Biomass Behavior upon Fast Pyrolysis in Inert and in CO2-Rich Atmospheres: Role of Lignin, Hemicellulose and Cellulose Content
Abstract
:1. Introduction
- with very fast heating rates (104 K/s);
- at very high temperature (up to 2000 K);
- in inert or CO2 rich atmospheres.
2. Materials and Methods
2.1. Materials
2.2. Thermogravimetric Analysis (TGA) of Raw Samples
2.3. Fast Pyrolysis in N2 and CO2
2.3.1. Heat Treatment in the Heated Strip Reactor (HSR)
2.3.2. Analysis of Primary Tar
2.3.3. Analysis of Char
3. Results
3.1. TG Pyrolysis of Raw Fuels
3.2. Heat Treatment in HRS in N2 and CO2
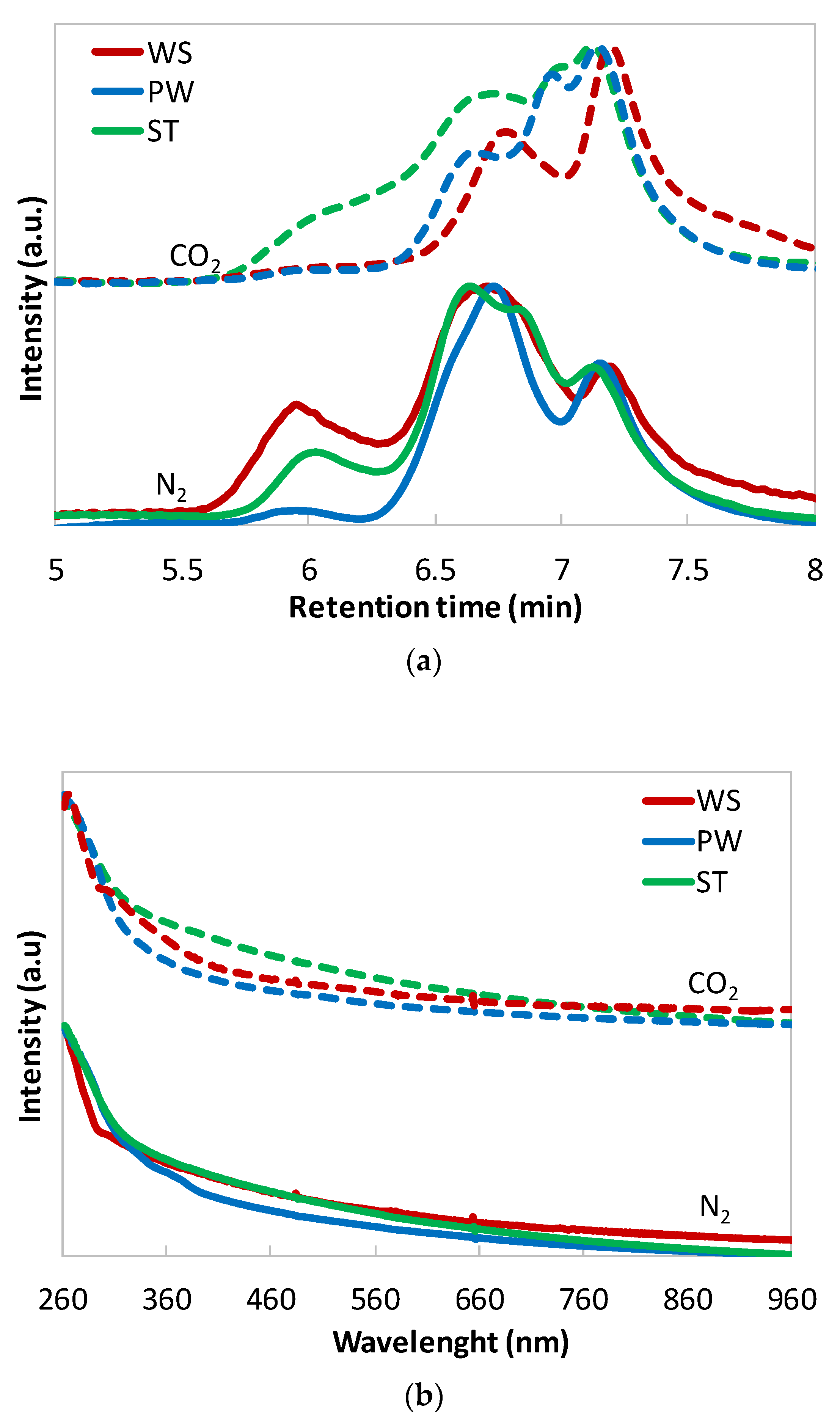
3.2.1. Tar Analysis
3.2.2. Char Analysis
4. Discussion and Conclusions
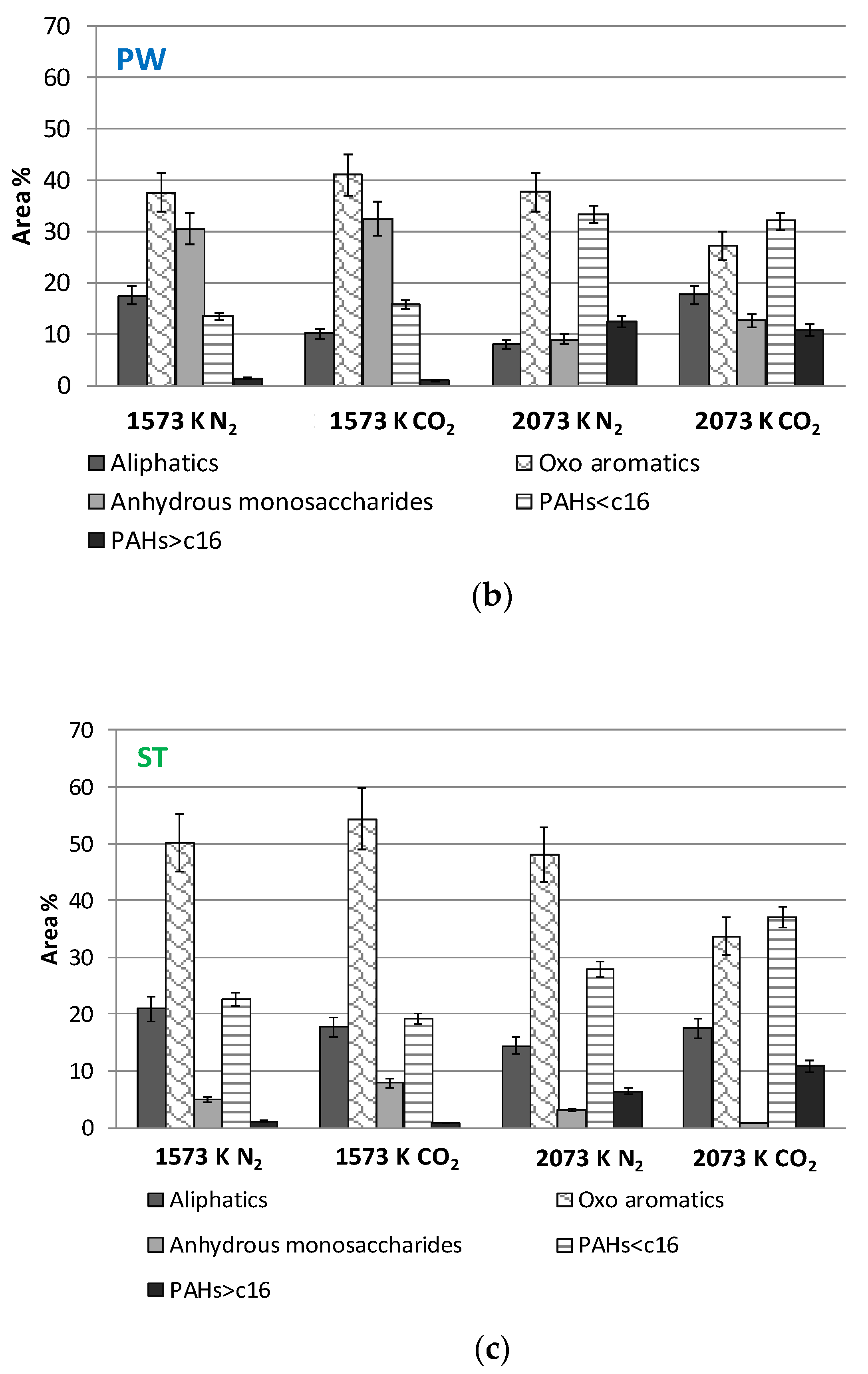
- In ref. [22], anhydrous monosaccharides were found to dominate tar from cellulose and hemicellulose. At odds, in the present work, anhydrous monosaccharides are scarce in ST, the biomass with the highest content of cellulose and hemicelluloses, and most abundant in tar from WS, the biomass with the largest fraction of lignin.
- In ref. [22], PAHs were found to be the most abundant species in tar from lignin pyrolysis, especially in CO2-rich atmospheres. Consistently, in the present work, PAHs are present in tars from all the examined biomasses but are particularly abundant in WS tar.
- In ref. [22], heavy tars were obtained from pyrolysis of both cellulose and lignin at high temperature. Consistently in the present work, they are produced from pyrolysis of all the examined biomasses at high temperature.
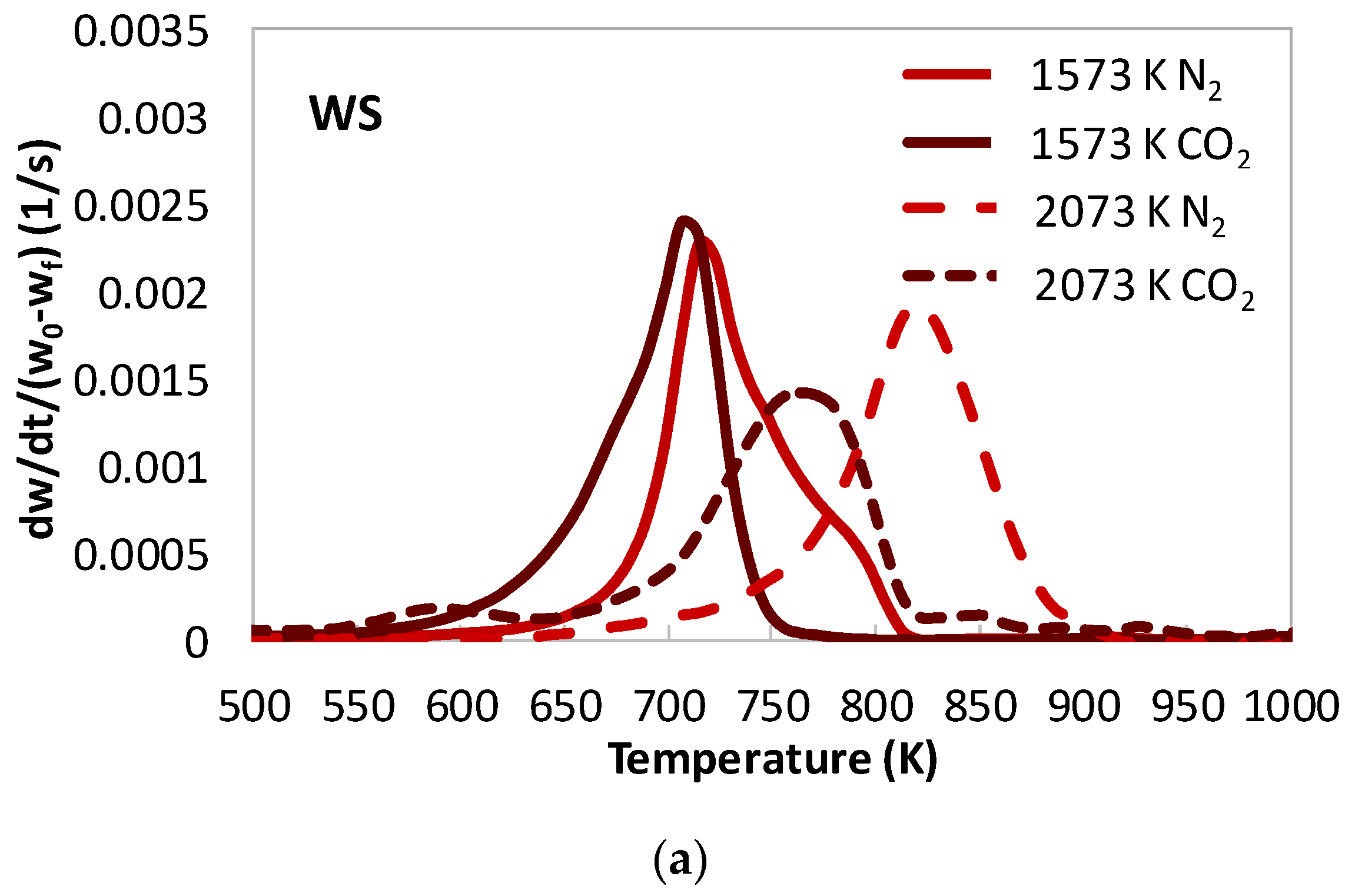
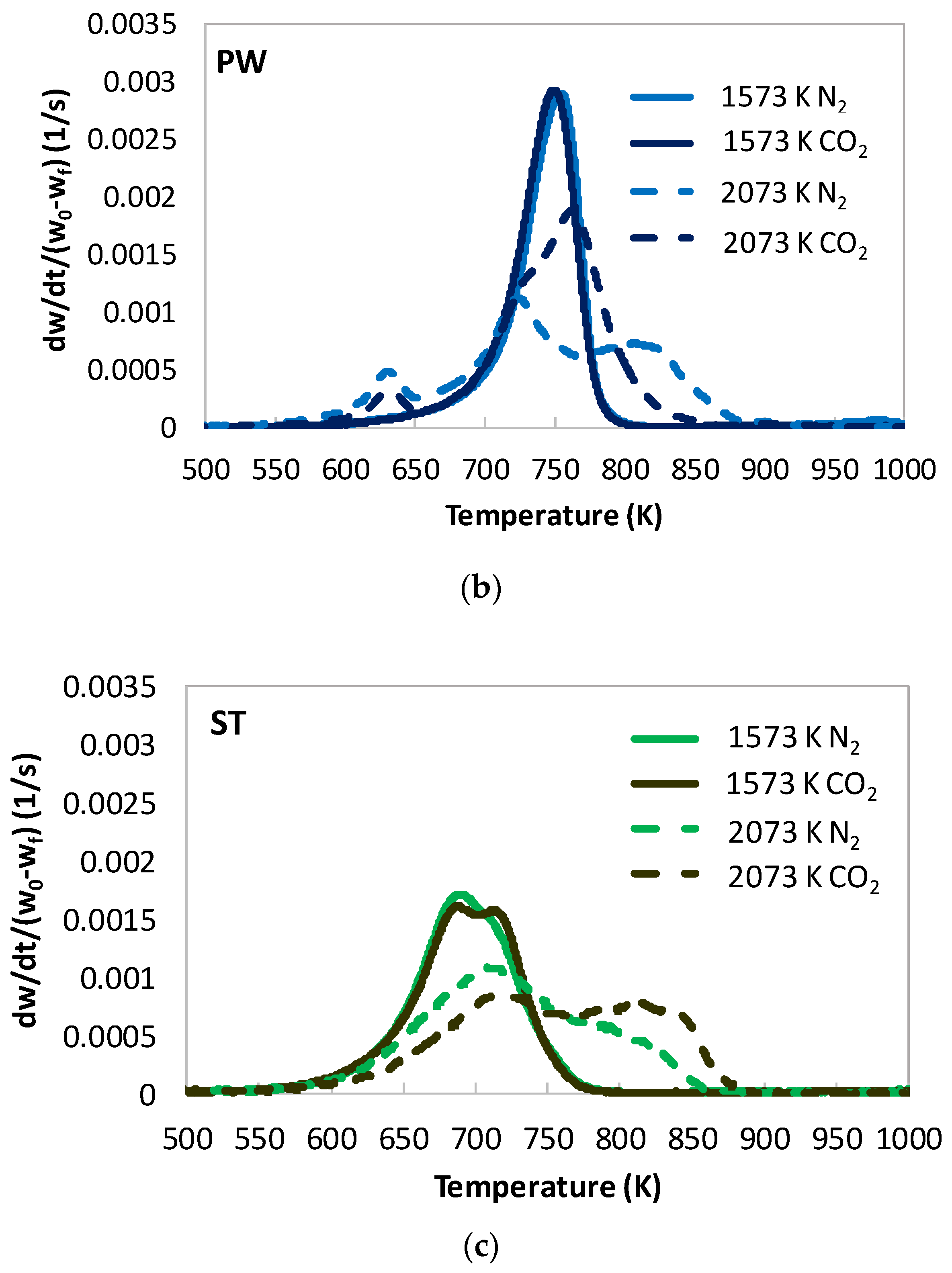
- In [22], cellulose and hemicelluloses did not produce relevant char residues, while lignin did. Its char appeared to be constituted by one or two components. Differently, all the biomasses investigated in the present work produced char. Chars were quite different from each other in terms of combustion reactivity and appeared to be constituted by several components. After heat treatment in N2 at 1573 K, the most reactive char was the one from ST, followed by WS and PW. However, the char of WS, the biomass with larger lignin content, appeared the most similar to the char produced from lignin in [22].
- In [22], lignin char had DTG combustion peaks in the range of 700–770 K. More severe heat treatment resulted in higher peak temperature values (lower combustion reactivity) and a higher degree of structural order/thermal annealing of the char. The presence of CO2 in the atmosphere upon heat treatment, however, hindered thermal annealing of lignin char; in fact, CO2 chars were more reactive than N2 chars. In the present work, severe heat treatment and the presence of CO2 in the atmospheres generated additional char components, which were both more reactive (with peaks at temperature as low as 630 K) and less reactive (with peak temperatures as high as 830 K). It is possible that the newly formed high reactive char component arose from interactions and cross-linking reactions within the solid matrix at the expense of anhydrous monosaccharides (which decrease sensibly in the corresponding tars), whereas the less reactive component arose from thermal annealing of the solid phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, D.; Xiao, R.; Gu, S.; Luo, K. The pyrolytic behavior of Cellulose in lignocellulosic biomass: A review. RSC Adv. 2011, 1, 1641–1660. [Google Scholar] [CrossRef]
- Shen, D.; Gu, S. The mechanism for thermal decomposition of Cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Shen, D.; Gu, S.; Bridgwater, A.V. The thermal performance of the polysaccharides extracted from hardwood: Cellulose and Hemicellulose. Carbohydr. Polym. 2010, 82, 39–45. [Google Scholar] [CrossRef]
- Kawamoto, H.; Morisaki, H.; Saka, S. Secondary decomposition of levoglucosan in pyrolytic production from cellulosic biomass. J. Anal. Appl. Pyrolysis 2009, 85, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Wang, B.; Ji, Y.; Xu, F.; Zong, P.; Zhang, J.; Tian, Y. Thermal decomposition of castor oil, corn starch, soy protein, Lignin, Xylan, and Cellulose during fast pyrolysis. Bioresour. Technol. 2019, 278, 287–295. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Ru, B.; Sun, W.; Wang, Y.; Luo, Z. Comparison of the pyrolysis behavior of pyrolytic Lignin and milled wood Lignin by using TG-FTIR analysis. J. Anal. Appl. Pyrolysis 2014, 108, 78–85. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, Xylan and Lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Shen, D.; Ma, Y.; Gea, X. CO2-looping in biomass pyrolysis or gasification. Sustain. Energy Fuels 2017, 1, 1700–1729. [Google Scholar] [CrossRef]
- Lee, J.; Yang, X.; Cho, S.H.; Kim, J.K.; Lee, S.S.; Tsang, D.C.W.; Ok, Y.S.; Kwon, E.E. Pyrolysis process of agricultural waste using CO2 for waste management energy recovery, and biochar fabrication. Appl. Energy 2017, 185, 214–222. [Google Scholar] [CrossRef]
- Lee, J.; Oh, J.I.; Ok, Y.S.; Kwon, E.E. Study on susceptibility of CO2-assisted pyrolysis of various biomass to CO2. Energy 2017, 137, 510–517. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Wang, G.; He, D.; Shao, S.; Zhang, J.; Zhong, Z. Biomass fast pyrolysis in a fuidized bed reactor under N2, CO2, CO, CH4 and H2 atmospheres. Bioresour. Technol. 2011, 102, 4258–4264. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment-a review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, J.; Tu, M.; Cheng, Y.S. Principles and development of lignocellulosic biomass pretreatment for biofuels. Adv. Bioenergy 2017, 2, 1–68. [Google Scholar]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomasspyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Biswas, B.; Singh, R.; Kumar, J.; Singh, R.; Gupta, P.; Krishna, B.B.; Bhaskar, T. Pyrolysis behavior of ricestraw under carbon dioxide for production of bio-oil. Renew. Energy 2018, 129, 686–694. [Google Scholar] [CrossRef]
- Cho, S.H.; Lee, J.; Kim, K.H.; Jeon, Y.J.; Kwon, E.E. Carbon dioxide assisted co-pyrolysis of coal and lingocellulosic biomass. Energy Convers. Manag. 2016, 118, 243–252. [Google Scholar] [CrossRef]
- Zellagui, S.; Schonnenbeck, C.; Zouaoui-Mahzoul, N.; Leyssens, G.; Authier, O.E.; Thunin, E.; Porcheron, L.; Brilhac, J.F. Pyrolysis of coal and woody biomass under N2 and CO2 atmospheres using a drop tube furnace -experimental study and kinetic modeling. Fuel Process. Technol. 2016, 148, 99–109. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F.; Heuer, S.; Ammendola, P. Slow pyrolysis of walnut shells in nitrogen and carbon dioxide. Fuel 2018, 225, 419–425. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F.; Cortese, L.; Heuer, S.; Schiemann, M.; Scherer, V. Effects of oxy-fuel conditions on walnut shells pyrolysis in a drop tube reactor. Fuel 2018, 229, 235–240. [Google Scholar] [CrossRef]
- Senneca, O.; Apicella, B.; Russo, C.; Cerciello, F.; Salatino, P.; Heuer, S.; Wutscher, A.; Schiemann, M.; Muhler, M.; Scherer, V. Pyrolysis and Thermal Annealing of Coal and Biomass in CO2 Rich Atmospheres. Energy Fuels 2018, 32, 10701–10708. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F.; Russo, C.; Wütscher, A.; Muhler, M.; Apicella, B. Thermal treatment on lignin, cellulose and hemicellulose in nitrogen and carbon dioxide. Fuel 2020, 271, 117656. [Google Scholar] [CrossRef]
- Arendt, P.; Jensen, A.D.; Jensen, A.D.; Garcia Llamas, A.D.; Umeki, K.; Glarborg, P. Effect of fast pyrolysis conditions on biomass solid residues at high temperatures. Fuel Proc. Technol. 2016, 43, 118–129. [Google Scholar]
- Senneca, O.; Urciuolo, M.; Chirone, R.; Cumbo, D. An experimental study of fragmentation of coals during fast pyrolysis at high temperature and pressure. Fuel 2011, 90, 2931–2938. [Google Scholar] [CrossRef]
- Apicella, B.; Senneca, O.; Russo, C.; Heuer, S.; Cortese, L.; Cerciello, F.; Scherer, V.; Schiemann, M.; Ciajolo, A. Separation and characterization of carbonaceous particulate (soot and char) produced from fast pyrolysis of coal in inert and CO2 atmospheres. Fuel 2017, 201, 118–123. [Google Scholar] [CrossRef]
- Senneca, O.; Chirone, R.; Salatino, P. Oxidative pyrolysis of solid fuels. J. Anal. Appl. Pyrolysis 2004, 71, 959–970. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Lu, Y.; He, Q.; Fan, G.; Cheng, Q.; Song, G. Extraction and modification of hemicellulose from lignocellulosic biomass: A review. Green Process. Synth. 2021, 10, 779–804. [Google Scholar] [CrossRef]
- Huang, D.; Li, R.; Xu, P.; Li, T.; Deng, R.; Chen, S.; Zhang, Q. The cornerstone of realizing lignin value-addition: Exploiting the native structure and properties of lignin by extraction methods. Chem. Eng. J. 2020, 402, 126237. [Google Scholar] [CrossRef]




| Volatiledb | Fix Carbondb | Ashdb | Cdb | Hdb | Ndb | |
|---|---|---|---|---|---|---|
| WS | 81.1 | 18.5 | 0.4 | 51.9 | 5.7 | 0.3 |
| PW | 83.1 | 16.8 | 0.1 | 49.3 | 6.6 | b.i.s. 1 |
| ST | 77.8 | 16.2 | 6.1 | 45.9 | 6.5 | 0.6 |
| Hemicelluloses (%) | Cellulose (%) | Lignin (%) | ||||
| WS | 22.2 | 25.5 | 52.3 | |||
| PW 2 | 17.8 | 38.3 | 31.4 | |||
| ST | 40.8 | 29.8 | 19.4 | |||
| Samples | Raw Biomass | HSR 1573 K N2 | HSR 1573 K CO2 | HSR 2073 K N2 | HSR 2073 K CO2 |
|---|---|---|---|---|---|
| Ashdry (%) | |||||
| WS | 0.4 (±0.1) | 5.8 (±0.6) | 8.8 (±1) | 5.4 (±0.6) | 17 (±2) |
| PW | 0.1 (±0.3) | 3.9 (±0.4) | 4.3 (±0.4) | 4.4 (±0.4) | 4.9 (±0.5) |
| ST | 6.1 (±0.5) | 29.8 (±3) | 32.2 (±3) | 36.8 (±4) | 42.8 (±4) |
| DTG peaks (K, ±5) | |||||
| WS | 520; 560; 690 | 720 | 650; 710 | 820 | 760 |
| PW | 590; 720 | 750 | 750 (±5) | 630; 720 *; 760 | 630; 720; 810 |
| ST | 530; 680 | 690; 710 * | 690; 710 | 710; 810 | 810 |
| Atmosphere | T (K) | Light Tar (GC–MS) | Heavy Tar (SEC) | Char DTG Peaks (K) |
|---|---|---|---|---|
| Walnut Shells | ||||
| N2 | 1573 | Anhydrous monosaccharides: 40% Oxo-aromatics: 40% Aliphatics: 10% Light PAHs: 10% | None | 720 |
| CO2 | 1573 | 650; 710 | ||
| N2 | 2073 | Heavy PAHs: 60% Light PAHs: 30% Anhydrous monosaccharides: 5% Oxo-aromatics: 5% | Trimodal distribution (higher MW) | 820 |
| CO2 | 2073 | Light PAHs: 55% Oxo-aromatics: 20% Heavy PAHs: 15% Anhydrous monosaccharides: 10% | 760 | |
| Pinewood | ||||
| N2 | 1573 | Oxo-aromatics: 40% Anhydrous monosaccharides 30% Aliphatics:15% Light PAHs: 15% | None | 750 |
| CO2 | 1573 | |||
| N2 | 2073 | Oxo-aromatics:40% Light PAHs: 30% Heavy PAHs: 10% Anhydrous monosaccharides 10% Aliphatics 10% | Trimodal distribution (higher MW) | 630; 720; 760 |
| CO2 | 2073 | Oxo-aromatics:30% Light PAHs: 30% Aliphatics: 20% Heavy PAHs: 10% Anhydrous monosaccharides: 10% | 630; 720; 810 | |
| Straw | ||||
| N2 | 1573 | Oxo-aromatics: 50% Light PAHs: 20% Aliphatics: 20% Anhydrous monosaccharides: 10% | None | |
| CO2 | 1573 | 690; 710 | ||
| N2 | 2073 | Oxo-aromatics: 50% Light PAHs: 30% Aliphatics: 15% Heavy PAHs: 5% | Trimodal distribution (higher MW) | 710; 810 |
| CO2 | 2073 | Light PAHs: 40% Oxo-aromatics: 35% Aliphatics: 15% Heavy PAHs: 10% | Trimodal distribution (higher MW) | 810 |
| Atmosphere | -T (K) | Light Tar (GC–MS) | Heavy Tar (SEC) | Char DTG Peaks (K) |
|---|---|---|---|---|
| Cellulose * | ||||
| N2 | 1573 | Anhydrous monosaccharides >90% | None | None |
| CO2 | 1573 | |||
| N2 | 2073 | Trimodal distribution (higher MW) | ||
| CO2 | 2073 | |||
| Hemicellulose (Xylan) ** | ||||
| N2 | 1573 | Anhydrous monosaccharides >90% | None | None |
| CO2 | 1573 | |||
| N2 | 2073 | |||
| CO2 | 2073 | |||
| Lignin *** | ||||
| N2 | 1573 | Oxo-aromatics: 50% Anhydrous monosaccharides: 30% Light PAHs: 20% | Bimodal distribution | 700; 750 |
| CO2 | 1573 | Anhydrous monosaccharides: 40% Oxo-aromatics: 30% Light PAHs: 30% | 700 | |
| N2 | 2073 | Anhydrous monosaccharides:60% Aliphatics: 20% Light PAHs: 15% Oxo-aromatics: 10% Heavy PAHs: 5% | Trimodal distribution (higher MW) | 760 |
| CO2 | 2073 | Light PAHs: 40% Oxo-aromatics: 30% Aliphatics: 20% Anhydrous monosaccharides:10% | Bimodal distribution | 700; 720 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senneca, O.; Apicella, B.; Russo, C.; Cerciello, F. Biomass Behavior upon Fast Pyrolysis in Inert and in CO2-Rich Atmospheres: Role of Lignin, Hemicellulose and Cellulose Content. Energies 2022, 15, 5430. https://doi.org/10.3390/en15155430
Senneca O, Apicella B, Russo C, Cerciello F. Biomass Behavior upon Fast Pyrolysis in Inert and in CO2-Rich Atmospheres: Role of Lignin, Hemicellulose and Cellulose Content. Energies. 2022; 15(15):5430. https://doi.org/10.3390/en15155430
Chicago/Turabian StyleSenneca, Osvalda, Barbara Apicella, Carmela Russo, and Francesca Cerciello. 2022. "Biomass Behavior upon Fast Pyrolysis in Inert and in CO2-Rich Atmospheres: Role of Lignin, Hemicellulose and Cellulose Content" Energies 15, no. 15: 5430. https://doi.org/10.3390/en15155430
APA StyleSenneca, O., Apicella, B., Russo, C., & Cerciello, F. (2022). Biomass Behavior upon Fast Pyrolysis in Inert and in CO2-Rich Atmospheres: Role of Lignin, Hemicellulose and Cellulose Content. Energies, 15(15), 5430. https://doi.org/10.3390/en15155430








