Feasibility of Thermal Utilization of Primary and Secondary Sludge from a Biological Wastewater Treatment Plant in Kaliningrad City
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
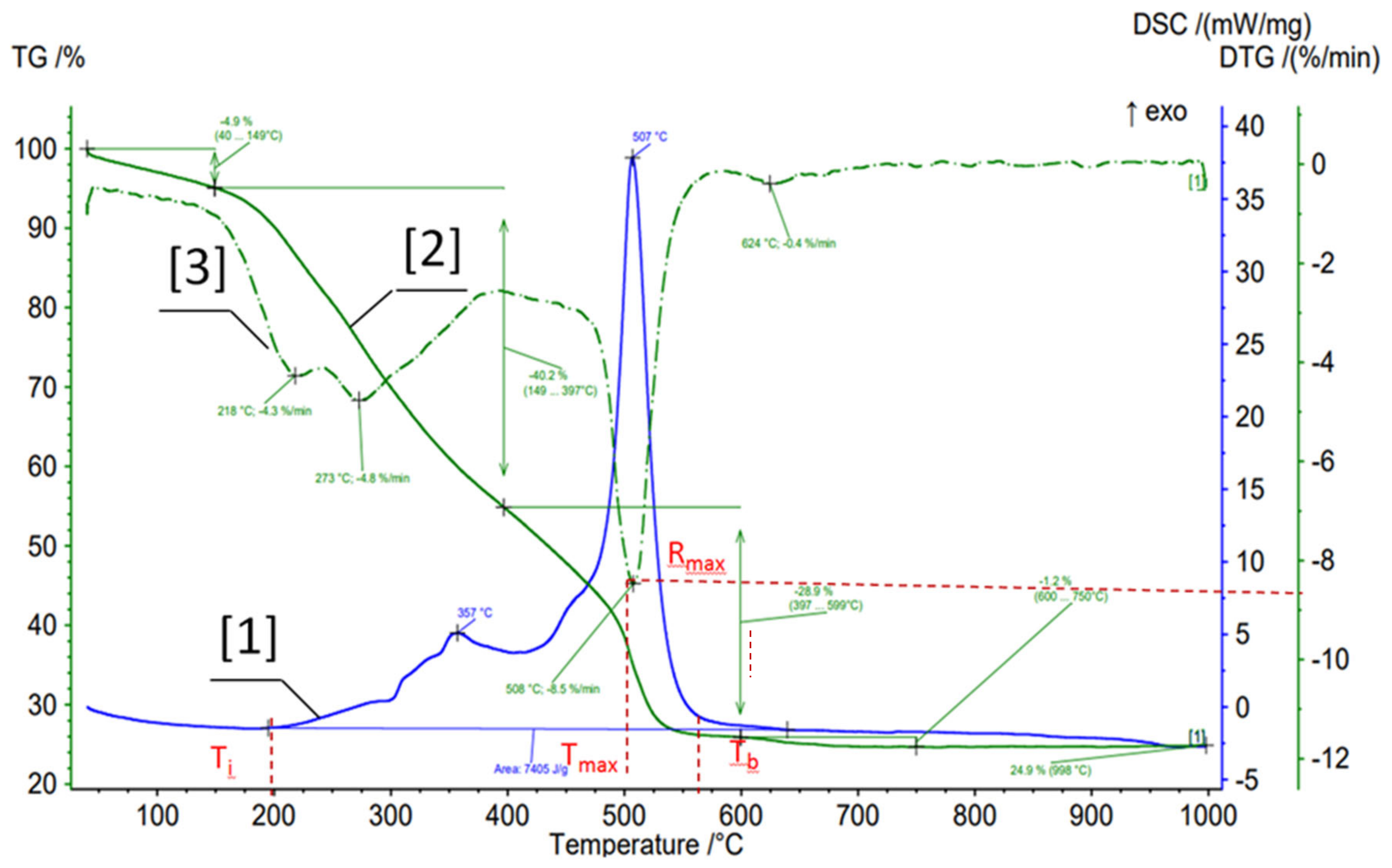
2.2. Sludge Characterization Methods
2.3. Hydrothermal Liquefaction Procedure
2.4. Products Characterization Methods
3. Results and Discussion
3.1. Primary and Secondary Sludge Characterization
| Parameter | Type II Kerogen [29] | Sludge from the Primary Clarifiers | Excess Secondary Sludge |
|---|---|---|---|
| C content, % | 70–73 | 54.58 ± 2.33 | 56.66 ± 0.31 |
| H content, % | 8–9 | 8.20 ± 0.28 | 8.93 ± 0.11 |
| O content, % | 9–11 | 25.30 ± 0.96 | 20.74 ± 0.18 |
| N content, % | 1–2 | 4.22 ± 0.25 | 10.22 ± 0.12 |
| S content, % | 0.78–1.27 | n/a | n/a |
| H/C ratio (atomic) | 0.5–1.25 | 1.8 | 1.89 |
| O/C ratio (atomic) | 0.1–0.3 | 0.35 | 0.27 |
| Ash content (% d.m.) | - | 26.93 ± 0.23 | 31.3 ± 0.13 |
| Total lipid content (%) | - | 5.2 ± 0.5 | 10.2 ± 0.7 |
| Total protein content (%) | - | 9.98 ± 0.21 | 20.32 ± 0.21 |
| Total carbohydrates content (%, calc.) | - | 57.89 ± 0.26 | 38.179 ± 0.25 |
| Parameter | Secondary Sludge | Primary Sludge | Sawdust [32] |
|---|---|---|---|
| Ignition temperature (Ti), °C | 220 | 200 | 308 |
| Maximum mass loss rate temperature (Tmax), °C | 299 | 508 | 443 |
| Ashing temperature (Tb), °C | 520 | 550 | 490 |
| Maximum mass loss rate (Rmax), %/min | 6.6 | 8.5 | 39 |
| Average mass loss rate (Raverege), %/min | 4.2 | 3.8 | 5.2 |
| Combustion index (S), ×10−6 | 1.1 | 1.5 | 4.36 |
| Ash content, % | 24.9 | 33.2 | 11.95 |
| HHV, MJ/kg (d.m.) | 8.69 | 7.41 | 15.86 |
| Initial water content, % | 92–98 | 92–95 |
3.2. Catalytic Sludge Hydrothermal Liquefaction
3.3. Bio-Oil Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christodoulou, A.; Stamatelatou, K. Overview of legislation on sewage sludge management in developed countries worldwide. Water Sci. Technol. 2015, 73, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, J.; Lee, D.-J.; Chang, Y.; Lee, Y.J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, N.A.; Pyrsikova, A.N. Ispol’zovanie osadkov stochnykh vod v kachestve udobrenii [The use of sewage sludge as fertilizers]. Int. Res. J. 2015, 3, 104–107. [Google Scholar]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Dyakov, M.S. Strategy for the management of solid waste from municipal wastewater treatment plants. Bull. PNRPU Appl. Ecol. Urban. Dev. 2019, 2, 35–48. [Google Scholar] [CrossRef]
- Bajbekov, R.F.; Merzlaya, G.E.; Vlasova, O.A. Organic waste utilization for the agrocenosis fertilization. Crop Husb. 2015, 2, 34–36. [Google Scholar]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A Review of Sludge-to-Energy Recovery Methods. Energies 2019, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Xu, D.; Hao, B.; Liu, L.; Wang, S.; Wu, Z. Thermochemical methods for the treatment of municipal sludge. J. Clean. Prod. 2021, 311, 127811. [Google Scholar] [CrossRef]
- Samolada, M.C.; Zabaniotou, A.A. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014, 34, 411–420. [Google Scholar] [CrossRef]
- Kulikova, Y.; Sukhikh, S.; Ivanova, S.; Babich, O.; Sliusar, N. Review of Studies on Joint Recovery of Macroalgae and Marine Debris by Hydrothermal Liquefaction. Appl. Sci. 2022, 12, 569. [Google Scholar] [CrossRef]
- Xu, D.; Lin, G.; Liu, L.; Wang, Y.; Jing, Z.; Wang, S. Comprehensive evaluation on product characteristics of fast hydrothermal liquefaction of sewage sludge at different temperatures. Energy 2018, 159, 686–695. [Google Scholar] [CrossRef]
- Basar, I.A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A review on key design and operational parameters to optimize and develop hydrothermal liquefaction of biomass for biorefinery applications. Green Chem. 2021, 23, 1404. [Google Scholar] [CrossRef]
- Yang, T.; Liu, X.; Li, R.; Li, B.; Kai, X. Hydrothermal liquefaction of sewage sludge to produce bio-oil: Effect of co-pretreatment with subcritical water and mixed surfactants. J. Supercrit. Fluids 2019, 144, 28–38. [Google Scholar] [CrossRef]
- Badrolnizam, R.; Elham, O.; Hadzifah, S.; Husain, M.; Hidayu, A.; Mohammad, N.; Mohamad Daud, A. Sewage sludge conversion via hydrothermal liquefaction (HTL)—A preliminary study. J. Phys. Conf. Ser. 2019, 1349, 012108. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Hu, M.; Du, G.; Tian, S.; He, Z.; Liu, B.; Ma, W. Hydrothermal Liquefaction of Sewage Sludge by Microwave Pretreatment. Energy Fuels 2019, 34, 1145–1152. [Google Scholar] [CrossRef]
- Mujahid, R.; Riaz, A.; Insyani, R.; Kim, J. A centrifugation-first approach for recovering high-yield bio-oil with high calorific values in biomass liquefaction: A case study of sewage sludge. Fuel 2020, 262, 116628. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y. Combustion, pyrolysis and char CO2-gasification characteristics of hydrothermal carbonization solid fuel from municipal solid wastes. Fuel 2016, 181, 905–915. [Google Scholar] [CrossRef]
- Nazari, L. Hydrothermal Liquefaction of High-Water Content Biomass and Waste Materials for the Production of Biogas and Bio-Crude Oil. Electronic Thesis and Dissertation Repository. 2016. Available online: https://ir.lib.uwo.ca/etd/4069 (accessed on 7 May 2022).
- Shah, A.A.; Toor, S.S.; Seehar, T.H.; Nielsen, R.S.; Nielsen, A.H.; Pedersen, T.H.; Rosendahl, L.A. Bio-Crude Production through Aqueous Phase Recycling of Hydrothermal Liquefaction of Sewage Sludge. Energies 2020, 13, 493. [Google Scholar] [CrossRef] [Green Version]
- Malins, K.; Kampars, V.; Brinks, J.; Neibolte, I.; Murnieks, R.; Kampare, R. Bio-oil from thermo-chemical hydro-liquefaction of wet sewage sludge. Bioresour. Technol. 2015, 187, 23–29. [Google Scholar] [CrossRef]
- ASTM-D7348; Standard Test Method for Ash in Biomass. ASTM: West Conshohocken, PA, USA, 2020; pp. 1–3.
- Kulikova, Y.; Sukhikh, S.; Babich, O.; Yuliya, M.; Krasnovskikh, M.; Noskova, S. Feasibility of Old Bark and Wood Waste Recycling. Plants 2022, 11, 1549. [Google Scholar] [CrossRef]
- Hwang, S.H.; Koo, M.; Jo, S.; Cho, Y.S. A comparison study of crude protein contents obtained utilizing the kjeldahl method and dumas combustion method in foods. J. Anal. Sci. Technol. 2020, 33, 143–150. [Google Scholar] [CrossRef]
- Thiex, N.; Anderson, S.; Gildemeister, B.; Adcock, W.; Boedigheimer, J.; Bogren, E.; Coffin, R.; Conway, K.; DeBaker, A.; Frankenius, E.; et al. Crude Fat, Diethyl Ether Extraction, in Feed, Cereal Grain, and Forage (Randall/Soxtec/Submersion Method): Collaborative Study. J. AOAC Int. 2003, 86, 888–898. [Google Scholar] [CrossRef] [Green Version]
- Rulkens, W.H. Sewage sludge as a biomass resource for the production of energy: Overview and assessment of the various options. Energy Fuels 2008, 22, 9–15. [Google Scholar] [CrossRef]
- Shchetinin, A.I. Elemental composition of activated sludge. Water Supply Sanit. Eng. 2010, 11, 49–54. (In Russian) [Google Scholar]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion: A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Whitehead, T.; Price, N.; Drake, H.; Cotta, M. Catabolic Pathway for the Production of Skatole and Indoleacetic Acid by the Acetogen Clostridium drakei, Clostridium scatologenes, and Swine Manure. Appl. Environ. Microbio. 2008, 74, 1950–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tissot, B.; Welte, D. The Fateof Orgunic Matteri n Sedimentar Byasins: Generation of Oil and Gus. In Petroleum Formation and Occurrence, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1984; pp. 69–130. [Google Scholar]
- Hernández, A.B.; Okonta, F.; Freeman, N. Thermal decomposition of sewage sludge under N2, CO2 and air: Gas characterization and kinetic analysis. J. Environ. Manag. 2017, 196, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, J.M.; Adegbite, S.; Pang, C.H.; Liu, H.; Parvez, A.M.; Wu, T. A novel index for the study of synergistic effects during the co-processing of coal and biomass. Appl. Energy 2017, 188, 215. [Google Scholar] [CrossRef]
- Guida, M.Y.; Lanaya, S.; Rbihi, Z.; Hannioui, A. Thermal degradation behaviors of sawdust wood waste: Pyrolysis kinetic and mechanism. J. Mater. Environ. Sci. 2019, 10, 742–755. [Google Scholar]
- Channiwala, S.; Parikh, P.P. A Unified Correlation for Estimating HHV of Solid, Liquid and Gaseous Fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]



| Raw Material | Conditions | Products | Yield | Reference |
|---|---|---|---|---|
| Primary sludge | Sludge:water ratio = 1:2, t = 250–400 °C, time 60 min | Oil | Max yield 52% at 350 °C | [14] |
| Primary sludge | Sludge:water ratio = 3:20, t = 300–350 °C, time 0–60 min, microwave pretreatment | Oil with HHV 26.3 MJ/kg | Max yield 35.4% at 350 °C | [15] |
| Primary sludge | Sludge:water ratio = 13:100, t = 300–400 °C and 12–14 MPa time 30–120 min | Oil | Max yield 34% at 325 °C, 30 min | [16] |
| Secondary sludge | Sludge:water ratio = 1:10, t = 260–350 °C time 10 min | Oil | Max yield 22.9% at 340 °C, 10 min | [11] |
| Secondary sludge | Sludge:water ratio = 1:10, t = 310 °C time 10 min, catalyst: KOH 0.13%; solvent: 15% ethanol | Oil | 30% | [17] |
| Secondary sludge | Sludge:water ratio = 1:10, t = 310 °C time 10 min, catalyst: KOH 0.5% | Oil | 23.1% | [18] |
| Primary and secondary sludge | Sludge:water ratio = 7:25, t = 350 °C time 15 min, catalyst: K2CO3 2.5% | Oil | 28% (+12%) | [19] |
| Primary and secondary sludge | Sludge:water ratio = 7:25, t = 350 °C time 15 min, catalyst: CH3COOH 2.5% | Oil | 26% (+8.3%) | [19] |
| Primary and secondary sludge | Sludge:water ratio = 1:5, t = 300 °C time 40 min, catalyst: FeSO4 5% | 46% (+15%) | [20] | |
| Primary sludge | Sludge:water ratio = 1:4, t = 300 °C, catalyst: K2CO3 2.5% | 48% (+140%) | [12] |
| Substrate | Catalyst | Oil Yield, % (Oil Yield Increase, %) | Solid Resedue Yield, % | Water Soluble Phase Yield, % |
|---|---|---|---|---|
| Secondary sludge | None | 29.75 ± 2.2 (0) | 62.1 ± 4.5 | 8.2 ± 0.8 |
| Primary sludge | None | 30.7 ± 2.2 (0) | 42.8 ± 3.5 | 26.6 ± 1.5 |
| Primary+Secondary sludge 1:1 | None | 35.3 ± 2.8 (+18.6%) | 46.6 ± 3.2 | 18.1 ± 1.1 |
| Homogeneous catalysis | ||||
| Secondary sludge | KOH | 21.6 ± 1.7 (−27.5%) | 32.5 ± 2.8 | 45.9 ± 3.3 |
| Secondary sludge | NaOH | 10.9 ± 1.7 (−63.4%) | 14.8 ± 0.9 | 74.3 ± 5.7 |
| Secondary sludge | NH4Fe(SO4)2 | 42.8 ± 4.3 (+43.6%) | 50.8 ± 4.1 | 6.4 ± 0.4 |
| Primary sludge | NH4Fe(SO4)2 | 36.6 ± 2.7 (+19.2%) | 47.1 ± 3.5 | 16.3 ± 0.9 |
| Secondary sludge | CoCl6 | 44.5 ± 3.6 (+49.3%) | 42.7 ± 3.3 | 12.8 ± 0.3 |
| Primary sludge | CoCl6 | 28.8 ± 1.6 (−6.2%) | 43.4 ± 2.8 | 27.8 ± 2.2 |
| Secondary sludge | NiSO4 | 48.7 ± 2.5 (+63.4%) | 47.6 ± 2.8 | 3.7 ± 0.2 |
| Primary sludge | NiSO4 | 41.4 ± 3.6 (+34.9%) | 44.0 ± 2.3 | 14.6 ± 0.9 |
| Secondary sludge | CuSO4 | 35.5 ± 2.2 (+19.1%) | 57.8 ± 1.9 | 6.7 ± 0.3 |
| Secondary sludge | ZnSO4 | 42.0 ± 3.8 (+40.9%) | 51.9 ± 3.1 | 6.1 ± 0.4 |
| Secondary sludge | MoO3 | 32.6 ± 1.9 (+9.4%) | 37.0 ± 2.4 | 30.4 ± 1.6 |
| Primary sludge | MoO3 | 23.8 ± 1.7 (−22.5%) | 53.0 ± 4.2 | 23.2 ± 1.7 |
| Heterogeneous catalysis | ||||
| Secondary sludge | MgO | 27.4 ± 2.4 (−8.1%) | 47.1 ± 3.6 | 25.5 ± 1.7 |
| Secondary sludge | Zeolite | 35.0 ± 2.6 (+17.4%) | 39.8 ± 2.7 | 25.2 ± 1.6 |
| Primary sludge | Zeolite | 33.3 ± 2.8 (+8.5%) | 37.4 ± 1.6 | 29.3 ± 1.4 |
| Secondary sludge | Al2O3 | 37.1 ± 2.1 (+24.5%) | 48.4 ± 2.5 | 14.5 ± 1.4 |
| Primary sludge | Al2O3 | 39.1 ± 3.1 (+27.4%) | 31.9 ± 4.4 | 29.0 ± 1.6 |
| Type of Bio-Oil | Boiling Point Temperature Range | |||
|---|---|---|---|---|
| 42–150 °C | 150–360 °C | 360–600 °C | Rest | |
| Bio-oil from HTL of primary sludge without catalyst, 4.4 MPa, 260 °C | 1.8 | 56.7 | 24.6 | 16.8 |
| Bio-oil from HTL of secondary sludge without catalyst, 4.4 MPa, 260 °C | 1.7 | 54.4 | 34.3 | 9.6 |
| Bio-oil from HTL of primary sludge, cat. 2 g NiSO4 4.4 MPa, 260 °C | 2.4 | 56.6 | 26.6 | 12.4 |
| Bio-oil from HTL of secondary sludge cat. 2 g NiSO4, 4.4 MPa, 260 °C | 2.7 | 65.32 | 26.9 | 5.0 |
| Parameter | Boiling Range | |||
|---|---|---|---|---|
| Primary Sludge Without Catalyst | Secondary Sludge Without Catalyst | Primary Sludge Cat. 2 g NiSO4 | Secondary Sludge Cat. 2 g NiSO4 | |
| Elemental composition (%, d.m.) | ||||
| C | 63.63 ± 2.15 | 67.22 ± 0.62 | 70.56 ± 0.84 | 72.97 ± 0.34 |
| H | 10.20 ± 0.23 | 9.17 ± 0.14 | 10.86 ± 0.11 | 11.21 ± 0.16 |
| N | 1.77 ± 0.15 | 6.85 ± 0.04 | 1.51 ± 0.03 | 4.29 ± 0.14 |
| S | 0.75 ± 0.09 | 1.06 ± 0.04 | 0.78 ± 0.04 | 1.05 ± 0.09 |
| O (calculated) | 6.85 | 6.10 | 3.89 | 5.44 |
| HHV, (MJ/kg, d.m.) | 33.22 | 33.45 | 36.82 | 38.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, Y.; Babich, O.; Tsybina, A.; Sukhikh, S.; Mokrushin, I.; Noskova, S.; Orlov, N. Feasibility of Thermal Utilization of Primary and Secondary Sludge from a Biological Wastewater Treatment Plant in Kaliningrad City. Energies 2022, 15, 5639. https://doi.org/10.3390/en15155639
Kulikova Y, Babich O, Tsybina A, Sukhikh S, Mokrushin I, Noskova S, Orlov N. Feasibility of Thermal Utilization of Primary and Secondary Sludge from a Biological Wastewater Treatment Plant in Kaliningrad City. Energies. 2022; 15(15):5639. https://doi.org/10.3390/en15155639
Chicago/Turabian StyleKulikova, Yuliya, Olga Babich, Anna Tsybina, Stanislav Sukhikh, Ivan Mokrushin, Svetlana Noskova, and Nikolaj Orlov. 2022. "Feasibility of Thermal Utilization of Primary and Secondary Sludge from a Biological Wastewater Treatment Plant in Kaliningrad City" Energies 15, no. 15: 5639. https://doi.org/10.3390/en15155639
APA StyleKulikova, Y., Babich, O., Tsybina, A., Sukhikh, S., Mokrushin, I., Noskova, S., & Orlov, N. (2022). Feasibility of Thermal Utilization of Primary and Secondary Sludge from a Biological Wastewater Treatment Plant in Kaliningrad City. Energies, 15(15), 5639. https://doi.org/10.3390/en15155639







