Enhancing Biomass-Exopolysaccharides Production of Lignosus rhinocerus in a High-Scale Stirred-Tank Bioreactor and Its Potential Lipid as Bioenergy
Abstract
:1. Introduction
2. Materials and Methods
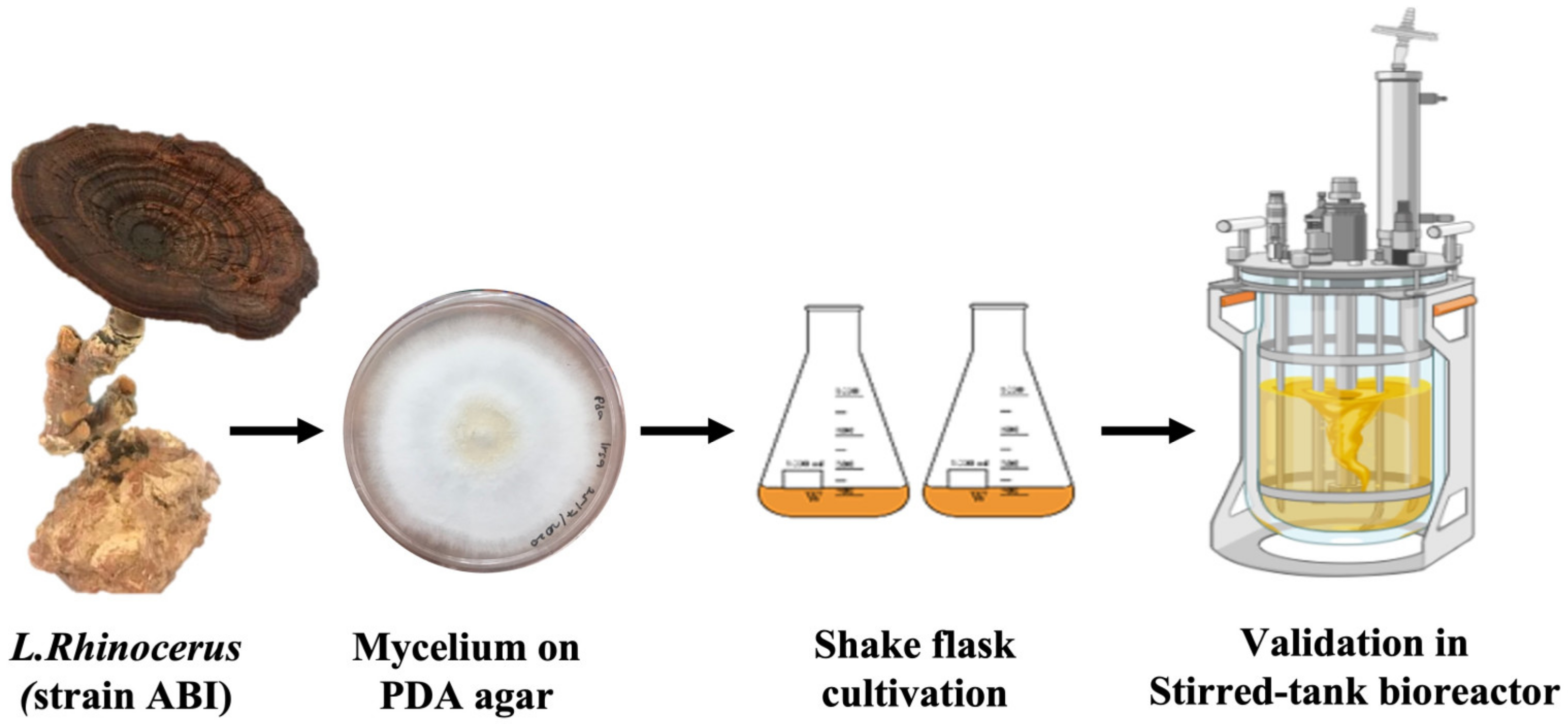
2.1. Fungal Source
2.2. Inoculum Preparation in Shake Flasks
2.3. Growth Curve Analysis
2.4. Media Optimization Using Statistical Tool Analysis
2.5. Bioreactor Validations
2.6. Analytical Methods
2.6.1. Mycelial Biomass (MB)
2.6.2. Exopolysaccharides (EPS)
2.7. Residual Glucose Determination
2.8. Kinetics Calculation
2.9. Image Analysis
2.9.1. Compound Microscope
2.9.2. SEM Microscopy
2.10. Lipid Extraction
2.11. Statistical Analysis
3. Results and Discussion
3.1. Growth Profiles
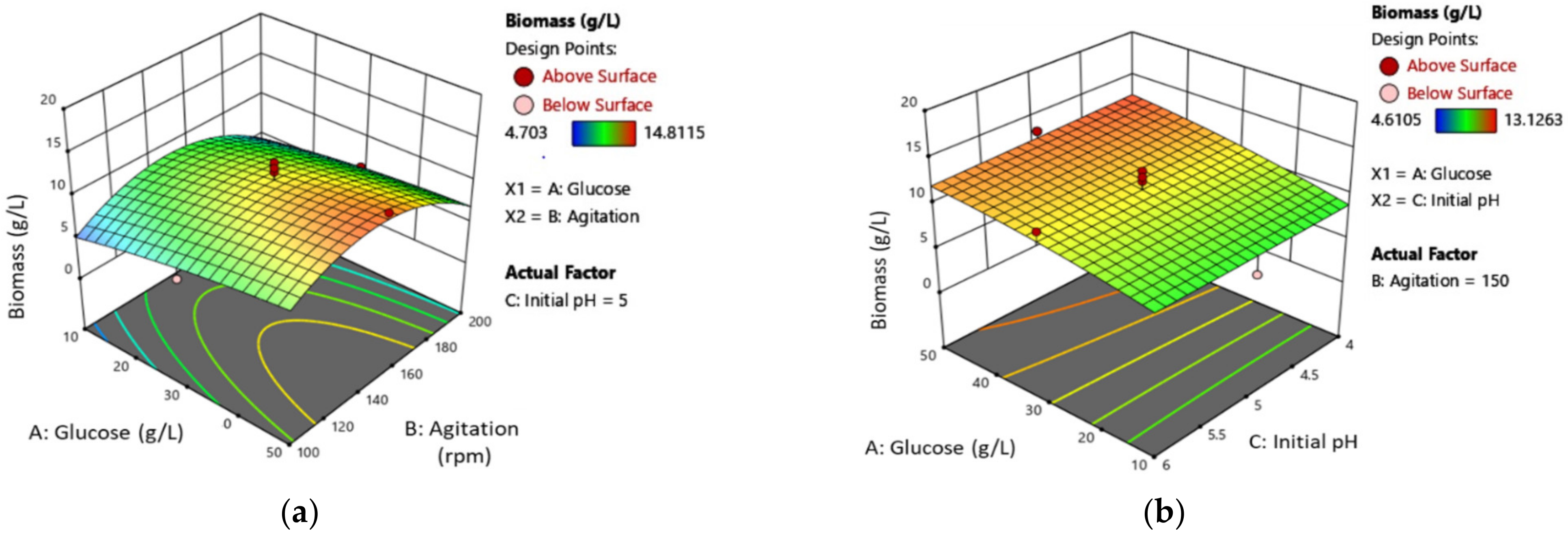
3.2. Mycelial Biomass (MB) Production Optimization
3.3. Exopolysaccharides (EPS) Production Optimisation
3.4. Optimized Conditions Verification
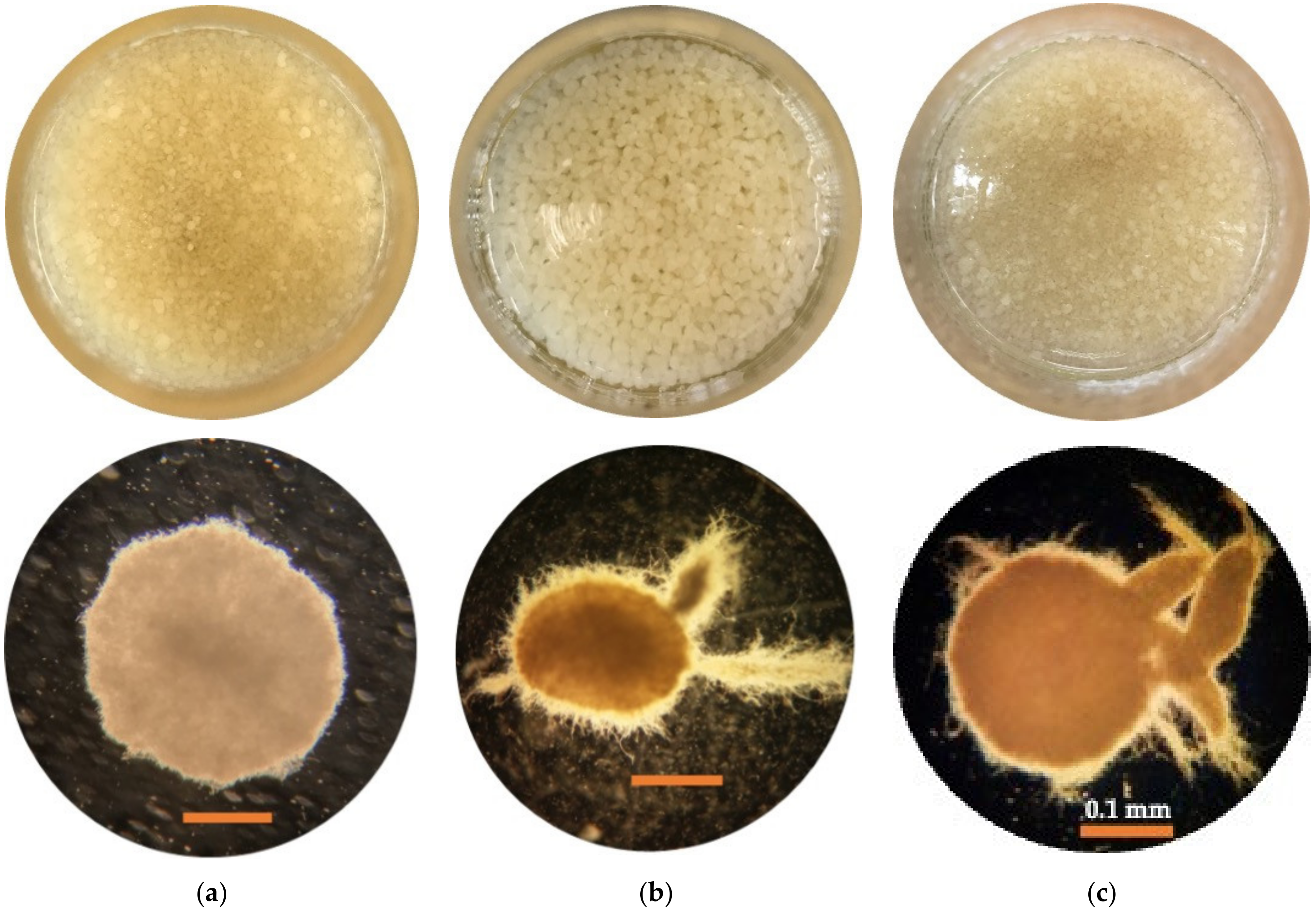
3.5. Image Analysis
3.5.1. Pellet Morphology
3.5.2. Microstructure Analysis
3.6. Lignosus Rhinocerus Lipid Yield and Its Potential as a Biodiesel
3.7. Comparison of the Present Study to Earlier Research
| Species | Origin | Temperature Range (°C) | Optimization Method | Cultivation Mode | Working Volume (L) | Initial pH | Glucose Concentration (g/L) | Agitation (rpm) | MB (g/L) | EPS (g/L) | Lipid Yield (% w/w) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. rhinocerus strain ABI | Lata Iskandar, Malaysia | 23–31 | Response surface methodology (RSM) | STR Bioreactor | 10 | 4.0 | 50 | 128.24 | 23.93 | 4.4 | 2.07 | Current study |
| L. rhinocerus strain ABI | Lata Iskandar, Malaysia | 23–31 | One-factor-at-a-time (OFAAT) | STR Bioreactor | 10 | 5.5 | 40 | 200 | * NA | NA | NA | [8] |
| L.rhinocerus | - | 25 | OFAAT | Shake flask | 2 | 5.0 | 20 | 120 | NA | NA | NA | [47] |
| STR bioreactor | 500; 20,000 | |||||||||||
| L. rhinocerus | Sanming, Fujian Province, China | 22–32 | OFAAT | Shake flask | 0.075 | NA | 40 | 180 | 15 | 5 | NA | [46] |
| L. rhinocerotis (KUM61075) | Kenaboi Forest Reserve, Negeri Sembilan, Malaysia | 22–30 | OFAAT | Shake flask | 0.1 | 6.0–6.5 | 15 | 150 | 0.062 | 0.294 | 2.3 | [48] |
| STR Bioreactor | 3 | 200 | NA | NA | ||||||||
| L. rhinocerus | Lata Iskandar, Malaysia | 23–31 | OFAAT and the orthogonal matrix method | Shake flask | 0.05 | 6.0 | 20 | 150 | 2.17 | 1.18 | NA | [45] |
| STR Bioreactor | 1.5 | 80 | 100 | 6.37 | 1.20 |
3.8. Challenges and Limitations of L. rhinocerus as a Potential Bioenergy
- i.
- Cost of the process: The cost of growing mycelium in a bioreactor and processing it into bioenergy will depend on several factors, such as the scale of production, the efficiency of the bioreactor system, and the cost of inputs such as water, nutrients, and energy.
- ii.
- Lipid content: The lipid content of the mycelium will also play a role in its potential as a source of bioenergy. Higher lipid content will result in higher energy output when converted into bioenergy. However, the lipid content can be affected by several factors, including the growing conditions, the mushroom strain, and the growth stage.
- iii.
- Stakeholders: The stakeholders in producing mycelium-based bioenergy will include growers, processors, investors, and government agencies. Ensuring the interests of all stakeholders are taken into account will be important in the successful development and implementation of this new source of bioenergy.
- iv.
- Readiness of industry: The bioenergy industry is rapidly evolving, and there may be challenges in establishing a market for mycelium-based bioenergy. The readiness of the industry to embrace this new source of bioenergy will play a role in its success.
- v.
- Change of fossil fuel to biodiesel: The shift from fossil fuels to bioenergy, including biodiesel, is important in reducing greenhouse gas emissions and promoting sustainability. However, this transition will need to consider the challenges and limitations of using mycelium-based bioenergy.
- vi.
- Food vs. energy: The United Nations Sustainable Development Goals (SDGs) provide a framework for promoting sustainable development. This study’s investigation aligns with two of these goals: SDG 7 (Affordable and Clean Energy) and SDG 12 (Responsible Consumption and Production). Additionally, the lipids extracted from L. rhinocerus could also contribute to SDG 2 (Zero Hunger), as mushrooms are a highly functional food. However, the debate around the risk of using food for biofuels is a long-standing and contentious issue that demands attention.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galanakis, C.M. The Vertigo of the Food Sector within the Triangle of Climate Change, the Post-Pandemic World, and the Russian-Ukrainian War. Foods 2023, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Ma, S.; Lei, T.; Meng, J.; Liang, X.; Guan, D. Global oil refining’s contribution to greenhouse gas emissions from 2000 to 2021. Innovation 2023, 4, 100361. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Sharma, A. Chapter 1—Climate change and the energy sector. In Advancement in Oxygenated Fuels for Sustainable Development; Kumar, N., Mathiyazhagan, K., Sreedharan, V.R., Kalam, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, K. CO2 Emissions in Asia-Pacific Region: Do Energy Use, Economic Growth, Financial Development, and International Trade Have Detrimental Effects? Sustainability 2022, 14, 5420. [Google Scholar] [CrossRef]
- Mujtaba, A.; Jena, P.K. Nexus Between the Energy Market, Industrialization, and Economic Growth in the Asia-Pacific Region. Energy Res. Lett. 2022, 3, 32631. [Google Scholar] [CrossRef]
- Rowan, N.J.; Casey, O. Empower Eco multiactor HUB: A triple helix ‘academia-industry-authority’ approach to creating and sharing potentially disruptive tools for addressing novel and emerging new Green Deal opportunities under a United Nations Sustainable Development Goals framework. Curr. Opin. Environ. Sci. Health 2021, 21, 100254. [Google Scholar] [CrossRef]
- Usuldin, S.R.A.; Mahmud, N.; Ilham, Z.; Ikram, N.K.K.; Ahmad, R.; Wan, W.A.A.Q.I. In-depth spectral characterization of antioxidative (1, 3)-β-D-glucan from the mycelium of an identified tiger milk mushroom Lignosus rhinocerus strain ABI in a stirred-tank bioreactor. Biocatal. Agric. Biotechnol. 2020, 23, 101455. [Google Scholar] [CrossRef]
- Alugoju, P.; Tencomnao, T. Tiger’s Milk Mushroom (Lignosus rhinocerus). In Mushrooms; CRC Press: Boca Raton, FL, USA, 2023; pp. 239–253. [Google Scholar]
- Ng, M.J.; Kong, B.H.; Teoh, K.H.; Yap, Y.H.-Y.; Ng, S.T.; Tan, C.S.; Razif, M.F.M.; Fung, S.Y. In vivo anti-tumor activity of Lignosus rhinocerus TM02® using a MCF7-xenograft NCr nude mice model. J. Ethnopharmacol. 2023, 304, 115957. [Google Scholar] [CrossRef]
- Moussaa, A.Y.; Xu, B. A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases. Food Sci. Hum. Wellness 2023, 12, 942–958. [Google Scholar] [CrossRef]
- Ng, M.J.; Razif, M.F.M.; Kong, B.H.; Ng, S.T.; Tan, C.S.; Yap, H.-Y.Y.; Fung, S.Y. The genome of Lignosus tigris: Uncovering its hidden nutraceutical potential. S. Afr. J. Bot. 2023, 154, 108–119. [Google Scholar] [CrossRef]
- Faris Taufeq, F.Y.; Habideen, N.H.; Rao, L.N.; Podder, P.K.; Katas, H. Potential Hemostatic and Wound Healing Effects of Thermoresponsive Wound Dressing Gel Loaded with Lignosus rhinocerotis and Punica granatum Extracts. Gels 2023, 9, 48. [Google Scholar] [CrossRef]
- Cheong, P.; Yong, Y.; Fatima, A.; Ng, S.; Tan, C.; Kong, B.; Tan, N.; Rajarajeswaran, J.; Fung, S. Cloning, overexpression, purification, and modeling of a lectin (Rhinocelectin) with antiproliferative activity from tiger milk mushroom, Lignosus rhinocerus. IUBMB Life 2019, 71, 1579–1594. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Purification and fractionation of bioactive peptides through membrane filtration: A critical and application review. Trends Food Sci. Technol. 2023, 131, 118–128. [Google Scholar] [CrossRef]
- Willetts, H.; Bullock, S. Developmental biology of sclerotia. Mycol. Res. 1992, 96, 801–816. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.-Y.; Li, H.-M.; Wan, D.-J.; Tang, Y.-J. Comparison of Lipid Content and Fatty Acid Composition between Tuber Fermentation Mycelia and Natural Fruiting Bodies. J. Agric. Food Chem. 2011, 59, 4736–4742. [Google Scholar] [CrossRef]
- Delattre, C.; Cabrera-Barjas, G.; Banerjee, A.; Rodriguez-Llamazares, S.; Pierre, G.; Dubessay, P.; Michaud, P.; Zamani, A. Production of fungal biopolymers and their advanced applications. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 497–532. [Google Scholar]
- Nallathamby, N.; Lakshmanan, H.; Vidyadaran, S.; Abd Malek, S.N.; Phan, C.-W.; Sabaratnam, V. Lipid-rich fraction of the sclerotium of Tiger Milk Mushroom Lignosus rhinocerotis (Agaricomycetes) attenuates LPS-induced inflammation in BV2 cells via Nrf2 pathway. Braz. J. Pharm. Sci. 2021, 56, 1–12. [Google Scholar] [CrossRef]
- Berovic, M.; Zhong, J.-J. Advances in Production of Medicinal Mushrooms Biomass in Solid State and Submerged Bioreactors; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Vehapi, M.; İnan, B.; Kayacan-Cakmakoglu, S.; Sagdic, O.; Özçimen, D. Production of Bacillus subtilis soil isolate as biocontrol agent under bioreactor conditions. Arch. Microbiol. 2023, 205, 52. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T.K. Large-Scale Culture of Mammalian Cells for Various Industrial Purposes. In Practical Approach to Mammalian Cell and Organ Culture; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–45. [Google Scholar]
- Egger, D.; Nebel, S.; Gensler, M.; Kreß, S.; Hansmann, J.; Kasper, C. Bioreactors: Enabling technologies for research and manufacturing. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 431–456. [Google Scholar]
- Contato, A.G.; Inácio, F.D.; Bueno, P.S.A.; Nolli, M.M.; Janeiro, V.; Peralta, R.M.; de Souza, C.G.M. Pleurotus pulmonarius: A protease-producing white rot fungus in lignocellulosic residues. Int. Microbiol. 2023, 26, 43–50. [Google Scholar] [CrossRef]
- Zhao, X.; Chai, J.; Wang, F.; Jia, Y. Optimization of Submerged Culture Parameters of the Aphid Pathogenic Fungus Fusarium equiseti Based on Sporulation and Mycelial Biomass. Microorganisms 2023, 11, 190. [Google Scholar] [CrossRef]
- Majid, S.; Ahmad, K.; Ali, D. Microbial detoxification of Pyrethroid insecticide Bifenthrin by selected Fungal strains and Optimizing conditions using Response Surface Methodology. Researchsquare, 2023; preprint. [Google Scholar] [CrossRef]
- Rahman, S.; Sharma, P.; Said, Z. Application of Response Surface Methodology Based D-Optimal Design for Modeling and Optimisation of Osmotic Dehydration of Zucchini. Digit. Chem. Eng. 2022, 4, 100039. [Google Scholar] [CrossRef]
- Yap, L.S.; Lee, W.L.; Ting, A.S.Y. Bioprocessing and purification of extracellular L-asparaginase produced by endophytic Colletotrichum gloeosporioides and its anticancer activity. Prep. Biochem. Biotechnol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Lamidi, S.; Olaleye, N.; Bankole, Y.; Obalola, A.; Aribike, E.; Adigun, I. Applications of Response Surface Methodology (RSM) in Product Design, Development, and Process Optimization. In Response Surface Methodology–Research Advances and Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Bouchelkia, N.; Tahraoui, H.; Amrane, A.; Belkacemi, H.; Bollinger, J.-C.; Bouzaza, A.; Zoukel, A.; Zhang, J.; Mouni, L. Jujube stones based highly efficient activated carbon for methylene blue adsorption: Kinetics and isotherms modeling, thermodynamics and mechanism study, optimization via response surface methodology and machine learning approaches. Process Saf. Environ. Prot. 2023, 170, 513–535. [Google Scholar] [CrossRef]
- Percy, A.J.; Edwin, M. Studies on the performance and emission characteristics of a dual fuel VCR engine using producer gas as secondary fuel: An optimization approach using response surface methodology. Energy 2023, 263, 125685. [Google Scholar] [CrossRef]
- Shah, S.A.R.; Kahla, N.B.; Atig, M.; Anwar, M.K.; Azab, M.; Mahmood, A. Optimization of fresh and mechanical properties of sustainable concrete composite containing ARGF and fly ash: An application of response surface methodology. Constr. Build. Mater. 2023, 362, 129722. [Google Scholar] [CrossRef]
- Reji, M.; Kumar, R. Response surface methodology (RSM): An overview to analyze multivariate data. Indian J. Microbiol. Res. 2022, 9, 241–248. [Google Scholar]
- Chong, D.J.S.; Chan, Y.J.; Arumugasamy, S.K.; Yazdi, S.K.; Lim, J.W. Optimisation and performance evaluation of response surface methodology (RSM), artificial neural network (ANN) and adaptive neuro-fuzzy inference system (ANFIS) in the prediction of biogas production from palm oil mill effluent (POME). Energy 2023, 266, 126449. [Google Scholar] [CrossRef]
- Supramani, S.; Ahmad, R.; Ilham, Z.; Annuar, M.S.M.; Klaus, A.; Wan, W.A.A.Q.I. Optimisation of biomass, exopolysaccharide and intracellular polysaccharide production from the mycelium of an identified Ganoderma lucidum strain QRS 5120 using response surface methodology. AIMS Microbiol. 2019, 5, 19. [Google Scholar] [CrossRef]
- Abdullah, N.R.; Sharif, F.; Azizan, N.H.; Hafidz, I.F.M.; Supramani, S.; Usuldin, S.R.A.; Ahmad, R.; Wan, W.A.A.Q.I. Pellet diameter of Ganoderma lucidum in a repeated-batch fermentation for the trio total production of biomass-exopolysaccharide-endopolysaccharide and its anti-oral cancer beta-glucan response. AIMS Microbiol. 2020, 6, 379. [Google Scholar] [CrossRef]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Elsadig, E.E.; Ali, A.E.B.; Abdelgadir, M.I. Comparative Study of some Physicochemical Properties of Extracted Oil from Modified and Non-modified Cottonseeds. J. Food Sci. 2023, 4, 1–11. [Google Scholar] [CrossRef]
- Gu, S.; Wang, Z.; Wang, J. Untargeted rapid differentiation and targeted growth tracking of fungal contamination in rice grains based on headspace-gas chromatography-ion mobility spectrometry. J. Sci. Food Agric. 2022, 102, 3673–3682. [Google Scholar] [CrossRef]
- Pokhrel, C.P.; Ohga, S. Submerged culture conditions for mycelial yield and polysaccharides production by Lyophyllum decastes. Food Chem. 2007, 105, 641–646. [Google Scholar] [CrossRef]
- Zheng, X.; Cairns, T.C.; Ni, X.; Zhang, L.; Zhai, H.; Meyer, V.; Zheng, P.; Sun, J. Comprehensively dissecting the hub regulation of PkaC on high-productivity and pellet macromorphology in citric acid producing Aspergillus niger. Microb. Biotechnol. 2022, 15, 1867–1882. [Google Scholar] [CrossRef]
- Lau, B.F.; Abdullah, N.; Aminudin, N. Chemical Composition of the Tiger’s Milk Mushroom, Lignosus rhinocerotis (Cooke) Ryvarden, from Different Developmental Stages. J. Agric. Food Chem. 2013, 61, 4890–4897. [Google Scholar] [CrossRef]
- Sergeeva, Y.; Galanina, L.; Andrianova, D.; Feofilova, E. Lipids of filamentous fungi as a material for producing biodiesel fuel. Appl. Biochem. Microbiol. 2008, 44, 523–527. [Google Scholar] [CrossRef]
- Li, G.; Hao, Y.; Yang, T.; Xiao, W.; Pan, M.; Huo, S.; Lyu, T. Enhancing Bioenergy Production from the Raw and Defatted Microalgal Biomass Using Wastewater as the Cultivation Medium. Bioengineering 2022, 9, 637. [Google Scholar] [CrossRef]
- Lai, W.H.; Salleh, S.M.; Daud, F.; Zainal, Z.; Othman, A.M.; Saleh, N.M. Optimization of submerged culture conditions for the production of mycelial biomass and exopolysaccharides from Lignosus rhinocerus. Sains Malays. 2014, 43, 73–80. [Google Scholar]
- Ma, Y.; Yu, X. Effects of aqueous extracts of different traditional Chinese medicines on triterpenoid and extracellular polysaccharide production in Lignosus rhinocerus fermentation process. Mycosystema 2017, 36, 1667–1673. [Google Scholar]
- Lin, J.; Zhao, C.; Wu, C.-J.; Chen, C. Evaluation of effectiveness of liquid fermented Lignosus rhinocerus mycelium against H1N1 Influenza virus infections. Hans J. Food Nutr. Sci. 2020, 9, 201–210. [Google Scholar] [CrossRef]
- Lau, B.F.; Abdullah, N.; Aminudin, N.; Lee, H.B.; Yap, K.C.; Sabaratnam, V. The Potential of Mycelium and Culture Broth of Lignosus rhinocerotis as Substitutes for the Naturally Occurring Sclerotium with Regard to Antioxidant Capacity, Cytotoxic Effect, and Low-Molecular-Weight Chemical Constituents. PLoS ONE 2014, 9, e102509. [Google Scholar] [CrossRef]





| Experimental Factors | Levels and Ranges | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Glucose concentration (g/L) | 10 | 30 | 50 |
| Speed of agitation (rpm) | 100 | 150 | 200 |
| Initial pH medium | 4 | 5 | 6 |
| No. | Experimental Factors | Responses | |||||
|---|---|---|---|---|---|---|---|
| Glucose Concentration (g/L) | Speed of Agitation (rpm) | Initial pH Medium | MB (g/L) | EPS (g/L) | |||
| Actual Value | Predicted Value | Actual Value | Predicted Value | ||||
| 1 | 30 | 150 | 5 | 12.47 | 11.08 | 2.60 | 2.33 |
| 2 | 30 | 100 | 5 | 6.43 | 7.75 | 2.53 | 2.53 |
| 3 | 30 | 150 | 6 | 12.01 | 10.94 | 1.92 | 2.04 |
| 4 | 10 | 150 | 5 | 6.83 | 8.91 | 1.74 | 1.56 |
| 5 | 30 | 200 | 5 | 6.21 | 6.21 | 1.18 | 1.32 |
| 6 | 30 | 150 | 5 | 12.00 | 11.08 | 2.85 | 2.33 |
| 7 | 50 | 100 | 6 | 7.90 | 8.43 | 3.26 | 3.18 |
| 8 | 30 | 150 | 5 | 11.00 | 11.08 | 2.20 | 2.33 |
| 9 | 30 | 150 | 5 | 10.5 | 11.08 | 2.10 | 2.33 |
| 10 | 50 | 100 | 4 | 11.1 | 10.63 | 3.54 | 3.50 |
| 11 | 10 | 100 | 4 | 6.85 | 5.80 | 1.22 | 1.29 |
| 12 | 50 | 200 | 4 | 6.72 | 6.71 | 1.82 | 1.73 |
| 13 | 10 | 200 | 6 | 5.17 | 5.32 | 0.59 | 0.60 |
| 14 | 30 | 150 | 4 | 8.87 | 11.26 | 2.22 | 2.25 |
| 15 | 30 | 150 | 5 | 13.13 | 11.08 | 2.15 | 2.33 |
| 16 | 10 | 100 | 6 | 4.80 | 4.48 | 1.19 | 1.25 |
| 17 | 50 | 150 | 5 | 13.13 | 12.36 | 2.72 | 3.04 |
| 18 | 50 | 200 | 6 | 6.67 | 7.39 | 1.45 | 1.35 |
| 19 | 30 | 150 | 5 | 10.00 | 11.08 | 2.38 | 2.33 |
| 20 | 10 | 200 | 4 | 4.61 | 3.75 | 0.66 | 0.70 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 137.37 | 9 | 15.26 | 6.1 | 0.0046 * | Significant |
| A: Glucose concentration | 29.76 | 1 | 29.76 | 11.9 | 0.0062 * | Significant |
| B: Speed of agitation | 5.94 | 1 | 5.94 | 2.37 | 0.1545 | |
| C: Initial pH medium | 0.2551 | 1 | 0.2551 | 0.102 | 0.756 | |
| AB | 1.76 | 1 | 1.76 | 0.702 | 0.4217 | |
| AC | 0.3898 | 1 | 0.3898 | 0.1558 | 0.7013 | |
| BC | 4.16 | 1 | 4.16 | 1.66 | 0.2264 | |
| A2 | 0.5323 | 1 | 0.5323 | 0.2128 | 0.6544 | |
| B2 | 46.2 | 1 | 46.2 | 18.47 | 0.0016 * | Significant |
| C2 | 0.0017 | 1 | 0.0017 | 0.0007 | 0.9798 | |
| Residual | 25.01 | 10 | 2.5 | |||
| Lack of fit | 17.67 | 5 | 3.53 | 2.41 | 0.1786 | Not significant |
| Pure error | 7.34 | 5 | 1.47 | |||
| Cor total | 162.38 | 19 | ||||
| Std. Dev. = 1.58 | R2 = 0.8460 | Adequate precision: 7.6973 | ||||
| Mean = 8.82 | Adj. R2 = 0.7073 | |||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 11.58263 | 9 | 1.286959 | 19.69009 | <0.0001 * | Significant |
| A: Glucose | 5.447916 | 1 | 5.447916 | 83.3515 | <0.0001 * | Significant |
| B: Speed of agitation | 3.666303 | 1 | 3.666303 | 56.09334 | <0.0001 * | Significant |
| C: Initial pH medium | 0.10983 | 1 | 0.10983 | 1.680372 | 0.223991 | |
| AB | 0.70152 | 1 | 0.70152 | 10.73305 | 0.008343 * | Significant |
| AC | 0.040186 | 1 | 0.040186 | 0.614836 | 0.451147 | |
| BC | 0.001653 | 1 | 0.001653 | 0.025292 | 0.876807 | |
| A2 | 0.003154 | 1 | 0.003154 | 0.048248 | 0.830559 | |
| B2 | 0.45747 | 1 | 0.45747 | 6.999156 | 0.024498* | Significant |
| C2 | 0.10176 | 1 | 0.10176 | 1.556903 | 0.240546 | |
| Residual | 0.653607 | 10 | 0.065361 | |||
| Lack of fit | 0.220607 | 5 | 0.044121 | 0.509486 | 0.7615 | Not significant |
| Pure error | 0.433 | 5 | 0.0866 | |||
| Cor total | 12.23624 | 19 | ||||
| Std. Dev. = 0.2557 | R2 = 0.9466 | Adequate precision: 16.0242 | ||||
| Mean = 2.02 | Adj. R2 = 0.8985 | |||||
| Run | Variables | Response | Fermentation Period (Day) | |||||
|---|---|---|---|---|---|---|---|---|
| Glucose (g/L) | Agitation (rpm) | Initial pH | Predicted | Actual | ||||
| MB (g/L) | EPS (g/L) | MB (g/L) | EPS (g/L) | |||||
| MB | 50.0 | 138 | 4.0 | 13.00 | - | 14.85 (SF) * | - | 11 |
| EPS | 49.7 | 103 | 4.6 | - | 3.55 | - | 4.71(SF) | 9 |
| MB + EPS | 50.0 | 128 | 4.0 | 12.85 | 3.33 | 14.31 (SF) | 1.87 (SF) | 11 |
| 15.60 (STR) * | 2.39 (STR) | |||||||
| MBconcentration (g/L) | MBProductivity (g/L/day) | EPSconcentration (g/L/day) | EPSProductivity (g/L/day) | EPSSpecific production rate (day−1) | YieldEPS (geps/gglucose) | YieldMB (gmb/gglucose) | |
|---|---|---|---|---|---|---|---|
| Shake flask (500 mL) * | 14.31 ± 0.1 | 1.30 ± 0.1 | 1.870 ± 0.005 | 0.170 ± 0.005 | 0.012 ± 0.05 | 0.133 ± 0.005 | 1.02 ± 0.1 |
| STR Bioreactor (5-L) * | 15.60 ± 0.1 | 1.42 ± 0.1 | 2.390 ± 0.005 | 0.217 ± 0.005 | 0.014 ± 0.05 | 0.171 ± 0.005 | 1.11 ± 0.1 |
| STR Bioreactor (13-L) | 29.93 ± 0.1 | 2.18 ± 0.1 | 4.40 ± 0.05 | 0.40 ± 0.005 | 0.017 ± 0.05 | 0.319 ± 0.005 | 1.73 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usuldin, S.R.A.; Ilham, Z.; Jamaludin, A.A.; Ahmad, R.; Wan-Mohtar, W.A.A.Q.I. Enhancing Biomass-Exopolysaccharides Production of Lignosus rhinocerus in a High-Scale Stirred-Tank Bioreactor and Its Potential Lipid as Bioenergy. Energies 2023, 16, 2330. https://doi.org/10.3390/en16052330
Usuldin SRA, Ilham Z, Jamaludin AA, Ahmad R, Wan-Mohtar WAAQI. Enhancing Biomass-Exopolysaccharides Production of Lignosus rhinocerus in a High-Scale Stirred-Tank Bioreactor and Its Potential Lipid as Bioenergy. Energies. 2023; 16(5):2330. https://doi.org/10.3390/en16052330
Chicago/Turabian StyleUsuldin, Siti Rokhiyah Ahmad, Zul Ilham, Adi Ainurzaman Jamaludin, Rahayu Ahmad, and Wan Abd Al Qadr Imad Wan-Mohtar. 2023. "Enhancing Biomass-Exopolysaccharides Production of Lignosus rhinocerus in a High-Scale Stirred-Tank Bioreactor and Its Potential Lipid as Bioenergy" Energies 16, no. 5: 2330. https://doi.org/10.3390/en16052330
APA StyleUsuldin, S. R. A., Ilham, Z., Jamaludin, A. A., Ahmad, R., & Wan-Mohtar, W. A. A. Q. I. (2023). Enhancing Biomass-Exopolysaccharides Production of Lignosus rhinocerus in a High-Scale Stirred-Tank Bioreactor and Its Potential Lipid as Bioenergy. Energies, 16(5), 2330. https://doi.org/10.3390/en16052330









