.7 |
| CO2 [%voldryN2free] | 18.3 | 16.4 | 14.4 | 20.7 | 17.7 | 13.9 | 12.1 | 11.3 |
| CH4 [%voldryN2free] | 2.6 | 3.1 | 2.9 | 9.4 | 8.6 | 2.0 | 1.4 | 0.6 |
| NH3 [ppm] | 447 | 260 | 66 | 1499 | 1032 | 112 | 87 | 57 |
| Average candle temperature [K] | 1022 | 1066 | 1113 | 1096 | 1124 | 1076 | 1108 | 1114 |
| Mass balance closure [%] | 1.1 | 1.7 | 5.2 | 1.3 | 4.6 | 2.5 | 2.2 | 0.6 |
| Test | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Toluene | 1856.3 | 1383.1 | 167.3 | 2001.5 | 1096.9 | 390.0 | 239.5 | 146.1 |
| Naphthalene | 861.3 | 636.0 | 186.0 | 1620.1 | 1366.4 | 254.0 | 136.7 | 47.5 |
| Phenol | 568.7 | 223.1 | 48.6 | 86.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1-ring compounds | 2311.7 | 1546.5 | 185.6 | 2489.0 | 1263.7 | 432.4 | 256.3 | 154.8 |
| 2-ring compounds | 571.8 | 274.5 | 58.9 | 609.6 | 228.9 | 53.4 | 24.6 | 15.0 |
| 3- and 4-ring compounds | 2803 | 1050 | 199 | 1412.6 | 393.3 | 58.3 | 15.2 | 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, A.A.; Savuto, E.; Di Carlo, A.; Tacconi, A.; Rapagnà, S. Synergic Effects of Bed Materials and Catalytic Filter Candle for the Conversion of Tar during Biomass Steam Gasification. Energies 2023, 16, 595. https://doi.org/10.3390/en16020595
Papa AA, Savuto E, Di Carlo A, Tacconi A, Rapagnà S. Synergic Effects of Bed Materials and Catalytic Filter Candle for the Conversion of Tar during Biomass Steam Gasification. Energies. 2023; 16(2):595. https://doi.org/10.3390/en16020595
Chicago/Turabian StylePapa, Alessandro Antonio, Elisa Savuto, Andrea Di Carlo, Alessandra Tacconi, and Sergio Rapagnà. 2023. "Synergic Effects of Bed Materials and Catalytic Filter Candle for the Conversion of Tar during Biomass Steam Gasification" Energies 16, no. 2: 595. https://doi.org/10.3390/en16020595
APA StylePapa, A. A., Savuto, E., Di Carlo, A., Tacconi, A., & Rapagnà, S. (2023). Synergic Effects of Bed Materials and Catalytic Filter Candle for the Conversion of Tar during Biomass Steam Gasification. Energies, 16(2), 595. https://doi.org/10.3390/en16020595








