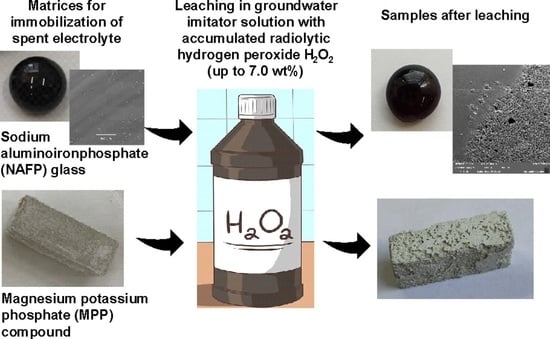
Behavior of Glass-like and Mineral-like Phosphate Compounds with an Immobilized Chloride Mixture in Hydrogen Peroxide Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Procedures
2.1.1. Synthesis of NAFP Glass
2.1.2. Synthesis of MPP Compound
2.2. Methods
3. Results and Discussion
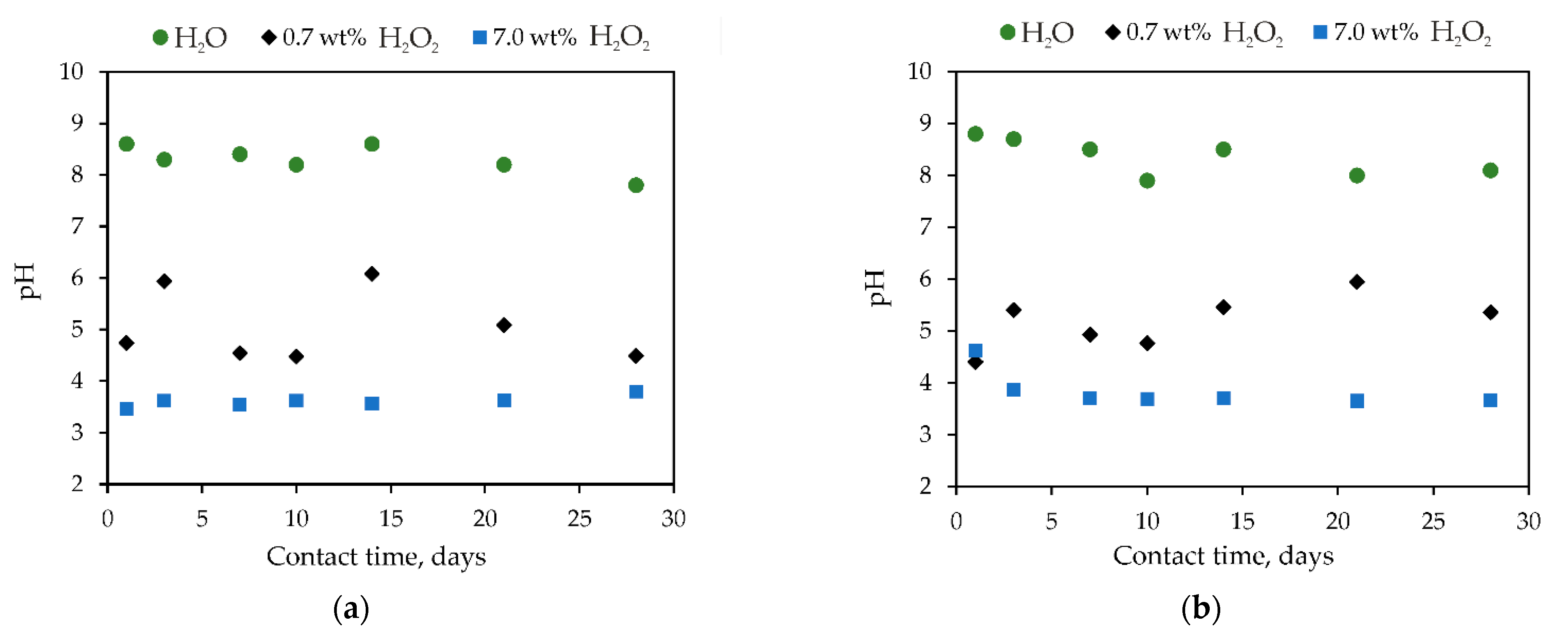
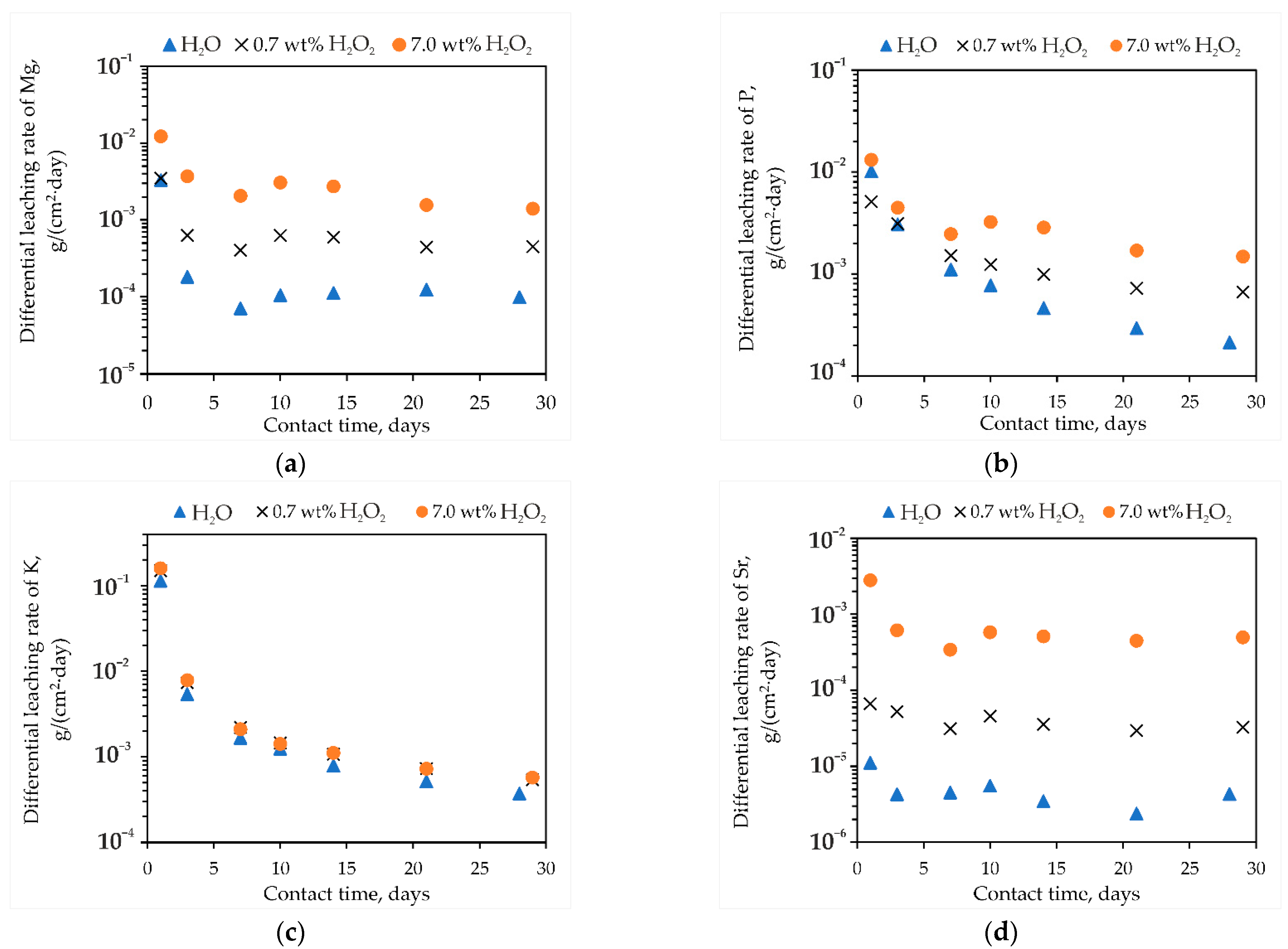
3.1. Study of NAFP Glass after Contact with Hydrogen Peroxide
3.1.1. Hydrolytic Stability of Samples
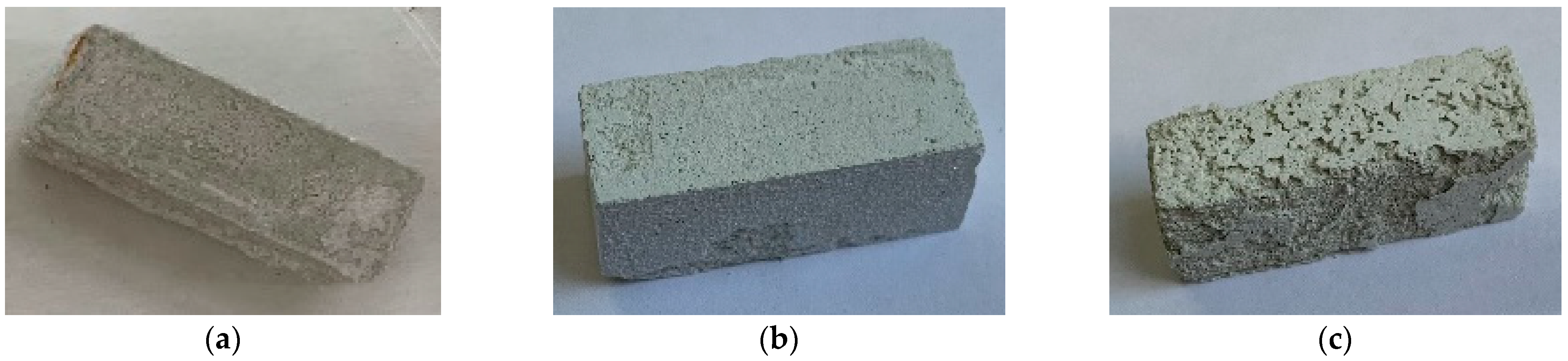
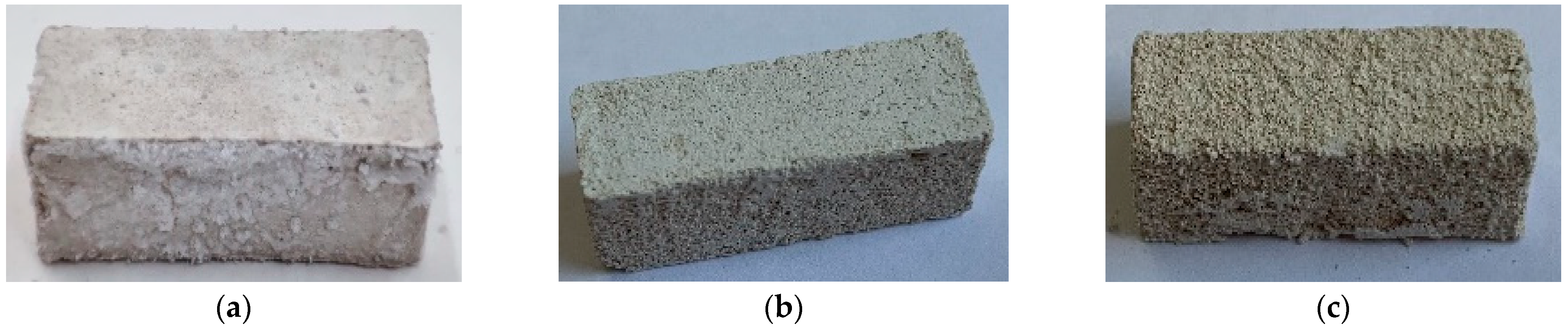
3.1.2. Surface of Samples
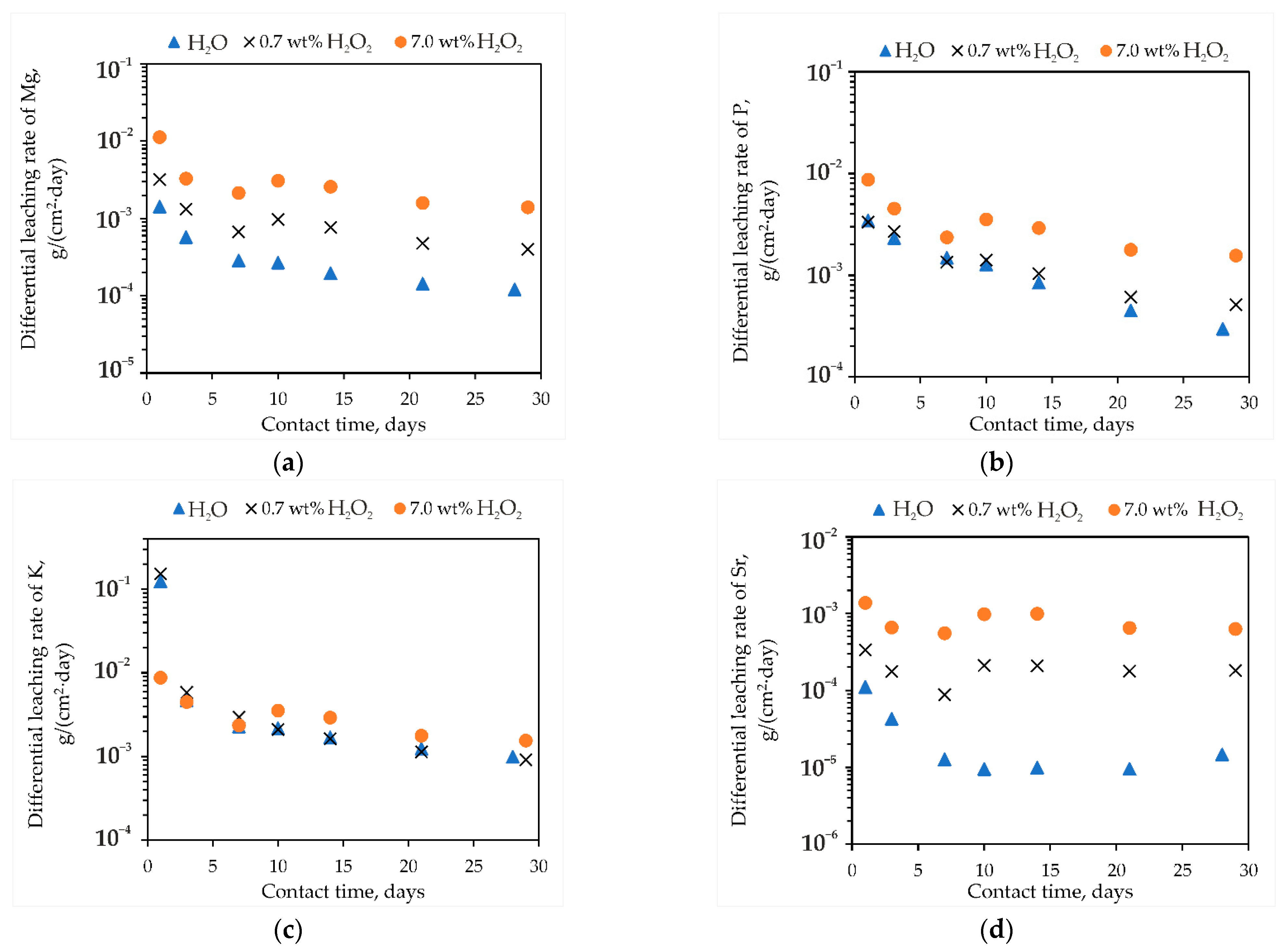
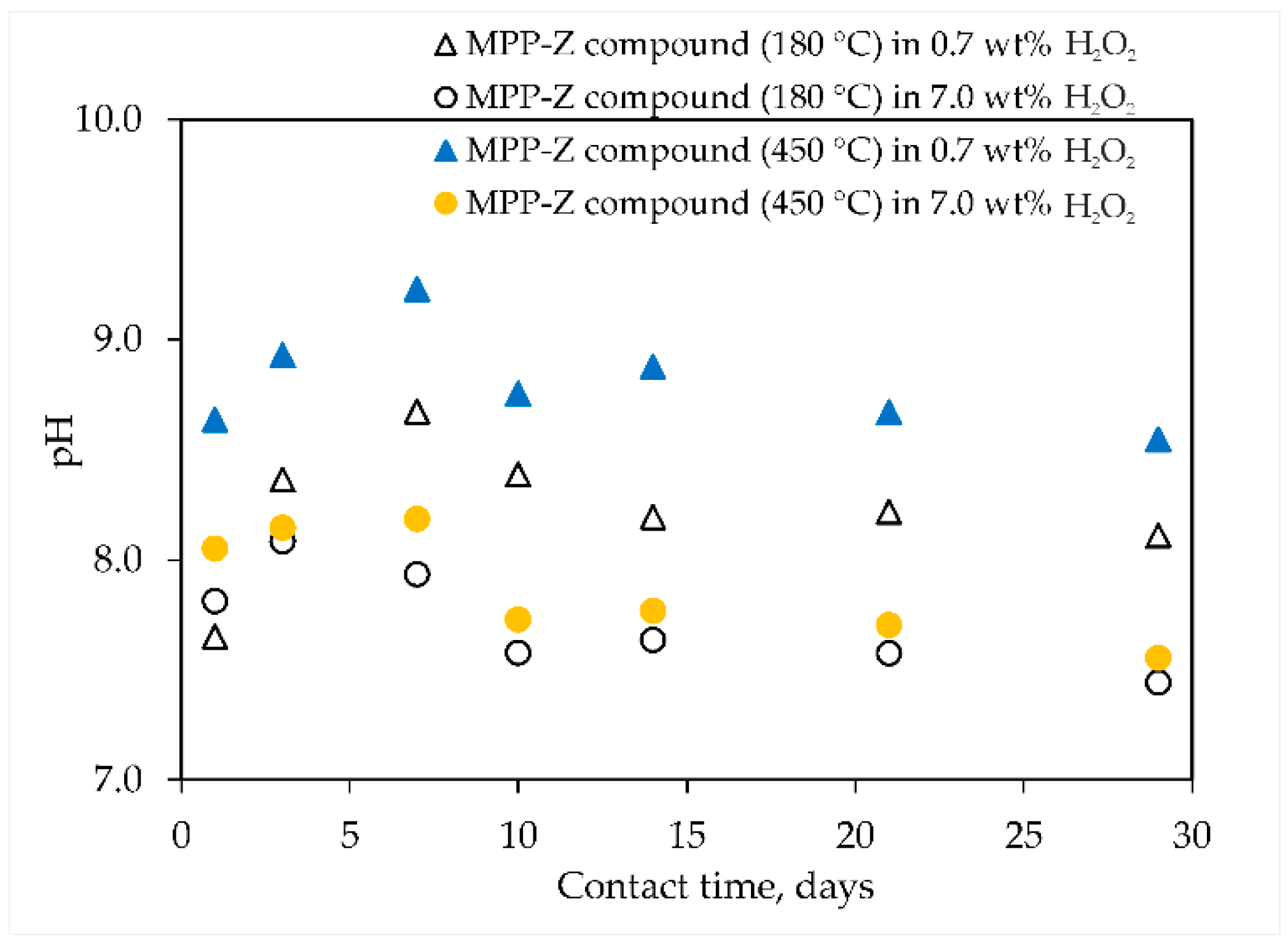
3.2. Study of MPP-Z Compound after Contact with Hydrogen Peroxide
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, B.; Charlet, L.; Fernandez-Martinez, A.; Kang, M.; Made, B. A review of the retention mechanisms of redox-sensitive radionuclides in multi-barrier systems. Appl. Geochem. 2019, 100, 414–431. [Google Scholar] [CrossRef]
- Donald, I.W. Waste Immobilization in Glass and Ceramic Based Hosts: Radioactive, Toxic and Hazardous Wastes; John Wiley & Sons: West Sussex, UK, 2010; pp. 1–507. ISBN 978-1-4443-1937-8. [Google Scholar]
- Ojovan, M.I.; Petrov, V.A.; Yudintsev, S.V. Glass Crystalline Materials as Advanced Nuclear Wasteforms. Sustainability 2021, 13, 4117. [Google Scholar] [CrossRef]
- McCloy, J.S.; Schuller, S. Vitrification of wastes: From unwanted to controlled crystallization, a review. Comptes Rendus Géosci. 2022, 354, 1–40, online first. [Google Scholar] [CrossRef]
- Poluektov, P.P.; Schmidt, O.V.; Kascheev, V.A.; Ojovan, M.I. Modelling aqueous corrosion of nuclear waste phosphate glass. J. Nucl. Mater. 2017, 484, 357–366. [Google Scholar] [CrossRef]
- Choi, J.; Um, W.; Choung, S. Development of iron phosphate ceramic waste form to immobilize radioactive waste solution. J. Nucl. Mater. 2014, 452, 16–23. [Google Scholar] [CrossRef]
- Shadrin, A.Y.; Dvoeglazov, K.N.; Maslennikov, A.G.; Kashcheev, V.A.; Tret’yakova, S.G.; Shmidt, O.V.; Vidanov, V.L.; Ustinov, O.A.; Volk, V.I.; Veselov, S.N.; et al. PH process as a technology for reprocessing mixed uranium-plutonium fuel from BREST-OD-300 reactor. Radiochemistry 2016, 58, 271–279. [Google Scholar] [CrossRef]
- Donald, I.W.; Metcalfe, B.L.; Fong, S.K.; Gerrard, L.A.; Strachan, D.M.; Scheele, R.D. A glass-encapsulated calcium phosphate wasteform for the immobilization of actinide-, fluoride-, and chloride-containing radioactive wastes from the pyrochemical reprocessing of plutonium metal. J. Nucl. Mater. 2007, 361, 78–93. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Danilov, S.S.; Matveenko, A.V.; Frolova, A.V.; Belova, K.Y.; Petrov, V.G.; Vinokurov, S.E.; Myasoedov, B.F. Perspective Compounds for Immobilization of Spent Electrolyte from Pyrochemical Processing of Spent Nuclear Fuel. Appl. Sci. 2021, 11, 1180. [Google Scholar] [CrossRef]
- Danilov, S.S.; Frolova, A.V.; Kulikova, S.A.; Vinokurov, S.E.; Maslakov, K.I.; Teterin, A.Y.; Teterin, Y.A.; Myasoedov, B.F. Immobilization of Rhenium as a Technetium Surrogate in Aluminum Iron Phosphate Glass. Radiochemistry 2021, 63, 99–106. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Belova, K.Y.; Tyupina, E.A.; Vinokurov, S.E. Conditioning of Spent Electro-lyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound. Energies 2020, 13, 1963. [Google Scholar] [CrossRef]
- Aloy, A.S.; Nikandrova, M.V. Leaching of borosilicate glasses containing simulated high-level waste in solutions of hydrogen peroxide as a substance simulating radiolysis products. Radiochemistry 2014, 56, 633–638. [Google Scholar] [CrossRef]
- McVay, G.L.; Pederson, L.R. Effect of gamma radiation on glass leaching. J. Am. Ceram. Soc. 1981, 64, 154–158. [Google Scholar] [CrossRef]
- Barkatt, A.; Barkatt, A.; Sousanpour, W. Gamma radiolysis of aqueous media and its effects on the leaching processes of nuclear waste disposal materials. Nucl. Technol. 1983, 60, 218–227. [Google Scholar] [CrossRef]
- Rolland, S.; Tribet, M.; Jollivet, P.; Jegou, C.; Broudic, V.; Marques, C.; Ooms, H.; Toulhoat, P. Influence of gamma irradiation effects on the residual alteration rate of the French SON68 nuclear glass. J. Nucl. Mater. 2013, 433, 382–389. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Glassy wasteforms for nuclear waste immobilisation. Metall. Mater. Trans. A 2011, 42, 837–851. [Google Scholar] [CrossRef]
- Abdelouas, A.; Ferrand, K.; Grambow, B.; Mennecart, T.; Fattahi, M.; Blondiaux, G.; Houee-Levin, C. Effect of gamma and alpha irradiation on the corrosion of the French borosilicate glass SON 68. MRS Online Proc. Libr. 2003, 807, 397–402. [Google Scholar] [CrossRef]
- Vance, E.R.; Davis, J.; Olufson, K.; Chironi, I.; Karatchevtseva, I.; Farnan, I. Candidate waste forms for immobilisation of waste chloride salt from pyroprocessing of spent nuclear fuel. J. Nucl. Mater. 2012, 420, 396–404. [Google Scholar] [CrossRef]
- Stefanovsky, S.V.; Stefanovskaya, O.I.; Vinokurov, S.E.; Danilov, S.S.; Myasoedov, B.F. Phase composition, structure, and hydrolytic durability of glasses in the Na2O-Al2O3-(Fe2O3)-P2O5 system at replacement of Al2O3 by Fe2O3. Radiochemistry 2015, 57, 348–355. [Google Scholar] [CrossRef]
- Stefanovskii, S.V.; Stefanovskaya, O.I.; Semenova, D.V.; Kadyko, M.I.; Danilov, S.S. Phase Composition, Structure, and Hydrolytic Stability of Sodium-Aluminum(Iron) Phosphate Glass Containing Rare-Earth Oxides. Glas. Ceram. 2018, 75, 89–94. [Google Scholar] [CrossRef]
- Maslakov, K.I.; Teterin, Y.A.; Stefanovsky, S.V.; Kalmykov, S.N.; Teterin, A.Y.; Ivanov, K.E.; Danilov, S.S. XPS study of neptunium and plutonium doped iron-bearing and iron-free sodium-aluminum-phosphate glasses. J. Non-Cryst. Solids 2018, 482, 23–29. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Vinokurov, S.E. The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste. Molecules 2019, 24, 3421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NP-019-15; Federal Norms and Rules in the Field of Atomic Energy Use “Collection, Processing, Storage and Conditioning of Liquid Radioactive Waste. Safety Requirements”. With Changes (Order No. 299 Dated 13.09.2021). Rostekhnadzor: Moscow, Russia, 2015; pp. 1–22.
- GOST R 52126-2003; Radioactive Waste. Long Time Leach Testing of Solidified Radioactive Waste Forms. Gosstandart 305: Moscow, Russia, 2003; pp. 1–8.
- Danilov, S.S.; Kulikova, S.A.; Frolova, A.V.; Vinokurov, S.S. Sodium alumino-iron phosphate glass application for immobilizing technetium containing waste. In Proceedings of the ChemCys 2020, Blankenberge, Belgium, 19–21 February 2020. [Google Scholar]



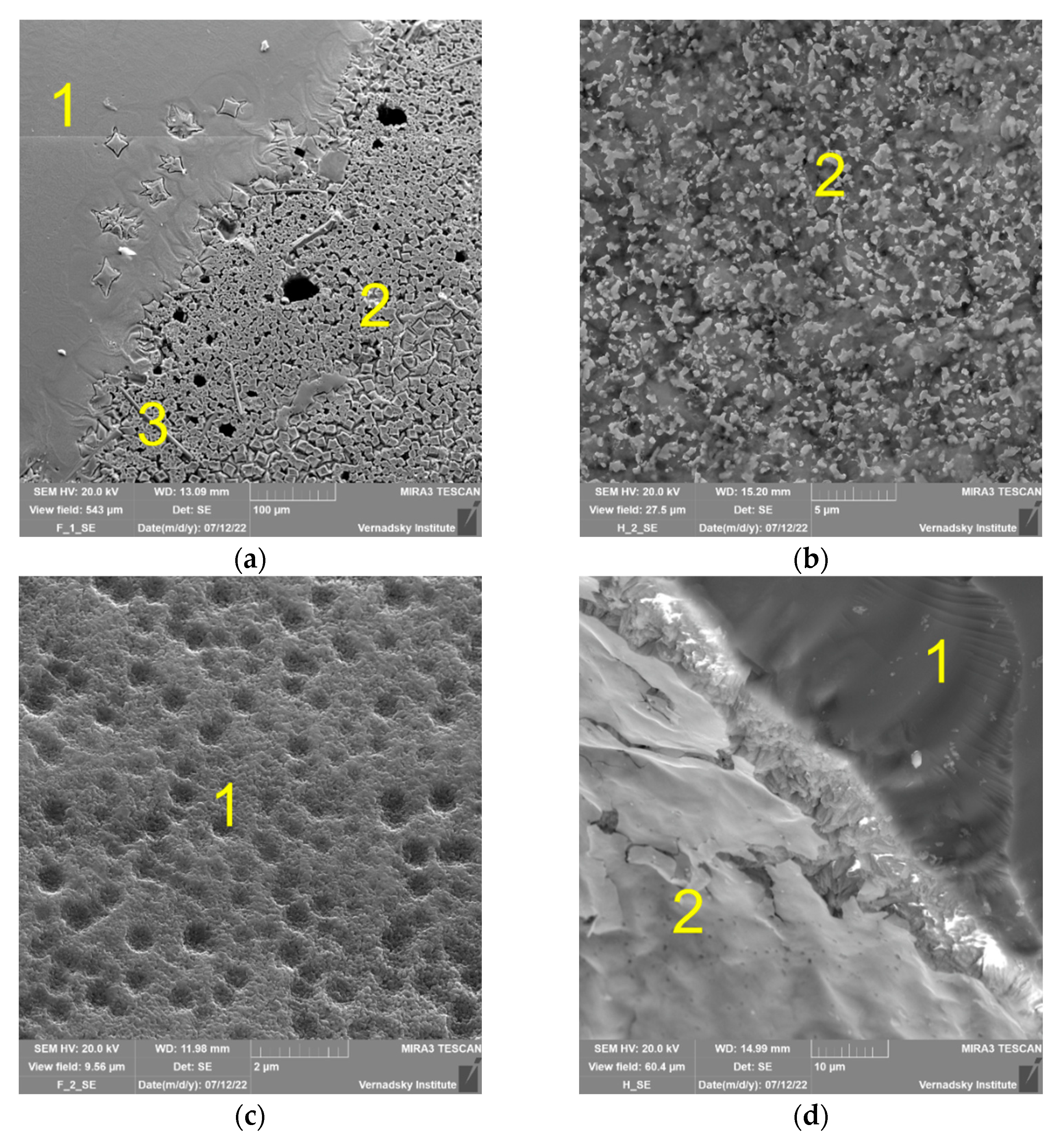
| Element | Na | P | Fe | Al | Cs | K | Li | La | Sr | Ba | O | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 15.4 | 20.7 | 9.3 | 4.5 | 0.3 | 1.6 | 0.4 | 1.7 | 0.2 | 0.3 | 40.2 | 5.4 |
| Element | Phase #1 | Phase #2 | Phase #3 |
|---|---|---|---|
| Na | 14.1 ± 2.1 | 12.0 ± 1.8 | 10.5 ± 1.6 |
| P | 21.5 ± 3.2 | 20.7 ± 3.1 | 22.3 ± 3.3 |
| Fe | 10.6 ± 1.6 | 12.9 ± 1.9 | 22.1 ± 3.3 |
| Al | 4.7 ± 0.7 | 4.8 ± 0.7 | 3.1 ± 0.5 |
| K | 1.8 ± 0.3 | 1.8 ± 0.3 | - |
| Cs | 0.1 ± 0.1 | 0.1 ± 0.1 | - |
| La | 2.4 ± 0.4 | 2.3 ± 0.4 | - |
| Sr | 0.2 ± 0.1 | 0.1 ± 0.1 | - |
| Ba | 0.3 ± 0.1 | 0.4 ± 0.1 | - |
| Cl | 0.9 ± 0.2 | 1.1 ± 0.2 | - |
| O | 42.3 ± 6.3 | 42.8 ± 6.4 | 41.8 ± 6.3 |
| Si | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.2 ± 0.1 |
| Element | Phase #1 | Phase #2 | Phase #3 |
|---|---|---|---|
| Na | 14.1 ± 2.1 | 12.1 ± 1.8 | 1.2 ± 0.2 |
| P | 21.2 ± 3.2 | 22.2 ± 3.3 | 8.5 ± 1.3 |
| Fe | 9.8 ± 1.5 | 20.6 ± 3.1 | 7.2 ± 1.1 |
| Al | 5.2 ± 0.8 | 3.1 ± 0.5 | 34.7 ± 5.2 |
| K | 1.5 ± 0.2 | - | 0.5 ± 0.1 |
| Cs | 0.1 ± 0.1 | - | - |
| La | 1.9 ± 0.3 | - | 2.2 ± 0.3 |
| Sr | 0.2 ± 0.1 | - | - |
| Ba | 0.3 ± 0.1 | - | - |
| Cl | 1.4 ± 0.2 | - | 0.2 ± 0.1 |
| O | 42.8 ± 6.4 | 41.8 ± 6.3 | 45.1 ± 6.8 |
| Si | 1.5 ± 0.2 | 0.2 ± 0.1 | 0.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolova, A.V.; Kulikova, S.A.; Belova, K.Y.; Danilov, S.S.; Vinokurov, S.E. Behavior of Glass-like and Mineral-like Phosphate Compounds with an Immobilized Chloride Mixture in Hydrogen Peroxide Solutions. Energies 2022, 15, 6477. https://doi.org/10.3390/en15176477
Frolova AV, Kulikova SA, Belova KY, Danilov SS, Vinokurov SE. Behavior of Glass-like and Mineral-like Phosphate Compounds with an Immobilized Chloride Mixture in Hydrogen Peroxide Solutions. Energies. 2022; 15(17):6477. https://doi.org/10.3390/en15176477
Chicago/Turabian StyleFrolova, Anna V., Svetlana A. Kulikova, Kseniya Y. Belova, Sergey S. Danilov, and Sergey E. Vinokurov. 2022. "Behavior of Glass-like and Mineral-like Phosphate Compounds with an Immobilized Chloride Mixture in Hydrogen Peroxide Solutions" Energies 15, no. 17: 6477. https://doi.org/10.3390/en15176477
APA StyleFrolova, A. V., Kulikova, S. A., Belova, K. Y., Danilov, S. S., & Vinokurov, S. E. (2022). Behavior of Glass-like and Mineral-like Phosphate Compounds with an Immobilized Chloride Mixture in Hydrogen Peroxide Solutions. Energies, 15(17), 6477. https://doi.org/10.3390/en15176477