Current CO2 Capture and Storage Trends in Europe in a View of Social Knowledge and Acceptance. A Short Review
Abstract
:1. State of the Art
2. Research Methods
3. Strategies and Mechanisms of CO2 Storage in Europe
3.1. CO2 Storage in Saline Aquifers
3.2. CO2 Storage within Depleted Oil and Gas Reservoirs
3.3. CO2 Storage in Coal Beds
3.4. CO2 Storage in Deep Ocean
3.5. CO2 Storage in Deep-Sea Sediments
CO2 Storage in Basalt Rock Formation in Europe
4. Carbon Capture and Utilization Pathways in Europe
4.1. CO2 in Chemicals, Fuels, and Durable Materials
4.2. CO2 for Mineral Carbonation and for Construction Materials
4.3. CO2 in Biological Algae Cultivation as Well as Enzymatic Conversion
5. Social Aspect and Statistical Analysis
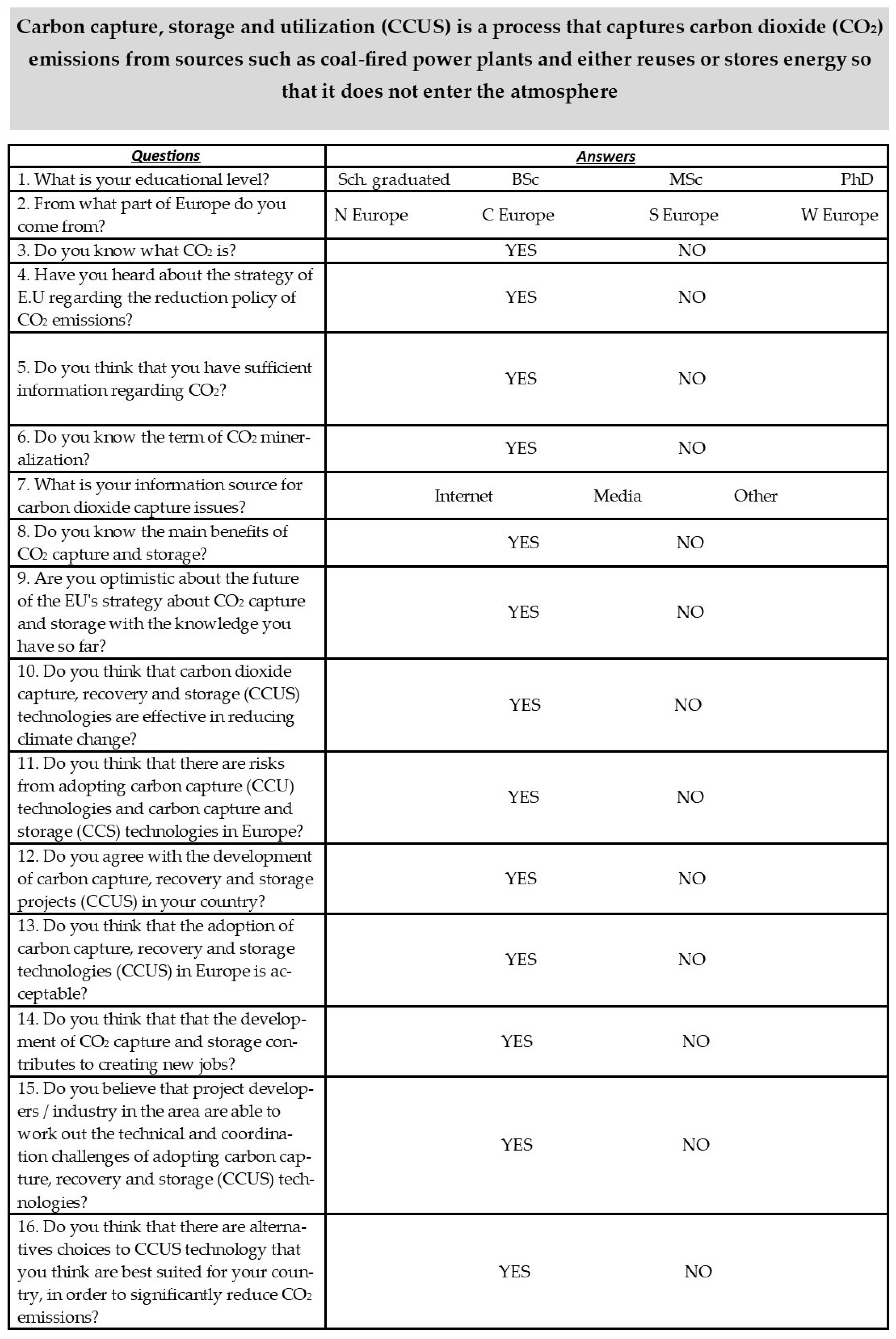
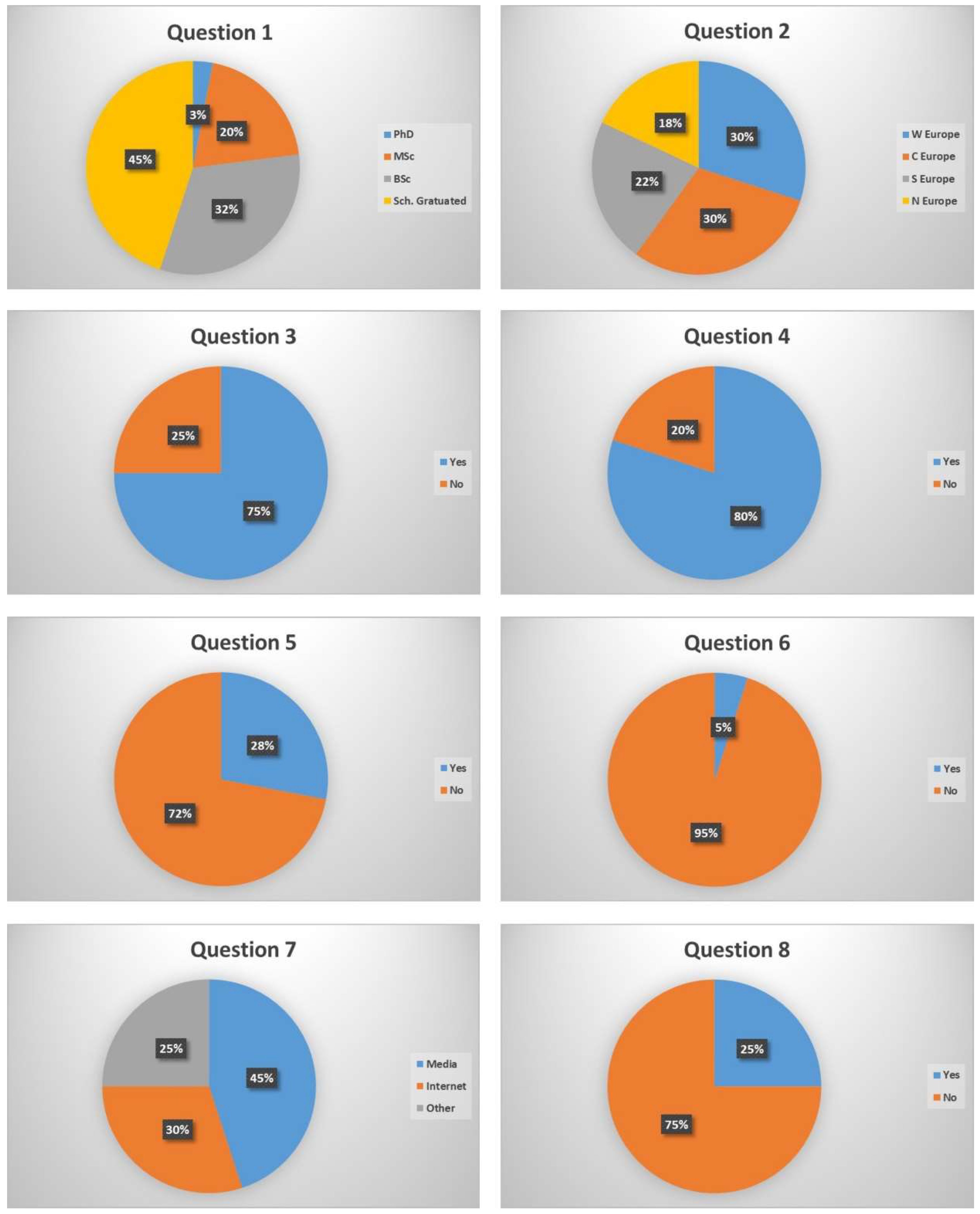
6. Questionnaire
7. Discussion and Suggestions for Future Research
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tai, C.Y.; Chen, W.-R.; Shih, S.-M. Factors affecting wollastonite carbonation under CO2 supercritical conditions. AIChE J. 2006, 52, 292–299. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Proceedings of the 14th Session of Working Group I and 54th Session of the IPCC, Virtual, 26 July–6 August 2021; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Hofmann, M.; Schellnhuber, H.J. Ocean acidification: A millennial challenge. Energy Environ. Sci. 2010, 3, 1883–1896. [Google Scholar] [CrossRef]
- BP. BP Statistical Review of World Energy; BP: Londok, UK, 2012. [Google Scholar]
- IEA. World Energy Outlook; OECD/IEA: Paris, France, 2010. [Google Scholar]
- Abu-Zahra, M.R.M.; Schneiders, L.H.J.; Niederer, J.P.M.; Feron, P.H.M.; Versteeg, G.F. CO2 capture from power plants. Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- IPCC. Carbon Dioxide Capture and Storage IPCC; IEA: Paris, France, 2005. [Google Scholar]
- Davison, J.; Freund, P.; Smith, A. Putting Carbon Back into the Ground; Climate Technology Centre and Network: Copenhagen, Denmark, 2001. [Google Scholar]
- Celia, M.A.; Bachu, S.; Nordbotten, J.M.; Bandilla, K.W. Status of CO2 storage in deep saline aquifers with emphasis on modeling approaches and practical simulations. Water Resour. Res. 2015, 51, 6846–6892. [Google Scholar] [CrossRef]
- Baran, P.; Zarębska, K.; Krzystolik, P.; Hadro, J.; Nunn, A. CO2-ECBM and CO2 Sequestration in Polish Coal Seam—Experimental Study. J. Sustain. Min. 2014, 13, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Jalili, P.; Saydam, S.; Cinar, Y. CO2 Storage in Abandoned Coal Mines. In Proceedings of the 11th Underground Coal Operators’ Conference, University of Wollongong & the Australasian, Institute of Mining and Metallurgy, Wollogong, Australia, 10–11 February 2011; pp. 355–360. [Google Scholar]
- Raza, A.; Gholami, R.; Rezaee, R.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. Well selection in depleted oil and gas fields for a safe CO2 storage practice: A case study from Malaysia. Petroleum 2017, 3, 167–177. [Google Scholar] [CrossRef]
- Györe, D.; Stuart, F.M.; Gilfillan, S.M.V.; Waldron, S. Tracing injected CO2 in the Cranfield enhanced oil recovery field (MS, USA) using He, Ne and Ar isotopes. Int. J. Greenh. Gas Control 2015, 42, 554–561. [Google Scholar] [CrossRef] [Green Version]
- Boschi, C.; Dini, A.; Dallai, L.; Ruggieri, G.; Gianelli, G. Enhanced CO2-mineral sequestration by cyclic hydraulic fracturing and Si-rich fluid infiltration into serpentinites at Malentrata (Tuscany, Italy). Chem. Geol. 2009, 265, 209–226. [Google Scholar] [CrossRef]
- García-Rios, M.; Luquot, L.; Soler, J.M.; Cama, J. Laboratory-Scale Interaction between CO2-Rich Brine and Reservoir Rocks (Limestone and Sandstone). Procedia Earth Planet. Sci. 2013, 7, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Ragnheidardottir, E.; Sigurdardottir, H.; Kristjansdottir, H.; Harvey, W. Opportunities and challenges for CarbFix: An evaluation of capacities and costs for the pilot scale mineralization sequestration project at Hellisheidi, Iceland and beyond. Int. J. Greenh. Gas. Control. 2011, 5, 1065–1072. [Google Scholar] [CrossRef]
- Kodama, S.; Nishimoto, T.; Yamamoto, N.; Yogo, K.; Yamada, K. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 2008, 33, 776–784. [Google Scholar] [CrossRef]
- Verduyn, M.; Geerlings, H.; Mossel, G.; Vijayakumari, S. Review of the various CO2 mineralization product forms. Energy Procedia 2011, 4, 2885–2892. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Yue, H.; Zhu, J.; Liang, B.; Li, C.; Wang, Y.; Xie, L.; Zhou, X. Scientific and Engineering Progress in CO2 Mineralization Using Industrial Waste and Natural Minerals. Engineering 2015, 1, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Lackner, K.S. A guide to CO2 sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef]
- Geerlings, H.; Zevenhoven, R. CO2 mineralization-bridge between storage and utilization of CO2. Annu. Rev. Chem. Biomol. Eng. 2013, 4, 103–117. [Google Scholar] [CrossRef]
- Koukouzas, N.; Ziogou, F.; Gemeni, V. Preliminary assessment of CO2 geological storage opportunities in Greece. Int. J. Greenh. Gas Control 2009, 3, 502–513. [Google Scholar] [CrossRef]
- Dichicco, M.C.; Laurita, S.; Paternoster, M.; Rizzo, G.; Sinisi, R.; Mongelli, G. Serpentinite carbonation for CO2 sequestration in the southern Apennines: Preliminary study. Energy Procedia 2015, 76, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation; ECN Publications: Petten, The Netherlands, 2003. [Google Scholar]
- The Intergovernmental Panel on Climate Change. IPCC Special Report on Carbon Dioxide Capture and Storage: Mineral Carbonation and Industrial Uses of Carbon Dioxide; IPCC: Geneva, Switzerland, 2005; pp. 320–335. [Google Scholar]
- Oelkers, E.H.; Gislason, S.R.; Matter, J. Mineral Carbonation of CO2. Elements 2008, 4, 333–337. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef] [Green Version]
- Little, M.G.; Jackson, R.B. Potential Impacts of Leakage from Deep CO2 Geosequestration on Overlying Freshwater Aquifer. Environ. Sci. Technol. 2010, 44, 9225–9232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celia, M.A.; Nordbotten, J.M.; Bachu, S.; Dobossy, M.; Court, B. Risk of Leakage versus Depth of Injection in Geological Storage. Energy Procedia 2009, 1, 2573–2580. [Google Scholar] [CrossRef] [Green Version]
- Zwaan, B.V.D.; Gerlagh, R. The Economics of Geological CO2 Storage and Leakage. Clim. Chang. Model. Policy 2008, 93, 285–309. [Google Scholar] [CrossRef] [Green Version]
- Bodnar, R.J.; Steele-MacInnis, M.; Capobianco, R.M.; Rimstidt, J.D. PVTX Properties of H2O-CO2-“salt” at PTX Conditions Applicable to Carbon Sequestration in Saline Formations. Rev. Miner. Geochem. 2013, 77, 123–152. [Google Scholar] [CrossRef]
- Gislason, S.R.; Broecker, W.S.; Gunnlaugsson, E.; Snæbjörnsdóttir, S.; Mesfin, K.G.; Alfredsson, H.A.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Stute, M.; et al. Rapid solubility and mineral storage of CO2 in basalt. Energy Procedia 2014, 63, 4561–4574. [Google Scholar] [CrossRef] [Green Version]
- Sigfusson, B.; Gislason, S.R.; Matter, J.M.; Stute, M.; Gunnlaugsson, E.; Gunnarsson, I.; Aradottir, E.S.; Sigurdardottir, H.; Mesfin, K.; Alfredsson, H.A.; et al. Solving the carbon-dioxide buoyancy challenge: The design and field testing of a dissolved CO2 injection system. Int. J. Greenh. Gas Control 2015, 37, 213–219. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, W.K.; Rush, G.E.; Dahlin, D.C. Laboratory Studies on the Carbonation Potential of Basalt: Applications to Geological Sequestration of CO2 in the Columbia River Basalt Group. In Proceedings of the AAPG Annual Meeting, Salt Lake City, UT, USA, 11–14 May 2003. [Google Scholar]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.G. Ophiolites; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Nicolas, A. Structures of Ophiolites and Dynamics of Oceanic Lithosphere; Springer: Dordrecht, The Netherlands, 1989; Volume 4. [Google Scholar]
- Tiseo, I. Carbon Dioxide Emissions in the European Union 1970–2020, by Selected Country; World Resources Institute: Washington, DC, USA, 2021. [Google Scholar]
- Shukla, R.; Ranjith, P.; Haque, A.; Choi, X. A review of studies on CO2 sequestration and caprock integrity. Fuel 2010, 89, 2651–2664. [Google Scholar] [CrossRef]
- Bachu, S.; Gunter, W.D.; Perkins, E.H. Aquifer disposal of CO2: Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279. [Google Scholar] [CrossRef]
- Bradshaw, J.; Bachu, S.; Bonijoly, D.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Issues and development of standards. Int. J. Greenh. Gas Control 2007, 1, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.Q.; Xiong, W.; Kasthuriarachchi, D.T.K.; Liu, Y.B. Phase equilibrium modeling for carbon dioxide solubility in aqueous sodium chloride solutions using an association equation of state. Ind. Eng. Chem. Res. 2019, 58, 10570–10578. [Google Scholar] [CrossRef]
- Sundal, A.; Hellevang, H.; Miri, R.; Dypvik, H.; Nystuen, J.P.; Aagaard, P. Variations in mineralization potential for CO2 related to sedimentary facies and burial depth—A comparative study from the North Sea. Energy Procedia 2014, 63, 5063–5070. [Google Scholar] [CrossRef] [Green Version]
- Gunter, W.D.; Bachu, S.; Benson, S. The role of hydrogeological and geochemical trapping in sedimentary basins for secure geological storage of carbon dioxide. Geol. Soc. Lond. Spec. Publ. 2004, 233, 129–145. [Google Scholar] [CrossRef]
- Bentham, M.; Kirby, G. CO2 Storage in Saline Aquifers. Oil Gas Sci. Technol. 2005, 60, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.A. Technical basis for carbon dioxide storage. Energy Procedia 2009, 1, 1727–1733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Huisingh, D. Carbon dioxide storage schemes: Technology, assessment and deployment. J. Clean. Prod. 2017, 142, 1055–1064. [Google Scholar] [CrossRef]
- Orlic, B. Geomechanical effects of CO2 storage in depleted gas reservoirs in the Netherlands: Inferences from feasibility studies and comparison with aquifer storage. J. Rock Mech. Geotech. Eng. 2016, 8, 846–859. [Google Scholar] [CrossRef]
- Williams, G.; Chadwick, A. Chimneys and channels: History matching the growing CO2 plume at the Sleipner storage site. In Proceedings of the Fifth CO2 Geological Storage Workshop, Utrecht, The Netherlands, 21–23 November 2018. [Google Scholar]
- Hansen, O.; Gilding, D.; Nazarian, B.; Osdal, B.; Ringrose, P.; Kristoffersen, J.B.; Eiken, O.; Hansen, H. Snøhvit: The history of injecting and storing 1 Mt CO2 in the fluvial Tubåen Fm. Energy Procedia 2013, 37, 3565–3573. [Google Scholar] [CrossRef] [Green Version]
- Hermanrud, C.; Eiken, O.; Hansen, O.R.; Nordgaard Bolaas, H.M.; Simmenes, T.; Teige, G.M.G.; Hansen, H.; Johansen, S. Importance of pressure management in CO2 storage. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2013. [Google Scholar]
- Martens, S.; Liebscher, A.; Möller, F.; Henninges, J.; Kempka, T.; Lüth, S.; Norden, B.; Prevedel, B.; Szizybalski, A.; Zimmer, M.; et al. CO2 Storage at the Ketzin Pilot Site, Germany: Fourth Year of Injection, Monitoring, Modelling and Verification. Energy Procedia 2013, 37, 6434–6443. [Google Scholar] [CrossRef] [Green Version]
- Brewer, P.G.; Friederich, G.; Peltzer, E.T.; Orr, F.M. Direct experiments on the ocean disposal of fossil fuel CO2. Science 1999, 284, 943–945. [Google Scholar] [CrossRef] [Green Version]
- Fer, I.; Haugan, P.M. Dissolution from a liquid CO2 Lake disposed in the deep ocean. Limnol. Oceanogr. 2003, 48, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Levine, J.S.; Matter, J.M.; Goldberg, D.; Cook, A.; Lackner, K.S. Gravitational trapping of carbon dioxide in deep sea sediments: Permeability, buoyancy, and geomechanical analysis. Geophy. Res. Lett. 2007, 34. [Google Scholar] [CrossRef] [Green Version]
- Koide, H.; Shindo, Y.; Tazaki, Y.; Iijima, M.; Ito, K.; Kimura, N.; Omata, K. Deep sub-seabed disposal of CO2—The most protective storage. Energy Convers. Manag. 1997, 38, S253–S258. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F.; Lackner, K.S. Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291–12295. [Google Scholar] [CrossRef] [Green Version]
- Schrag, D.P. Storage of carbon dioxide in offshore sediments. Science 2009, 325, 1658–1659. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Zhang, D. Long-term viability of carbon sequestration in deep-sea sediments. Sci. Adv. 2018, 4, eaao6588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. Carbon Dioxide Capture and Storage; IPCC Special Report. New York, NY, USA. 2005. Available online: https://www.researchgate.net/publication/239877190_IPCC_Special_Report_on_Carbon_dioxide_Capture_and_Storage (accessed on 15 June 2019).
- Adams, E.E.; Caldeira, K. Ocean Storage of CO2. Elements 2008, 4, 319–324. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An overview of the status and challenges of CO2 storage in minerals and geological formations. Front. Clim. 2019, 1, 1–20. [Google Scholar] [CrossRef]
- Koukouzas, N.; Koutsovitis, P.; Tyrologou, P.; Karkalis, C.; Arvanitis, A. Potential for Mineral Carbonation of CO2 in Pleistocene Basaltic Rocks in Volos Region (Central Greece). Minerals 2019, 9, 627. [Google Scholar] [CrossRef] [Green Version]
- Dessert, C.; Dupré, B.; Gaillardet, J.; François, L.M.; Allegre, C.J. Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chem. Geol. 2003, 202, 257–273. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Ho, A.M.; Chien, Y.J.; Dooley, J.J.; Davidson, C.L. Potential for carbon dioxide sequestration in flood basalts. J. Geophys. Res. Solid Earth 2006, 111. [Google Scholar] [CrossRef]
- Hähnchen, M.; Prigiobbe, V.; Storti, G.; Seward, T.M.; Mazzotti, M. Dissolution kinetics of fosteritic olivine at90–150 degrees C including effects of the presence of CO2. Geochim. Cosmochim. Acta 2006, 70, 4403–4416. [Google Scholar] [CrossRef]
- Teir, S.; Kuusik, R.; Fogelholm, C.-J.; Zevenhoven, R. Production of magnesium carbonates from serpentinite for long–term storage of CO2. Int. J. Miner. Process. 2007, 85, 1–15. [Google Scholar] [CrossRef]
- Palandri, J.L.; Kharaka, Y.K. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geochemical Modeling; U.S. Geological Survey Open File Report (of 2004–1068); National Energy Technology Laboratory—United States Department of Energy: Menlo Park, CA, USA, 2004; p. 71. [Google Scholar]
- Kelemen, P.B.; Matter, J.; Streit, L.; Rugde, J.; Curry, B.; Blusztajn, J. Annual Review of Earth and Planetary Sciences. Rates and mechanisms of mineral carbonation in peridotite: Natural processes and recipes for enhanced, in situ CO2 capture and storage. Annu. Rev. Earth Planet. Sci. 2011, 39, 545–576. [Google Scholar] [CrossRef]
- Knauss, K.; Nguyen, S.; Weed, H. Diopside dissolution kinetics as a function of pH, CO2, temperature, and time. Geochim. Cosmochim. Acta 1993, 57, 285–294. [Google Scholar] [CrossRef]
- Stockmann, G.; Wolff-Boenisch, D.; Gislason, S.; Oelkers, E. The effect of carbonate coating on the dissolution rate of basaltic glass and diopside—Implications for CO2 injection at Hellisheidi. In Proceedings of the Nordic Geological Winter Meeting, Reykjavík, Iceland, 9–12 January 2012. [Google Scholar]
- Pokrovsky, O.S.; Schott, J. Forsterite surface composition in aqueous solutions: A combined potentiometric, electrokinetic, and spectroscopic approach. Geochim. Cosmochim. Acta 2000, 64, 3299–3312. [Google Scholar] [CrossRef]
- Oelkers, E.; Gislason, S. The mechanism, rates and consequences of basaltic glass dissolution: I. An experimental study of the dissolution rates of basaltic glass as a function of aqueous Al, Si and oxalic acid concentration at 25 °C and pH = 3 and 11. Geochim. Cosmochim. Acta 2001, 65, 3671–3681. [Google Scholar] [CrossRef]
- Miranda-Barbosa, E.; Sigfússon, B.; Carlsson, J.; Tzimas, E. Advantages from Combining CCS with Geothermal Energy. Energy Procedia 2017, 114, 6666–6676. [Google Scholar] [CrossRef]
- Gíslason, S.R.; Sigurdardóttir, H.; Aradóttir, E.S.; Oelkers, E.H. A brief history of CarbFix: Challenges and victories of the project’s pilot phase. Energy Procedia 2018, 146, 103–114. [Google Scholar] [CrossRef]
- Callow, B.; Falcon-Suarez, I.; Ahmed, S.; Matter, J. Assessing the carbon sequestration potential of basalt using X-ray micro-CT and rock mechanics. Int. J. Greenh. Gas Control 2018, 70, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Matter, J.M.; Stute, M.; Snæbjörnsdóttir, S.Ó.; Oelkers, E.H.; Gislason, S.; Aradottir, E.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunnarson, I.; Aradóttir, E.S.; Oelkers, E.H.; Clark, D.E.; Arnarson, M.Þ.; Sigfússon, B.; Snæbjörnsdóttir, S.Ó.; Matter, J.M.; Stute, M.; Júlíusson, B.M.; et al. The rapid and cost-effective capture and subsurface mineral storage of carbon and sulfur at the CarbFix2 site. Int. J. Greenh. Gas Control 2018, 79, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Reguera, D.F.; Stute, M.; Matter, J. Laboratory experiments on CO2 dissolution in water for carbon sequestration; GC31C-0899. In Proceedings of the 2010 Fall Meeting, AGU, San Francisco, CA, USA, 13–17 December 2010. [Google Scholar]
- Clark, D.E.; Gunnarsson, I.; Aradóttir, E.S.P.; Arnarson, M.T.; Þorgeirsson, T.A.; Sigurðardóttir, S.S.; Sigfússon, B.; Snæbjörnsdóttir, S.Ó.; Oelkers, E.H.; Gislason, S.R. The chemistry and potential reactivity of the CO2-H2S charged injected waters at the basaltic CarbFix2 site, Iceland. Energy Procedia 2018, 146, 121–128. [Google Scholar] [CrossRef]
- Khalilabad, M.R.; Axelsson, G.; Gislason, S.R. Aquifer characterization with tracer test technique; permanent CO2 sequestration into basalt, SW Iceland. Mineral. Magaz. 2008, 72, 121–125. [Google Scholar] [CrossRef]
- Menez, B.; Campion, P.; Trias, R.E.; Aradóttir, S.P.; Gunnarsson, I.; Gislason, S.R.; Snæbjörnsdóttir, S.Ó.; Alfredsson, H.A.; Mesfin, K.G.; Gerard, E. Reactivity of deep ecosystems inhabiting basalt following CO2 (±H2S-H2) injection and associated consequences for mineral storage. In Proceedings of the International Carbon Conference, Reykjavík, Iceland, 27–28 June 2014. [Google Scholar]
- Trias, R.; Ménez, B.; le Campion, P.; Zivanovic, Y.; Lecourt, L.; Lecoeuvre, A.; Schmitt-Kopplin, P.; Uhl, J.; Gislason, S.R.; Alfreðsson, H.A.; et al. High reactivity of deep biota under anthropogenic CO2 injection into basalt. Nat. Commun. 2017, 8, 1063. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Takahashi, T.; Slagle, A.L. Carbon dioxide sequestration in deep-sea basalt. Proc. Natl. Acad. Sci. USA 2008, 105, 9920–9925. [Google Scholar] [CrossRef] [Green Version]
- Ratouis, T.M.P.; Snæbjörnsdóttir, S.Ó.; Voigt, M.J.V.; Sigfússon, B.; Gunnarsson, G.; Aradóttir, E.S.; Hjörleifsdóttir, V. Carbfix 2: A transport model of long-term CO2 and H2S injection into basaltic rocks at Hellisheidi, SW-Iceland. Int. J. Greenh. Gas Contr. 2022, 114, 103586. [Google Scholar] [CrossRef]
- Clark, D.E.; Oelkers, E.H.; Gunnarsson, I.; Sigfússon, B.; Snæbjörnsdóttir, S.Ó.; Aradóttir, E.S.; Gíslason, S.R. CarbFix2: CO2 and H2S mineralization during 3.5 years of continuous injection into basaltic rocks at more than 250 °C. Geochim. Cosmochim. Acta 2020, 279, 45–66. [Google Scholar] [CrossRef]
- Alfredsson, H.A.; Oelkers, E.H.; Hardarsson, B.S.; Franzson, H.; Gunnlaugsson, E.; Gislason, S.R. The geology and water chemistry of the Hellisheidi, SW-Iceland carbon storage site. Int. J. Greenh. Gas Control 2013, 12, 399–418. [Google Scholar] [CrossRef] [Green Version]
- Snæbjörnsdóttir, S.O.; Oelkers, E.H.; Mesfin, K.; Aradóttir, E.S.; Dideriksen, K.; Gunnarsson, I.; Gunnlaugsson, E.; Matter, J.M.; Stute, M.; Gislason, S.R. The chemistry and saturation states of subsurface fluids during the in situ mineralisation of CO2 and H2S at the CarbFix site in SW-Iceland. Int. J. Greenh. Gas Control 2017, 58, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Gislason, S.R.; Oelkers, E.H. Carbon storage in basalt. Science 2014, 344, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.; Slagle, A.L. A global assessment of deep-sea basalt sites for carbon sequestration. Energy Procedia 2009, 1, 3675–3682. [Google Scholar] [CrossRef] [Green Version]
- Snæbjörnsdóttir, S.Ó.; Wiese, F.; Fridriksson, T.; Ármansson, H.; Einarsson, G.M.; Gislason, S.R. CO2 storage potential of basaltic rocks in Iceland and the oceanic ridges. Energy Procedia 2014, 63, 4585–4600. [Google Scholar] [CrossRef] [Green Version]
- Aradóttir, E.S.P.; Sonnenthal, E.L.; Björnsson, G.; Jónsson, H. Multidimensional reactive transport modeling of CO2 mineral sequestration in basalts at the Hellisheidi geothermal field, Iceland. Int. J. Greenh. Gas Control 2012, 9, 24–40. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Liu, L.; Yiman, l.; Zeng, R. Capacity assessment of CO2 storage in deep saline aquifers by mineral trapping and the implications for Songliao Basin, Northeast China. Energy Sci. Eng. 2017, 5, 81–89. [Google Scholar] [CrossRef]
- Koukouzas, N.; Kypritidou, Z.; Purser, G.; Rochelle, C.A.; Vasilatos, C.; Tsoukalas, N. Assessment of the impact of CO2 storage in sandstone formations by experimental studies and geochemical modeling: The case of the Mesohellenic Trough, NW Greece. Int. J. Greenh. Gas Control 2018, 71, 116–132. [Google Scholar] [CrossRef]
- Petrounias, P.; Giannakopoulou, P.P.; Rogkala, A.; Kalpogiannaki, M.; Koutsovitis, P.; Damoulianou, M.-E.; Koukouzas, N. Petrographic Characteristics of Sandstones as a Basis to Evaluate Their Suitability in Construction and Energy Storage Applications. A Case Study from Klepa Nafpaktias (Central Western Greece). Energies 2020, 13, 1119. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Nianmin, Z.; Xu, C.; Yingchang, C.; Guanghui, Y.; Gluyas, J.G.; Miruo, L. Geologic CO2 storage in arkosic sandstones with CaCl2-rich formation water. Chem. Geol. 2020, 558, 119867. [Google Scholar]
- Christopoulou, M.A.; Koutsovitis, P.; Kostoglou, N.; Paraskevopoulou, C.; Sideridis, A.; Petrounias, P.; Rogkala, A.; Stock, S.; Koukouzas, N. Evaluation of the CO2 Storage Capacity in Sandstone Formations from the Southeast Mesohellenic trough (Greece). Energies 2022, 15, 3491. [Google Scholar] [CrossRef]
- Allen, K.G.; von Backström, T.W.; Kröger, D.G.; Kisters, A.F.M. Rock bed storage for solar thermal power plants: Rock characteristics, suitability, and availability. Sol. Energy Mater. Sol. Cells 2014, 126, 170–183. [Google Scholar] [CrossRef]
- Tiskatine, R.; Eddemani, A.; Gourdo, L.; Abnay, B.; Ihlal, A.; Aharoune, A.; Bouirden, L. Experimental evaluation of thermo-mechanical performances of candidate rocks for use in high temperature thermal storage. Appl. Energy 2016, 171, 243–255. [Google Scholar] [CrossRef]
- Clark, S. Constraining Diagenetic Timings, Processes and Reservoir Quality in Igneous-Affected Basins. Ph.D Thesis, Durham University, Durham, UK, 2014; p. 377. [Google Scholar]
- Sætrea, C.; Hellevang, H.; Dennehyc, C.; Dypvika, H.; Clark, S. A diagenetic study of intrabasaltic siliciclastics sandstones from the Rosebank field. Mar. Pet. Geol. 2018, 98, 335–355. [Google Scholar] [CrossRef]
- Izgec, O.; Demiral, B.; Bertin, H.; Akin, S. CO2 injection into saline carbonate aquifer formations I: Laboratory investigation. Transp. Porous Media 2008, 72, 1–24. [Google Scholar] [CrossRef]
- Romanov, V.; Soong, Y.; Carney, C.; Gilbert, E.; Rush, G.E.; Nielsen, B.; O’Connor, W. Mineralization of Carbon Dioxide: A Literature Review. Chem. Biomol. Eng. Rev. 2015, 2, 231–256. [Google Scholar]
- Tassianas, A.; Koukouzas, N. CO2 Storage Capacity Estimate in the Lithology of the Mesohellenic Trough, Greece. Energy Procedia 2016, 86, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Hangx, S.J.; Spiers, C.J. Reaction of plagioclase feldspars with CO2 under hydrothermal conditions. Chem. Geol. 2009, 265, 88–98. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2-rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Buddenberg, T.; Wu, S.; Sigurbjörnsson, Ó.; Tran, K.C.; Kakaras, E. The Challenge of Energy Storage in Europe: Focus on Power to Fuel. J. Energy Resour. Technol. 2016, 138, 042002. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Alper, E.; Yuksel Orhan, O. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Bareschino, P.; Mancusi, E.; Urciuolo, M.; Paulillo, A.; Chirone, R.; Pepe, F. Life cycle assessment and feasibility analysis of a combined chemical looping combustion and power-to-methane system for CO2 capture and utilization. Renew. Sustain. Energy Rev. 2020, 130, 109962. [Google Scholar] [CrossRef]
- Irabien, A.; Alvarez-Guerra, M.; Albo, J.; Dominguez-Ramos, A. Electrochemical conversion of CO2 to Value-Added Products. In Electrochemical Water and Wastewater Treatment; Butterworth-Heinemann: Oxford, UK, 2018; pp. 29–59. [Google Scholar]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Küngas, R. Review—Electrochemical CO2 Reduction for CO Production: Comparison of Low- and High-Temperature Electrolysis Technologies. J. Electrochem. Soc. 2020, 167, 044508. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Muthuraj, R.; Mekonnen, T. Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef] [Green Version]
- Bocin-Dumitriu, A.; Perez, M.; Sveen, T.; Bocin-dumitriu, A. Carbon Capture and Utilisation Workshop Background and Proceedings; Publications Office of the European Union: Luxembourg, 2013.
- Naraharisetti, P.K.; Yeo, T.Y.; Bu, J. New classification of CO2 mineralization processes and economic evaluation. J. Renew. Sustain. Energy Rev. 2019, 99, 220–233. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A review on carbon dioxide mineral carbonation through pH-swing process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.B.; Jarvis, M.; Hitchins, T. The technology of CO2 sequestration by mineral carbonation: Current status and future prospects. Can. Metall. Q. 2018, 57, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2020, 105, 103446. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Butera, A.; Le, K.N.; Li, W. Utilising CO2 technologies for recycled aggregate concrete: A critical review. Constr. Build. Mater. 2020, 250, 118903. [Google Scholar] [CrossRef]
- Jang, J.G.; Kim, G.M.; Kim, H.J.; Lee, H.K. Review on recent advances in CO2 utilization and sequestration technologies in cement-based materials. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Eloneva, S.; Said, A.; Fogelholm, C.-J.; Zevenhoven, R. Preliminary assessment of a method utilizing carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl. Energy 2012, 90, 329–334. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chen, Y.H.; Fan, L.S.; Kim, H.; Gao, X.; Ling, T.C.; Chiang, P.C.; Pei, S.L.; Gu, G. CO2 Mineralization and Utilization by Alkaline Solid Wastes for Potential Carbon Reduction. Nat. Sustain. 2020, 3, 399–405. [Google Scholar] [CrossRef]
- He, Z.; Wang, S.; Mahoutian, M.; Shao, Y. Flue gas carbonation of cement-based building products. J. CO2 Util. 2020, 37, 309–319. [Google Scholar] [CrossRef]
- Kassim, M.A.; Meng, T.K. Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production. Sci. Total Environ. 2017, 584–585, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Velea, S.; Dragos, N.; Serban, S.; Ilie, L.; Stalpeanu, D.; Nicoara, A.; Stepan, E. Biological sequestration of carbon dioxide from thermal power plant emmisions, by absorption in microalgal culture media. Rom. Biotechnol. Lett. 2009, 14, 4485–4500. [Google Scholar]
- Wu, W.; Lin, K.-H.; Chang, J.-S. Economic and life-cycle greenhouse gas optimization of microalgae-to-biofuels chains. Bioresour. Technol. 2018, 267, 550–559. [Google Scholar] [CrossRef]
- Anwar, M.N.; Fayyaz, A.; Sohail, N.F.; Khokhar, M.F.; Baqar, M.; Yasar, A.; Rasool, K.; Nazir, A.; Raja, M.U.F.; Rehan, M.; et al. CO2 utilization: Turning greenhouse gas into fuels and valuable products. J. Environ. Manag. 2020, 260, 110059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Microalgae Removal of CO2 from Flue Gas; IEA Clean Coal Centre: London, UK, 2015. [Google Scholar]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae biofuel: Current status and future applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Schlager, S.; Dibenedetto, A.; Aresta, M.; Apaydin, D.H.; Dumitru, L.M.; Neugebauer, H.; Sariciftci, N.S. Biocatalytic and Bioelectrocatalytic Approaches for the Reduction of Carbon Dioxide using Enzymes. Energy Technol. 2017, 5, 812–821. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Bulut, M.; Breugelmans, T.; Patil, S.A.; Pant, D. Current trends in enzymatic electrosynthesis for CO2 reduction. Curr. Opin. Green Sustain. Chem. 2019, 16, 65–70. [Google Scholar] [CrossRef]
- Bian, B.; Bajracharya, S.; Xu, J.; Pant, D.; Saikaly, P.E. Microbial electrosynthesis from CO2: Challenges, opportunities and perspectives in the context of circular bioeconomy. Bioresour. Technol. 2020, 302, 122863. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.R.; Olfe-Kräutlein, B.; Naims, H.; Armstrong, K. The Social Acceptance of Carbon Dioxide Utilisation: A Review and Research Agenda. Front. Energy Res. 2017, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- d’Amore, F.; Lovisotto, L.; Bezzo, F. Introducing social acceptance into the design of CCS supply chains: A case study at a European level. J. Clean. Prod. 2020, 249, 119337. [Google Scholar] [CrossRef]
- Jones, C.R.; Kaklamanou, D.; Stuttard, W.M.; Radford, R.L.; Burley, J. Investigating public perceptions of carbon dioxide utilisation (CDU) technology: A mixed methods study. Faraday Discuss. 2015, 183, 327–347. [Google Scholar] [CrossRef] [Green Version]
- Arning, K.; van Heek, J.; Ziefle, M. Acceptance profiles for a carbon-derived foam mattress. Exploring and segmenting consumer perceptions of a carbon capture and utilization product. J. Clean. Prod. 2018, 188, 171–184. [Google Scholar] [CrossRef]
- Arning, K.; Offermann-van Heek, J.; Linzenich, A.; Kaetelhoen, A.; Sternberg, A.; Bardow, A.; Ziefle, M. Same or different? Insights on public perception and acceptance of carbon capture and storage or utilization in Germany. Energy Policy 2019, 125, 235–249. [Google Scholar] [CrossRef]
- Swennenhuis, F.; Mabon, L.; Flach, T.A.; de Coninck, H. What role for CCS in delivering just transitions? An evaluation in the North Sea region. Int. J. Greenh. Gas Control 2020, 94, 102903. [Google Scholar] [CrossRef]
- Haug, J.K.; Stigson, P. Local acceptance and communication as crucial elements for realizing CCS in the Nordic region. Energy Procedia 2016, 86, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Whitmarsh, L.; Xenias, D.; Jones, C.R. Framing effects on public support for carbon capture and storage. Palgrave Commun. 2019, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Tcvetkov, P.; Cherepovitsyn, A.; Fedoseev, S. Public perception of carbon capture and storage: A state-of-the-art overview. Heliyon 2019, 5, e02845. [Google Scholar] [CrossRef] [Green Version]
- Glanz, S.; Schönauer, A.-L. Towards a Low-Carbon Society via Hydrogen and Carbon Capture and Storage: Social Acceptance from a Stakeholder Perspective. J. Sustain. Dev. Energy Water Environ. Syst. 2021, 9, 9. [Google Scholar] [CrossRef]
- Dütschke, E.; Wohlfarth, K.; Höller, S.; Viebahn, P.; Schumann, D.; Pietzner, K. Differences in the public perception of CCS in Germany depending on CO2 source, transport option and storage location. Int. J. Greenh. Gas Control 2016, 53, 149–159. [Google Scholar] [CrossRef]
- Heek, J.O.-V.; Arning, K.; Sternberg, A.; Bardow, A.; Ziefle, M. Assessing public acceptance of the life cycle of CO2-based fuels: Does information make the difference? Energy Policy 2020, 143, 111586. [Google Scholar] [CrossRef]
- IEA. Technology Roadmap: High-Efficiency, Low-Emissions Coal-Fired Power Generation; OECD/IEA: Paris, France, 2012. [Google Scholar]
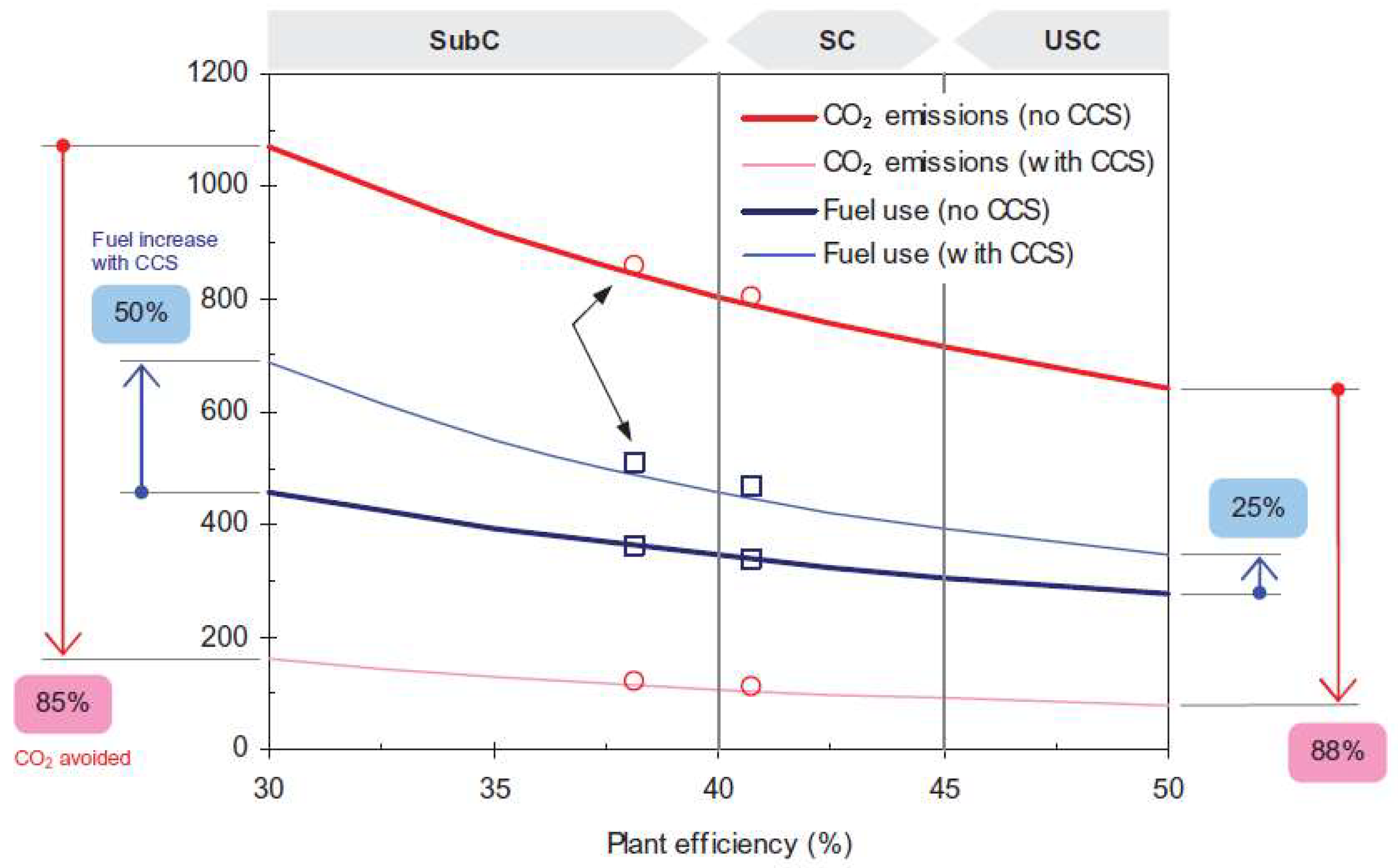
- Cebrucean, D.; Cebrucean, V.; Ionel, I. CO2 Capture and Storage from Fossil Fuel Power Plants. Energy Procedia 2014, 63, 18–26. [Google Scholar] [CrossRef] [Green Version]


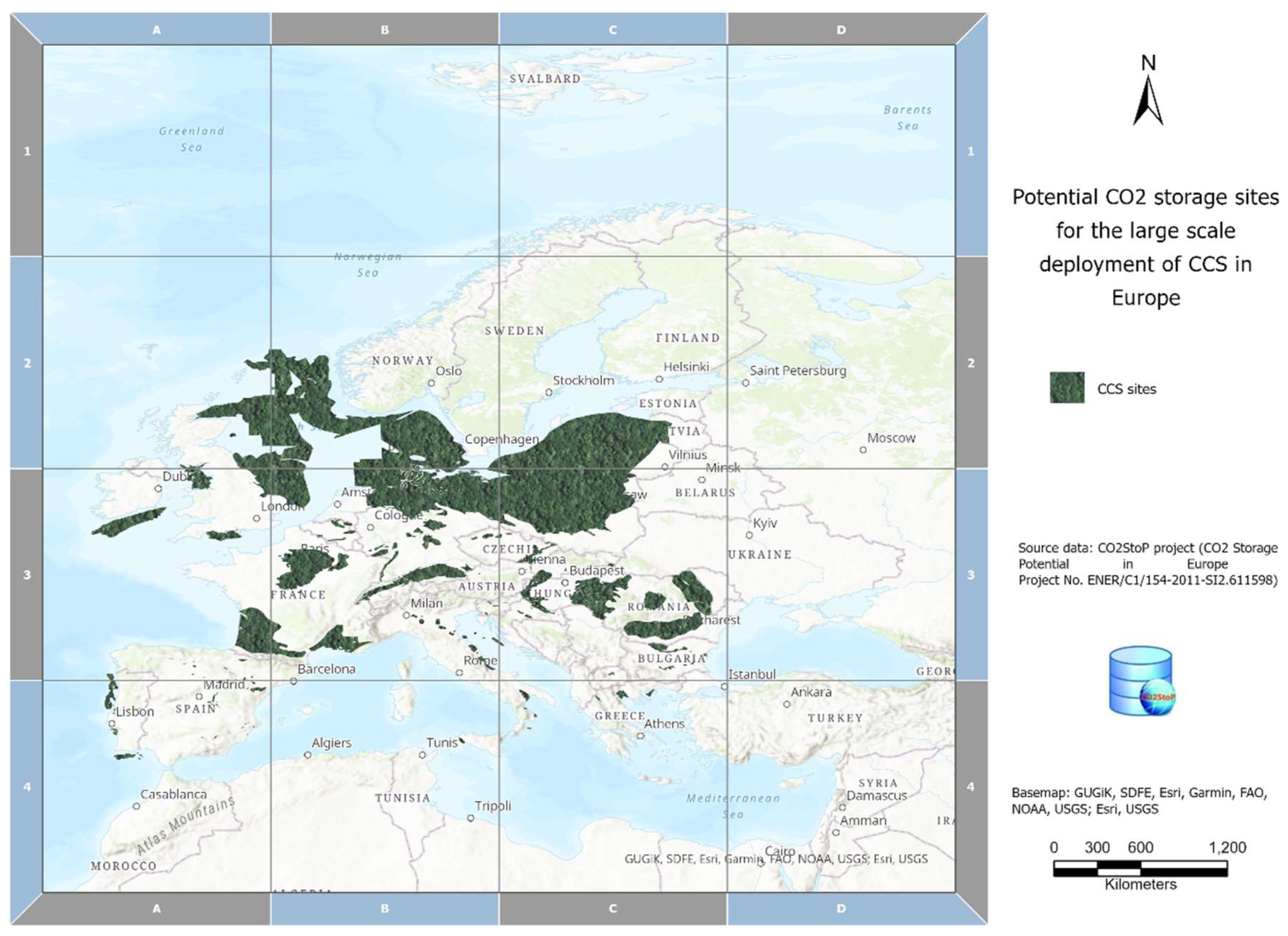
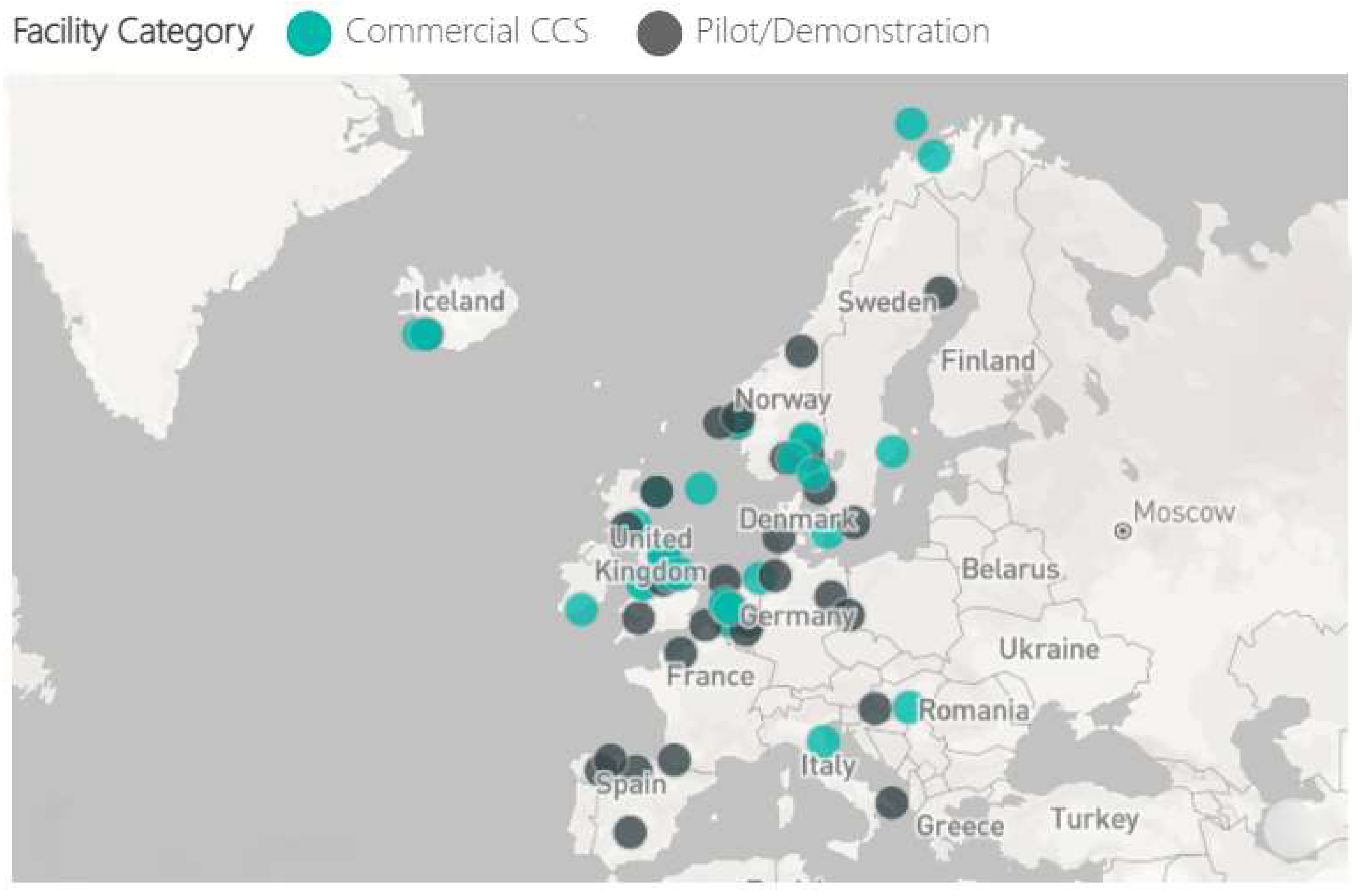

| Project | Location | Category | Status/Started | Operational | Facility Industry | Storage |
|---|---|---|---|---|---|---|
| Hafslund Oslo Celsio-Klemetsrud Waste to Energy Plant | Norway | Commercial CCS Facility | In Construction | 2025 | Waste Incineration | Yes |
| Acorn | UK | Commercial CCS Facility | Early Development | Mid 2020s | Oil Refining | Yes |
| Acorn (Minimum Viable CCS Development) | UK | Pilot and Demonstration CCS Facility | Advanced Development | 2021–2022 | Various | Yes |
| Acorn Direct Air Capture Facility | UK | Commercial CCS Facility | Early Development | 2026 | N/A | No |
| Acorn | UK | Commercial CCS Facility | Early Development | 2025 | Hydrogen Production | Yes |
| Adriatic Blue-ENI Hydrogen CCS | Italy | Commercial CCS Facility | Early Development | 2026 | Hydrogen Production | Yes |
| Adriatic Blue-ENI Power CCS | Italy | Commercial CCS Facility | Early Development | 2026 | Power Generation | Yes |
| Air Liquide Refinery Rotterdam CCS | The Netherlands | Commercial CCS Facility | Advanced Development | 2024 | Hydrogen Production | Yes |
| Air Products Refinery Rotterdam CCS | The Netherlands | Commercial CCS Facility | Advanced Development | 2024 | Hydrogen Production | Yes |
| Antwerp@C-BASF Antwerp CCS | Belgium | Commercial CCS Facility | Advanced Development | 2030 | Chemical Production | - |
| Antwerp@C-Borealis Antwerp CCS | Belgium | Commercial CCS Facility | Early Development | 2030 | Chemical Production | - |
| Antwerp@C-Exxonmobil Antwerp CCS | Belgium | Commercial CCS Facility | Early Development | 2030 | Chemical Production | - |
| Antwerp@C-Ineos Antwerp CCS | Belgium | Commercial CCS Facility | Early Development | 2030 | Chemical Production | No |
| Barents Blue | Norway | Commercial CCS Facility | Early Development | Mid 2020s | Fertilizer Production | No |
| Borg CO2 | Norway | Pilot and Demonstration CCS Facility | Early Development | Various | Yes | |
| Caledonia Clean Energy | UK | Commercial CCS Facility | Early Development | 2024 | Power Generation | Yes |
| Carbfix Project | Iceland | Pilot and Demonstration CCS Facility | Operational | 2012 | Power Generation | Yes |
| Cinfracap–Pipeline | Sweden | Pilot and Demonstration CCS Facility | Early Development | Various | No | |
| Cinfracap–Shipping Pipeline | Sweden | Pilot and Demonstration CCS Facility | Early Development | Various | No | |
| CIUDEN: CO2 Storage Technology Development Plant | Spain | Pilot and Demonstration CCS Facility | Operational | 2015 | N/A | Yes |
| CODA Shipping | Iceland | Commercial CCS Facility | Advanced Development | 2026 | Various | No |
| CODA Terminal Onshore Infrastructure | Iceland | Commercial CCS Facility | Advanced Development | 2026 | Various | No |
| CODA Terminal Pipeline | Iceland | Commercial CCS Facility | Advanced Development | 2026 | No | |
| CODA Terminal Storage | Iceland | Commercial CCS Facility | Advanced Development | 2026 | Yes | |
| Copenhill (Amager Bakker) Waste to Energy CCS | Denmark | Commercial CCS Facility | Advanced Development | 2025 | Waste Incineration | No |
| DMXTM Demonstration in Dunkirk | France | Pilot and Demonstration CCS Facility | Advanced Development | 2022 | Iron and Steel Production | No |
| Drax BECCS Project | UK | Commercial CCS Facility | Early Development | 2027 | Power Generation | Yes |
| Drax bioenergy carbon capture pilot plant | UK | Pilot and Demonstration CCS Facility | Operational | 2019 | Power Generation | No |
| Ervia Cork CCS | Ireland | Commercial CCS Facility | Early Development | 2028 | Power Generation Refining | Yes |
| ExxonMobil Benelux Refinery CCS | The Netherlands | Commercial CCS Facility | Advanced Development | 2024 | Hydrogen Production | Yes |
| Fortum Oslo Varme–Shipping Route | Norway | Pilot and Demonstration CCS Facility | Early Development | 2025 | Waste Incineration | Yes |
| Fortum Oslo Varme–Truck Route | Norway | Commercial CCS Facility | Advanced Development | 2025 | Waste Incineration | Yes |
| Geothermal Plant with CO2 Re-injection | Croatia | Pilot and Demonstration CCS Facility | Operational | 2018 | Power Generation | Yes |
| Humber Zero–Phillips 66 Humber Refinery CCS | UK | Commercial CCS Facility | Advanced Development | 2028 | Hydrogen Production | Yes |
| Humber Zero–VPI Immingham Power Plant CCS | UK | Commercial CCS Facility | Advanced Development | 2027 | Power Generation | Yes |
| Hydrogen 2 Magnum (H2M) | The Netherlands | Commercial CCS Facility | Early Development | 2024 | Power Generation | No |
| Hydrogen to Humber Saltend | UK | Commercial CCS Facility | Early Development | 2026–2027 | Hydrogen Production | No |
| HyNet North West | UK | Commercial CCS Facility | Early Development | Mid 2020s | Hydrogen Production | Yes |
| HyNet North West–Hanson Cement CCS | UK | Commercial CCS Facility | Early Development | 2026 | Cement Production | Yes |
| LEILAC | Belgium | Pilot and Demonstration CCS Facility | In Construction | 2020′s | Cement Production | No |
| MOL Szank field CO2 EOR | Hungary | Commercial CCS Facility | Operational | 1992 | Natural Gas Processing | Yes |
| Net Zero Teesside–BP H2Teesside | UK | Commercial CCS Facility | Early Development | 2027 | Hydrogen Production | Yes |
| Net Zero Teesside-CCGT Facility | UK | Commercial CCS Facility | Early Development | 2025 | Power Generation | Yes |
| Net Zero Teesside-Net Power Plant | UK | Commercial CCS Facility | Early Development | Late 2020s | Power Generation | Yes |
| Net Zero Teesside-Suez Waste to Energy CCS | UK | Commercial CCS Facility | Early Development | 2027 | Waste Incineration | Yes |
| Norcem Brevik–Cement Plant | Norway | Commercial CCS Facility | In Construction | 2024 | Cement Production | No |
| Norcem Brevik-Shipping Route | Norway | Commercial CCS Facility | In Construction | 2024 | Cement Production | Yes |
| Northern Gas Network H21 North of England | UK | Commercial CCS Facility | Early Development | 2026 | Hydrogen Production | Yes |
| Nothern Lights–Pipeline | Norway | Pilot and Demonstration CCS Facility | Early Development | 2024 | Various | No |
| Nothern Lights–Storage | Norway | Pilot and Demonstration CCS Facility | Early Development | 2024 | Various | Yes |
| Orca | Iceland | Commercial CCS Facility | Operational | 2021 | Direct Air Capture | Yes |
| Polaris Carbon Storage | Norway | Commercial CCS Facility | Advanced Development | 2024 | Hydrogen Production | Yes |
| Porthos–Compressor Station | The Netherlands | Commercial CCS Facility | Advanced Development | 2024 | Various | No |
| Porthos–Offshore Pipeline | The Netherlands | Commercial CCS Facility | Advanced Development | 2024 | Various | No |
| Porthos–Onshore Pipeline | The Netherlands | Commercial CCS Facility | Advanced Development | 2024 | Various | No |
| Porthos–Storage | The Netherlands | Commercial CCS Facility | Advanced Development | 2024 | Various | Yes |
| Preem Refinery CCS | Sweden | Commercial CCS Facility | Early Development | 2025 | Hydrogen Production | No |
| Shell Refinery Rotterdam CCS | The Netherlands | Commercial CCS Facility | Advanced Development | 2024 | Hydrogen Production | No |
| Sleipner CO2 Storage | Norway | Commercial CCS Facility | Operational | 1996 | Natural Gas Processing | Yes |
| Snohvit CO2 Storage | Norway | Commercial CCS Facility | Operational | 2008 | Natural Gas Processing | Yes |
| STEPWISE Pilot of SEWGS Technology at Swerea/Mefos | Sweden | Pilot and Demonstration CCS Facility | Operational | 2017 | Iron and Steel Production | No |
| Stockholm Exergi BECCS | Sweden | Commercial CCS Facility | Advanced Development | 2025 | Bioenergy | - |
| Stockholm Exergi BECCS–Shipping Route | Sweden | Commercial CCS Facility | Advanced Development | 2025 | Bioenergy | - |
| Technology Centre Mongstad (TCM) | Norway | Pilot and Demonstration CCS Facility | Operational | 2012 | Oil Refining | No |
| UKCCSRC Pilot-scale Advanced Capture Technology (PACT) | UK | Pilot and Demonstration CCS Facility | Operational | Power Generation | No | |
| ZERO Carbon Humber–Keady 3 CCS Power Station | UK | Commercial CCS Facility | Early Development | 2027 | Power Generation | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukouzas, N.; Christopoulou, M.; Giannakopoulou, P.P.; Rogkala, A.; Gianni, E.; Karkalis, C.; Pyrgaki, K.; Krassakis, P.; Koutsovitis, P.; Panagiotaras, D.; et al. Current CO2 Capture and Storage Trends in Europe in a View of Social Knowledge and Acceptance. A Short Review. Energies 2022, 15, 5716. https://doi.org/10.3390/en15155716
Koukouzas N, Christopoulou M, Giannakopoulou PP, Rogkala A, Gianni E, Karkalis C, Pyrgaki K, Krassakis P, Koutsovitis P, Panagiotaras D, et al. Current CO2 Capture and Storage Trends in Europe in a View of Social Knowledge and Acceptance. A Short Review. Energies. 2022; 15(15):5716. https://doi.org/10.3390/en15155716
Chicago/Turabian StyleKoukouzas, Nikolaos, Marina Christopoulou, Panagiota P. Giannakopoulou, Aikaterini Rogkala, Eleni Gianni, Christos Karkalis, Konstantina Pyrgaki, Pavlos Krassakis, Petros Koutsovitis, Dionisios Panagiotaras, and et al. 2022. "Current CO2 Capture and Storage Trends in Europe in a View of Social Knowledge and Acceptance. A Short Review" Energies 15, no. 15: 5716. https://doi.org/10.3390/en15155716
APA StyleKoukouzas, N., Christopoulou, M., Giannakopoulou, P. P., Rogkala, A., Gianni, E., Karkalis, C., Pyrgaki, K., Krassakis, P., Koutsovitis, P., Panagiotaras, D., & Petrounias, P. (2022). Current CO2 Capture and Storage Trends in Europe in a View of Social Knowledge and Acceptance. A Short Review. Energies, 15(15), 5716. https://doi.org/10.3390/en15155716












