The Composition and Origin of PM1-2 Microspheres in High-Calcium Fly Ash from Pulverized Lignite Combustion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
3. Results and Discussion
3.1. Characterization of Fine Narrow Fraction
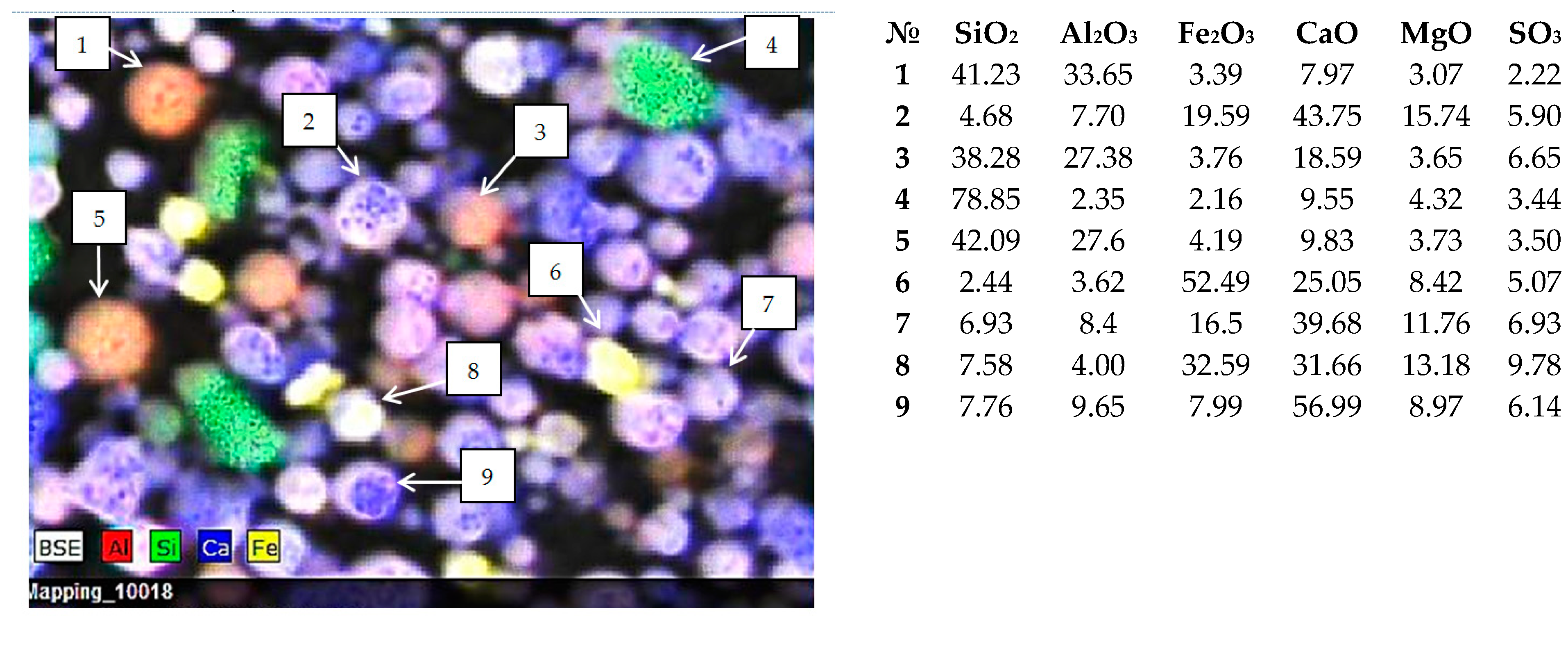
3.2. Single-Particle SEM-EDS Analysis
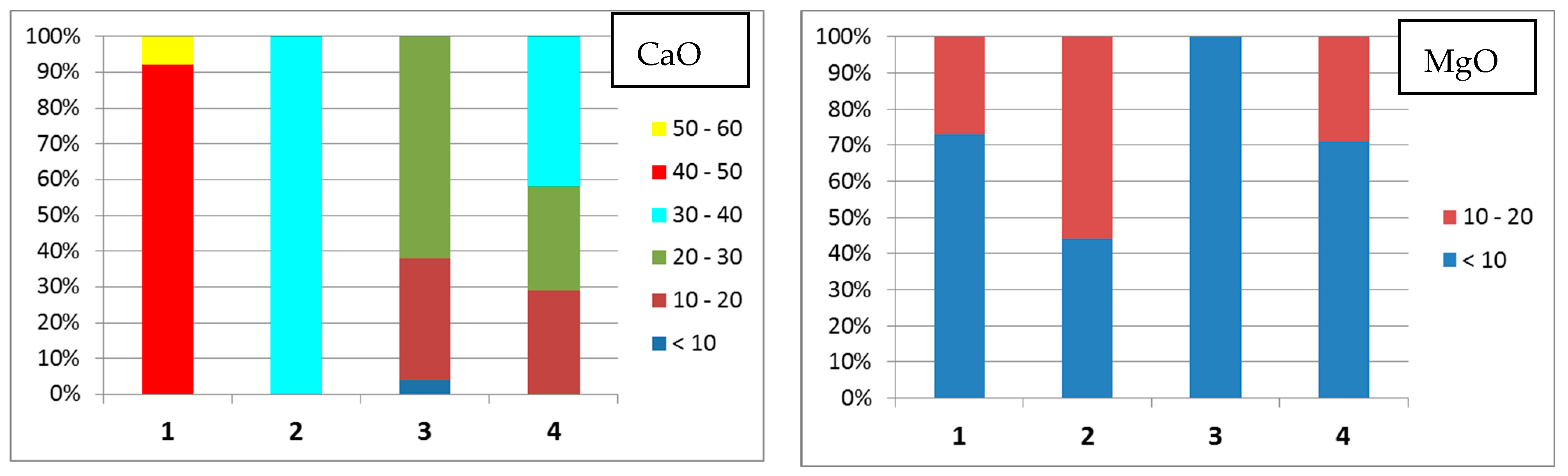
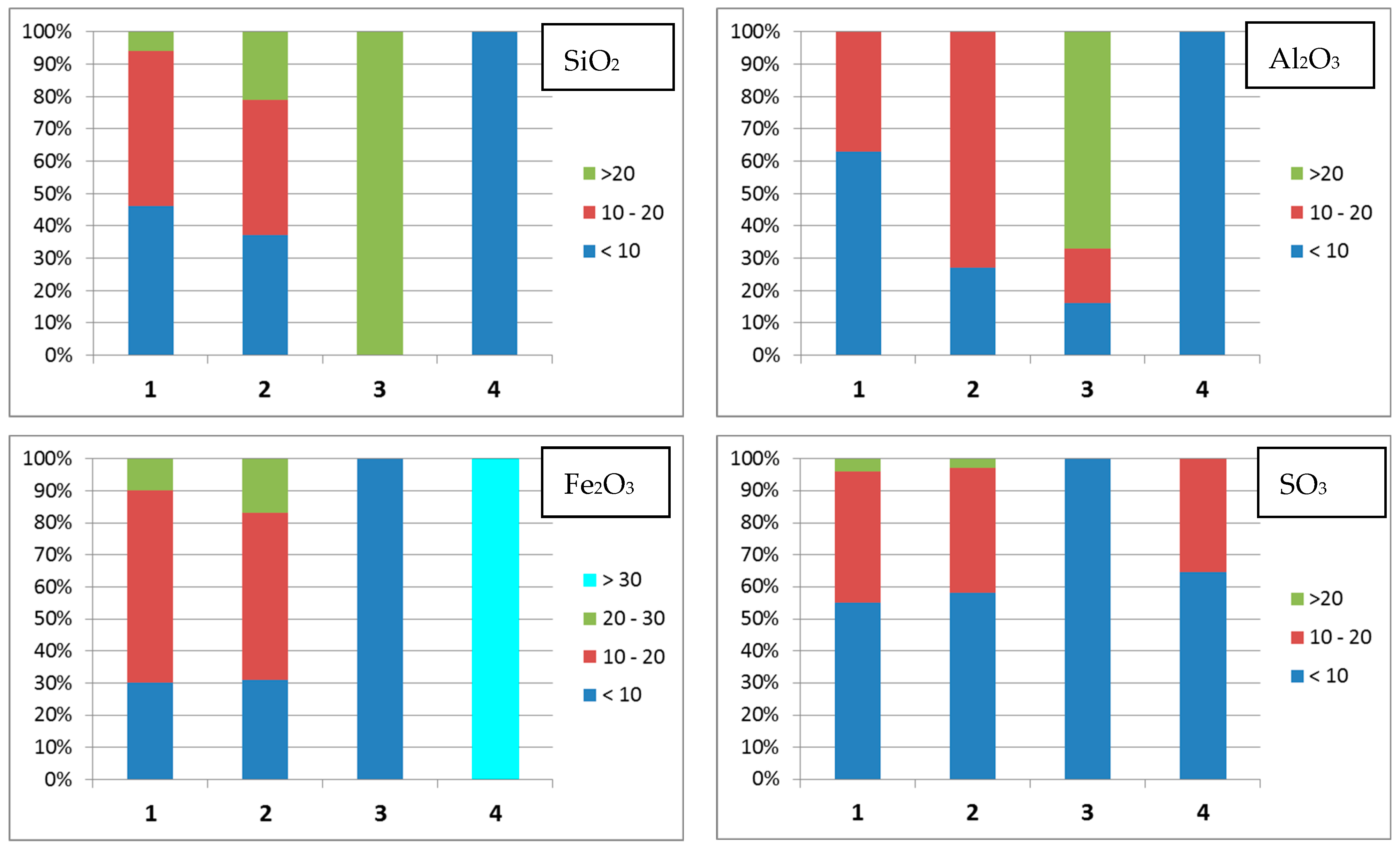
- Group 1 includes the microspheres with CaO content > 40 wt % and SiO2 + Al2O3 ≤ 35 wt %; 36% of all the studied particles meet these composition criteria;
- Group 2 contains the microspheres with a smaller CaO content compared to that in Group 1 (30–40 wt %) but higher contents of MgO (up to 21 wt %) and Fe2O3 (up to 27 wt %); the silicon and aluminum contents are also increased. The total content of oxides of these elements increases to 40 wt %; this group contains 35% of all microspheres;
- Group 3 consists of microspheres with increased SiO2 and Al2O3 contents; the total content of these oxides in globules significantly increases from 40 to 75 wt %; the content of other major components is considerably lower than that for the Group 1 and Group 2 microspheres: CaO ≤ 30, Fe2O3 < 10, MgO ≤ 10, and SO3 ≤ 10 wt %; the contents of Na2O and K2O noticeably increase to reach 11 and 4 wt %, respectively; 25% of microspheres have this composition;
- Group 4 contains microspheres with a high Fe2O3 content (30–60 wt %) and with a reduced content of SiO2 and Al2O3 (SiO2 + Al2O3 < 14 wt %), MgO ≤ 14 wt %; it is the smallest group that includes 4% of all the studied particles.
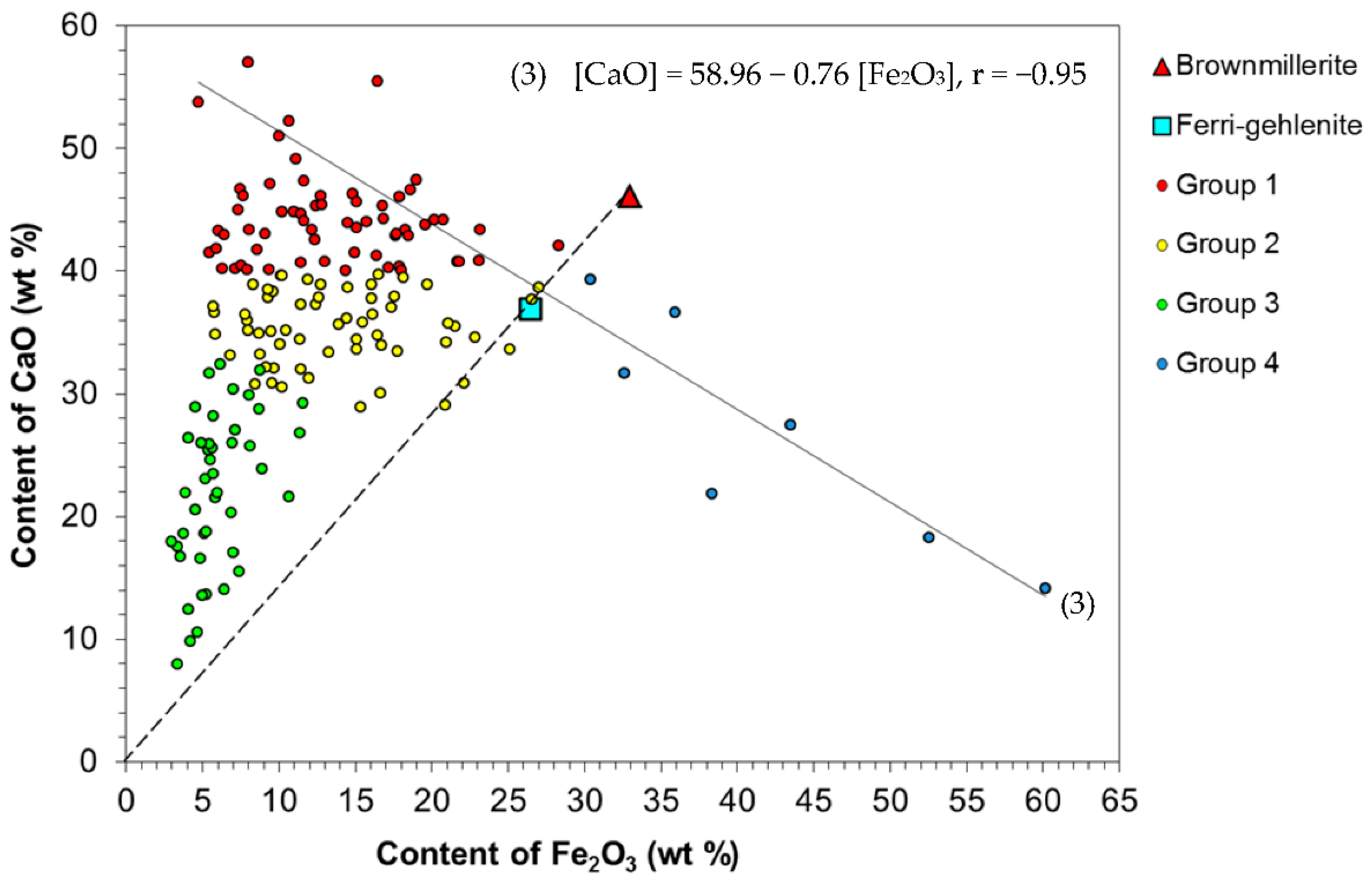
3.3. Identification of Mineral Precursors
4. Conclusions
- A systematic study of the compositions of dispersed PM1-2 microspheres formed in high-calcium fly ash during Irsha–Borodinsky lignite combustion was conducted for the first time. Individual microspheres (sized 1–2 μm) of the narrow fraction of the fly ash sampled from the fourth field of the electrostatic precipitator at the Krasnoyarsk TPP-2 burning coal mined at the Kansk–Achinsk basin (Russia) were analyzed by scanning electron microscopy and energy-dispersive X-ray spectroscopy.
- CaO, SiO2, Al2O3, Fe2O3, and MgO were found to be the major components of the analyzed microspheres; their gross content ranges from 75–95 wt %. The compositions of all the microspheres under study follow the general dependence with a high correlation coefficient: [SiO2 + Al2O3] = 88.80 − 1.02 [CaO + Fe2O3 + MgO], r = −0.97. The formation pathway for fine microspheres with different compositions is parallel to the general trend: anorthite CaAlSi2O8; gehlenite Ca2Al2SiO7; esseneite CaFeAlSiO6; tricalcium aluminate Ca3Al2O6; ferrigehlenite Ca2FeAlSiO7; and brownmillerite Ca4Al2Fe2O10.
- The microspheres were classified into four groups depending on the content of major components: Group 1 (CaO > 40, SiO2 + Al2O3 ≤ 35, Fe2O3 < 23, MgO < 16 wt %) and Group 2 (30 < CaO < 40, SiO2 + Al2O3 ≤ 40, Fe2O3 < 27, MgO < 21 wt %) contain a considerable portion of PM1-2 (71% of globules), which are characterized by a high CaO content (30–57 wt %); Group 3 (CaO ≤ 30, 40 ≤ SiO2 + Al2O3 ≤ 75, Fe2O3 < 10, MgO < 10 wt %) contains 25% of microspheres with a typically increased content of aluminosilicate components and alkali metal oxides; and the smallest Group 4 contains 4% of microspheres with a high Fe2O3 content (30–60 wt %).
- A comparative analysis of the relationship between major component concentrations suggests the routes of formation of environmentally hazardous PM1-2 from feldspars and Ca–, Mg–, and Fe–humate complexes during lignite combustion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.worldcoal.org/coal-facts/coal-electricity/ (accessed on 1 July 2022).
- Bryers, R.W. Fireside slagging, fouling, and high-temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels. Prog. Energy Combust. Sci. 1996, 22, 29–120. [Google Scholar] [CrossRef]
- Raask, E. Mineral Impurities in Coal Combustion: Behavior, Problems and Remedial Measures; Taylor & Francis: Sydney, Australia, 1985. [Google Scholar]
- Xu, M.; Yu, D.; Yao, H.; Liu, X.; Qiao, Y. Coal combustion-generated aerosols: Formation and properties. Proc. Combust. Inst. 2011, 33, 1681–1697. [Google Scholar] [CrossRef]
- Sloss, L.L. The Importance of PM10/2.5 Emissions; IEA Clean Coal Centre: London, UK, 2004. [Google Scholar] [CrossRef]
- Smith, K.R.; Veranth, J.M.; Hu, A.A.; Lighty, J.S.; Aust, A.E. Interleukin-8 Levels in Human Lung Epithelial Cells Are Increased in Response to Coal Fly Ash and Vary with the Bioavailability of Iron, as a Function of Particle Size and Source of Coal. Chem. Res. Toxicol. 2000, 13, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, C.; Wang, Z.; Pang, X.; Zhong, Y.; Han, X.; Ning, P. Chemical composition and source apportionment of PM2.5 in a border city in southwest China. Atmosphere 2021, 13, 7. [Google Scholar] [CrossRef]
- Neville, M.; Quann, R.; Haynes, B.; Sarofim, A. Vaporization and condensation of mineral matter during pulverized coal combustion. Symp. (Int.) Combust. 1981, 18, 1267–1274. [Google Scholar] [CrossRef]
- Flagan, R.C.; Friedlander, S.K. Particle formation in pulverized coal combustion: A review. Recent Dev. Aerosol Sci. 1978, 2, 25–59. [Google Scholar]
- Linak, W.P.; Miller, C.A.; Seames, W.S.; Wendt, J.O.; Ishinomori, T.; Endo, Y.; Miyamae, S. On trimodal particle size distributions in fly ash from pulverized-coal combustion. Proc. Combust. Inst. 2002, 29, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Seames, W.S. An initial study of the fine fragmentation fly ash particle mode generated during pulverized coal combustion. Fuel Process. Technol. 2003, 81, 109–125. [Google Scholar] [CrossRef]
- Buhre, B.; Hinkley, J.; Gupta, R.; Nelson, P.; Wall, T. Fine ash formation during combustion of pulverised coal–coal property impacts. Fuel 2006, 85, 185–193. [Google Scholar] [CrossRef]
- Yan, L.; Gupta, R.; Wall, T. The implication of mineral coalescence behaviour on ash formation and ash deposition during pulverised coal combustion. Fuel 2001, 80, 1333–1340. [Google Scholar] [CrossRef]
- Yu, D.; Xu, M.; Yao, H.; Liu, X.; Zhou, K.; Li, L.; Wen, C. Mechanisms of the central mode particle formation during pulverized coal combustion. Proc. Combust. Inst. 2008, 32, 2075–2082. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, D.; Luo, G.; Yao, H. Temperature Effect on Central-Mode Particulate Matter Formation in Combustion of Coals with Different Mineral Compositions. Energy Fuels 2015, 29, 5245–5252. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Sato, A.; Ninomiya, Y.; Yamashita, T. Effects of coal blending on the reduction of PM10 during high-temperature combustion 1. Mineral transformations. Fuel 2008, 87, 2997–3005. [Google Scholar] [CrossRef]
- Shah, K.V.; Cieplik, M.K.; Betrand, C.I.; van de Kamp, W.L.; Vuthaluru, H.B. A kinetic-empirical model for particle size distribution evolution during pulverised fuel combustion. Fuel 2010, 89, 2438–2447. [Google Scholar] [CrossRef]
- Dai, S.; Hower, J.C.; Finkelman, R.B.; Graham, I.T.; French, D.; Ward, C.R.; Eskenazy, G.; Wei, Q.; Zhao, L. Organic associations of non-mineral elements in coal: A review. Int. J. Coal Geol. 2019, 218, 103347. [Google Scholar] [CrossRef]
- Gupta, R.; Wall, T.; Kajigaya, I.; Miyamae, S.; Tsumita, Y. Computer-controlled scanning electron microscopy of minerals in coal—Implications for ash deposition. Prog. Energy Combust. Sci. 1998, 24, 523–543. [Google Scholar] [CrossRef]
- Yan, L.; Gupta, R.; Wall, T. A mathematical model of ash formation during pulverized coal combustion. Fuel 2002, 81, 337–344. [Google Scholar] [CrossRef]
- Fix, G.; Seames, W.; Mann, M.; Benson, S.; Miller, D. The effect of oxygen-to-fuel stoichiometry on coal ash fine-fragmentation mode formation mechanisms. Fuel Process. Technol. 2011, 92, 793–800. [Google Scholar] [CrossRef]
- Fix, G.; Seames, W.; Mann, M.; Benson, S.; Miller, D. The effect of combustion temperature on coal ash fine-fragmentation mode formation mechanisms. Fuel 2013, 113, 140–147. [Google Scholar] [CrossRef]
- Zhang, L.; Ninomiya, Y.; Yamashita, T. Formation of submicron particulate matter (PM1) during coal combustion and influence of reaction temperature. Fuel 2006, 85, 1446–1457. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Sato, A.; Ninomiya, Y.; Yamashita, T. Interactions among inherent minerals during coal combustion and their impacts on the emission of PM10. 2. emission of submicrometer-sized particles. Energy Fuels 2007, 21, 766–777. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, C. Correlation of the sub-micrometer ash yield from pulverized coal combustion with coal ash composition. Energy Fuels 2018, 32, 9961–9970. [Google Scholar] [CrossRef]
- Yu, D.; Xu, M.; Zhang, L.; Yao, H.; Wang, A.Q.; Ninomiya, Y. Computer-controlled scanning electron microscopy (CCSEM) investigation on the heterogeneous nature of mineral matter in six typical chinese coals. Energy Fuels 2006, 21, 468–476. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Sato, A.; Ninomiya, Y.; Yamashita, T. Interactions among inherent minerals during coal combustion and their impacts on the emission of PM10. 1. emission of micrometer-sized particles. Energy Fuels 2007, 21, 756–765. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Garcia-Perez, M.; Wu, H. Roles of inherent fine included mineral particles in the emission of PM10 during pulverized coal combustion. Energy Fuels 2012, 26, 6783–6791. [Google Scholar] [CrossRef]
- Ma, Z.; Bai, J.; Li, W.; Bai, Z.; Kong, L. Mineral transformation in char and its effect on coal char gasification reactivity at high temperatures, Part 1: Mineral transformation in char. Energy Fuels 2013, 27, 4545–4554. [Google Scholar] [CrossRef]
- Gao, X.; Rahim, M.U.; Chen, X.; Wu, H. Significant contribution of organically-bound Mg, Ca, and Fe to inorganic PM10 emission during the combustion of pulverized Victorian brown coal. Fuel 2013, 117, 825–832. [Google Scholar] [CrossRef]
- Wen, C.; Gao, X.; Yu, Y.; Wu, J.; Xu, M.; Wu, H. Emission of inorganic PM10 from included mineral matter during the combustion of pulverized coals of various ranks. Fuel 2015, 140, 526–530. [Google Scholar] [CrossRef]
- Bykadorov, V.S.; Pekarets, P.A.; Radchenko, G.P.; Ryabokon, N.F.; Tkalich, S.M. Geology of Coal and Combustible Shale Deposits in the USSR; Nedra: Moscow, Russia, 1964; Volume 8, p. 790. Available online: https://www.geokniga.org/bookfiles/geokniga-geologiya-mestorozhdeniy-uglya-i-goryuchih-slancev-sssr-tom-8.pdf (accessed on 1 July 2022).
- Kuchumova, A. Krasnoyarsk HPP-2: We Speak and Show; Industrial pages of Siberia: Krasnoyarsk, Russia, 2017; p. 125. Available online: http://www.epps.ru/journal/detail.php?id=1892 (accessed on 1 July 2022).
- Fomenko, E.V.; Akimochkina, G.V.; Anshits, A.G. Narrow dispersed fractions of high-calcium fly ash produced from the pulverized combustion of irsha-borodinsky coal. Therm. Eng. 2019, 66, 560–568. [Google Scholar] [CrossRef]
- GOST 5382-91; Cements and Materials for Cement Production. Chemical Analysis Methods. Publishing House of Standards: Moscow, Russia, 1991. Available online: http://docs.cntd.ru/document/901704800 (accessed on 1 July 2022).
- Fomenko, E.; Anshits, N.; Solovyov, L.; Mikhaylova, O.A.; Anshits, A. Composition and morphology of fly ash cenospheres produced from the combustion of kuznetsk coal. Energy Fuels 2013, 27, 5440–5448. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Tian, C.; Li, H.; Shao, X.; Zheng, C. Mineralogy and Chemical Composition of High-Calcium Fly Ashes and Density Fractions from a Coal-Fired Power Plant in China. Energy Fuels 2010, 24, 834–843. [Google Scholar] [CrossRef]
- Korobetskiy, I.A.; Shpirt, M.Y. Genesis and Properties of Mineral Components of Coals; Nauka. Sib. Otdelenie: Novosibirsk, Russia, 1988; p. 227. [Google Scholar]
- Berezhnoy, A.S. Multicomponent Systems of Oxides; Academy of Sciences Ukr. SSR, Institute of General and Inor-ganic Chemistry; Science Thought: Kyiv, Russia, 1970; p. 544. [Google Scholar]
- Anshits, N.N.; Fedorchak, M.A.; Zhizhaev, A.M.; Anshits, A.G. Composition–structure relationship of skeletal–dendritic ferrospheres formed during industrial combustion of lignite and coal. Energy Fuels 2019, 33, 6788–6796. [Google Scholar] [CrossRef]
- Anshits, N.N.; Fedorchak, M.A.; Fomenko, E.V.; Mazurova, E.V.; Anshits, A.G. Composition, structure, and formation routes of blocklike ferrospheres separated from coal and lignite fly ashes. Energy Fuels 2020, 34, 3743–3754. [Google Scholar] [CrossRef]
- Anshits, N.N.; Fomenko, E.V.; Anshits, A.G. Composition–structure relationship and routes of formation of blocklike ferrospheres produced by pulverized combustion of two coal types. ACS Omega 2021, 6, 26004–26015. [Google Scholar] [CrossRef]
- Anshits, N.N.; Fedorchak, M.A.; Sharonova, O.M.; Kirik, N.P.; Shishkina, N.N.; Zhizhaev, A.M.; Anshits, A.G. Structure–composition Relationship of platelike ferrospheres in calcium-rich power plant ash. Inorg. Mater. 2018, 54, 466–472. [Google Scholar] [CrossRef]
- Matsuoka, K.; Rosyadi, E.; Tomita, A. Mode of occurrence of calcium in various coals. Fuel 2002, 81, 1433–1438. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the combined chemical and mineral classification of the inorganic matter in coal. 1. Chemical and mineral classification systems. Fuel 2009, 88, 235–245. [Google Scholar] [CrossRef]
- Huffman, G.; Huggins, F.; Shah, N.; Shah, A. Behavior of basic elements during coal combustion. Prog. Energy Combust. Sci. 1990, 16, 243–251. [Google Scholar] [CrossRef]
- Zavorin, A.S.; Buvakov, K.V.; Gladkov, V.E.; Krasilnikova, L.G. Identification of mineral macrocomponents of the inorganic part of the Kansk-Achinsk coals. Proc. Tomsk. Polytech. Univ. 2006, 309, 123–129. Available online: https://www.elibrary.ru/download/elibrary_9549097_72993905.pdf (accessed on 1 July 2022).
- Wen, C.; Xu, M.; Zhou, K.; Yu, D.; Zhan, Z.; Mo, X. The melting potential of various ash components generated from coal combustion: Indicated by the circularity of individual particles using CCSEM technology. Fuel Process. Technol. 2015, 133, 128–136. [Google Scholar] [CrossRef]
- Huggins, F.E.; Kosmack, D.A.; Huffman, G.P.; Lee, R.J. Coal mineralogy by SEM analysis. Scanning Electron Microsc. 1980, 1, 531–540. [Google Scholar]
- Zygarlicke, C.J.; Steadman, E.N. Advanced SEM Techniques to Characterize Coal Minerals. Scanning Electron Microsc. 1990, 4, 579–590. Available online: https://digitalcommons.usu.edu/microscopy/vol4/iss3/8 (accessed on 1 July 2022).
- Wen, C.; Gao, X.; Xu, M. A CCSEM study on the transformation of included and excluded minerals during coal devolatilization and char combustion. Fuel 2016, 172, 96–104. [Google Scholar] [CrossRef]
- Wu, H.; Gao, X.; Wee, H.; Ngu, L.-N.; Ninomiya, Y.; Wang, Q. Occurrence and characteristics of abundant fine included mineral particles in Collie coal of Western Australia. Fuel 2018, 216, 53–60. [Google Scholar] [CrossRef]
- Miller, S.F.; Schobert, H.H. Effect of the occurrence and composition of silicate and aluminosilicate compounds on ash formation in pilot-scale combustion of pulverized coal and coal-water slurry fuels. Energy Fuels 1994, 8, 1197–1207. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Vasilieva, N.G.; Mikhaylova, O.A.; Rogovenko, E.S.; Zhizhaev, A.M.; Anshits, A.G. Characterization of fly ash cenospheres produced from the combustion of ekibastuz coal. Energy Fuels 2015, 29, 5390–5403. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Kushnerova, O.A.; Akimochkina, G.V.; Kukhtetskiy, S.V.; Anshits, A.G. Separation of nonmagnetic fine narrow fractions of PM10 from coal fly ash and their characteristics and mineral precursors. Energy Fuels 2019, 33, 3584–3593. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Yumashev, V.V.; Kukhtetskiy, S.V.; Zhizhaev, A.M.; Anshits, A.G. Scanning electron microscopy–energy-dispersive X-ray spectrometry (SEM–EDS) analysis of PM1–2 microspheres located in coal char particles with different morphologies. Energy Fuels 2020, 34, 8848–8856. [Google Scholar] [CrossRef]
- Betekhtin, A.G. Course of Mineralogy; State Publishing House of Geological Literature: Moscow, Russia, 1951; p. 543. [Google Scholar]
- Querol, X.; Turiel, J.L.F.; Soler, A.L. The behaviour of mineral matter during combustion of Spanish subbituminous and brown coals. Miner. Mag. 1994, 58, 119–133. [Google Scholar] [CrossRef]




| Bulk Density, g/cm3 | Particle Size Distribution, µm | ||||||||||
| dav | d10 | d50 | d90 | d99 | |||||||
| 0.89 | 1.6 | 0.5 | 1.3 | 3.1 | 5.4 | ||||||
| Chemical composition, wt % | |||||||||||
| LOI | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | TiO2 | ||
| 5.30 | 13.98 | 9.17 | 13.96 | 38.50 | 8.20 | 0.32 | 0.18 | 9.60 | 0.32 | ||
| Phase composition, wt % | |||||||||||
| glass phase | Ca4Al2Fe2O10 | Ca3Al2O6 | CaSO4 | CaCO3 | CaO | Ca(OH)2 | MgO | quartz | Fe spinel | ||
| 41.3 | 14.5 | 8.7 | 14.2 | 0.9 | 1.6 | 8.6 | 7.0 | 1.5 | 1.7 | ||
| Chemical composition of the glass phase, wt % | |||||||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | TiO2 | |||
| 32.88 | 15.51 | 9.70 | 33.30 | 3.16 | 0.84 | 0.47 | 3.29 | 0.84 | |||
| Group | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | K2O | TiO2 | SiO2/Al2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CaO > 40, SiO2 + Al2O3 ≤ 35 wt %; 64 microspheres | |||||||||
| min | 40.01 | 4.68 | 2.54 | 4.70 | 3.76 | 3.80 | <0.01 | <0.01 | <0.01 | 0.42 |
| max | 56.99 | 29.29 | 17.98 | 23.17 | 16.11 | 22.58 | 2.72 | 0.46 | 2.52 | 4.01 |
| 2 | 30 < CaO < 40, SiO2 + Al2O3 ≤ 40 wt %; 62 microspheres | |||||||||
| min | 30.03 | 3.40 | 2.93 | 5.68 | 5.54 | 3.72 | 0.22 | <0.01 | <0.01 | 0.30 |
| max | 39.68 | 33.26 | 19.75 | 27.04 | 20.75 | 20.67 | 2.78 | 1.31 | 2.00 | 2.38 |
| 3 | CaO ≤ 30, 40 ≤ SiO2 + Al2O3 ≤ 75, Fe2O3 < 10, MgO < 10, SO3 < 10 wt %; 45 microspheres | |||||||||
| min | 7.97 | 23.34 | 2.99 | 2.99 | 2.37 | 2.21 | 0.61 | <0.01 | <0.01 | 1.01 |
| max | 30.37 | 54.72 | 33.65 | 8.87 | 10.09 | 10.09 | 11.00 | 4.18 | 0.87 | 15.54 |
| 4 | 14 < CaO < 40, SiO2 + Al2O3 < 14, Fe2O3 > 30, MgO ≤ 14 wt %; 7 microspheres | |||||||||
| min | 14.11 | 4.71 | 4.00 | 30.39 | 5.89 | 4.58 | 0.99 | <0.01 | 0.05 | 0.70 |
| max | 39.34 | 8.39 | 6.74 | 60.19 | 14.26 | 14.67 | 2.15 | 0.20 | 0.71 | 1.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomenko, E.; Anshits, N.; Akimochkina, G.; Solovyov, L.; Kukhteskiy, S.; Anshits, A. The Composition and Origin of PM1-2 Microspheres in High-Calcium Fly Ash from Pulverized Lignite Combustion. Energies 2022, 15, 5551. https://doi.org/10.3390/en15155551
Fomenko E, Anshits N, Akimochkina G, Solovyov L, Kukhteskiy S, Anshits A. The Composition and Origin of PM1-2 Microspheres in High-Calcium Fly Ash from Pulverized Lignite Combustion. Energies. 2022; 15(15):5551. https://doi.org/10.3390/en15155551
Chicago/Turabian StyleFomenko, Elena, Natalia Anshits, Galina Akimochkina, Leonid Solovyov, Sergey Kukhteskiy, and Alexander Anshits. 2022. "The Composition and Origin of PM1-2 Microspheres in High-Calcium Fly Ash from Pulverized Lignite Combustion" Energies 15, no. 15: 5551. https://doi.org/10.3390/en15155551
APA StyleFomenko, E., Anshits, N., Akimochkina, G., Solovyov, L., Kukhteskiy, S., & Anshits, A. (2022). The Composition and Origin of PM1-2 Microspheres in High-Calcium Fly Ash from Pulverized Lignite Combustion. Energies, 15(15), 5551. https://doi.org/10.3390/en15155551







