Individual Phenolic Acids in Distillery Stillage Inhibit Its Biomethanization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Substrate
2.2. Experimental Design for Methane Fermentation
2.3. Analytical Methods
2.4. Abundance of Methanogenic Microorganisms
2.5. Data Analysis
3. Results and Discussion
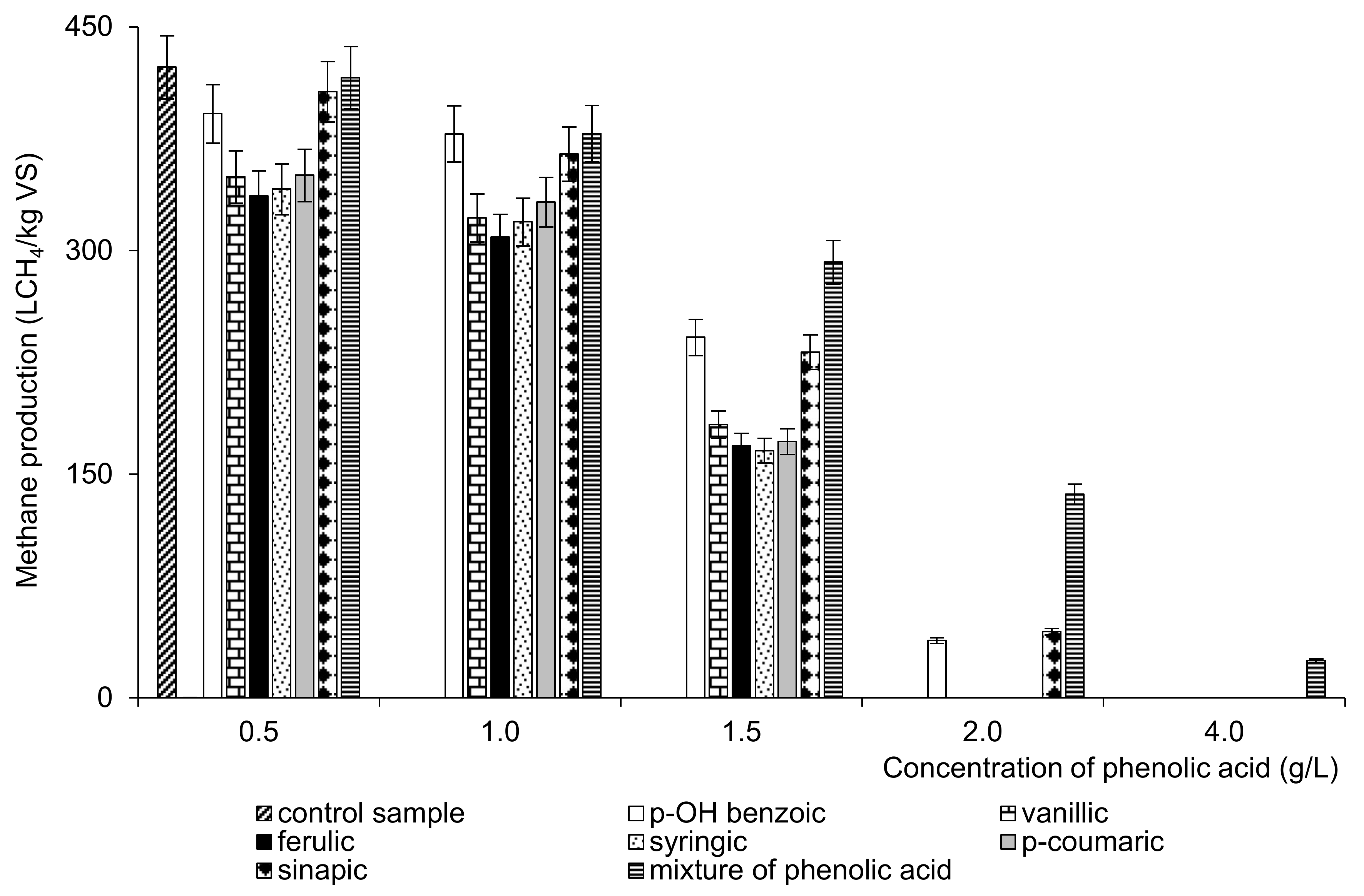
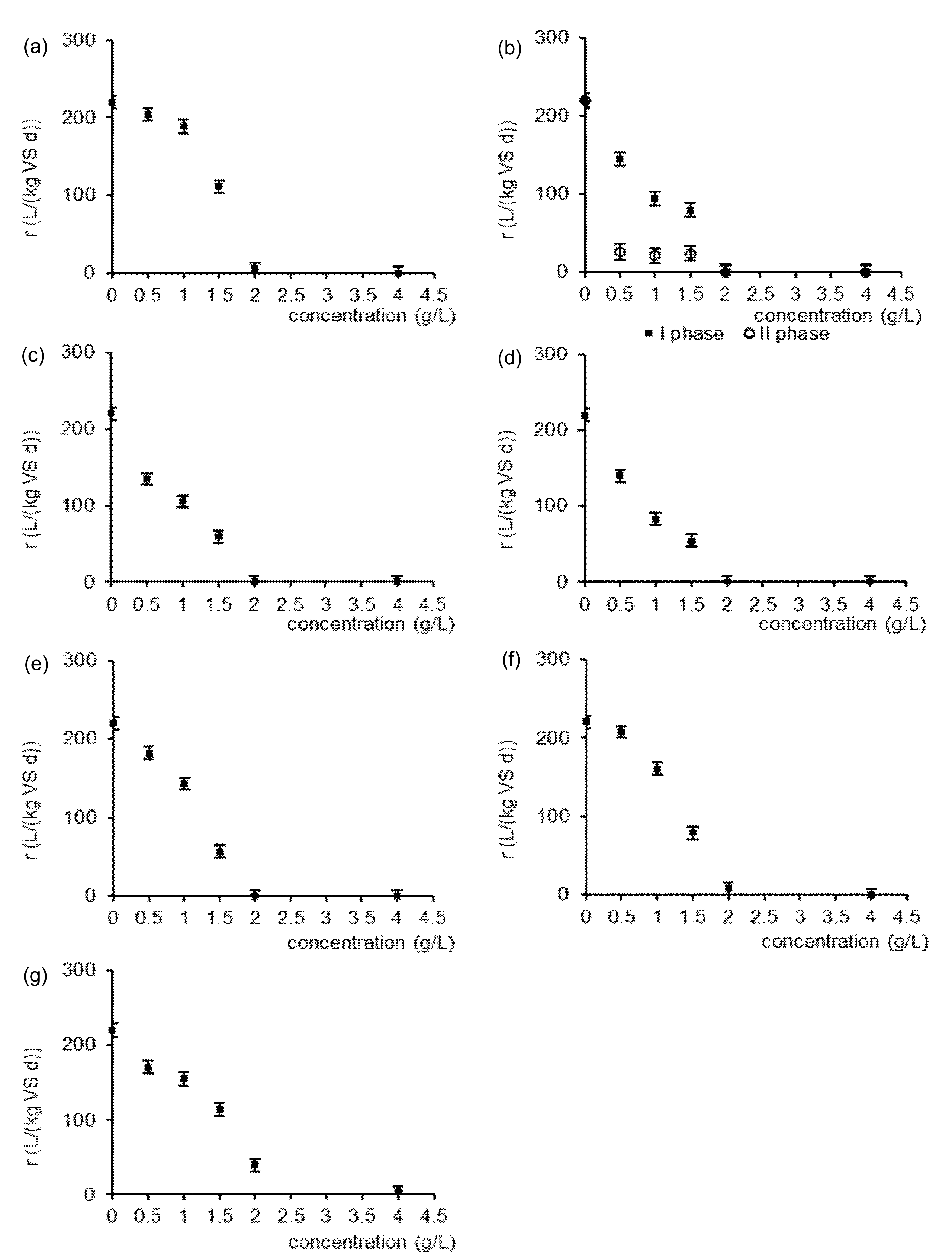
3.1. Methane Production
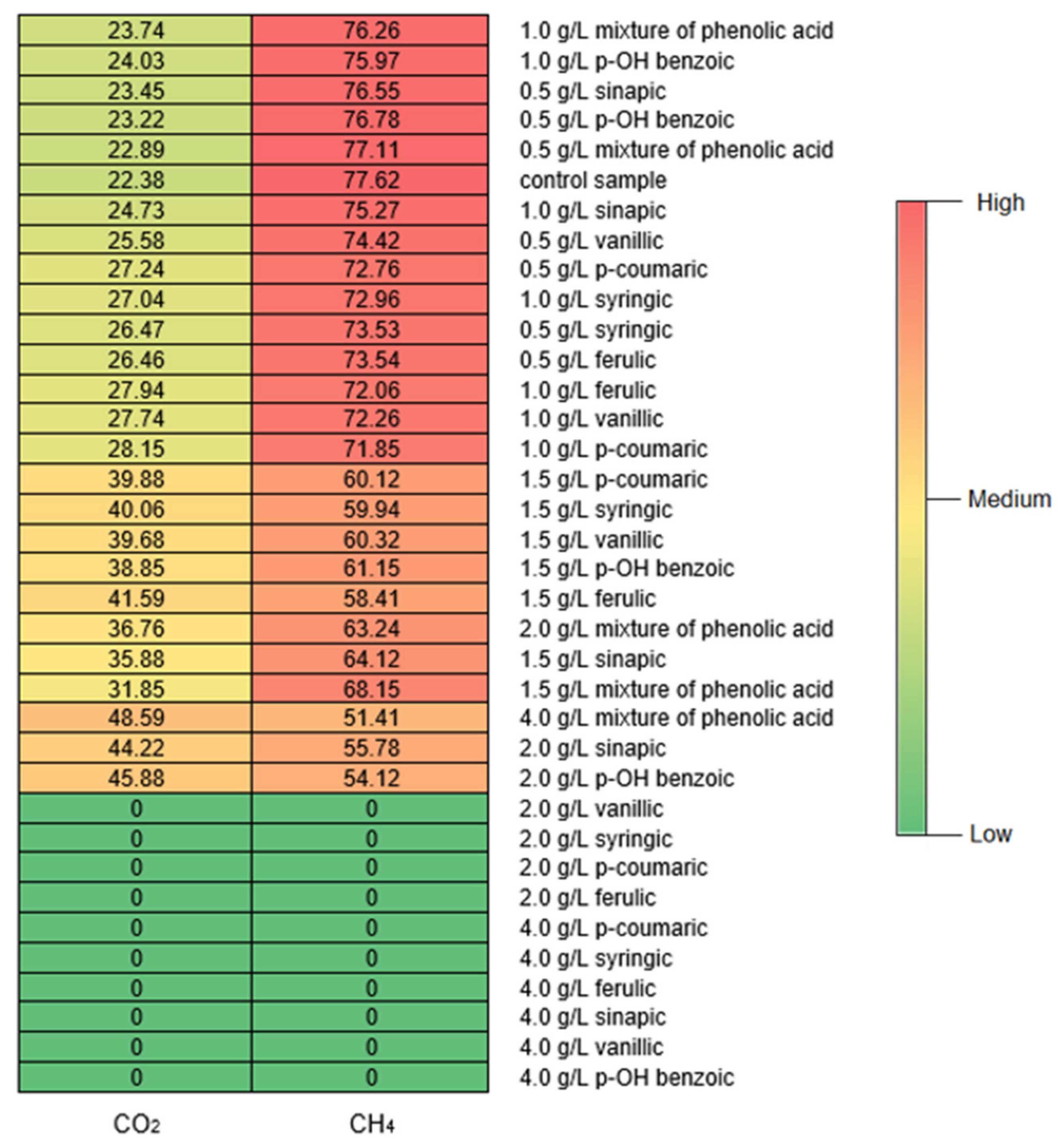
3.2. Characteristics of Digestate
3.3. Abundance of Methanogenic Archaea
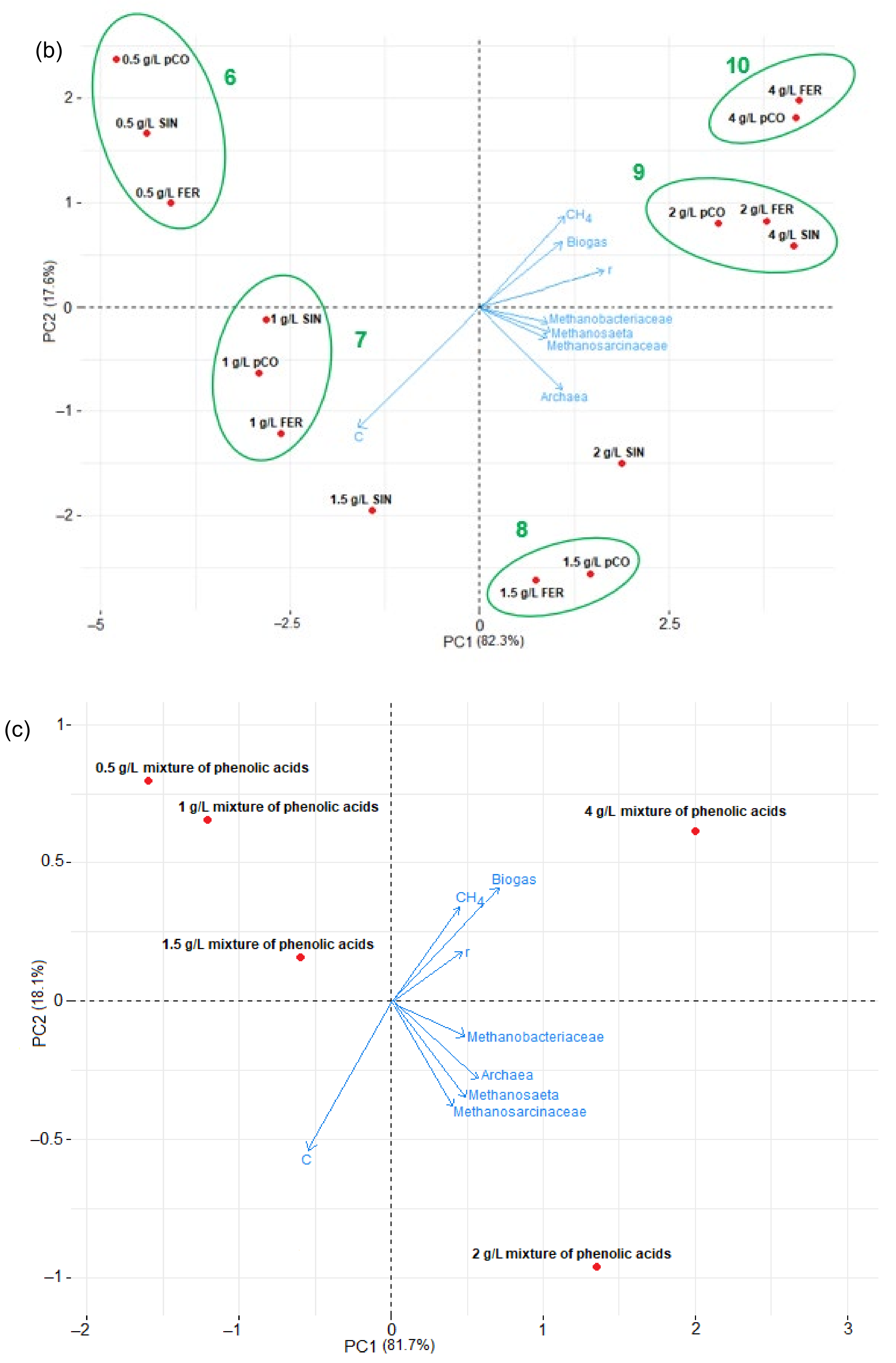
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fito, J.; Tefera, N.; Kloos, H.; van Hulle, S.W.H. Physicochemical properties of the sugar industry and ethanol distillery wastewater and their impact on the environment. Sugar Tech. 2019, 21, 265–277. [Google Scholar] [CrossRef]
- Jimenez, A.M.; Borja, R.; Martin, A. Aerobic/anaerobic biodegradation of beet molasses alcoholic fermentation wastewater. Process Biochem. 2003, 38, 275–1284. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, D.A.; Hill, L.E.; Zambiazi, R.C.; Borges, C.D.; Vizzotto, M.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Effect of nanoencapsulation using PLGA on antioxidant and antimicrobial activities of guabiroba fruit phenolic extract. Food Chem. 2018, 240, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Elalami, D.; Carrere, H.; Abdelouahdi, K.; Garcia-Bernet, D.; Peydecastaing, J.; Vaca-Medina, G.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Mild microwaves, ultrasonic and alkaline pretreatments for improving methane production: Impact on biochemical and structural properties of olive pomace. Bioresour. Technol. 2020, 299, 122591. [Google Scholar] [CrossRef]
- Caroca, E.; Serrano, A.; Borja, R.; Jimenez, A.; Carvajal, A.; Braga, A.F.M.; Rodriguez-Gutierrez, G.; Fermoso, F.G. Influence of phenols and furans released during thermal pretreatment of olive mill solid waste on its anaerobic digestion. Waste Manag. 2021, 120, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.E.; Edyvean, R.G.J. Inhibition of biogas production and biodegradability by substituted phenolic compounds in anaerobic sludge. J. Hazard. Mater. 2008, 160, 20–28. [Google Scholar] [CrossRef]
- El Gnaoui, Y.; Sounni, F.; Bakraoui, M.; Karouach, F.; Benlemlih, M.; Barz, M.; El Bari, H. Anaerobic co-digestion assessment of olive mill wastewater and food waste: Effect of mixture ratio on methane production and process stability. J. Environ. Chem. Eng. 2020, 8, 103874. [Google Scholar] [CrossRef]
- El Achkar, J.H.; Lendormi, T.; Hobaika, Z.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.-L. Anaerobic digestion of nine varieties of grape pomace: Correlation between biochemical composition and methane production. Biomass Bioenergy 2017, 107, 335–344. [Google Scholar] [CrossRef]
- Dong, D.; Wang, R.; Geng, P.; Li, C.; Zhao, Z. Enhancing effects of activated carbon supported nano zero-valent iron on anaerobic digestion of phenol-containing organic wastewater. J. Environ. Manag. 2019, 244, 1–12. [Google Scholar] [CrossRef]
- Baddi, G.A.; Cegarra, J.; Merlina, G.; Revel, J.C.; Hafidi, M. Qualitative and quantitative evolution of polyphenolic compounds during composting of an olive-mill waste-wheat straw mixture. J. Hazard. Mater. 2009, 165, 1119–1123. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Rodríguez-Gutierrez, G.; Fernandez-Bolanos, J.; Borja, R. Biomethanization of olive mill solid waste after phenols recovery through low-temperature thermal pre-treatment. Waste Manag. 2017, 61, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbera, M. Reuse of food waste and wastewater as a source of polyphenolic compounds to use as food additives. J. AOAC Int. 2020, 103, 906–914. [Google Scholar] [CrossRef]
- Ifie, I.; Marshall, L.J. Food processing and its impact on phenolic constituents in food. Cogent Food Agric. 2018, 4, 1507782. [Google Scholar] [CrossRef]
- Kaushik, A.; Basu, S.; Raturi, S.; Batra, V.S.; Balakrishnan, M. Recovery of antioxidants from sugarcane molasses distillery wastewater and its effect on biomethanation. J. Water Process. Eng. 2018, 25, 205–211. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielinska, M.; Bulkowska, K.; Witonska, I. Recovery of polyphenols from distillery stillage by microwave-assisted, ultrasound-assisted and conventional solid-liquid extraction. Sci. Rep. 2022, 12, 3232. [Google Scholar] [CrossRef] [PubMed]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.C.; Collier, P.J. The impact and mode of action of phenolic compounds extracted from brown seaweed on mixed anaerobic microbial cultures. J. Appl. Microbiol. 2013, 114, 964–973. [Google Scholar] [CrossRef]
- Oliveira, J.V.; Costa, J.C.; Cavaleiro, A.J.; Pereira, M.A.; Alves, M.M. Effect of Endogenous Methane Production: A Step Forward in the Validation of Biochemical Methane Potential (BMP) Tests. Energies 2022, 15, 4696. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Kopsahelis, A.; Zagklis, D.; Zafiri, C.; Kornaros, M. Used disposable nappies and expired food products valorisation through one- & two-stage anaerobic co-digestion. Renew. Energy 2020, 147, 610–619. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Water Works Association (AWWA) and Water Pollution Control Federation (WPCF): Washington, DC, USA, 1992. [Google Scholar]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, A.; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sust. Energ. Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Singletion, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 52–178. [Google Scholar] [CrossRef]
- Nielsen, P.H.; Nguyen, H.T.T.; McIlroy, S.; Mielczarek, A.T.; Seviour, R. Identification and quantification of microorganisms in activated sludge and biofilms by FISH. In FISH Handbook for Biological Wastewater Treatment: Identification and Quantification of Microorganisms in Activated Sludge and Biofilms; Nielsen, P.D., Daim, H., Eds.; IWA Publishing: London, UK, 2009; pp. 25–31. [Google Scholar]
- Stahl, D.A.; Amann, R. Development and application of nucleic acid probes in bacterial systematics. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1991; pp. 205–248. [Google Scholar]
- Raskin, L.; Poulsen, L.K.; Noguera, D.R.; Rittmann, B.E.; Stahl, D.A. Quantification of methanogenic groups in anaerobic biological reactors by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 1994, 60, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Westerholm, M.; Hansson, M.; Schnürer, A. Improved biogas production from whole stillage by co-digestion with cattle manure. Bioresour. Technol. 2012, 114, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Eskicioglu, C.; Kennedy, K.J.; Marin, J.; Strehler, B. Anaerobic digestion of whole stillage from dry-grind corn ethanol plant under mesophilic and thermophilic conditions. Bioresour. Technol. 2011, 102, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Lin, R.; Li, L.; Wu, B.; Deng, C.; O’Shea, R.; Sun, Y.; Murphy, J.D. Assessment of pretreatment and digestion temperature on anaerobic digestion of whiskey byproducts and microbial taxonomy. Energy Convers. Manag. 2021, 243, 114331. [Google Scholar] [CrossRef]
- Kang, X.; Lin, R.; Wu, B.; Li, L.; Deng, C.; Rajendran, K.; Sun, Y.; O’Shea, R.; Murphy, J.D. Towards green whiskey production: Anaerobic digestion of distillery by-products and the effects of pretreatment. J. Clean. Prod. 2022, 357, 131844. [Google Scholar] [CrossRef]
- Wu, L.; Hu, X.; Wang, S.; Hasan, M.M.; Jiang, S.; Zhang, L.; Li, C.Z. Reaction behaviour of light and heavy components of bio-oil in methanol and in water. Fuel 2018, 232, 645–652. [Google Scholar] [CrossRef]
- Chapleur, O.; Madigou, C.; Civade, R.; Rodolphe, Y.; Mazéas, L.; Bouchez, T. Increasing concentrations of phenol progressively affect anaerobic digestion of cellulose and associated microbial communities. Biodegradation 2016, 27, 15–27. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fòlino, A.; Tamburino, V.; Zappia, G.; Zema, D.A. Increasing the tolerance to polyphenols of the anaerobic digestion of olive wastewater through microbial adaptation. Biosyst. Eng. 2018, 172, 19–28. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Angelidaki, I. Optimization of biogas production from wheat straw stillage in UASB reactor. Appl. Energy 2010, 87, 3779–3783. [Google Scholar] [CrossRef]
- Djukic-Vukovic, A.P.; Mojovic, L.V.; Vukasinovic-Sekulic, M.S.; Nikolic, S.B.; Pejin, J.D. Integrated production of lactic acid and biomass on distillery stillage. Bioprocess Biosyst. Eng. 2013, 36, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Battimelli, A.; Torrijos, M.; Moletta, R.; Delgenès, J.P. Slaughterhouse fatty waste saponification to increase biogas yield. Bioresour. Technol. 2010, 101, 3388–3393. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, J.R.; Kim, Y. Enhanced digestion of waste activated sludge using microbial electrolysis cells at ambient temperature. Water Res. 2015, 87, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. Bioconversions of ferulic acid, an hydroxycinnamic acid. Crit. Rev. Microbiol. 2006, 32, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Shi, J.; Li, Y.B. Comparison of solid state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour. Technol. 2012, 384, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, V.N. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F.; Schieber, A.; Ganzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Nutr. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Adeboye, P.T.; Bettiga, M.; Olsson, L. The chemical nature of phenolic compounds determines their toxicity and induces distinct physiological responses in Saccharomyces cerevisiae in lignocellulose hydrolysates. AMB Express 2014, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrere, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Olguin-Lora, P.; Puig-Grajales, L.; Razo-Flores, E. Inhibition of the acetoclastic methanogenic activity by phenol and alkyl phenols. Environ. Technol. 2003, 24, 999–1006. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption properties of congo red from aqueous solution onto N,O-carboxymethyl-chitosan. Bioresour. Technol. 2008, 99, 1403–1408. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, P.; Zhang, G.; Jin, S.; Li, D.; Zhang, M.; Xu, X. Effect of alkaline addition on anaerobic sludge digestion with combined pretreatment of alkaline and high pressure homogenization. Bioresour. Technol. 2014, 168, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Dogan, T.; Ince, O.; Oz, N.Y.; Ince, B. Inhibition of volatile fatty acid production in granular sludge from a UASB reactor. Environ. Technol. 2005, 40, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Yen, H.W.; Yang, Y.C.; Yu, Y.H. Using crude glycerol and thin stillage for the production of microbial lipids through the cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2012, 114, 453–456. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Xue, L.; Chen, C.; Liu, G.; Zhang, R. Effects of ammonia on anaerobic digestion of food waste: Process performance and microbial community. Energy Fuels 2016, 30, 5749–5757. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Karakashev, D.; Batstone, D.J.; Trably, E.; Angelidaki, I. Acetate oxidation is the dominant methanogenic pathway from acetate in the absence of Methanosaetaceae. Appl. Environ. Microbiol. 2006, 72, 5138–5141. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Yan, H.; Li, N.; He, J.; Ding, Y.; Dai, L.; Dong, B. Metabolic adaptation of microbial communities to ammonium stress in a high solid anaerobic digester with dewatered sludge. Sci. Rep. 2016, 6, 28193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, S.; Bize, A.; Bureau, C.; Bouchez, T.; Chapleur, O. Community shifts within anaerobic digestion microbiota facing phenol inhibition: Towards early warning microbial indicators? Water Res. 2016, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Alsouleman, K.; Linke, B.; Klang, J.; Klocke, M.; Krakat, N.; Theuerl, S. Reorganisation of a mesophilic biogas microbiome as response to a stepwise increase of ammonium nitrogen induced by poultry manure supply. Bioresour. Technol. 2016, 208, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, P.; Chen, Y.; Liu, X.; Li, D. The role of endogenous and exogenous hydrogen in the microbiology of biogas production systems. World J. Microbiol. Biotechnol. 2020, 36, 79. [Google Scholar] [CrossRef] [PubMed]
- Cazier, E.A.; Trably, E.; Steyer, J.P.; Escudie, R. Reversibility of hydrolysis inhibition at high hydrogen partial pressure in dry anaerobic digestion processes fed with wheat straw and inoculated with anaerobic granular sludge. Waste Manag. 2019, 85, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Hardle, W.; Simar, L. Applied Multivariate Statistical Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Xiao, L.; Zheng, S.; Lichtfouse, E.; Luo, M.; Tan, Y.; Liu, F. Carbon nanotubes accelerate acetoclastic methanogenesis: From bure cultures to anaerobic soils. Soil Biol. Biochem. 2020, 150, 107938. [Google Scholar] [CrossRef]



| Characteristic | Unit | Distillery Stillage | Inoculum |
|---|---|---|---|
| Total solids (TS) | g/kg | 79.9 ± 3.0 | 61.5 ± 2.9 |
| Ash | g/kg | 3.7 ± 0.4 | 6.1 ± 1.4 |
| Volatile solids (VS) | g/kg | 76.2 ± 2.4 | 55.4 ± 1.9 |
| Organics content | % | 95.4 | 90.1 |
| pH | - | 6.52 ± 0.04 | 7.21 ± 0.01 |
| Total COD | mg/L | 109,000 ± 11,554 | n.m. |
| Soluble COD | mg/L | 43,600 ± 4830 | 8700 ± 347 |
| Volatile fatty acids (VFAs) | mg/L | 789.7 ± 0.8 | 571.2 ± 26.1 |
| Ammonium nitrogen | mg/L | 293.8 ± 4.7 | 162.4 ± 2.5 |
| Total phosphorus (TP) | mg/L | 212.4 ± 0.6 | 331.6 ± 2.0 |
| Alkalinity | meq/L | 182.8 ± 1.4 | 225.2 ± 1.1 |
| Type of Sample | Percentage of Maximum Theoretical Methane Potential | Type of Sample | Percentage of Maximum Theoretical Methane Potential |
|---|---|---|---|
| control sample | 86.8 | ||
| 0.5 g/L p-OH benzoic | 80.4 | 0.5 g/L ferulic | 69.1 |
| 1 g/L p-OH benzoic | 77.7 | 1 g/L ferulic | 63.4 |
| 1.5 g/L p-OH benzoic | 49.6 | 1.5 g/L ferulic | 34.7 |
| 2 g/L p-OH benzoic | 7.9 | 2 g/L ferulic | 0.0 |
| 4 g/L p-OH benzoic | 0.0 | 4 g/L ferulic | 0.0 |
| 0.5 g/L vanillic | 71.7 | 0.5 g/L syringic | 70.0 |
| 1 g/L vanillic | 66.1 | 1 g/L syringic | 65.5 |
| 1.5 g/L vanillic | 37.6 | 1.5 g/L syringic | 34.0 |
| 2 g/L vanillic | 0.0 | 2 g/L syringic | 0.0 |
| 4 g/L vanillic | 0.0 | 4 g/L syringic | 0.0 |
| 0.5 g/L p-coumaric | 72.0 | 0.5 g/L sinapic | 83.5 |
| 1 g/L p-coumaric | 68.2 | 1 g/L sinapic | 74.9 |
| 1.5 g/L p-coumaric | 35.3 | 1.5 g/L sinapic | 47.6 |
| 2 g/L p-coumaric | 0.0 | 2 g/L sinapic | 9.1 |
| 4 g/L p-coumaric | 0.0 | 4 g/L sinapic | 0.0 |
| 0.5 g/L mixture | 85.4 | ||
| 1 g/L mixture | 77.7 | ||
| 1.5 g/L mixture | 60.0 | ||
| 2 g/L mixture | 28.0 | ||
| 4 g/L mixture | 5.1 |
| Type of Sample | k (1/d) | R2 | C0 (L/kg VS) | Type of Sample | k (1/d) | R2 | C0 (L/kg VS) |
|---|---|---|---|---|---|---|---|
| control sample | 0.52 ± 0.03 | 0.99 | 423 ± 11 | ||||
| 0.5 g/L p-OH benzoic | 0.52 ± 0.02 | 0.99 | 392 ± 9 | 0.5 g/L ferulic | 0.40 ± 0.01 | 0.99 | 337 ± 10 |
| 1 g/L p-OH benzoic | 0.50 ± 0.02 | 0.99 | 378 ± 10 | 1 g/L ferulic | 0.34 ± 0.02 | 0.99 | 309 ± 8 |
| 1.5 g/L p-OH benzoic | 0.46 ± 0.01 | 0.99 | 242 ± 6 | 1.5 g/L ferulic | 0.35 ± 0.01 | 0.98 | 169 ± 6 |
| 2 g/L p-OH benzoic | 0.13 ± 0.01 | 0.99 | 38 ± 1 | 2 g/L ferulic | 0 | 0 | 0 |
| 4 g/L p-OH benzoic | 0 | 0 | 0 | 4 g/L ferulic | 0 | 0 | 0 |
| 0.5 g/L vanillic phase I | 0.98 ± 0.04 | 0.99 | 148 ± 7 | 0.5 g/L syringic | 0.41 ± 0.02 | 0.99 | 341 ± 7 |
| 1 g/L vanillic phase I | 0.72 ± 0.03 | 0.98 | 131 ± 4 | 1 g/L syringic | 0.26 ± 0.01 | 0.99 | 319 ± 8 |
| 1.5 g/L vanillic phase I | 1.38 ± 0.04 | 0.98 | 58 ± 2 | 1.5 g/L syringic | 0.33 ± 0.01 | 0.99 | 166 ± 4 |
| 2 g/L vanillic phase I | 0 | 0 | 0 | 2 g/L syringic | 0 | 0 | 0 |
| 4 g/L vanillic phase I | 0 | 0 | 0 | 4 g/L syringic | 0 | 0 | 0 |
| 0.5 g/L vanillic phase II | 0.13 ± 0.01 | 0.98 | 201 ± 9 | 0.5 g/L p-coumaric | 0.52 ± 0.03 | 0.99 | 350 ± 9 |
| 1 g/L vanillic phase II | 0.11 ± 0.01 | 0.98 | 191 ± 7 | 1 g/L p-coumaric | 0.43 ± 0.02 | 0.99 | 332 ± 7 |
| 1.5 g/L vanillic phase II | 0.19 ± 0.01 | 0.98 | 125 ± 5 | 1.5 g/L p-coumaric | 0.33 ± 0.02 | 0.99 | 172 ± 7 |
| 2 g/L vanillic phase II | 0 | 0 | 0 | 2 g/L p-coumaric | 0 | 0 | 0 |
| 4 g/L vanillic phase II | 0 | 0 | 0 | 4 g/L p-coumaric | 0 | 0 | 0 |
| 0.5 g/L sinapic | 0.51 ± 0.02 | 0.99 | 407 ± 10 | 0.5 g/L mixture | 0.41 ± 0.02 | 0.99 | 416 ± 13 |
| 1 g/L sinapic | 0.44 ± 0.02 | 0.99 | 365 ± 8 | 1 g/L mixture | 0.41 ± 0.01 | 0.99 | 378 ± 8 |
| 1.5 g/L sinapic | 0.34 ± 0.01 | 0.99 | 232 ± 6 | 1.5 g/L mixture | 0.39 ± 0.01 | 0.99 | 292 ± 9 |
| 2 g/L sinapic | 0.19 ± 0.01 | 0.98 | 44 ± 1 | 2 g/L mixture | 0.29 ± 0.01 | 0.99 | 136 ± 4 |
| 4 g/L sinapic | 0 | 0 | 0 | 4 g/L mixture | 0.13 ± 0.01 | 0.99 | 25 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikucka, W.; Zielinska, M. Individual Phenolic Acids in Distillery Stillage Inhibit Its Biomethanization. Energies 2022, 15, 5377. https://doi.org/10.3390/en15155377
Mikucka W, Zielinska M. Individual Phenolic Acids in Distillery Stillage Inhibit Its Biomethanization. Energies. 2022; 15(15):5377. https://doi.org/10.3390/en15155377
Chicago/Turabian StyleMikucka, Wioleta, and Magdalena Zielinska. 2022. "Individual Phenolic Acids in Distillery Stillage Inhibit Its Biomethanization" Energies 15, no. 15: 5377. https://doi.org/10.3390/en15155377






