Turning Waste Cooking Oils into Biofuels—Valorization Technologies: A Review
Abstract
1. Introduction
1.1. Consumption of Edible Oils in Europe
1.2. Waste Cooking Oils
2. Production of Alternative Fuels through Thermochemical Conversion Processes
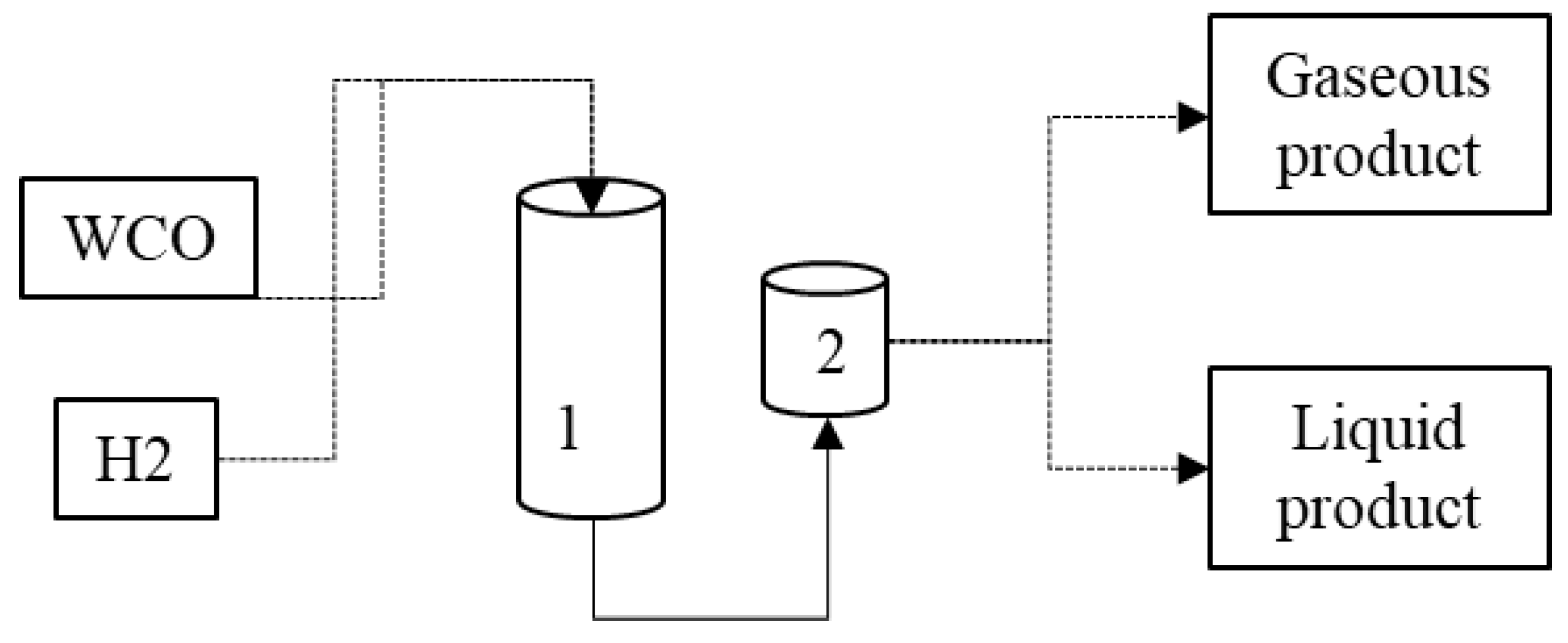
2.1. Hydrocracking
2.2. Gasification
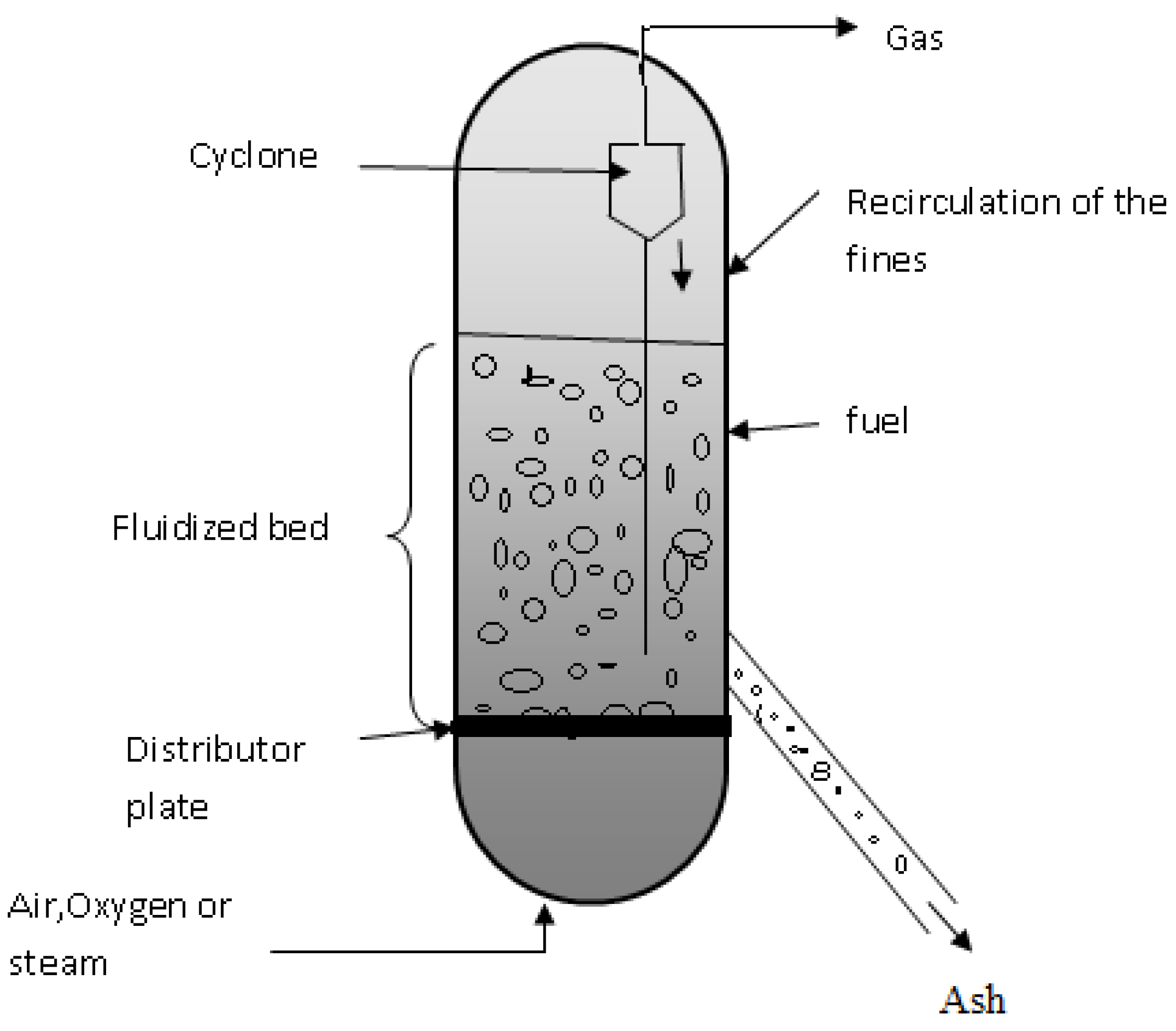
2.2.1. Fluidized Bed Gasifier
2.2.2. Fixed Bed Gasifier
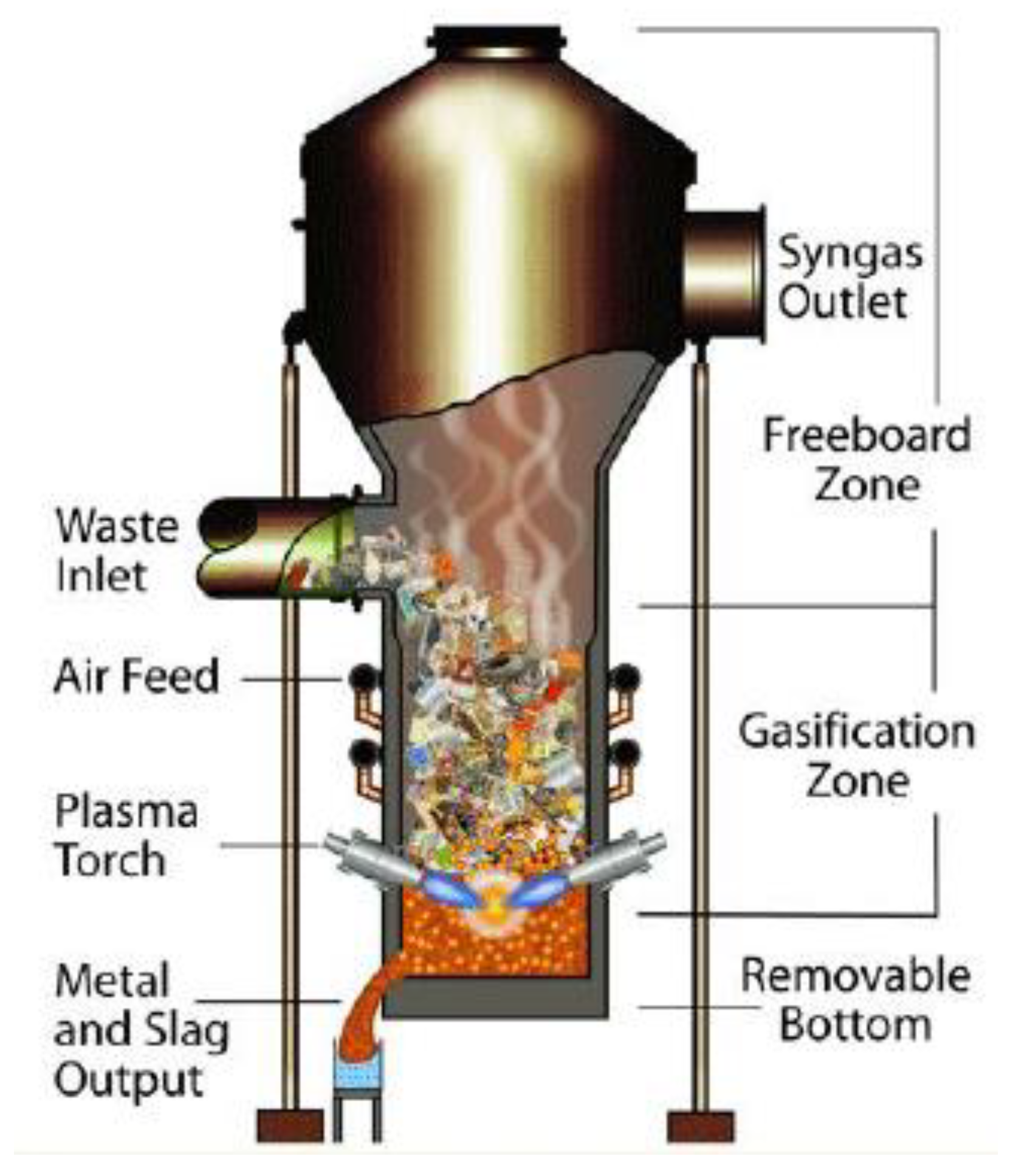
2.2.3. Plasma Gasifier
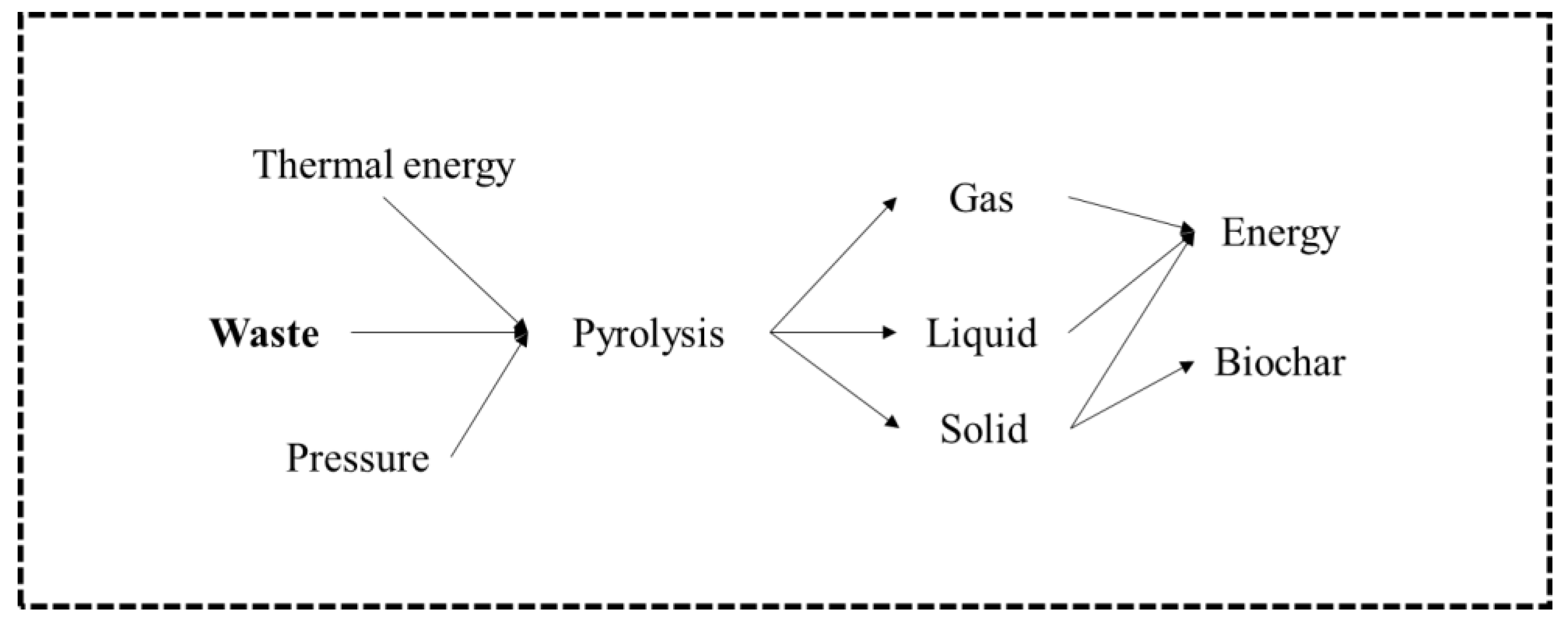
3. Pyrolysis
3.1. Studies of Recovery by Slow Pyrolysis
3.2. Valorisation Studies by Fast Pyrolysis
3.3. Valorization Studies by Catalytic Pyrolysis
4. Final Remarks on the Production of Alternative Biofuels through the WCO
Author Contributions
Funding
Conflicts of Interest
References
- Peters, J.; Thielmann, S. Promoting biofuels: Implications for developing countries. Energy Policy 2008, 36, 1538–1544. [Google Scholar] [CrossRef]
- European Commission. Study on the Implementation of Conformity Checks in the Olive Oil Sector throughout the European Union; European Union. Publications Office: Brussels, Belgium, 2020; ISBN 9789276092643. [Google Scholar]
- EU. European Union Oil and Protein Meal Industry, International Olive Council; European Union: Brussels, Belgium, 2020. [Google Scholar]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Anderssen, I.M.; Webber, C.; Kelly, R.; Andersen, O. Localized Production and Supply of Biodiesel from Used Cooking Oils. State of the Art in Europe; European Commission: Brussels, Belgium, 2007; ISSN nr: 0803-4354. Available online: https://www.vestforsk.no/sites/default/files/migrate_files/rapport12-07-biodienet_wp2_rpt_v12.pdf (accessed on 1 October 2021).
- EUBIA. Transformation of Used Cooking Oil into Biodiesel: From Waste to Resource; European Biomass Industry Association: Brussels, Belgium, 2015; Available online: https://www.eubren.com/UCO_to_Biodiesel_2030_01.pdf (accessed on 1 October 2021).
- Tsoutsos, T.D.; Tournaki, S.; Paraíba, O.; Kaminaris, S.D. The Used Cooking Oil-to-biodiesel chain in Europe assessment of best practices and environmental performance. Renew. Sustain. Energy Rev. 2016, 54, 74–83. [Google Scholar] [CrossRef]
- GREENA. “Market Watch”. 2020. Available online: https://www.greenea.com/wp-content/uploads/2020/10/Greenea-Market-Watch-October-2020-_-EN.pdf (accessed on 1 October 2021).
- European Biomass Industry Association. Available online: https://www.eubia.org/ (accessed on 1 October 2021).
- Teixeira, M.R.; Nogueira, R.; Nunes, L.M. Quantitative assessment of the valorisation of used cooking oils in 23 countries. Waste Manag. 2018, 78, 611–620. [Google Scholar] [CrossRef]
- Foster, W.; Azimov, U.; Gauthier-Maradei, P.; Molano, L.C.; Combrinck, M.; Munoz, J.; Esteves, J.J.; Patino, L. Waste-to-energy conversion technologies in the UK: Processes and barriers—A review. Renew. Sustain. Energy Rev. 2021, 135, 110226. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2021; 112285, in press. [Google Scholar] [CrossRef] [PubMed]
- El-Araby, R.; Abdelkader, E.; El Diwani, G.; Hawash, S.I. Bio-aviation fuel via catalytic hydrocracking of waste cooking oils. Bull. Natl. Res. Cent. 2020, 44, 177. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels securing the planet’s future energy needs. Energy Convers. Manag. 2009, 50, 2239–2249. [Google Scholar] [CrossRef]
- Energy Efficiency & Renewable Energy, Biofuel Basics-Biofuel BasicsBioenergy Technologies Office. Available online: https://www.energy.gov/eere/bioenergy/biofuel-basics (accessed on 5 October 2021).
- Saladini, F.; Patrizi, N.; Pulselli, F.M.; Marchettini, N.; Bastianoni, S. Guidelines for emergy evaluation of first, second and third generation biofuels. Renew. Sustain. Energy Rev. 2016, 66, 221–227. [Google Scholar] [CrossRef]
- Juniper. IOFUELS: A Decision Maker’s Guide to Opportunities for Converting Biomass and Wastes into Transport Fuels Independt Waste Technology Review; Juniper Consultancy Services Limited: Bolton, UK, 2009. [Google Scholar]
- Stocker Lago, S.M.; Freire da Rocha, W., Jr. Logística reversa, legislação e sustentabilidade: O óleo de fritura residual como matéria-prima para produção de biodiesel. Gestão E Soc. 2016, 10, 1458. [Google Scholar] [CrossRef]
- Paraiba, O.; Tournaki, S.; Tsoutsos, T.; Antunes, D. Used cooking oil to biodiesel: Practical Guide. In Used Cooking Oil Recycling for Sustainable Biodiesel Production; Sustainable Cities: Designing for People and the Planet; Energy and Environment Agency of Arrábida: Coimbra, Portugal, 2013. [Google Scholar]
- Dias, D.J.B. Processos de Valorização Energética de Óleos e Gorduras. 2013. Available online: http://hdl.handle.net/10362/11340 (accessed on 1 October 2021).
- Nascimento, A.C.; Nascimento, R.M.; Caetano, R. Manual: A logística reversa do óleo de fritura usado como solução para problemas ambientais. 2011. Available online: https://www.setorreciclagem.com.br/images/oleo.pdf (accessed on 1 October 2021).
- Tsita, K.G.; Kiartzis, S.J.; Ntavos, N.K.; Pilavachi, P.A. Next generation biofuels derived from thermal and chemical conversion of the Greek transport sector. Therm. Sci. Eng. Prog. 2020, 17, 100387. [Google Scholar] [CrossRef]
- Bezergianni, S.; Voutetakis, S.; Kalogianni, A. Catalytic Hydrocracking of Fresh and Used Cooking Oil. Ind. Eng. Chem. Res. 2009, 48, 8402–8406. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kalogianni, A. Hydrocracking of used cooking oil for biofuels production. Bioresour. Technol. 2009, 100, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.; Oliveira, M.R.; Gondim, A.D.; Souza, J.D.; Araujo, A.S. Thermal and catalytic pyrolysis of sunflower oil using AlMCM-41. Renew. Energy 2017, 101, 900–906. [Google Scholar] [CrossRef]
- Lam, S.S.; Mahari, W.A.W.; Ok, Y.S.; Peng, W.; Chong, C.T.; Ma, N.L.; Chase, H.A.; Liew, Z.; Yusup, S.; Kwon, E.E.; et al. Microwave vacuum pyrolysis of waste plastic and used cooking oil for simultaneous waste reduction and sustainable energy conversion: Recovery of cleaner liquid fuel and techno-economic analysis. Renew. Sustain. Energy Rev. 2019, 115, 109359. [Google Scholar] [CrossRef]
- Li, Q.; Zhengshun, W.; Shaofei, X.; Xiaoyan, L.; Tingting, S.; Qiangxian, W.; Yu, Z. Catalytic Cracking of Waste Cooking Oil for the Production of Synthesis Gas. J. Biobased Mater. Bioenergy 2013, 7, 357–366. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Kaewkannetra, P. Production of bio-hydrogenated kerosene by catalytic hydrocracking from refined bleached deodorised palm/palm kernel oils. Renew. Energy 2020, 147, 464–472. [Google Scholar] [CrossRef]
- Nanda, S.; Rana, R.; Hunter, H.N.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Hydrothermal catalytic processing of waste cooking oil for hydrogen-rich syngas production. Chem. Eng. Sci. 2019, 195, 935–945. [Google Scholar] [CrossRef]
- Kim, Y.-D.; Jung, S.-H.; Jeong, J.-Y.; Yang, W.; Lee, U.-D. Production of producer gas from waste cooking oil in a fluidized bed reactor: Influence of low-temperature oxidation of fuel. Fuel 2015, 146, 125–131. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Tacconi, D. Sustainable bio kerosene: Process routes and industrial demonstration activities in aviation biofuels. Appl. Energy 2014, 136, 767–774. [Google Scholar] [CrossRef]
- Singhabhandhu, A.; Tezuka, T. Prospective framework for collection and exploitation of waste cooking oil as feedstock for energy conversion. Energy 2010, 35, 1839–1847. [Google Scholar] [CrossRef]
- Wiggers, V.R.; Meier, H.F.; Wisniewski Jr, A.; Barros, A.A.C.; Maciel, M.R.W. Biofuels from continuous fast pyrolysis of soybean oil: A pilot plant study. Bioresour. Technol. 2009, 100, 6570–6577. [Google Scholar] [CrossRef]
- Meier, H.F.; Wiggers, V.R.; Zonta, G.R.; Scharf, D.R.; Simionatto, E.L.; Ender, L. A kinetic model for thermal cracking of waste cooking oil based on chemical lumps. Fuel 2015, 144, 50–59. [Google Scholar] [CrossRef]
- Tiwari, R.; Rana, B.S.; Kumar, R.; Verma, D.; Kumar, R.; Joshi, R.K.; Garg, M.O.; Sinha, A.K. Hydrotreating and hydrocracking catalysts for processing of waste soya-oil and refinery-oil mixtures. Catal. Commun. 2011, 12, 559–562. [Google Scholar] [CrossRef]
- Gregório, A. Catálise de Hydrocracking baseado em Zeólitos. Master’s Thesis, Department Engenharia Quimica Instituto Superior de Engenharia de Lisboa, Lisboa, Portugal, 2015. Available online: https://repositorio.ipl.pt/bitstream/10400.21/5392/1/Disserta%C3%A7%C3%A3o.pdf (accessed on 1 October 2021).
- Melo, B.M.d.C. Hidrogenação Catalítica de Resíduos Para Produção de Biocombustíveis Líquidos; Department Ciências. University: Lisboa, Portugal, 2016; Available online: http://hdl.handle.net/10451/25623 (accessed on 1 October 2021).
- Li, T.; Cheng, J.; Huang, R.; Zhou, J.; Cen, K. Conversion of waste cooking oil to jet biofuel with nickel-based mesoporous zeolite Y catalyst. Bioresour. Technol. 2015, 197, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, K.; Syoufian, A.; Ariantika, S.D. Hydrocracking of Used Cooking Oil into Biofuel Catalyzed by Nickel-Bentonite. Asian J. Chem. 2014, 26, 3785–3789. [Google Scholar] [CrossRef]
- Guran, S. Sustainable Waste-to-Energy Technologies: Gasification and Pyrolysis. In Sustainable Food Waste-To-Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 141–158. [Google Scholar] [CrossRef]
- Alauddin, Z.A.B.Z.; Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Gasification of lignocellulosic biomass in fluidized beds for renewable energy development: A review. Renew. Sustain. Energy Rev. 2010, 14, 2852–2862. [Google Scholar] [CrossRef]
- Caputo, A.C.; Pelagagge, P.M. RDF production plants: II Economics and profitability. Appl. Therm. Eng. 2002, 22, 439–448. [Google Scholar] [CrossRef]
- Higman, C. “Gasification”. In Combustion Engineering Issues for Solid Fuel Systems, 1st ed.; Miller, B.G., Tillman, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 423–468. [Google Scholar]
- Oliveira, P.; Cruz, S.F.; De, R. Recuperação energética e eliminação urbanos, resíduos sólidos. Master’s Thesis, Faculdade de Tecnologia de São Paulo-FATEC, São Paulo, Brazil, 2016. [Google Scholar]
- Monir, M.U.; Aziz, A.A.; Kristanti, R.A.; Yousuf, A. Gasification of lignocellulosic biomass to produce syngas in a 50 kW downdraft reactor. Biomass Bioenergy 2018, 119, 335–345. [Google Scholar] [CrossRef]
- Monir, M.U.; Khatun, F.; Aziz, A.A.; Vo, D.-V.N. Thermal treatment of tar generated during co-gasification of coconut shell and charcoal. J. Clean. Prod. 2020, 256, 120305. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Williams, P.T.; Shi, J.; Huang, J. Hydrogen production from biomass gasification with Ni/MCM-41 catalysts: Influence of Ni content. Appl. Catal. B Environ. 2011, 108–109, 6–13. [Google Scholar] [CrossRef]
- Govender, N.S.; Janse van Vuuren, M.; Claeys, M.; van Steen, E. Importance of the usage ratio in iron-based Fischer−Tropsch synthesis with recycle. Ind. Eng. Chem. Res. 2006, 45, 8629–8633. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L.; Jones, D.D.; Hanna, M.A. Contemporary issues in thermal gasification of biomass and its application to electricity and fuel production. Biomass Bioenergy 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Ahrenfeldt, J.; Thomsen, T.P.; Henriksen, U.; Clausen, L.R. Biomass gasification cogeneration–A review of state of the art technology and near future perspectives. Appl. Therm. Eng. 2013, 50, 1407–1417. [Google Scholar] [CrossRef]
- Oliveira, P.; Cruz, F. Gaseificação: Processo alternativo para a recuperação energética e eliminação de resíduos sólidos urbanos. Master’s Thesis, Faculdade de Tecnologia de São Paulo -FATEC, São Paulo, Brazil, 2016. [Google Scholar]
- Sivakumar, L.; Anithamary, X. Lower Order Modeling and Control of Alstom Fluidized Bed Gasifier. In Gasification for Practical Applications; IntechOpen: London, UK, 2012; pp. 313–338. [Google Scholar] [CrossRef][Green Version]
- Tamošiūnas, A.; Jeguirim, M. Char gasification. In Char and Carbon Materials Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2019; pp. 187–228. [Google Scholar]
- Costa, L.; Silva, H.; Oliveira, J.; Fernandes, S.; Freitas, E.; Hilliou, L. Plastic waste use as aggregate and binder modifier in open-graded asphalts. In Proceedings of the 3rd Int Conference on Wastes: Solutions, Treatments, and Opportunities, Viana Do Castelo, Portugal, 14–16 September 2015; pp. 67–72. Available online: http://hdl.handle.net/1822/39125 (accessed on 1 October 2021).
- Li, J.; Yin, Y.; Zhang, X.; Liu, J.; Yan, R. Hydrogen-rich gas production by steam gasification of palm oil wastes over supported tri-metallic catalyst. Int. J. Hydrogen Energy 2009, 34, 9108–9115. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Watkinson, A.P.; Ellis, N. Steam Gasification of Bio-Oil and Bio-Oil/Char Slurry in a Fluidized Bed Reactor. Energy Fuels 2010, 24, 5181–5189. [Google Scholar] [CrossRef]
- Bacovsky, D.; Dallos, M.; Wörgetter, M. Status of 2nd Generation Biofuels Demonstration Facilities in June 2010; Report T-39; IEA Bioenergy: Vienna, Austria, 2010. [Google Scholar]
- Ahmad, J.; Rashid, U.; Patuzzi, F.; Alamoodi, N.; Choong, T.S.Y.; Soltani, S.; Ngamcharussrivichai, C.; Nehdi, I.A.; Baratieri, M. Mesoporous Acidic Catalysts Synthesis from Dual-Stage and Rising Co-Current Gasification Char: Application for FAME Production from Waste Cooking Oil. Materials 2020, 13, 871. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Rashid, U.; Patuzzi, F.; Baratieri, M.; Taufiq-Yap, Y.H. Synthesis of char-based acidic catalyst for methanolysis of waste cooking oil: An insight into a possible valorization pathway for the solid by-product of gasification. Energy Convers. Manag. 2018, 158, 186–192. [Google Scholar] [CrossRef]
- Rafiq, M.H.; Hustad, J.E. Biosyngas production by autothermal reforming of waste cooking oil with propane using a plasma-assisted gliding arc reactor. Int. J. Hydrogen Energy 2011, 36, 8221–8233. [Google Scholar] [CrossRef]
- Costa, P. Produção de Hidrocarbonetos Líquidos e Gasosos por Pirólise de Resíduos Plásticos. Ph.D. Thesis, Faculdade de Ciências e Tecnologia, Univercity, Nova Lisboa, Lisboa, 2006. [Google Scholar]
- Breeze, P. Advanced Waste-to-Energy Technologies: Gasification, Pyrolysis, and Plasma Gasification. In Energy from Waste; Elsevier: Amsterdam, The Netherlands, 2018; pp. 65–75. [Google Scholar] [CrossRef]
- Pereira, B.M.S.P. Reaproveitamento de óleo queimado através do processo de Pirolise. Master’s Thesis, Department Mechanical Engenharia Mecanica Instituto Politécnico de Tomar, Tomar, Portugal, 2016. Available online: https://comum.rcaap.pt/handle/10400.26/28883?locale=en (accessed on 1 October 2021).
- Dong, R.; Zhao, M. Research on the pyrolysis process of crumb tire rubber in waste cooking oil. Renew. Energy 2018, 125, 557–567. [Google Scholar] [CrossRef]
- Moreira, R. Estudo da pirólise lenta da casca da castanha de caju. Master’s Thesis, Instituto de Pesquisas Energéticas e Nucleares, Universidade de São Paulo, São Paulo, Brazil, 2015. Available online: https://teses.usp.br/teses/disponiveis/85/85134/tde-07102015-090727/pt-br.php (accessed on 1 October 2021).
- Borges, F.A.; Oliveira, T.J.P.D.E.; Costa, K.L.S. Estudo Experimental De Pirólise Lenta De Pellets De Casca De Café Em Um Reator De Leito Fixo. Blucher Chem. Eng. Proc. 2019, VI, 672–678. [Google Scholar]
- Demirbas, A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Alcalá, A.; Bridgwater, A. V Upgrading fast pyrolysis liquids: Blends of biodiesel and pyrolysis oil. Fuel 2013, 109, 417–426. [Google Scholar] [CrossRef]
- Wang, Y.; Darensbourg, D.J. Carbon dioxide-based functional polycarbonates: Metal catalyzed copolymerization of CO2 and epoxides. Coord. Chem. Rev. 2018, 372, 85–100. [Google Scholar] [CrossRef]
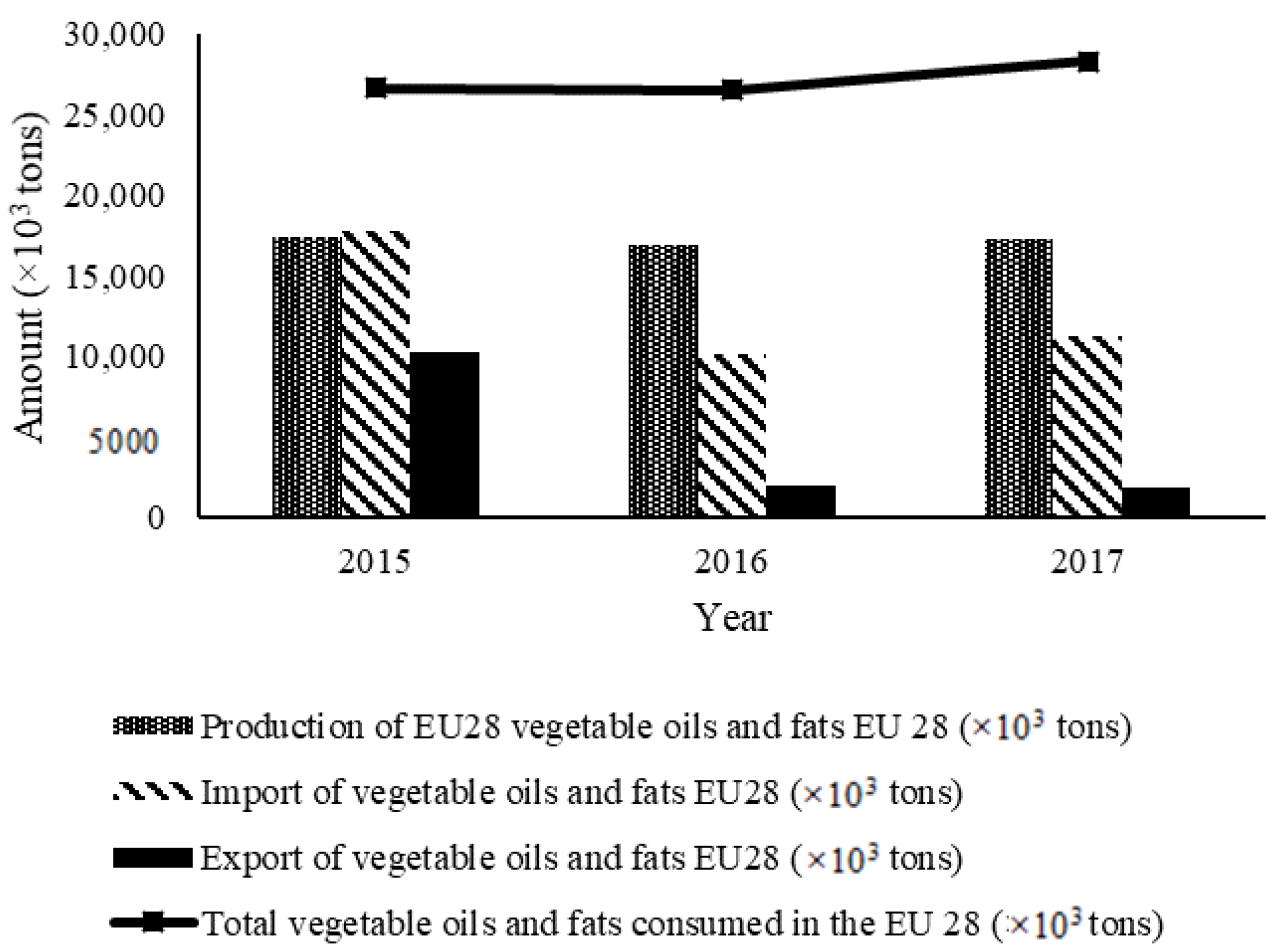

| Year | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|
| Total vegetable oils and fats consumed in the EU 28 (×103 tons) | 26,625 | 26,523 | 28,312 | 27,978 |
| Production of EU28 vegetable oils and fats EU 28 (×103 tons) | 17,399 | 16,942 | 17,341 | 17,635 |
| Import of vegetable oils and fats EU28 (×103 tons) | 17,808 | 10,175 | 11,223 | 10,728 |
| Export of vegetable oils and fats EU28 (×103 tons) | 10,257 | 1999 | 1836 | 1885 |
| First Generation | Second Generation | Third Generation |
|---|---|---|
| Biodiesel from vegetable and animal oils and fats by transesterification. | Ethanol from cellulosic materials by fermentation. | Biofuels derived from algae. |
| Ethanol from sugar crops and cereals via conventional fermentation. | Biomass alcohol via gasification and catalytic or enzymatic conversion. | Hydrogen biofuel. |
| Biogas from biodegradable waste by anaerobic digestion. | Fuels from biomass via gasification and Fischer–Tropsch. | Biofuels derived from mixed waste (e.g., MSW). |
| Biodiesel via pyrolysis, depolymerization, and cracks. | ||
| Biofuels from vegetable oils and fats through existing chemical and petrochemical processes. |
| Technology | Product | Reference |
|---|---|---|
| Hidrocracking | Biodiesel, bio-oil; biokerosene | [23,24,27,28] |
| Gasification | Syngas + H | [29,30] |
| Fast pyrolysis | Biokerosene; Bio-oil | [31,32,33,34] |
| Pyrolysis | Bio-oil | [25,26] |
| Catalytic pyrolysis | Bio-oil | [25] |
| Company | Local | Typology of Biofuels | Feedstock |
|---|---|---|---|
| Oleotorres | Portugal | Biodiesel, lubricants | WCO, vegetable oil, and animal fat |
| PRIO | Portugal | Biodiesel, syngas and lubricants | WCO |
| Recivalongo | Portugal | Bio syngas | Recovered solid fuel |
| CHOREN | Germany | Bio syngas | Forest biomass (waste), miscanthus and straw |
| Fuel Frontiers | USA | Bio syngas | Coffee husk, food waste, MSW, biomass |
| NSE Biofuels | Finland | Bio syngas, biodiesel and bio-oil | Biomass |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, L.; Ribeiro, A.; Ferreira, A.; Valério, N.; Pinheiro, V.; Araújo, J.; Vilarinho, C.; Carvalho, J. Turning Waste Cooking Oils into Biofuels—Valorization Technologies: A Review. Energies 2022, 15, 116. https://doi.org/10.3390/en15010116
Nascimento L, Ribeiro A, Ferreira A, Valério N, Pinheiro V, Araújo J, Vilarinho C, Carvalho J. Turning Waste Cooking Oils into Biofuels—Valorization Technologies: A Review. Energies. 2022; 15(1):116. https://doi.org/10.3390/en15010116
Chicago/Turabian StyleNascimento, Lucas, André Ribeiro, Ana Ferreira, Nádia Valério, Vânia Pinheiro, Jorge Araújo, Cândida Vilarinho, and Joana Carvalho. 2022. "Turning Waste Cooking Oils into Biofuels—Valorization Technologies: A Review" Energies 15, no. 1: 116. https://doi.org/10.3390/en15010116
APA StyleNascimento, L., Ribeiro, A., Ferreira, A., Valério, N., Pinheiro, V., Araújo, J., Vilarinho, C., & Carvalho, J. (2022). Turning Waste Cooking Oils into Biofuels—Valorization Technologies: A Review. Energies, 15(1), 116. https://doi.org/10.3390/en15010116





