Biogas and Biohydrogen Production Using Spent Coffee Grounds and Alcohol Production Waste
Abstract
:1. Introduction
2. Methods
2.1. Batch Culturing
2.2. Feedstock, Treatment, and Medium Preparation
2.3. Gas Analysis
2.4. Reagents and Data Processing
3. Results and Discussion
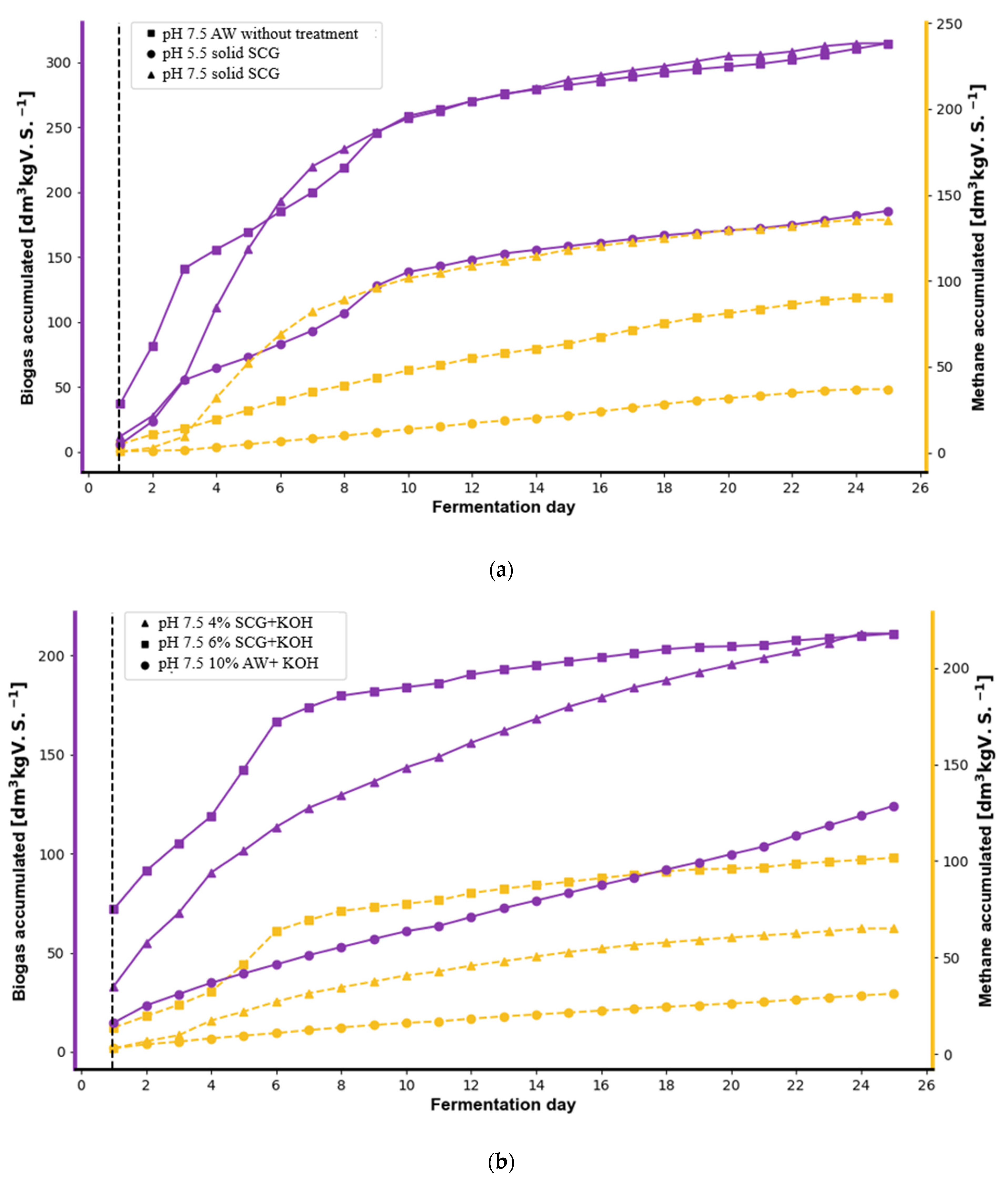
3.1. Biogas and Biohydrogen Production from SCG and AW
3.2. The Effect of Pretreatment on Biogas and Biohydrogen Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trchounian, K.; Trchounian, A. Hydrogen production from glycerol by Escherichia coli and other bacteria: An overview and perspectives. Appl. Energy 2015, 156, 174–184. [Google Scholar] [CrossRef]
- Mahmoodi-Eshkaftaki, M.; Mockaitis, G. Structural optimization of biohydrogen production: Impact of pretreatments on volatile fatty acids and biogas parameters. Int. J. Hydrogen Energy 2022, 47, 7072–7081. [Google Scholar] [CrossRef]
- Abrahama, A.; Mathewa, A.K.; Parka, H.; Choia, O.; Sindhub, R.; Parameswaranb, B.; Pandeyc, A.; Parkd, J.H.; Sang, B.-I. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsana, T.; Jayamuthunagai, J.; Praveenkumar, R.; Chozhavendhan, S.; Iyyappan, J. Biogas production—A review on composition, fuel properties, feed stock and principles of anaerobic digestion. Renew. Sust. Energ. Rev. 2018, 90, 570–582. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Buhainc, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sust. Energ. Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of brewery wastes in food industry. Peer J. 2020, 8, 9427. [Google Scholar] [CrossRef] [PubMed]
- Poladyan, A.; Trchounian, K.; Vassilian, A.; Trchounian, A. Hydrogen production by Escherichia coli using brewery waste: Optimal pretreatment of waste and role of different hydrogenases. Renew. Energy 2018, 115, 931. [Google Scholar] [CrossRef]
- Available online: https://www.statista.com/forecasts/232925/volume-of-global-spirit-consumption (accessed on 9 July 2022).
- Kharayat, Y. Distillery wastewater: Bioremediation approaches. J. Integr. Environ. Sci. 2012, 9, 69–91. [Google Scholar] [CrossRef]
- Guo, S.; Kumar, A.M.; Wang, Y.; Xu, P. Current understanding in conversion and application of tea waste biomass: A review. Bioresour. Technol. 2021, 338, 125530. [Google Scholar] [CrossRef]
- Yun, B.Y.; Cho, H.M.; Kim, Y.U.; Lee, S.C.; Berardi, U.; Kim, S. Circular reutilization of coffee waste for sound absorbing panels: A perspective on material recycling. Environ. Res. 2020, 184, 109281. [Google Scholar] [CrossRef]
- Petrosyan, H.; Vanyan, L.; Mirzoyan, S.; Trchounian, A.; Trchounian, K. Roasted coffee wastes as a substrate for Escherichia coli to grow and produce hydrogen. FEMS Lett. 2020, 367, fnaa088. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Progress in microbiology for fermentative hydrogen production from organic wastes. Crit. Rev. Environ. Sci. Tec. 2019, 49, 1–41. [Google Scholar] [CrossRef]
- Raghulchandrana, R.; Tamizhini, A.; Snehya, A.V.; Pandey, R.P.; Raghulchandrana, R.; Tamizhini, A.; Snehya, A.; Pandey, R.P. Pretreatment Studies of Biohydrogen Production from Agro-Industrial Waste. Preprints 2020, 2020110320. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Wei, H.; Junhong, T.; Yongfeng, L. Utilization of food waste for fermentative hydrogen production. Phys. Sci. Rev. 2016, 1, 20160050. [Google Scholar]
- Sekoai, P.T.; Daramola, M.O. Effect of metal ions on dark fermentative biohydrogen production using suspended and immobilized cells of mixed bacteria. Chem. Eng. Commun. 2018, 205, 1011–1022. [Google Scholar] [CrossRef]
- Trchounian, K.; Sawers, R.G.; Trchounian, A. Improving biohydrogen productivity by microbial dark- and photo-fermentations: Novel data and future approaches. Renew. Sust. Energ. Rev. 2017, 80, 1201–1216. [Google Scholar] [CrossRef]
- Rezania, S.; Din, M.F.M.; Mohamad, S.E.; Sohaili, J.; Taib, S.M.; Yusof, M.B.M.; Kamyab, H.; Darajeh, N.; Ahsan, A. Review on Pretreatment Methods and Ethanol Production from Cellulosic Water Hyacinth. BioResources 2017, 12, 2108–2124. [Google Scholar] [CrossRef]
- Kamperidou, V.; Terzopoulou, P. Anaerobic Digestion of Lignocellulosic Waste Materials. Sustainability 2021, 13, 12810. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Steyer, J.P.; Carrere, H. Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour. Technol. 2012, 120, 241–247. [Google Scholar] [CrossRef]
- Montoya-Rosales, J.J.; Peces, M.; González-Rodríguez, L.M.; Alatriste-Mondragón, F.; Villa-Gómez, D.K. A broad overview comparing a fungal, thermal and acid pre-treatment of bean straw in terms of substrate and anaerobic digestion effect. Biomass Bioenergy 2020, 142, 105775. [Google Scholar] [CrossRef]
- Meng, J.; Duan, H.; Li, H.; Watts, S.; Liu, P.; Shrestha, S.; Zheng, M.; Yu, W.; Chen, Z.; Song, Y.; et al. Free nitrous acid pre-treatment enhances anaerobic digestion of waste activated sludge and rheological properties of digested sludge: A pilot-scale study. Water Res. 2020, 172, 115515. [Google Scholar] [CrossRef] [PubMed]
- Blok, J.; De Morsier, A.; Gerike, P. Harmonisation of ready biodegradability tests. Chemosphere 1985, 14, 1805–1820. [Google Scholar] [CrossRef]
- Raposo, F.; De la Rubia, M.A.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sust. Energ. Rev. 2012, 16, 861–877. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Methane and hydrogen production from cotton waste by dark fermentation under anaerobic and micro-aerobic conditions. Biomass Bioenergy 2020, 138, 105576. [Google Scholar] [CrossRef]
- Taylor, M.J.; Alabdrabalameer, H.A.; Skoulou, V. Choosing Physical, Physicochemical and Chemical Methods of Pre-Treating Lignocellulosic Wastes to Repurpose into Solid Fuels. Sustainability 2019, 11, 3604. [Google Scholar] [CrossRef]
- Mirzoyan, S.; Toleugazykyzy, A.; Bekbayev, K.; Trchounian, A.; Trchounian, K. Enhanced hydrogen gas production from mixture of beer spent grains (BSG) and distiller’s grains (DG) with glycerol by Escherichia coli. Int. J. Hydrog. Energy 2020, 45, 17233–17240. [Google Scholar] [CrossRef]
- Trchounian, K.; Sanchez-Torres, V.; Wood, T.K.; Trchounian, A. Escherichia coli hydrogenase activity and H2 production under glycerol fermentation at a low pH. Int. J. Hydrog. Energy 2011, 36, 4323–4331. [Google Scholar] [CrossRef]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Anaerobic digestion of coffee grounds soluble fraction at laboratory scale: Evaluation of the biomethane potential. Appl. Energy 2017, 207, 166–175. [Google Scholar] [CrossRef]
- Vasmara, C.; Marchetti, R. Spent coffee grounds from coffee vending machines as feedstock for biogas production. Environ. Eng. Manag. J. 2018, 17, 2401–2408. [Google Scholar]
- Girotto, F.; Lavagnolo, M.C.; Pivato, A. Spent Coffee Grounds Alkaline Pre-treatment as Biorefinery Option to Enhance their Anaerobic Digestion Yield. Waste Biomass Valor. 2018, 9, 2565–2570. [Google Scholar] [CrossRef]
- Neves, L.; Oliveira, R.; Alves, M.M. Anaerobic co-digestion of coffee waste and sewage sludge. J. Waste Manag. 2006, 26, 176–181. [Google Scholar] [CrossRef]
- Passos, F.; Cordeiro, P.H.M.; Baeta, B.E.L.; de Aquino, S.F.; Perez-Elvira, S.I. Anaerobic co-digestion of coffee husks and microalgal biomass after thermal hydrolysis. Bioresour. Technol. 2018, 253, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, M.; Zilouei, H.; Asadinezhad, A. Enhanced Biogas and Biohydrogen Production from Cotton Plant Wastes Using Alkaline Pretreatment. Energy Fuels 2016, 30, 10484–10493. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Buitrón, G. Biohydrogen and methane production via a two-step process using an acid pretreated native microalgae consortium. Bioresour. Technol. 2016, 221, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for biogas upgrading to biomethane: A review. Bioengineer 2019, 6, 9. [Google Scholar] [CrossRef]
- Dimitrellos, G.; Lyberatos, G.; Antonopoulou, G. Does Acid Addition Improve Liquid Hot Water Pretreatment of Lignocellulosic Biomass towards Biohydrogen and Biogas Production? Sustainability 2020, 12, 8935. [Google Scholar] [CrossRef]
- Cetecioglu, Z.; Atasoy, M.; Cenian, A.; Sołowski, G.; Trcek, J.; Ugurlu, A.; Sedlakova-Kadukova, J. Bio-Based Processes for Material and Energy Production from Waste Streams under Acidic Conditions. Fermentation 2022, 8, 115. [Google Scholar] [CrossRef]




| Material | pH | Consistency | TS [%FM] | VSS [%TS] |
|---|---|---|---|---|
| Inoculum | 7.5 | liquid | 3.52 | 69.00 |
| Coffee waste without treatment | - | solid | 41.58 | 97.53 |
| 4% (w/v) coffee waste treated with 0.4% (v/v) sulfuric acid | 1–2 | liquid | 1.98 | 92.31 |
| 6% (w/v) coffee waste treated with 0.4% (v/v) sulfuric acid | 1–2 | liquid | 2.72 | 91.86 |
| Alcohol waste without treatment | 5 | liquid | 3.27 | 86.81 |
| 10% (w/v) AW treated with 1.5% (v/v) sulfuric acid | 1–2 | liquid | 3.48 | 80.21 |
| 20% (w/v) AW treated with 1.5% (v/v) sulfuric acid | 1–2 | liquid | 3.80 | 95.85 |
| Substrate | Inoculum | Cumulative H2 Volume Dm3 Kg V.S−1 | Cumulative CH4 Volume Dm3 Kg V.S−1 | Biogas Volume Dm3 Kg V.S−1 | Reference |
|---|---|---|---|---|---|
| Boll pretreated with 8% (w/w) sodium hydroxide solution for 10 min at 100 °C. | Wastewater treatment sludge heat-shocked at 85 °C for 45 min | 17.1 | 246.4 | - | [35] |
| Native consortium of microalgal biomass without treatment | Treated anaerobic sludge | 15.0 | 245 | - | [36] |
| SCG | a liquid fraction of pig manure | - | 290 | - | [31] |
| SCG pretreated with 8% NaOH | Granular sludge (5.2 g VS/L, VS/TS ratio = 0.6) collected from a full-scale upflow anaerobic sludge blanket (UASB) digester of a brewery factory | - | 392 | - | [32] |
| ‘‘coffee’’ waste from instant coffee substitute production | The granular sludge collected from a UASB (upflow anaerobic sludge blanket) reactor treating brewery effluent | - | 280 | - | [33] |
| Coffee husks harvested from agricultural land in the municipality of Lavras. Pretreatment at 120 °C for 60 min | Microalgal biomass was harvested from a full-scale wastewater treatment raceway pond | - | 196 | - | [34] |
| Spent coffee water | fresh cow manure | - | 167.80 | - | [30] |
| Cotton waste | Inoculum from a mesophilic digester mainly used to treat maize silage and manure | 1.1 | 780 | - | [26] |
| Alcohol waste without treatment | Sewage sludge from wastewater treatment plant | - | 135 | 240 | This study |
| SCG without treatment | Sewage sludge from wastewater treatment plant | - | 190 | 320 | This study |
| SCG treated with 0.4% sulfuric acid | Sewage sludge from wastewater treatment plant | 3.85 | 1.3 | 43 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanyan, L.; Cenian, A.; Trchounian, K. Biogas and Biohydrogen Production Using Spent Coffee Grounds and Alcohol Production Waste. Energies 2022, 15, 5935. https://doi.org/10.3390/en15165935
Vanyan L, Cenian A, Trchounian K. Biogas and Biohydrogen Production Using Spent Coffee Grounds and Alcohol Production Waste. Energies. 2022; 15(16):5935. https://doi.org/10.3390/en15165935
Chicago/Turabian StyleVanyan, Liana, Adam Cenian, and Karen Trchounian. 2022. "Biogas and Biohydrogen Production Using Spent Coffee Grounds and Alcohol Production Waste" Energies 15, no. 16: 5935. https://doi.org/10.3390/en15165935
APA StyleVanyan, L., Cenian, A., & Trchounian, K. (2022). Biogas and Biohydrogen Production Using Spent Coffee Grounds and Alcohol Production Waste. Energies, 15(16), 5935. https://doi.org/10.3390/en15165935







