1. Introduction
In recent decades, there has been increasing interest in energy conservation technologies and materials. A wide variety of solutions have been created for reducing the cost of energy and limiting the consumption of fossil fuels. Nowadays, research efforts have notably shifted towards energy efficiency and the use of renewable energy sources.
According to the U.S. Energy Information Association [
1], around 40 percent of total U.S. end-use energy consumption in 2020 was related to the residential and commercial building sectors, and approximately one third of this was used for heating, ventilation, and cooling purposes. To increase energy usage efficiency in these applications, various methods have been adopted for heat recovery, as well as for active and passive cooling and heating. Among the most compelling technologies, given their potential for seamless integration and high impact, are Latent Heat Thermal Energy Storage Systems (LHTES), which include phase change materials (PCMs). The increased interest in PCMs as energy storage media is due to their reasonably high thermal energy storing capacity and almost isothermal heat conduction during charge and discharge processes. PCMs can absorb significant amounts of heat while maintaining stable temperatures until the material is fully melted. As soon as the ambient temperature around the material decreases, the PCM start to solidify again and releases the stored latent heat. This operation principle is at the core of the design and development of PCM–air heat exchangers, PCM solar power generators and water heating systems, PCM building envelopes, etc. [
2,
3].
Our research team has been working on a variety of PCM chemical formulations and the development of different types of thermally dynamic building systems, including solar thermal and photovoltaic modules, PCM-enhanced envelopes, space conditioning and water heating systems, etc. A wide diversity of current research efforts have demonstrated that PCMs used in buildings can be effectively utilized to shave and shift time daily peak electricity demands for heating and cooling purposes. Mechanical ventilation systems can be aided by using integrated PCM heat exchangers. In their recent work, Kosny et al. [
4] showed that using passively working PCM in roof-deck-installed thermal disconnect systems can significantly reduce and shift overall and peak roof-generated thermal loads. They also showed substantial reductions in peak roof temperatures, nightly heat losses, and daily heat gains. Kuznik et al. [
5] proposed a methodology that uses dimensionless analysis of the equations describing PCM heat exchanger systems. The approach was used to identify the best PCM for the chosen usage scenario and the configuration of heat storage for the purpose of electricity peak-load shaving. Medrano et al. [
6] experimentally explored five PCM heat exchangers with varying inlet temperatures and flow rates. They identified the best performing among the proposed designs and showed that increasing the inlet temperature closer to the PCM phase change temperature positively affected phase change performance. Lazaro et al. [
7] investigated the efficacy of PCM–air heat exchanger slab designs using real-scale experiments. They showed that an increase of heat exchanger performance can be achieved by design enhancements instead of by the thermal conductivity of the PCM material, as the latter is generally associated with increased PCM cost.
Several recent reports have focused on the numerical investigation of PCM heat exchangers. In numerical investigations, there are two main approaches for modeling phase change in LHTES, using models respectively based either on temperature or on enthalpy as the solution variable. Models based on temperature generally require separate equations for the different phases (solid and liquid) and solid–liquid interface tracking. In contrast, models based on enthalpy typically utilize one energy conservation equation written in terms of enthalpy. Alternatively, the apparent heat capacity method can be used for modeling phase change. This method solves a single energy equation and employs effective material properties to model the phase-change interface. Raj et al. [
8] used this method to perform a computational fFluid dynamics (CFD) analysis of heat transfer and pressure drop in a PCM–air heat exchanger for free convective cooling applications. They experimentally and computationally analyzed the thermal performance of a PCM-enhanced heat-exchanger module, showing that increasing air inlet velocity had a detrimental effect on PCM solidification time. Dolado et al. [
9] characterized a real-scale PCM–air heat exchanger by using computational modeling and experimentation. Phase change was simulated using an experimentally determined enthalpy–temperature curve to model heat storage capacity as a function of temperature. They proved the validity of the model for a wide range of inlet air velocities and temperatures. Also, they showed that PCM charge–discharge times were proportional to the PCM enthalpy values.
This paper presents the computational analysis of five stackable panel PCM–air heat exchanger designs for building applications, and focuses on finding the best performing PCM pouch shapes and placement patterns. A representative design using pyramidical PCM capsules for a stack of four panels is presented in
Figure 1. Air flows through the stacked layers of plastic panels containing arrays of PCM pouches. Air and PCM are not in direct contact, as the PCM material is encased in pouches of various forms, sizes, and relative positions. A thin layer of packaging material makes a barrier between PCM and the air.
The PCM formulation analyzed in this paper was mixture of calcium chloride hexahydrate (CaCl
2·6H
2O) + sodium chloride (NaCl) + strontium chloride (SrCl
2·6H
2O), 93% + 5% + 2% by mass. In the numerical analyses, we used PCM latent enthalpy values obtained by differential scanning calorimeter (DSC) measurements, as depicted in
Figure 2 and in thermal cycles data in
Table 1. The PCM formulation considered in this study exhibited a melting point of 26.2 °C, peak temperature of 29.6 °C, and phase-change enthalpy of around 180 J/g.
The various advantages of the proposed PCM–air heat exchanger include its layered structure, the packing of the PCM material, the modular configuration of the panels, the flexibility to use different plate configurations, and the possibility of mechanical and chemical cleaning of individual panels. This type of heat exchanger design is typically used when overall heat transfer coefficients are low either on one side or both sides of the heat transfer surfaces, such as air in the present PCM–air heat exchanger [
10].
In the present work, we performed three-dimensional (3D) CFD analyses of transient conjugate heat transfer with phase change processes for five different designs of PCM–air heat exchangers. The numerical model included single-phase convection on the gas (air) domain, together with conduction solid-to-liquid phase change (i.e., negligible effect of natural convection) in the PCM domain. Gas flows were set to specified inlet velocities, thermally interacting with the PCM pouches throughout the length of the heat exchanger. In this model we assumed constant properties, which can be considered a limitation of the model as most fluid properties vary with temperature. Nevertheless, given the temperature ranges of interest, the changes in properties with temperature were negligible, and therefore the assumption of constant properties was reasonable. Moreover, since the simulations were time-dependent and 3D, the assumed constancy of material properties drastically reduced the computational cost of the simulations.
In view of the goal of determining the optimal PCM–air heat exchanger design configuration from among the five considered, we first investigated isothermal pressure drops for various inlet velocities. Subsequently, fully-coupled thermal-fluid simulations with conjugate heat transfer and non-isothermal incompressible flow were employed to analyze the thermal performance of the different PCM–air heat exchanger configurations, to assess their performance.
2. Materials and Methods
In the building applications under consideration, the heat exchangerconsisted of multiple layers of PCM panels stacked on top of each other, as depicted in
Figure 1b. In each panel, PCM was packaged inside equilateral square pyramids with a truncated apex (frustum), or cylinders, and encased with plastic material. For all considered pyramidical and cylindrical pouch designs, the height of PCM varied between 6.35 and 17.78 mm (i.e., ¼ and 0.7 inches). In the pyramidical configurations, the distance between two frustums, base length, and the angle between the base and lateral surface were constant and were equal to 4.78 mm (0.19 inches), 38.1 mm (1.5 inches), and 45 degrees, correspondingly. Complete schematics and dimensions of the PCM panels for the pyramidical (PY) configuration are presented in
Figure 3.
For the other pyramidical designs, namely pyramidical with offset (PYO) and pyramidical rotated (PYR), the same values were used for pouch height, base length, angle between base and lateral sides, and inter-frustum distance. In the case of the PYO design, each even row was shifted in the direction perpendicular to the inlet flow, by the base length of the pyramidical pouch divided by two. For the PYR design, each pouch was rotated 45 degrees clockwise, using the vector normal to the panel as the axis of rotation. For the cylindrical designs (CY and CYO), the diameter of the pouches was equal to the base length of the pyramidical pouches, i.e., 38.1 mm (1.5 inches), and the cylinders were offset by the same length as in the PYO.
The initial computational assessment of the PCM pouch design was based on thermal simulations where PCM was initially solid at a temperature of 20 °C. Inlet air was at a temperature of 35 °C, higher than the melting temperature of PCM. Upon entering the domain, the air stream heated the PCM pouches, with their subsequent phase change occurring in the temperature range of 23 to 31 °C, and latent heat of melting of 180 kJ/kg (from the DSC analysis, see
Figure 2). The density of the PCM in the solid phase was 1.71 × 10
3 kg/m
3 and the specific heat at constant pressure was 1.6 kJ/kg/K; values for the liquid phase were 1.56 × 10
3 kg/m
3 and 1.9 kJ/kg/K, respectively. Other assumptions used in the computational model included:
Air flow was incompressible due to the small variations of density for the considered inlet velocities.
Material properties were constant for the liquid and solid phases of the PCM.
Natural (free) convection within the encapsulated PCM was considered negligible, hence the pouches experienced heat conduction-dominated phase change.
Five PCM-–air heat exchanger panel designs were considered, namely pyramidical (PY), pyramidical with offset (PYO), pyramidical rotated (PYR), cylindrical (CY) and cylindrical with offset (CYO), and their schematics are shown in
Figure 4.
Table 2 summarizes the key geometric characteristics of the PCM pouches, including the volume of PCM within a pouch, the surface areas exposed to air, and the ratio of volume to heat-exchange surface area.
The computational analysis framework was composed of two coupled models: laminar incompressible non-isothermal flow, and conjugate heat transfer between fluid and PCM. The models describe the flow of air and its interaction with the PCM pouches leading to the thermal evolution (temperature and phase fraction) of the PCM–air heat exchanger.
The flow of air is described by the equations of mass conservation for incompressible flow, momentum conservation, and energy conservation. These are given by:
where ρ,
u, p,
τ, c
p, T,
q are mass density, velocity, pressure, stress tensor, specific heat at constant pressure, temperature, and heat flux, respectively. The stress tensor in the case of Newtonian fluid is given by:
and the heat flux is described using Fourier’s law:
where κ is thermal conductivity. In the energy conservation equation, we neglected thermal expansion, viscous dissipation, and pressure work. The values of transport and material properties used in simulations are listed in
Table 3.
The first phase of the computational analysis was constituted by steady-state simulations under isothermal conditions (i.e., Equations (1) and (2) only) in order to determine the pressure drop across a PCM–air heat exchanger panel. The second phase of the analysis consisted of simulations of coupled conjugate heat transfer with phase change and incompressible fluid flow in order to assess the thermal performance of the designs.
Because our model was time-dependent and 3D, for the reduction of computational cost we neglected convective motion caused by buoyancy. The thermal evolution of the PCM can be described by the nonlinear heat conduction model given by:
where the heat capacity c
p and thermal conductivity κ are (nonlinear) functions of temperature T. To describe phase change within the PCM pouches, we use the apparent heat capacity formulation. This formulation assumes that specific heat is equal to the phase-fraction-averaged specific heats plus the latent heat of melting multiplied by the temperature derivative of the mass fraction, namely:
In the above expressions, quantities with subscripts s and l correspond to values in solid and liquid states, respectively, and θ denotes phase fraction. We have only two phases:
Thus, the mass fraction is given by:
whereas effective thermal conductivity is also phase fraction averaged:
Equations (6)–(11) describe heat transfer and phase change in the PCM part of the simulation domain, and Equations (1)–(5) model fluid flow and conjugate heat transfer in the air part.
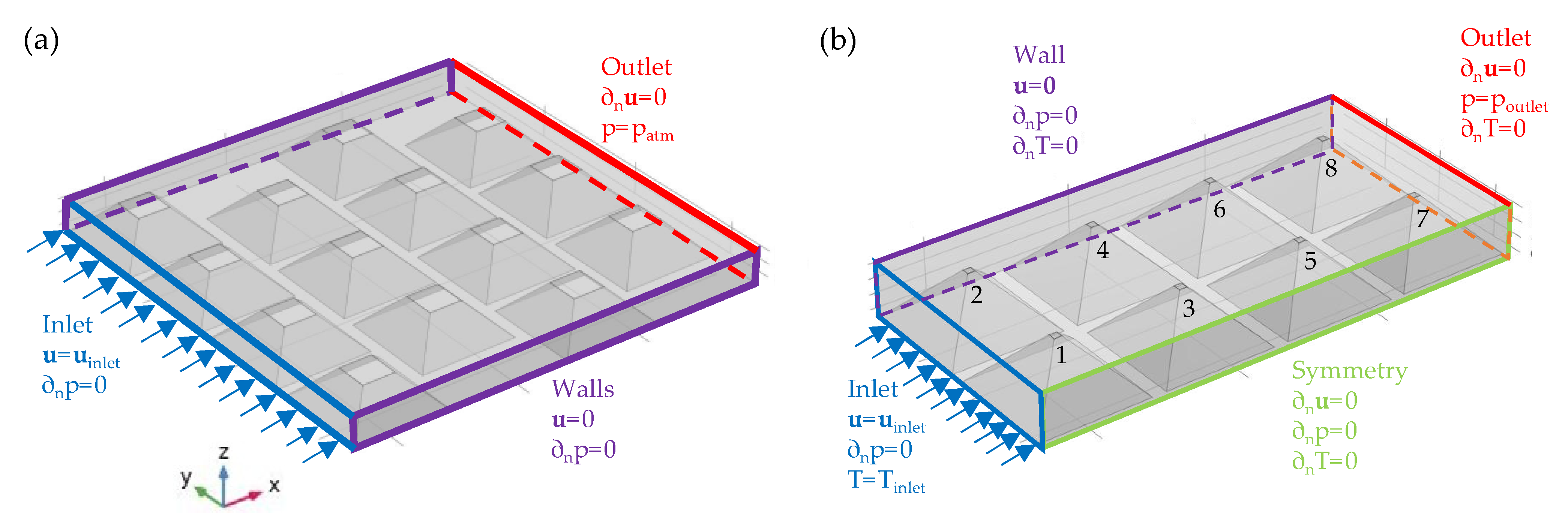
For the first set of simulations, we modelled the full 3D domain of the PCM–air heat exchanger. The computational domain and the set of boundary conditions used are schematically depicted in
Figure 5. In the second set of simulations, we used a symmetry plane in the direction of air flow and simulated only half of the domain to decrease the computational cost for the coupled fluid flow and conjugate heat transfer simulations.
The boundary conditions used in the set of numerical experiments are listed in
Table 4. In the simulations, we set inlet velocities and temperatures at fixed values with zero pressure gradient. For the outflow boundary, zero gradient boundary conditions were used for velocity and temperature, while the value of pressure was specified equal to 1 atm. At the wall boundaries, velocity was set to zero (i.e., no-slip condition) and zero gradients were used for temperature and pressure. The same values were used on the surfaces of the PCM pouches, except for temperature, for which the value was coupled to the temperature of the air following the conjugate transfer model.
Model set-up, geometry specification, meshing, and analysis were performed using COMSOL
® software [
15], specifically its Heat Transfer module. COMSOL is widely used for the computational modeling of various coupled physics problems, including conjugate heat transfer and phase change. There are numerous studies that verify and validate COMSOL’s Heat Transfer module for these purposes. Kylili et al. [
16] used it to characterize the thermal performance of PCM-enhanced building elements, validating their results with experiments. Kant et al. [
17] used and validated COMSOL for the prediction of melting–solidification of five different fatty acids in an aluminum container. Moreover, COMSOL has been validated with PCM heat transfer problems similar to the present study [
18,
19,
20]. COMSOL employs the finite element method (FEM) for solving the set of partial differential equations comprising the model. COMSOL’s capability of coupling and modeling different multiphysics makes it a convenient and highly flexible CFD tool. Because coupled 3D simulations are computationally intensive to speed-up simulations, the client-server capability of COMSOL to run computations on a remote high-performance cluster was used. Simulations were run for 10 h of simulation time using 15 cores and took several hours of wall-clock time on average.
4. Discussion
In this work, we performed computational optimization analysis for several PCM–air heat exchanger designs. The analysis consisted of two parts: isothermal incompressible steady-state fluid flow pressure drop, and transient conjugated heat transfer with phase change and coupled non-isothermal flow.
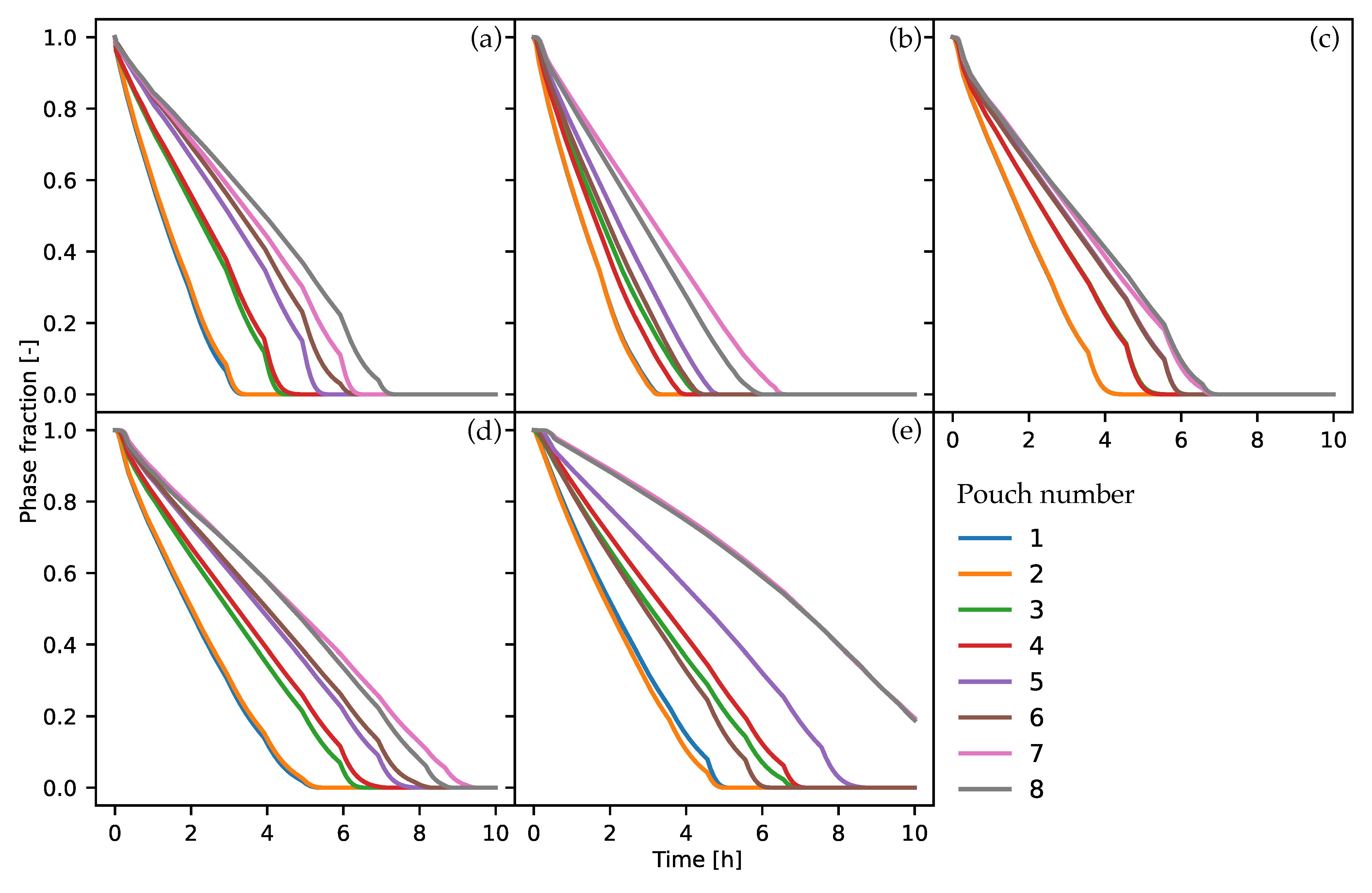
The results revealed that the more distant rows of PCM pouches began phase change much later than the front rows. This fact can be used for to improve proposed PCM–air heat exchanger designs, by placing packaged PCM material with decreasing melting temperatures in each subsequent row. This will allow synchronization of the phase change process in the heat exchanger, thus decreasing the time required for charge and discharge of the PCM panel and thereby increasing its efficiency. The combination of different PCMs with different melting temperatures has proven quite successful in other applications, and can improve the performance of panel PCM–air heat exchangers [
21,
22].
Finally, a lower ratio of PCM volume with surface area exposed to air was associated with faster PCM charging times. In this resecpt, the fastest pyramidical design had a value of 4.51, while cylindrical configurations had a ratio of 9.52. Therefore, to maximize the heat transfer process between the air and the PCM panels, volume-to-surface ratio should be minimized.
From the first part of the optimization analysis, we concluded that in terms of pressure drop and air residence time, the CY design was optimal because it had the lowest value of pressure drop for varying heights, ranging from 27 to 43 Pa for the highest inlet velocity of 2 m/s. The second-best design was the CYO, with values of 28 to 61 Pa for the same velocity range. Similar values for all pyramidical designs (PY, PYR, PYO) were on average higher by the same order of magnitude.
For the second part of the numerical investigation, we determined that the PYO design was optimal in terms of charging speed, as its charging was approximately 51% faster than the CY design. The second-fastest panel was the PYR design, which charged 5% slower than the PYO design and was followed by the PY, CY, and CYO panels. The worst design in terms of charging was the CYO configuration, which had not completed the phase-change process by the end of 10 h simulation time. It is important to notice that the PYO configuration had two fewer PCM pouches, which contributed to the faster completion of its phase change.
5. Conclusions
A parametric study of the design of a panel PCM–air heat exchanger design composed of stackable layers has been presented in this paper. Using CFD modeling, the study focused on evaluating different shapes and patterns of encapsulated PCM pouches packaged in stackable panels. The pouches under consideration had pyramidical or cylindrical shapes and their patterns included regular, regular-rotated, and offset staggered arrays; the shapes and patterns were chosen due to their suitability for mass manufacture. A total of five panel designs were evaluated: pyramidical (PY), pyramidical with offset (PYO), pyramidical rotated (PYR), cylindrical (CY), and cylindrical with offset (CYO).
First, an investigation of steady-state pressure drop as function of height and air inlet velocity was performed for the different designs. The obtained results indicated that the optimal configuration was the CYO, which showed a significantly lower pressure drop than the other designs.
In the second part of the work, transient simulations of coupled conjugate heat transfer with phase change and incompressible fluid flow were carried out to determine the best-performing PCM panel in terms of thermal charging speed, from among the five considered designs. Results show that the PYO design had the fastest charging speed of 6.8 h, followed by the PY, PYR, and CY designs with charging times of 7.1, 7.5 and 9.6 h, respectively. In the case of CYO configuration, the phase-change process had not finished by the end of the 10 h simulation time.
This study can be used as a basis for the design optimization of panel PCM–air heat exchangers. For design optimization purposes, the full charging–discharging cycle should be modeled for a wider range of PCM pouch shapes (e.g., prisms, truncated cones) and placement patterns (e.g., staggered with variable spacing). Additionally, cost benefit analysis should be performed for determining optimal configurations for HVAC and energy-saving applications.