Novel Trends in Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction
- -
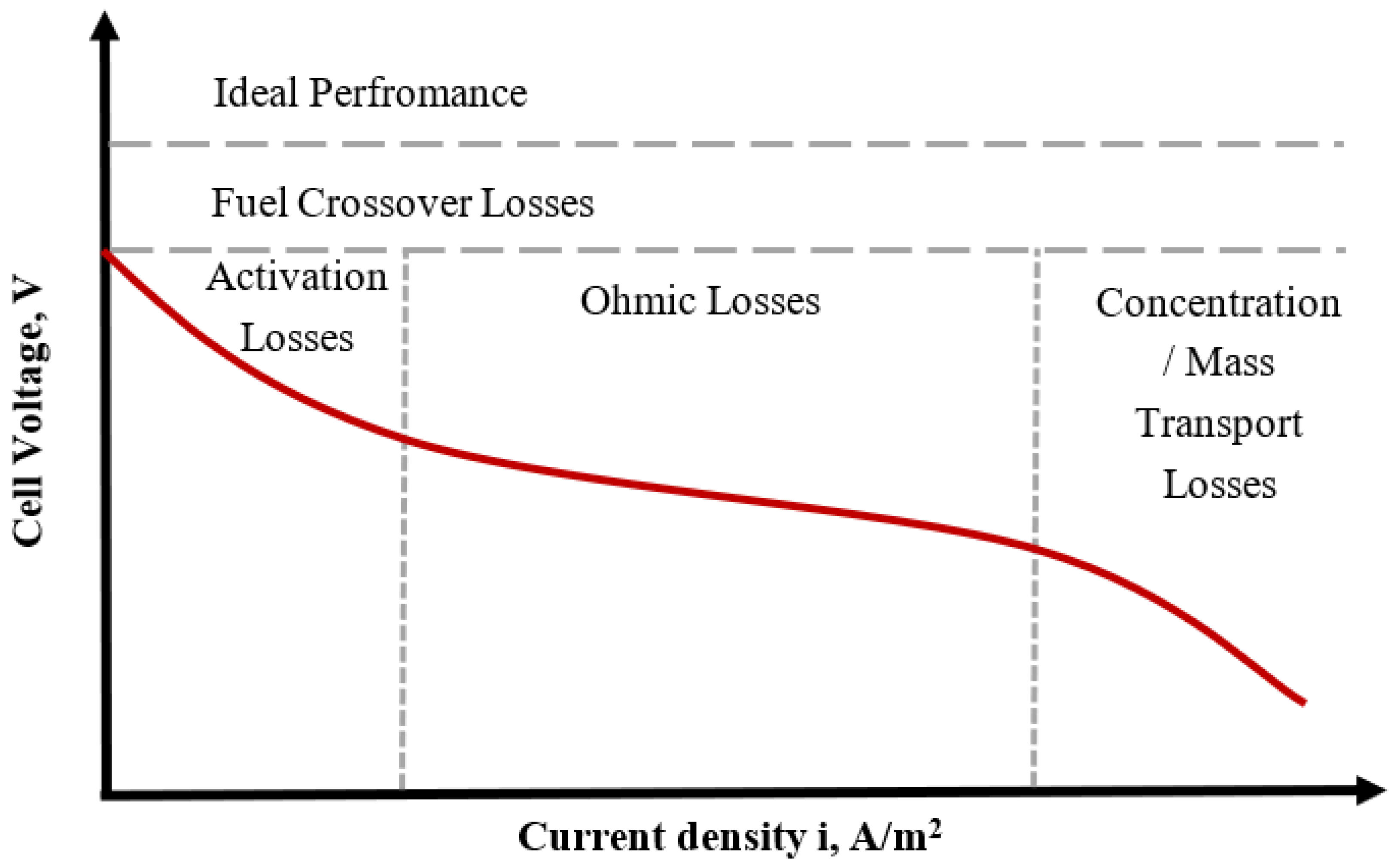
- Activation losses: These indicate that PEMFC requires specific energy to cause excitation of the electrons via the external circuit, which depends mainly on the catalyst material, loading, and utilization, and are predominant at low currents [17].
- -
- Ohmic losses: These occur due to the flow of electrons or protons and depend on the material of the membrane electrode assembly (MEA), more specifically the membrane conductivity and thickness. This region is a desirable operating zone as the maximum output power of the PEMFC exists here.
- -
- Mass transfer losses: These are due to the rate of consumption of the reactant in the electrochemical reaction and are characterized by a high voltage drop at high currents [21]. These losses depend on the porosity of the gas diffusion layer (GDL) as well as the hydrophobicity characteristics of the membrane and GDL.
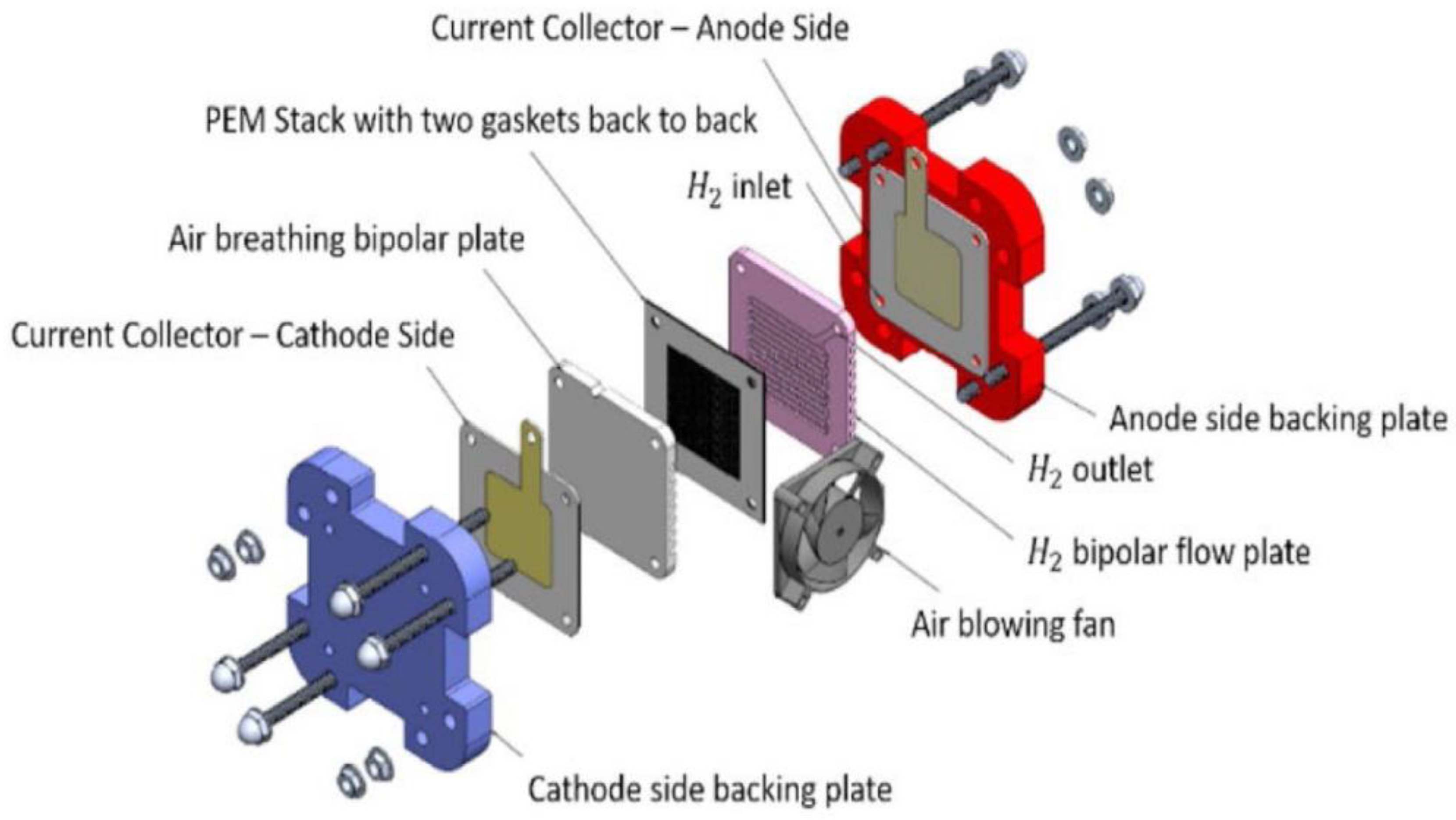
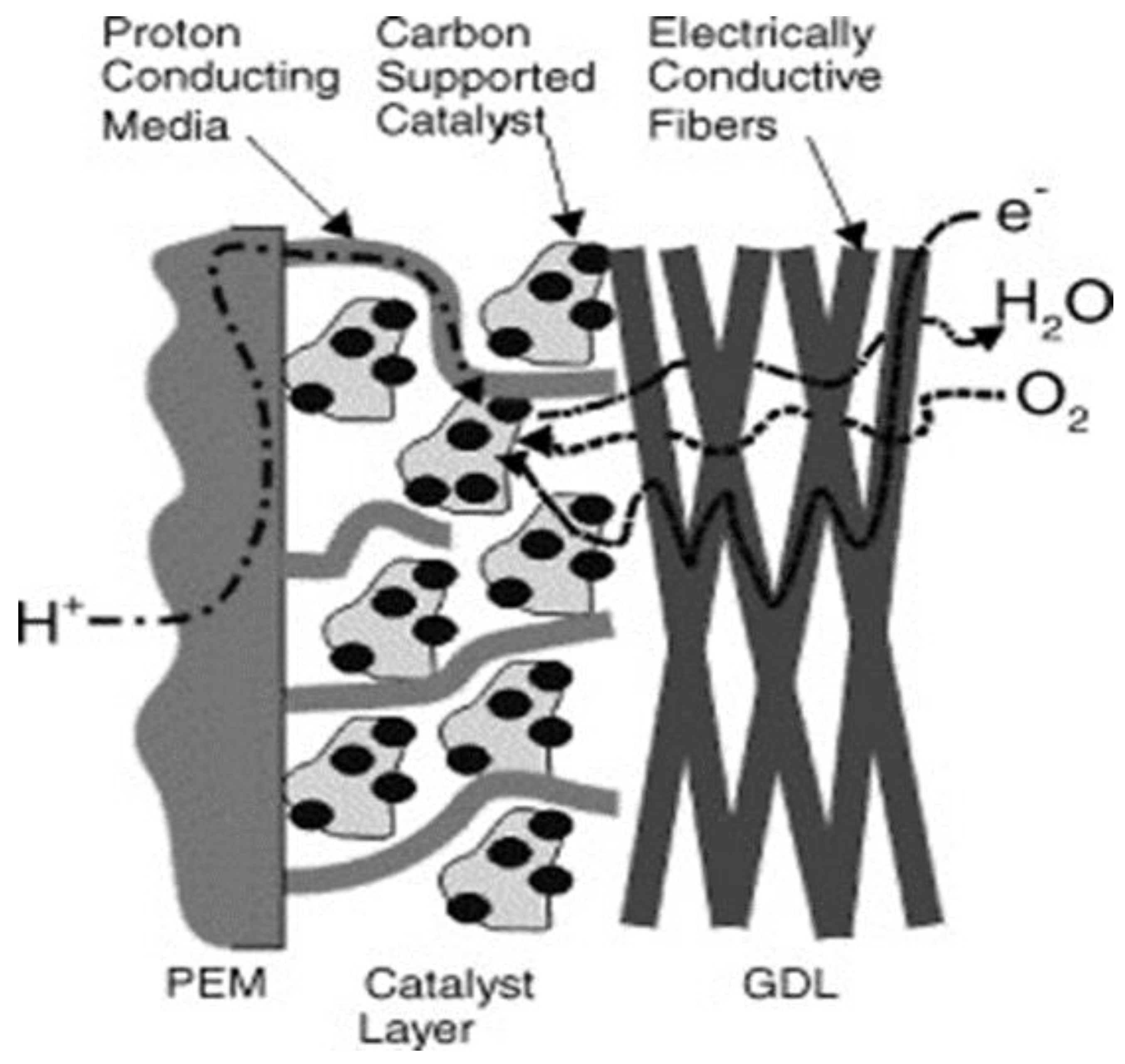
2. Components of Fuel Cells/Stacks
2.1. Membrane Electrode Assembly (MEA)
- Perfluorinated membranes, such as perfluorosulfonic acid;
- Partially fluorinated, such as styrene grafted and sulfonated poly(vinylidene fluoride);
- Non-fluorinated, such as the incorporation of a sulfonic acid group into aromatic polymers, e.g., sulfonated polybenzimidazole, polyimides, and polyphenylene;
- A non-fluorinated composite, such as acid-doped poly benzimidazoles;
- A perfluorinated composite, such as compositing PTEE with Nafion material.
2.2. Catalyst
- -
- The reduction of catalyst cost per kW, e.g., the economic use of the catalyst content, increasing catalyst durability, finding alternative catalyst materials, etc.
- -
- A tolerance to carbon monoxide and sulfur species present in hydrogen feed as impurities [87].
2.3. Gas Diffusion Layer
- Product permeability: It serves as an exit point for the elimination of by-products from the cell.
- Electronic conductivity: It allows the flow of electrons.
- Heat conductivity: This allows heat dissipation to other components in the cell.
- Mechanical strength: This supports the MEA mechanically, especially when a pressure drop of reactants fluctuates between the anode and cathode flow fields’ channels, hence maintaining sufficient contact force to avoid intrusion into the channels.
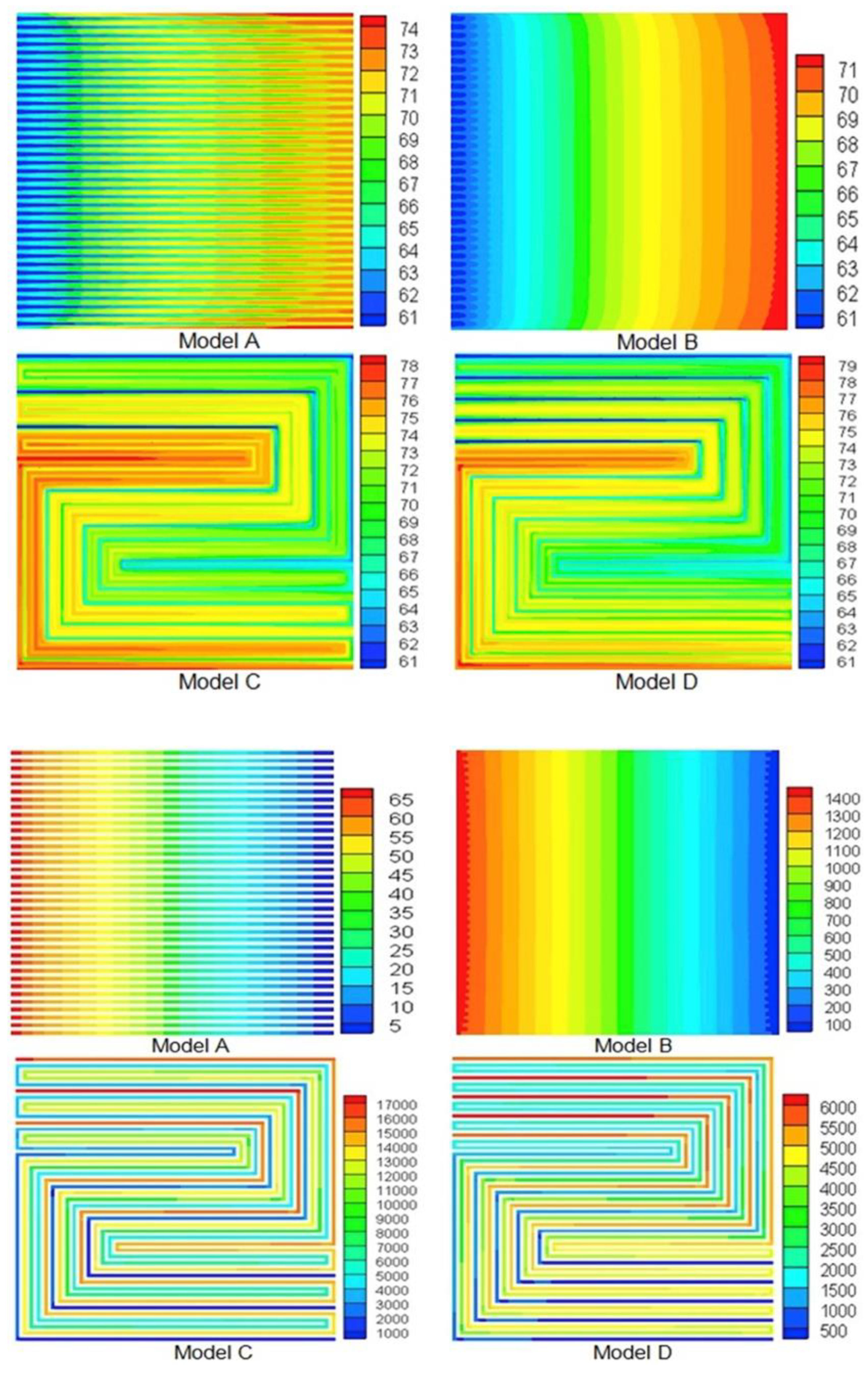
2.4. Bipolar Plates
- Allowing the easy flow of electrons.
- Being impermeable to gases: to prevent leakage of reactants and, thus, increasing the utilization level.
- Providing adequate strength: to prevent the cracking and crushing of plates at a high compressive force.
- Being thermally conductive: by housing drilled internal cooling channels for the heat exchange fluid to flow and, thus, allowing the generated heat to be removed from the system.
- Having corrosion resistance: due to the acidic condition of the MEA, the BPs are more likely to be corroded easily and, hence, must be able to sustain these conditions.
2.4.1. Graphite Material
2.4.2. Metallic Bipolar Plates
2.4.3. Carbon Polymer Composite Material
2.4.4. Foam-Based Bipolar Plate
2.5. End Plate
- Low-density material, to make the stack lighter and, hence, achieve high power density (W/Kg).
- High rigidity to ensure the end plates do not bend when a high tightening force is applied.
- Good chemical and electrochemical stability.
- Electrically insulating to resist the flow of electrons to ensure safety and that the electrical power is not transmitted through the material, especially at high output power (50 cells or more).
- Thermally insulating to keep the heat produced within the FC and also to prevent heat dissipation at the start.
2.6. Gaskets
2.6.1. Silicon Rubber
2.6.2. Ethylene-Propylene-Diene Monomer (EPDM)
2.6.3. Fluroelastomers
3. The Manufacturing Method of PEMFC
3.1. Membrane Electrode Assembly MEA
3.1.1. Hand Painting
3.1.2. Spray Painting
3.1.3. Sputtering
3.1.4. Inkjet Printing
3.1.5. Electrospraying
3.1.6. Electron Beam Reduction
3.1.7. Sonicated Spray
3.2. Fabrication of Bipolar Plates
3.2.1. Micro-Stamping Process
3.2.2. Hydroforming Process
3.2.3. Rubber Pad Forming Process
3.2.4. Selective Laser Sintering (SLS)
3.3. End Plate Fabrication
3.4. Gasket Fabrication
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Proton exchange membrane fuel cells | |
| MEA | Membrane electrode assembly |
| Gas diffusion layer | |
| Fuel cell | |
| Kilowatt | |
| Balance of plant | |
| United States Department of Energy | |
| TFE | Tetrafluoroethylene |
| Direct methanol FCs | Direct methanol fuel cells |
| PFSA | Perfuorosulfonic acid |
| e-PTFE | Polytetrafluorethylene |
| EIS | Electrochemical impedance spectroscopy |
| HOR | Hydrogen oxidation reaction |
| Carbon monoxide | |
| Oxygen reduction reaction | |
| Platinum | |
| HCC | Hybrid cathode catalyst |
| NCA | Nitrogen-doped carbon aerogel |
| CP | Carbon paper |
| CC | Carbon cloth |
| PTFE | Fluorinated ethylene propylene |
| Micro porous layer | |
| BP | Bipolar plate |
| Computational fluid dynamics | |
| High temperature | |
| AB-PEMFC | Air-breathing PEMFC |
| EPDM | Ethylene-propylene-diene monomer |
| VF2 | Vinylidene fluoride |
| CTFE | Chlorotrifluoroethylene |
| CCM | Catalyst-coated membrane |
| Decal transfer method | |
| PVD | Physical vapor deposition |
| Transmission electron microscope | |
| Near net shape casting | |
| Metal matrix composite | |
| FIPG | Form-in-place gasket |
| CNC | Computerized numerical control |
References
- Olabi, A.G. Developments in sustainable energy and environmental protection. Energy 2012, 39, 2–5. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Sayed, E.T.; Abdelkareem, M.A.; Bahaa, A.; Eisa, T.; Alawadhi, H.; Al-Asheh, S.; Chae, K.-J.; Olabi, A.G. Synthesis and performance evaluation of various metal chalcogenides as active anodes for direct urea fuel cells. Renew. Sustain. Energy Rev. 2021, 150, 111470. [Google Scholar] [CrossRef]
- Tanveer, W.H.; Abdelkareem, M.A.; Kolosz, B.W.; Rezk, H.; Andresen, J.; Cha, S.W.; Sayed, E.T. The role of vacuum based technologies in solid oxide fuel cell development to utilize industrial waste carbon for power production. Renew. Sustain. Energy Rev. 2021, 142, 110803. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge transfer and biocompatibility aspects in conducting polymer-based enzymatic biosensors and biofuel cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Progress and insights in the application of MXenes as new 2D nano-materials suitable for biosensors and biofuel cell design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef] [PubMed]
- Andriukonis, E.; Celiesiute-Germaniene, R.; Ramanavicius, S.; Viter, R.; Ramanavicius, A. From microorganism-based amperometric biosensors towards microbial fuel cells. Sensors 2021, 21, 2442. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting polymers in the design of biosensors and biofuel cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Wilberforce, T.; Abdelkareem, M.A.; Elsaid, K.; Olabi, A.G.; Sayed, E.T. Role of carbon-based nanomaterials in improving the performance of microbial fuel cells. Energy 2022, 240, 122478. [Google Scholar] [CrossRef]
- Sayed, E.T.; Abdelkareem, M.A.; Alawadhi, H.; Elsaid, K.; Wilberforce, T.; Olabi, A.G. Graphitic carbon nitride/carbon brush composite as a novel anode for yeast-based microbial fuel cells. Energy 2021, 221, 119849. [Google Scholar] [CrossRef]
- Abdallah, M.; Feroz, S.; Alani, S.; Sayed, E.T.; Shanableh, A. Continuous and scalable applications of microbial fuel cells: A critical review. Rev. Environ. Sci. Bio./Technol. 2019, 18, 3543–3578. [Google Scholar] [CrossRef]
- Sayed, E.T.; Abdelkareem, M.A.; Alawadhi, H.; Olabi, A.G. Enhancing the performance of direct urea fuel cells using Co dendrites. Appl. Surf. Sci. 2021, 555, 149698. [Google Scholar] [CrossRef]
- Alawadhi, H.; Abdelkareem, M.A.; Hussain, N.; Wilberforce, T.; Sayed, E.T. A composite of graphitic carbon nitride and Vulcan carbon as an effective catalyst support for Ni in direct urea fuel cells. J. Taiwan Inst. Chem. Eng. 2020, 116, 160–168. [Google Scholar] [CrossRef]
- Cao, Q.; Hao, S.; Wu, Y.; Pei, K.; You, W.; Che, R. Interfacial charge redistribution in interconnected network of Ni2P–Co2P boosting electrocatalytic hydrogen evolution in both acidic and alkaline conditions. Chem. Eng. J. 2021, 424, 130444. [Google Scholar] [CrossRef]
- Park, J.-S. Hydrogen-based energy conversion: Polymer electrolyte fuel cells and electrolysis. Energies 2021, 14, 5068. [Google Scholar] [CrossRef]
- Hagen, A.; Caldogno, R.; Capotondo, F.; Sun, X. Metal supported electrolysis cells. Energies 2022, 15, 2045. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Shabani, B. Proton exchange membrane fuel cells heat recovery opportunities for combined heating/cooling and power applications. Energy Convers. Manag. 2020, 204, 112328. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Prospects of fuel cell combined heat and power systems. Energies 2020, 13, 4104. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, Q.-A.; Wang, Y.-J.; Zhang, F.; Li, W.; Li, A.; Zhang, L.; Zhang, J. Recent progress in the use of electrochemical impedance spectroscopy for the measurement, monitoring, diagnosis and optimization of proton exchange membrane fuel cell performance. J. Power Sources 2020, 468, 228361. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Ye, F.; Ma, C.F. Baffle shape effects on mass transfer and power loss of proton exchange membrane fuel cells with different baffled flow channels. Int. J. Energy Res. 2019, 43, 72737–72755. [Google Scholar] [CrossRef]
- Wee, J.-H. Applications of proton exchange membrane fuel cell systems. Renew. Sustain. Energy Rev. 2007, 11, 81720–81738. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)–A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Ijaodola, O.; El-Hassan, Z.; Ogungbemi, E.; Khatib, F.; Wilberforce, T.; Thompson, J.; Olabi, A. Energy efficiency improvements by investigating the water flooding management on proton exchange membrane fuel cell (PEMFC). Energy 2019, 179, 246–267. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A. Fuel cell membranes–Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Wilberforce, T.; El Hassan, Z.; Ogungbemi, E.; Ijaodola, O.; Khatib, F.; Durrant, A.; Thompson, J.; Baroutaji, A.; Olabi, A. A comprehensive study of the effect of bipolar plate (BP) geometry design on the performance of proton exchange membrane (PEM) fuel cells. Renew. Sustain. Energy Rev. 2019, 111, 236–260. [Google Scholar] [CrossRef]
- Jouin, M.; Gouriveau, R.; Hissel, D.; Péra, M.-C.; Zerhouni, N. Prognostics and health management of PEMFC—State of the art and remaining challenges. Int. J. Hydrogen Energy 2013, 38, 15307–15317. [Google Scholar] [CrossRef] [Green Version]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.N.; Al Makky, A.; Mooney, J.; Barouaji, A.; Carton, J.G.; Olabi, A.-G. Development of Bi-polar plate design of PEM fuel cell using CFD techniques. Int. J. Hydrogen Energy 2017, 42, 25663–25685. [Google Scholar] [CrossRef] [Green Version]
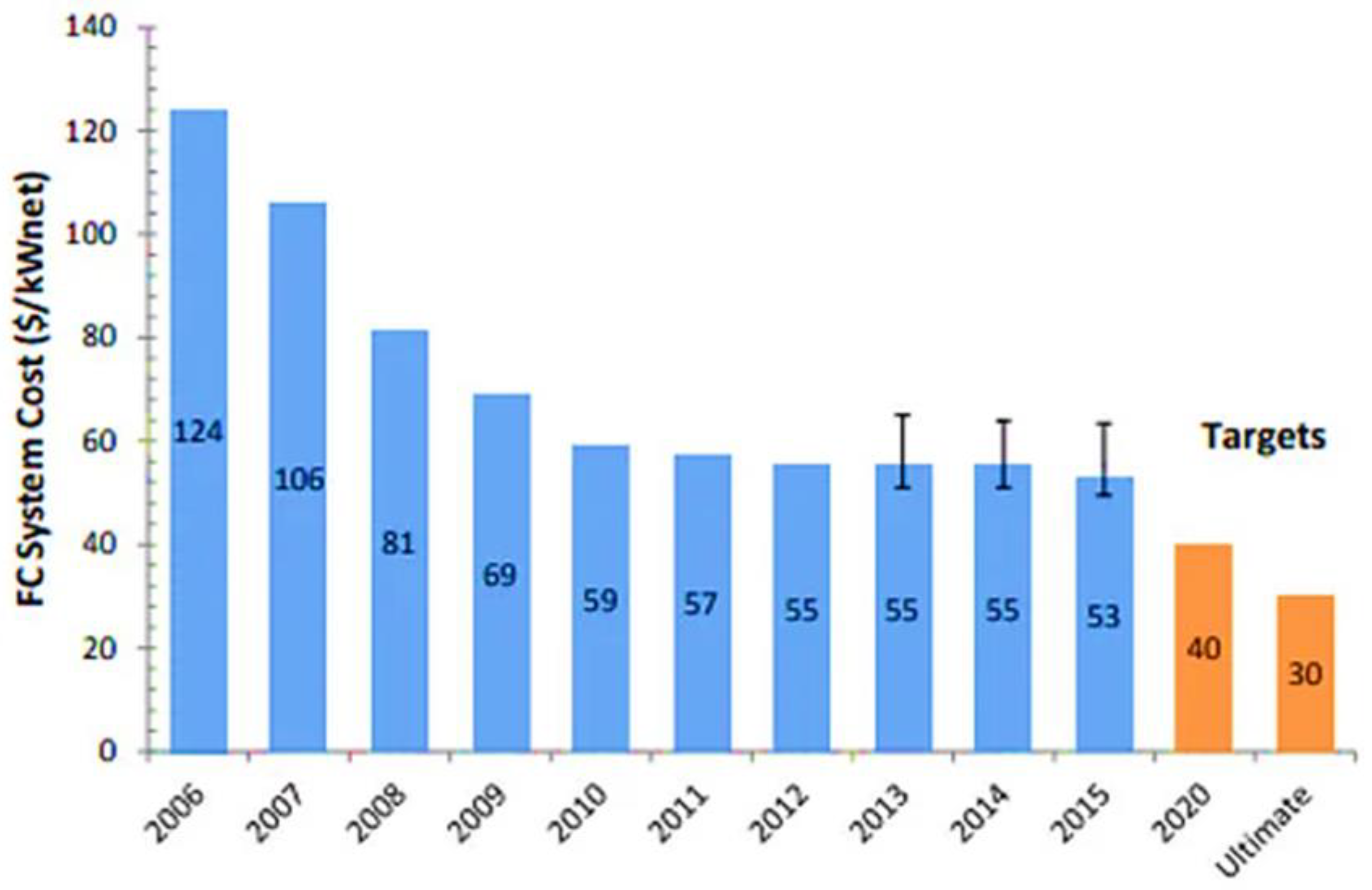
- Manufacturing Cost Analysis of PEM Fuel Cell Systems for 5- and 10-kW Backup Power Applications; Battelle Memorial Institute: Columbus, OH, USA, 2016.
- Wilson, A.; Marcinkoski, J.; Papageorgopoulos, D. Fuel Cell System Cost–2016, DOE Hydrogen and Fuel Cells Program Record# 16020; 2016.
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Daud, W.R.W. Coating of stainless steel and titanium bipolar plates for anticorrosion in PEMFC: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Gao, P.; Xie, Z.; Wu, X.; Ouyang, C.; Lei, T.; Yang, P.; Liu, C.; Wang, J.; Ouyang, T.; Huang, Q. Development of Ti bipolar plates with carbon/PTFE/TiN composites coating for PEMFCs. Int. J. Hydrogen Energy 2018, 43, 20947–20958. [Google Scholar] [CrossRef]
- Sun, X.; Simonsen, S.C.; Norby, T.; Chatzitakis, A. Composite membranes for high temperature PEM fuel cells and electrolysers: A critical review. Membranes 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatib, F.N.; Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; El-Hassan, Z.; Durrant, A.; Thompson, J.; Olabi, A.G. Material degradation of components in polymer electrolyte membrane (PEM) electrolytic cell and mitigation mechanisms: A review. Renew. Sustain. Energy Rev. 2019, 111, 1–14. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 132–153. [Google Scholar] [CrossRef]
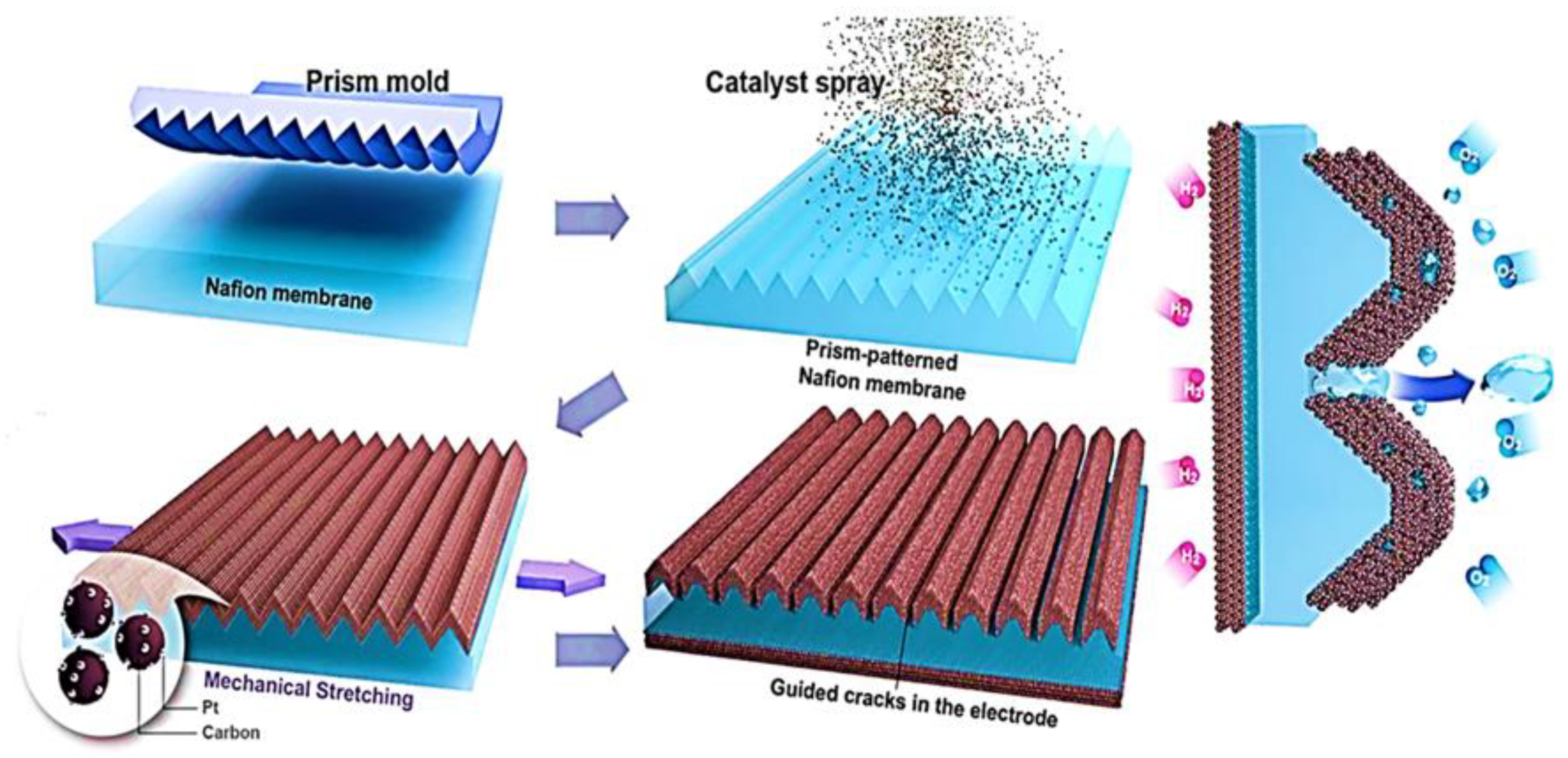
- Ahn, C.-Y.; Jang, S.; Cho, Y.-H.; Choi, J.; Kim, S.; Kim, S.M.; Sung, Y.-E.; Choi, M. Guided cracking of electrodes by stretching prism-patterned membrane electrode assemblies for high-performance fuel cells. Sci. Rep. 2018, 8, 1257. [Google Scholar] [CrossRef] [PubMed]
- Wilberforce, T.; Khatib, F.; Ijaodola, O.; Ogungbemi, E.; El-Hassan, Z.; Durrant, A.; Thompson, J.; Olabi, A. Numerical modelling and CFD simulation of a polymer electrolyte membrane (PEM) fuel cell flow channel using an open pore cellular foam material. Sci. Total Environ. 2019, 678, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Teng, H. Overview of the development of the fluoropolymer industry. Appl. Sci. 2012, 2, 496–512. [Google Scholar] [CrossRef]
- Kreuer, K. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 129–139. [Google Scholar] [CrossRef]
- Peinemann, K.-V.; Nunes, S.P. Membranes for Energy Conversion; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Tsujiguchi, T.; Abdelkareem, M.A.; Kudo, T.; Nakagawa, N.; Shimizu, T.; Matsuda, M. Development of a passive direct methanol fuel cell stack for high methanol concentration. J. Power Sources 2010, 195, 5975–5979. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Allagui, A.; Sayed, E.T.; El Haj Assad, M.; Said, Z.; Elsaid, K. Comparative analysis of liquid versus vapor-feed passive direct methanol fuel cells. Renew. Energy 2019, 131, 563–584. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Masdar, M.S.; Tsujiguchi, T.; Nakagawa, N.; Sayed, E.T.; Barakat, N.A.M. Elimination of toxic products formation in vapor-feed passive DMFC operated by absolute methanol using air cathode filter. Chem. Eng. J. 2014, 240, 38–44. [Google Scholar] [CrossRef]
- Peron, J.; Mani, A.; Zhao, X.; Edwards, D.; Adachi, M.; Soboleva, T.; Shi, Z.; Xie, Z.; Navessin, T.; Holdcroft, S. Properties of Nafion® NR-211 membranes for PEMFCs. J. Membr. Sci. 2010, 356, 44–51. [Google Scholar] [CrossRef]
- Alnaqbi, H.; Sayed, E.T.; Al-Asheh, S.; Bahaa, A.; Alawadhi, H.; Abdelkareem, M.A. Current progression in graphene-based membranes for low temperature fuel cells. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Ke, Y.; Yuan, W.; Zhou, F.; Guo, W.; Li, J.; Zhuang, Z.; Su, X.; Lu, B.; Zhao, Y.; Tang, Y.; et al. A critical review on surface-pattern engineering of nafion membrane for fuel cell applications. Renew. Sustain. Energy Rev. 2021, 145, 110860. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in hybrid composite Nafion®-based membranes for proton exchange fuel cell application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Huo, Y.; Li, Q.; Rui, Z.; Ding, R.; Liu, J.; Li, J.; Liu, J. A highly stable reinforced PEM assisted by resveratrol and polydopamine-treated PTFE. J. Membr. Sci. 2021, 635, 119453. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Z.; Wang, L.; Chen, X.; Shi, S. Fracture property of Nafion XL composite membrane determined by R-curve method. J. Power Sources 2018, 398, 34–41. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Bae, J.W.; Cho, Y.-H.; Lim, J.W.; Ahn, M.; Yoon, W.-S.; Kwon, N.-H.; Jho, J.Y.; Sung, Y.-E. Performance enhancement of membrane electrode assemblies with plasma etched polymer electrolyte membrane in PEM fuel cell. Int. J. Hydrogen Energy 2010, 35, 10452–10456. [Google Scholar] [CrossRef]
- Zakil, F.A.; Kamarudin, S.K.; Basri, S. Modified Nafion membranes for direct alcohol fuel cells: An overview. Renew. Sustain. Energy Rev. 2016, 65, 841–852. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Panero, S.; Navarra, M.A. Polymer electrolyte membranes based on nafion and a superacidic inorganic additive for fuel cell applications. Polymers 2019, 11, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.; Oh, K.; Park, J.; Park, S.; Lee, T.G.; Son, B. Morphological structure of silica sulfuric acid and Nafion composite membrane using electrostatic force microscopy. Int. J. Energy Res. 2021, 45, 21195–21208. [Google Scholar] [CrossRef]
- Zanchet, L.; da Trindade, L.G.; Trombetta, F.; Martins, A.D.; Martini, E.M.A.; Becker, M.R.; de Souza, M.O. Improving Nafion/zeolite nanocomposite with a CF3SO3 based ionic liquid for PEMFC application. Ionics 2021, 27, 2027–2036. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Barbir, F. Main cell components, materials properties and processes. In PEM Fuel Cells: Theory and Practice; Elsevier Academic Press: New York, NY, USA, 2005; pp. 73–113. [Google Scholar]
- Malekian, A.; Salari, S.; Stumper, J.; Djilali, N.; Bahrami, M. Effect of compression on pore size distribution and porosity of PEM fuel cell catalyst layers. Int. J. Hydrogen Energy 2019, 44, 23396–23405. [Google Scholar] [CrossRef]
- Litster, S.; McLean, G. PEM fuel cell electrodes. J. Power Sources 2004, 130, 61–76. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Abdelghani, E.A.M.; Olabi, A.G. Transition metal carbides and nitrides as oxygen reduction reaction catalyst or catalyst support in proton exchange membrane fuel cells (PEMFCs). Int. J. Hydrogen Energy 2021, 46, 23529–23547. [Google Scholar] [CrossRef]
- Al-Dhaifallah, M.; Abdelkareem, M.A.; Rezk, H.; Alhumade, H.; Nassef, A.M.; Olabi, A.G. Co-decorated reduced graphene/titanium nitride composite as an active oxygen reduction reaction catalyst with superior stability. Int. J. Energy Res. 2021, 45, 1587–1598. [Google Scholar] [CrossRef]
- Wee, J.-H.; Lee, K.-Y.; Kim, S.H. Fabrication methods for low-Pt-loading electrocatalysts in proton exchange membrane fuel cell systems. J. Power Sources 2007, 165, 667–677. [Google Scholar] [CrossRef]
- Jayasayee, K.; Veen, J.A.R.V.; Manivasagam, T.G.; Celebi, S.; Hensen, E.J.M.; de Bruijn, F.A. Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: Influence of particle size and non-noble metals. Appl. Catal. B Environ. 2012, 111, 515–526. [Google Scholar] [CrossRef]
- Obradović, M.D.; Gojković, S.L. CO tolerant Pt/Ru0.7Ti0.3O2 nanocatalyst for hydrogen oxidation reaction. Zaštita Mater. 2018, 59, 265–272. [Google Scholar] [CrossRef]
- Lin, R.; Che, L.; Shen, D.; Cai, X. High durability of Pt-Ni-Ir/C ternary catalyst of PEMFC by stepwise reduction synthesis. Electrochim. Acta 2020, 330, 135251. [Google Scholar] [CrossRef]
- Kurdin, K.A.; Kuznetsov, V.V.; Sinitsyn, V.V.; Galitskaya, E.A.; Filatova, E.A.; Belina, C.A.; Stevenson, K.J. Synthesis and characterization of Pt-HxMoO3 catalysts for CO-tolerant PEMFCs. Catal. Today 2020, 388–389, 147–157. [Google Scholar]
- Zhou, Z.-M.; Shao, Z.-G.; Qin, X.-P.; Chen, X.-G.; Wei, Z.-D.; Yi, B.-L. Durability study of Pt–Pd/C as PEMFC cathode catalyst. Int. J. Hydrogen Energy 2010, 35, 1719–1726. [Google Scholar] [CrossRef]
- Mani, P.; Srivastava, R.; Strasser, P. Dealloyed binary PtM3 (M = Cu, Co, Ni) and ternary PtNi3M (M = Cu, Co, Fe, Cr) electrocatalysts for the oxygen reduction reaction: Performance in polymer electrolyte membrane fuel cells. J. Power Sources 2011, 196, 666–673. [Google Scholar] [CrossRef]
- Madkikar, P.; Menga, D.; Harzer, G.S.; Mittermeier, T.; Siebel, A.; Wagner, F.E.; Merz, M.; Schuppler, S.; Nagel, P.; Muñoz-García, A.B.; et al. Nanometric Fe-Substituted ZrO2 on carbon black as PGM-Free ORR catalyst for PEMFCs. J. Electrochem. Soc. 2019, 166, F3032–F3043. [Google Scholar] [CrossRef] [Green Version]
- Roh, G.; Lee, H.; Jeong, Y.; Kim, J.H.; Kim, H. Preparation of carbon-supported Pt–Ru core-shell nanoparticles using carbonized polydopamine and ozone for a CO tolerant electrocatalyst. Int. J. Hydrogen Energy 2019, 44, 21588–21596. [Google Scholar] [CrossRef]
- Yang, D.; Yan, Z.; Li, B.; Higgins, D.C.; Wang, J.; Lv, H.; Chen, Z.; Zhang, C. Highly active and durable Pt–Co nanowire networks catalyst for the oxygen reduction reaction in PEMFCs. Int. J. Hydrogen Energy 2016, 41, 18592–18601. [Google Scholar] [CrossRef]
- Antoniassi, R.M.; Quiroz, J.; Barbosa, E.C.M.; Parreira, L.S.; Isidoro, R.A.; Spinacé, E.V.; Silva, J.C.M.; Camargo, P.H.C. Improving the electrocatalytic activities and CO tolerance of Pt NPs by incorporating TiO2 nanocubes onto carbon supports. ChemCatChem 2021, 13, 1931–1939. [Google Scholar] [CrossRef]
- Xu, F.; Wang, D.; Sa, B.; Yu, Y.; Mu, S. One-pot synthesis of Pt/CeO2/C catalyst for improving the ORR activity and durability of PEMFC. Int. J. Hydrogen Energy 2017, 42, 13011–13019. [Google Scholar] [CrossRef]
- Iezzi, R.C.; Santos, R.D.; Da Silva, G.; Paganin, V.A.; Ticianelli, E.A. CO tolerance and stability of proton exchange membrane fuel cells with Nafion® and Aquivion® membranes and Mo-based anode electrocatalysts. J. Braz. Chem. Soc. 2018, 29, 1094–1104. [Google Scholar] [CrossRef]
- Mittermeier, T.; Madkikar, P.; Wang, X.; Gasteiger, H.A.; Piana, M. ZrO2 Based oxygen reduction catalysts for PEMFCs: Towards a better understanding. J. Electrochem. Soc. 2016, 163, F1543–F1552. [Google Scholar] [CrossRef]
- Yazici, M.S.; Dursun, S.; Borbáth, I.; Tompos, A. Reformate gas composition and pressure effect on CO tolerant Pt/Ti0.8Mo0.2O2–C electrocatalyst for PEM fuel cells. Int. J. Hydrogen Energy 2021, 46, 13524–13533. [Google Scholar] [CrossRef]
- Pizzutilo, E.; Knossalla, J.; Geiger, S.; Grote, J.-P.; Polymeros, G.; Baldizzone, C.; Mezzavilla, S.; Ledendecker, M.; Mingers, A.; Cherevko, S.; et al. The Space Confinement Approach Using Hollow Graphitic Spheres to Unveil Activity and Stability of Pt-Co Nanocatalysts for PEMFC. Adv. Energy Mater. 2017, 7, 1700835. [Google Scholar] [CrossRef]
- Todoroki, N.; Kato, T.; Hayashi, T.; Takahashi, S.; Wadayama, T. Pt–Ni nanoparticle-stacking thin film: Highly active electrocatalysts for oxygen reduction reaction. Acs Catal. 2015, 5, 2209–2212. [Google Scholar] [CrossRef]
- Neergat, M.; Shukla, A.; Gandhi, K. Platinum-based alloys as oxygen–reduction catalysts for solid–polymer–electrolyte direct methanol fuel cells. J. Appl. Electrochem. 2001, 31, 373–378. [Google Scholar] [CrossRef]
- Min, M.-K.; Cho, J.; Cho, K.; Kim, H. Particle size and alloying effects of Pt-based alloy catalysts for fuel cell applications. Electrochim. Acta 2000, 45, 4211–4217. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active sites and mechanism of oxygen reduction reaction electrocatalysis on nitrogen-doped carbon materials. Adv. Mater. 2019, 31, e1804297. [Google Scholar] [CrossRef] [PubMed]
- Jose, V.; Nsanzimana, J.M.V.; Hu, H.; Choi, J.; Wang, X.; Lee, J.-M. Highly efficient oxygen reduction reaction activity of N-Doped carbon–Cobalt boride heterointerfaces. Adv. Energy Mater. 2021, 11, 2100157. [Google Scholar] [CrossRef]
- Tan, H.; Tang, J.; Kim, J.; Kaneti, Y.V.; Kang, Y.-M.; Sugahara, Y.; Yamauchi, Y. Rational design and construction of nanoporous iron- and nitrogen-doped carbon electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2019, 7, 1380–1393. [Google Scholar] [CrossRef]
- Liu, Z.; Su, F.; Zhang, X.; Tay, S.W. Preparation and characterization of PtRu nanoparticles supported on nitrogen-doped porous carbon for electrooxidation of methanol. ACS Appl. Mater. Interfaces 2011, 3, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-P.; Lee, M.; Lee, Y.J.; Bae, S.; Song, H.D.; Song, I.K.; Yi, J. Polymer-mediated synthesis of a nitrogen-doped carbon aerogel with highly dispersed Pt nanoparticles for enhanced electrocatalytic activity. Electrochim. Acta 2016, 193, 137–144. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.-S.; Wang, H.; Shen, J. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. J. Power Sources 2007, 165, 2739–2756. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC: Part I. physico-chemical and electronic interaction between Pt and carbon support, and activity enhancement of Pt/C catalyst. J. Power Sources 2007, 172, 133–144. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Pérez, G.; Pastor, E.; Zinola, C. A novel Pt/Cr/Ru/C cathode catalyst for direct methanol fuel cells (DMFC) with simultaneous methanol tolerance and oxygen promotion. Int. J. Hydrogen Energy 2009, 34, 9523–9530. [Google Scholar] [CrossRef]
- Kiani, M.; Tian, X.Q.; Zhang, W. Non-precious metal electrocatalysts design for oxygen reduction reaction in polymer electrolyte membrane fuel cells: Recent advances, challenges and future perspectives. Coord. Chem. Rev. 2021, 441, 213954. [Google Scholar] [CrossRef]
- Zhang, J.; Shuihua, T.; Longyu, L.; Weifei, Y. Progress in non-platinum catalysts with applications in low temperature fuel cells. Chin. J. Catal. 2013, 34, 1051–1065. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Sayed, E.T.; Mohamed, H.O.; Obaid, M.; Rezk, H.; Chae, K.-J. Nonprecious anodic catalysts for low-molecular-hydrocarbon fuel cells: Theoretical consideration and current progress. Prog. Energy Combust. Sci. 2020, 77, 100805. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Sayed, E.T.; Nakagawa, N. Significance of diffusion layers on the performance of liquid and vapor feed passive direct methanol fuel cells. Energy 2020, 209, 118492. [Google Scholar] [CrossRef]
- Qiu, D.; Janßen, H.; Peng, L.; Irmscher, P.; Lai, X.; Lehnert, W. Electrical resistance and microstructure of typical gas diffusion layers for proton exchange membrane fuel cell under compression. Appl. Energy 2018, 231, 127–137. [Google Scholar] [CrossRef]
- Moreira, J.; Ocampo, A.; Sebastian, P.; Smit, M.A.; Salazar, M.; Del Angel, P.; Montoya, J.; Pérez, R.; Martınez, L. Influence of the hydrophobic material content in the gas diffusion electrodes on the performance of a PEM fuel cell. Int. J. Hydrogen Energy 2003, 28, 625–627. [Google Scholar] [CrossRef]
- Lee, W.-K.; Ho, C.-H.; Van Zee, J.; Murthy, M. The effects of compression and gas diffusion layers on the performance of a PEM fuel cell. J. Power Sources 1999, 84, 45–51. [Google Scholar] [CrossRef]
- Ge, J.; Higier, A.; Liu, H. Effect of gas diffusion layer compression on PEM fuel cell performance. J. Power Sources 2006, 159, 922–927. [Google Scholar] [CrossRef]
- Kandlikar, S.; Lu, Z.; Lin, T.; Cooke, D.; Daino, M. Uneven gas diffusion layer intrusion in gas channel arrays of proton exchange membrane fuel cell and its effects on flow distribution. J. Power Sources 2009, 194, 328–337. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Rapaport, P.A.; Ji, C.; Kumar, V. Channel intrusion of gas diffusion media and the effect on fuel cell performance. J. Power Sources 2008, 184, 120–128. [Google Scholar] [CrossRef]
- Li, H.; Tang, Y.; Wang, Z.; Shi, Z.; Wu, S.; Song, D.; Zhang, J.; Fatih, K.; Zhang, J.; Wang, H. A review of water flooding issues in the proton exchange membrane fuel cell. J. Power Sources 2008, 178, 103–117. [Google Scholar] [CrossRef]
- Santoro, C.; Agrios, A.; Pasaogullari, U.; Li, B. Effects of gas diffusion layer (GDL) and micro porous layer (MPL) on cathode performance in microbial fuel cells (MFCs). Int. J. Hydrogen Energy 2011, 36, 13096–13104. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-W.; Popov, B.N. A review of gas diffusion layer in PEM fuel cells: Materials and designs. Int. J. Hydrogen Energy 2012, 37, 5850–5865. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Zhang, J.; Xu, H.; Tian, Z.; Chen, J.; Zhong, H.; Liang, Y.; Yi, B. Micro-porous layer with composite carbon black for PEM fuel cells. Electrochim. Acta 2006, 51, 4909–4915. [Google Scholar] [CrossRef]
- Mustafa, M. Design and Manufacturing of a (PEMFC) Proton Exchange Membrane Fuel Cell. Ph.D. Thesis, Coventry University, Coventry, UK, 2009. [Google Scholar]
- Mathur, R.; Dhakate, S.; Gupta, D.; Dhami, T.; Aggarwal, R. Effect of different carbon fillers on the properties of graphite composite bipolar plate. J. Mater. Processing Technol. 2008, 203, 184–192. [Google Scholar] [CrossRef]
- Wilberforce, T.; El-Hassan, Z.; Khatib, F.; Al Makky, A.; Baroutaji, A.; Carton, J.G.; Olabi, A.G. Developments of electric cars and fuel cell hydrogen electric cars. Int. J. Hydrogen Energy 2017, 42, 25695–25734. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-L.; He, S.-M.; Lan, C.-H. Protective graphite coating on metallic bipolar plates for PEMFC applications. Electrochimica Acta 2012, 62, 30–35. [Google Scholar] [CrossRef]
- US Department of Energy (DOE). Technical Targets for Polymer Electrolyte Membrane Fuel Cell Components; US Department of Energy: Washington, DC, USA, 2019.
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.-G. Advances in stationary and portable fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Turner, J.A. Ferritic stainless steels as bipolar plate material for polymer electrolyte membrane fuel cells. J. Power Sources 2004, 128, 193–200. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Liang, X.; Luo, F.; Cheng, R.; Gong, F. Fabrication of metallic bipolar plate for proton exchange membrane fuel cells by using polymer powder medium based flexible forming. J. Mater. Processing Technol. 2018, 262, 32–40. [Google Scholar] [CrossRef]
- Lee, S.-J.; Huang, C.-H.; Lai, J.-J.; Chen, Y.-P. Corrosion-resistant component for PEM fuel cells. J. Power Sources 2004, 131, 162–168. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Zeng, C.; Shen, J.; Lin, H. Corrosion behavior of TiN coated type 316 stainless steel in simulated PEMFC environments. Corros. Sci. 2004, 46, 1369–1380. [Google Scholar] [CrossRef]
- Mi, B.; Chen, Z.; Wang, Q.; Li, Y.; Qin, Z.; Wang, H. Properties of C-doped CrTiN films on the 316L stainless steel bipolar plate for PEMFC. Int. J. Hydrogen Energy 2021, 46, 32645–32654. [Google Scholar] [CrossRef]
- Chanda, U.K.; Behera, A.; Roy, S.; Pati, S. Evaluation of Ni-Cr-P coatings electrodeposited on low carbon steel bipolar plates for polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energy 2018, 43, 223430–223440. [Google Scholar] [CrossRef]
- Maeda, K.; Childs, T. Laser sintering (SLS) of hard metal powders for abrasion resistant coatings. J. Mater. Processing Technol. 2004, 149, 609–615. [Google Scholar] [CrossRef]
- Woodman, A.; Jayne, K.; Anderson, E.; Kimble, M.C. Development of Corrosion-Resistant Coatings for Fuel Cell Bipolar Plates; American Electroplaters and Surface Finishers Society Inc.: Orlando, FL, USA, 1999; pp. 717–726. [Google Scholar]
- Li, Q.; Liu, Z.; Sun, Y.; Yang, S.; Deng, C. A review on temperature control of proton exchange membrane fuel cells. Processes 2021, 9, 235. [Google Scholar] [CrossRef]
- Thiruchitrambalam, M.; Kumar, D.B.; Shanmugam, D.; Jawaid, M. A review on PEEK composites—Manufacturing methods, properties and applications. Mater. Today Proc. 2020, 33, 1085–1092. [Google Scholar] [CrossRef]
- Lim, J.W. Carbon composite hybrid bipolar plates with bypass-connected gas diffusion layers for PEM fuel cells. Compos. Struct. 2013, 95, 557–563. [Google Scholar] [CrossRef]
- Tseng, C.-J.; Tsai, B.T.; Liu, Z.-S.; Cheng, T.-C.; Chang, W.-C.; Lo, S.-K. A PEM fuel cell with metal foam as flow distributor. Energy Convers. Manag. 2012, 62, 14–21. [Google Scholar] [CrossRef]
- Afshari, E.; Ziaei-Rad, M.; Shariati, Z. A study on using metal foam as coolant fluid distributor in the polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energy 2016, 41, 1902–1912. [Google Scholar] [CrossRef]
- Tseng, C.-J.; Heush, Y.-J.; Chiang, C.-J.; Lee, Y.-H.; Lee, K.-R. Application of metal foams to high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 16196–16204. [Google Scholar] [CrossRef]
- Baroutaji, A.; Carton, J.; Stokes, J.; Olabi, A.-G. Application of open pore cellular foam for air breathing PEM fuel cell. Int. J. Hydrogen Energy 2017, 42, 25630–25638. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; Khatib, F.; Leslie, T.; El-Hassan, Z.; Thomposon, J.; Olabi, A. Technical evaluation of proton exchange membrane (PEM) fuel cell performance—A review of the effects of bipolar plates coating. Renew. Sustain. Energy Rev. 2019, 113, 109286. [Google Scholar] [CrossRef]
- Awin, Y.; Dukhan, N. Experimental performance assessment of metal-foam flow fields for proton exchange membrane fuel cells. Appl. Energy 2019, 252, 113458. [Google Scholar] [CrossRef]
- Awin, Y.A. Design and Evaluation of Bipolar Plates of a Proton Exchange Membrane Fuel Cell Using Metal Foam. Ph.D. Thesis, University of Detroit Mercy, Detroit, MI, USA, 2019. [Google Scholar]
- Zhang, Y.; Tao, Y.; Shao, J. Application of porous materials for the flow field in polymer electrolyte membrane fuel cells. J. Power Sources 2021, 492, 229664. [Google Scholar] [CrossRef]
- Ting, F.-P.; Hsieh, C.-W.; Weng, W.-H.; Lin, J.-C. Effect of operational parameters on the performance of PEMFC assembled with Au-coated Ni-foam. Int. J. Hydrogen Energy 2012, 37, 13696–13703. [Google Scholar] [CrossRef]
- Afshari, E.; Houreh, N.B. Performance analysis of a membrane humidifier containing porous metal foam as flow distributor in a PEM fuel cell system. Energy Convers. Manag. 2014, 88, 612–621. [Google Scholar] [CrossRef]
- Carton, J.; Olabi, A.-G. Three-dimensional proton exchange membrane fuel cell model: Comparison of double channel and open pore cellular foam flow plates. Energy 2017, 136, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Carton, J.; Olabi, A.-G. Representative model and flow characteristics of open pore cellular foam and potential use in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2015, 40, 5726–5738. [Google Scholar] [CrossRef] [Green Version]
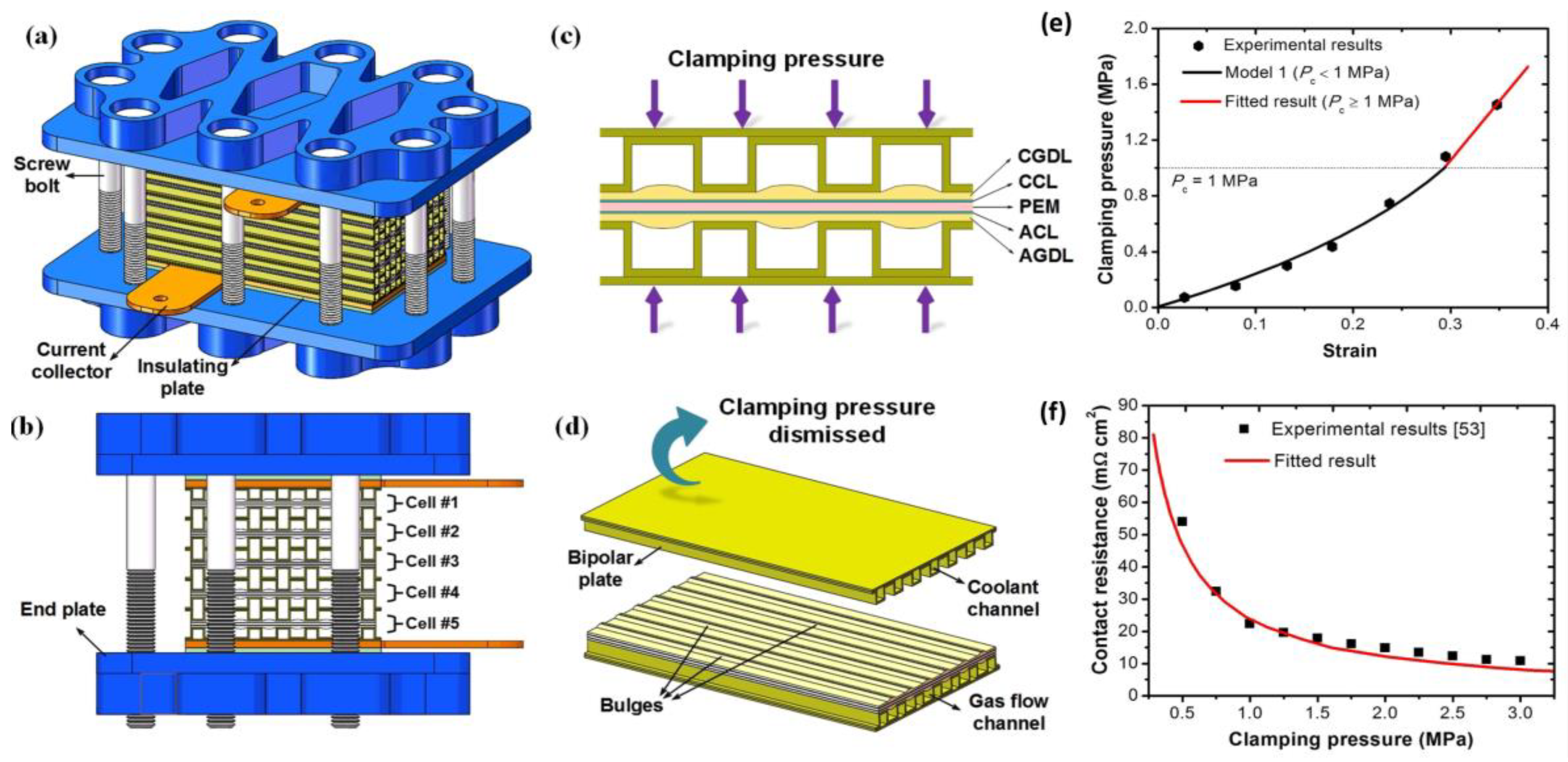
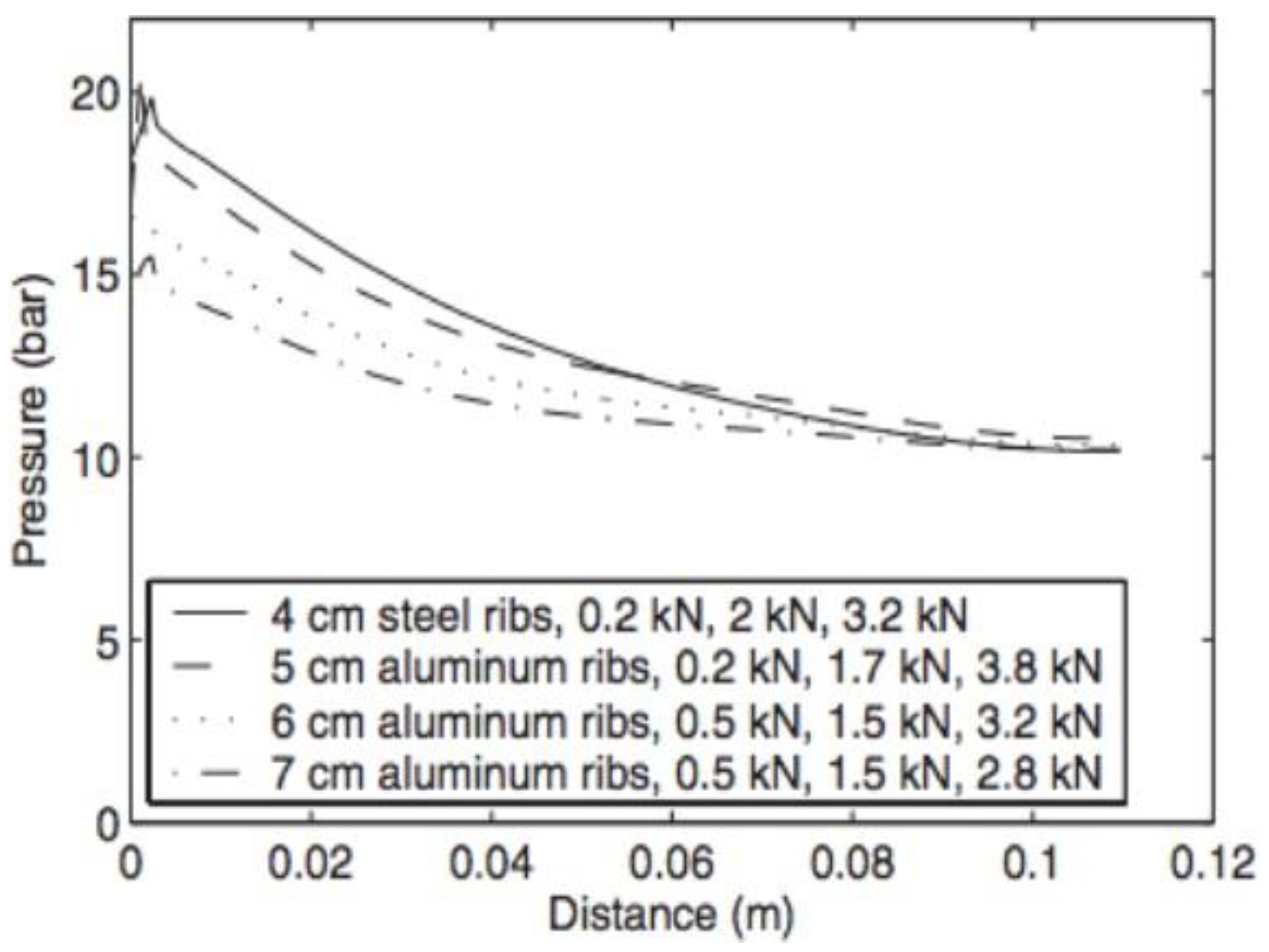
- Yan, X.; Lin, C.; Zheng, Z.; Chen, J.; Wei, G.; Zhang, J. Effect of clamping pressure on liquid-cooled PEMFC stack performance considering inhomogeneous gas diffusion layer compression. Appl. Energy 2020, 258, 114073. [Google Scholar] [CrossRef]
- Asghari, S.; Shahsamandi, M.; Khorasani, M.A. Design and manufacturing of end plates of a 5 kW PEM fuel cell. Int. J. Hydrogen Energy 2010, 35, 9291–9297. [Google Scholar] [CrossRef]
- Montanini, R.; Squadrito, G.; Giacoppo, G. Experimental evaluation of the clamping pressure distribution in a PEM fuel cell using matrix-based piezoresistive thin-film sensors. In Proceedings of the XIX IMEKO World Congress Fundamental and Applied Metrology, Lisbon, Portugal, 6–11 September 2009. [Google Scholar]
- Giacoppo, G.; Hovland, S.; Barbera, O. 2 kW Modular PEM fuel cell stack for space applications: Development and test for operation under relevant conditions. Appl. Energy 2019, 242, 1683–1696. [Google Scholar] [CrossRef]
- Jo, M.; Cho, H.-S.; Na, Y. Comparative analysis of circular and square end plates for a highly pressurized proton exchange membrane water electrolysis stack. Appl. Sci. 2020, 10, 6315. [Google Scholar] [CrossRef]
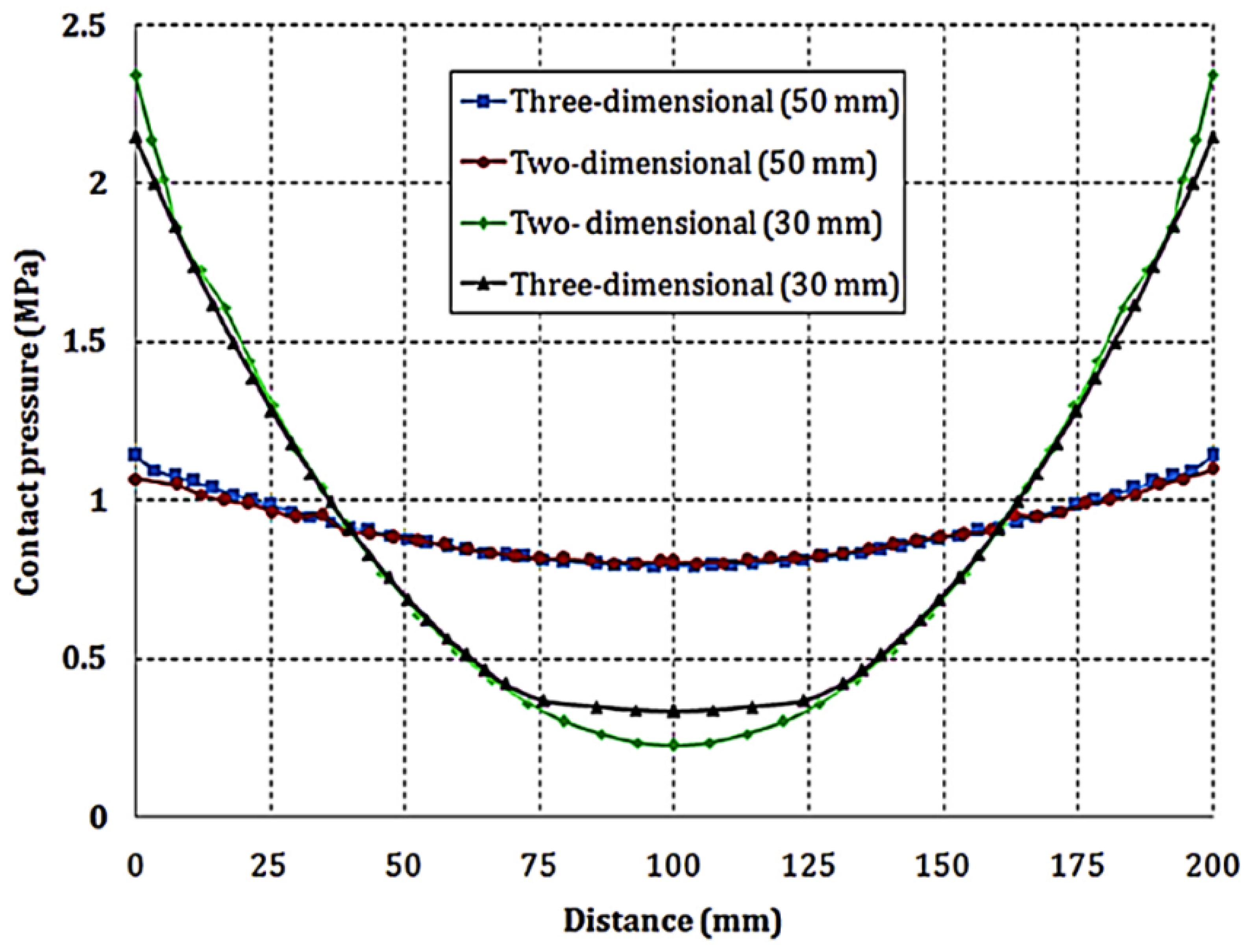
- Zhou, P.; Lin, P.; Wu, C.; Li, Z. Effect of nonuniformity of the contact pressure distribution on the electrical contact resistance in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 6039–6044. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Song, H.; Wang, S.; Zhou, Y.; Hu, S.J. Estimation of contact resistance in proton exchange membrane fuel cells. J. Power Sources 2006, 162, 1165–1171. [Google Scholar] [CrossRef]
- Bouziane, K.; Lachat, R.; Zamel, N.; Meyer, Y.; Candusso, D. Impact of cyclic mechanical compression on the electrical contact resistance between the gas diffusion layer and the bipolar plate of a polymer electrolyte membrane fuel cell. Renew. Energy 2020, 153, 349–361. [Google Scholar] [CrossRef]
- Liang, P.; Qiu, D.; Peng, L.; Yi, P.; Lai, X.; Ni, J. Contact resistance prediction of proton exchange membrane fuel cell considering fabrication characteristics of metallic bipolar plates. Energy Convers. Manag. 2018, 169, 334–344. [Google Scholar] [CrossRef]
- Thompson, G. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Film. 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Lai, X.; Peng, L.; Ni, J. A mechanical–electrical finite element method model for predicting contact resistance between bipolar plate and gas diffusion layer in PEM fuel cells. J. Power Sources 2008, 182, 153–159. [Google Scholar] [CrossRef]
- Alizadeh, E.; Barzegari, M.; Momenifar, M.; Ghadimi, M.; Saadat, S. Investigation of contact pressure distribution over the active area of PEM fuel cell stack. Int. J. Hydrogen Energy 2016, 41, 3062–3071. [Google Scholar] [CrossRef]
- Karvonen, S.; Hottinen, T.; Ihonen, J.; Uusalo, H. Modeling of polymer electrolyte membrane fuel cell stack end plates. J. Fuel Cell Sci. Technol. 2008, 5, 041009. [Google Scholar] [CrossRef]
- Stein, T.; Ein-Eli, Y. Challenges and Perspectives of Metal-Based Proton Exchange Membrane’s Bipolar Plates: Exploring Durability and Longevity. Energy Technol. 2020, 8, 2000007. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lim, J.W. Composite sandwich endplates with a compliant pressure distributor for a PEM fuel cell. Compos. Struct. 2015, 119, 505–512. [Google Scholar] [CrossRef]
- Yu, H.N.; Kim, S.S.; Suh, J.D.; Lee, D.G. Composite endplates with pre-curvature for PEMFC (polymer electrolyte membrane fuel cell). Compos. Struct. 2010, 92, 1498–1503. [Google Scholar] [CrossRef]
- Patermarakis, G.; Papandreadis, N. Effect of the structure of porous anodic Al2O3 films on the mechanism of their hydration and pore closure during hydrothermal treatment. Electrochim. Acta 1993, 38, 1413–1420. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Chaedir, B.A.; Sasmito, A.P.; Shamim, T. Progress on open cathode proton exchange membrane fuel cell: Performance, designs, challenges and future directions. Appl. Energy 2020, 283, 116359. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.-Y.; Han, M.; Yong, R.-Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Han, J.; Nguyen, X.L.; Yu, S.; Goo, Y.-M.; Le, D.D. Review of the durability of polymer electrolyte membrane fuel cell in long-term operation: Main influencing parameters and testing protocols. Energies 2021, 14, 4048. [Google Scholar] [CrossRef]
- Ahmadi Sarbast, V. Modeling of Proton Exchange Membrane Fuel Cell Performance Degradation and Operation Life. Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 2021. [Google Scholar]
- Javed, S.; Noreen, R.; Kamal, S.; Rehman, S.; Yaqoob, N.; Abrar, S. Polymer blends as matrix materials for the preparation of the nanocomposites. In Bionanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–54. [Google Scholar]
- Yang, S.; Jiang, K. Elastomer application in microsystem and microfluidics. In Advanced Elastomers-Technology, Properties and Applications; IntechOpen: London, UK, 2012. [Google Scholar]
- Bieringer, R.; Adler, M.; Geiss, S.; Viol, M. Gaskets: Important durability issues. In Polymer Electrolyte Fuel Cell Durability; Springer: Berlin/Heidelberg, Germany, 2009; pp. 271–281. [Google Scholar]
- Lee, D.; Lim, J.W.; Nam, S.; Choi, I. Gasket-integrated carbon/silicone elastomer composite bipolar plate for high-temperature PEMFC. Compos. Struct. 2015, 128, 284–290. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, M.; Kim, K.H. Innovative gasketless carbon composite bipolar plates for PEM fuel cells. Int. J. Hydrogen Energy 2012, 37, 19018–19026. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Nayoze-Coynel, C.; Shaigan, N.; Fisher, D.; Zhao, N.; Zamel, N.; Gazdzicki, P.; Ulsh, M.; Friedrich, K.A.; Girard, F. A review of functions, attributes, properties and measurements for the quality control of proton exchange membrane fuel cell components. J. Power Sources 2021, 491, 229540. [Google Scholar] [CrossRef]
- Tan, J.; Chao, Y.; Van Zee, J.; Lee, W. Degradation of elastomeric gasket materials in PEM fuel cells. Mater. Sci. Eng. A 2007, 445, 669–675. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chien, C.-H.; Tan, J.; Chao, Y.J.; Van Zee, J. Chemical degradation of five elastomeric seal materials in a simulated and an accelerated PEM fuel cell environment. J. Power Sources 2011, 196, 1955–1966. [Google Scholar] [CrossRef]
- Frisch, L. PEM fuel cell stack sealing using silicone elastomers. Seal. Technol. 2001, 2001, 7–9. [Google Scholar] [CrossRef]
- Öztürk, A.; Özçelik, N.; Yurtcan, A.B. Platinum/graphene nanoplatelets/silicone rubber composites as polymer electrolyte membrane fuel cell catalysts. Mater. Chem. Phys. 2021, 260, 124110. [Google Scholar] [CrossRef]
- Bhargava, S.; O’Leary, K.; Jackson, T.; Lakshmanan, B. Durability testing of silicone materials for proton exchange membrane fuel cell use. Rubber Chem. Technol. 2013, 86, 128–137. [Google Scholar] [CrossRef]
- Choudhury, B.; Escobedo, G.; Curtin, D.E.; Banerjee, S. PEM hydrogen fuel cell durability: A perspective review and recent advances. Transw. Res. Netw. 2008, 37661, 695–723. [Google Scholar]
- Prasad, K.; Nikzad, M.; Sbarski, I. Permeability control in polymeric systems: A review. J. Polym. Res. 2018, 25, 11–20. [Google Scholar] [CrossRef]
- Wu, F.; Chen, B.; Yan, Y.; Chen, Y.; Pan, M. Degradation of silicone rubbers as sealing materials for proton exchange membrane fuel cells under temperature cycling. Polymers 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basuli, U.; Jose, J.; Lee, R.H.; Yoo, Y.H.; Jeong, K.-U.; Ahn, J.-H.; Nah, C. Properties and degradation of the gasket component of a proton exchange membrane fuel cell—A review. J. Nanosci. Nanotechnol. 2012, 12, 7641–7657. [Google Scholar] [CrossRef]
- Herring, A.M.; Motz, A.R.; Pandey, T.P.; Kuo, M.-C. Fuel Cell Membranes with Enhanced Durability and Performance Based on Fluoroelastomers Functionalized with Heteropoly Acids. In Proceedings of the 2017 AIChE Annual Meeting, Minneapolis, MN, USA, 3 November 2017. [Google Scholar]
- Motz, A.R.; Kuo, M.-C.; Horan, J.L.; Yadav, R.; Seifert, S.; Pandey, T.P.; Galioto, S.; Yang, Y.; Dale, N.V.; Hamrock, S.J. Heteropoly acid functionalized fluoroelastomer with outstanding chemical durability and performance for vehicular fuel cells. Energy Environ. Sci. 2018, 11, 1499–1509. [Google Scholar] [CrossRef]
- Tailor, R.B.; Ramachandran, M.; Raichurkar, P. Review on Non-Woven polymeric Gaskets their Characteristics and Applications. Int. J. Text. Eng. Process. 2017, 3, 14–21. [Google Scholar]
- Moore, A.L.; Drobny, J.G. Fluoroelastomers Handbook: The Definitive User’s Guide and Databook; Taylor & Francis: Oxfordshire, UK, 2006. [Google Scholar]
- Klingender, R.C. Handbook of Specialty Elastomers; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Yang, C.; Fischer, L.; Maranda, S.; Worlitschek, J. Rigid polyurethane foams incorporated with phase change materials: A state-of-the-art review and future research pathways. Energy Build. 2015, 87, 25–36. [Google Scholar] [CrossRef]
- Li, G.; Gong, J.-M.; Tan, J.-Z.; Zhu, D.-S.; Jia, W.-H. Stress relaxation behavior and life prediction of gasket materials used in proton exchange membrane fuel cells. J. Cent. South Univ. 2019, 26, 623–631. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, J.; Wang, Y.; Liu, Z.; Feng, Q. Chemical and mechanical degradation of silicone rubber under two compression loads in simulated proton-exchange membrane fuel-cell environments. J. Appl. Polym. Sci. 2019, 136, 47855. [Google Scholar] [CrossRef]
- Guzmán-Sánchez, M.A. Comparative study of stress relaxation phenomenological constitutive modeling of carbon black-reinforced natural rubber-based compounds. Dyna 2021, 88, 55–61. [Google Scholar] [CrossRef]
- Qiu, D.; Liang, P.; Peng, L.; Yi, P.; Lai, X.; Ni, J. Material behavior of rubber sealing for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2020, 45, 5465–5473. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The temperature dependence of relaxation mechanisms in amorphous polymers and other glass-forming liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Unsworth, G.; Li, X. Development of a low temperature decal transfer method for the fabrication of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 11813–11822. [Google Scholar] [CrossRef]
- Xu, Z.; Qiu, D.; Yi, P.; Peng, L.; Lai, X. Towards mass applications: A review on the challenges and developments in metallic bipolar plates for PEMFC. Prog. Nat. Sci. Mater. Int. 2020, 30, 815–824. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Li, X. Impact of manufacturing processes on proton exchange membrane fuel cell performance. Appl. Energy 2018, 225, 1022–1032. [Google Scholar] [CrossRef]
- Passalacqua, E.; Squadrito, G.; Lufrano, F.; Patti, A.; Giorgi, L. Effects of the diffusion layer characteristics on the performance of polymer electrolyte fuel cell electrodes. J. Appl. Electrochem. 2001, 31, 449–454. [Google Scholar] [CrossRef]
- Bonifácio, R.N.; Paschoal, J.O.A.; Linardi, M.; Cuenca, R. Catalyst layer optimization by surface tension control during ink formulation of membrane electrode assemblies in proton exchange membrane fuel cell. J. Power Sources 2011, 196, 4680–4685. [Google Scholar] [CrossRef] [Green Version]
- Holdcroft, S. Fuel cell catalyst layers: A polymer science perspective. Chem. Mater. 2014, 26, 381–393. [Google Scholar] [CrossRef]
- Cavarroc, M.; Ennadjaoui, A.; Mougenot, M.; Brault, P.; Escalier, R.; Tessier, Y.; Durand, J.; Roualdès, S.; Sauvage, T.; Coutanceau, C. Performance of plasma sputtered fuel cell electrodes with ultra-low Pt loadings. Electrochem. Commun. 2009, 11, 859–861. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Garcia-Ybarra, P.; Castillo, J. Electrospray deposition of catalyst layers with ultra-low Pt loadings for PEM fuel cells cathodes. J. Power Sources 2010, 195, 2443–2449. [Google Scholar] [CrossRef]
- Benitez, R.; Soler, J.; Daza, L. Novel method for preparation of PEMFC electrodes by the electrospray technique. J. Power Sources 2005, 151, 108–113. [Google Scholar] [CrossRef]
- Bladergroen, B.; Su, H.; Pasupathi, S.; Linkov, V. Overview of Membrane Electrode Assembly Preparation Methods for Solid Polymer Electrolyte Electrolyzer; InTech: Houston, TX, USA, 2012. [Google Scholar]
- Fofana, D.; Natarajan, S.K.; Bénard, P.; Hamelin, J. High performance PEM fuel cell with low platinum loading at the cathode using magnetron sputter deposition. Int. Sch. Res. Not. 2013, 2013, 174834. [Google Scholar] [CrossRef]
- Towne, S.; Viswanathan, V.; Holbery, J.; Rieke, P. Fabrication of polymer electrolyte membrane fuel cell MEAs utilizing inkjet print technology. J. Power Sources 2007, 171, 575–584. [Google Scholar] [CrossRef]
- Pai, Y.-H.; Huang, H.-F.; Chang, Y.-C.; Chou, C.-C.; Shieu, F.-S. Electron-beam reduction method for preparing electrocatalytic particles for membrane electrode assemblies (MEA). J. Power Sources 2006, 159, 878–884. [Google Scholar] [CrossRef]
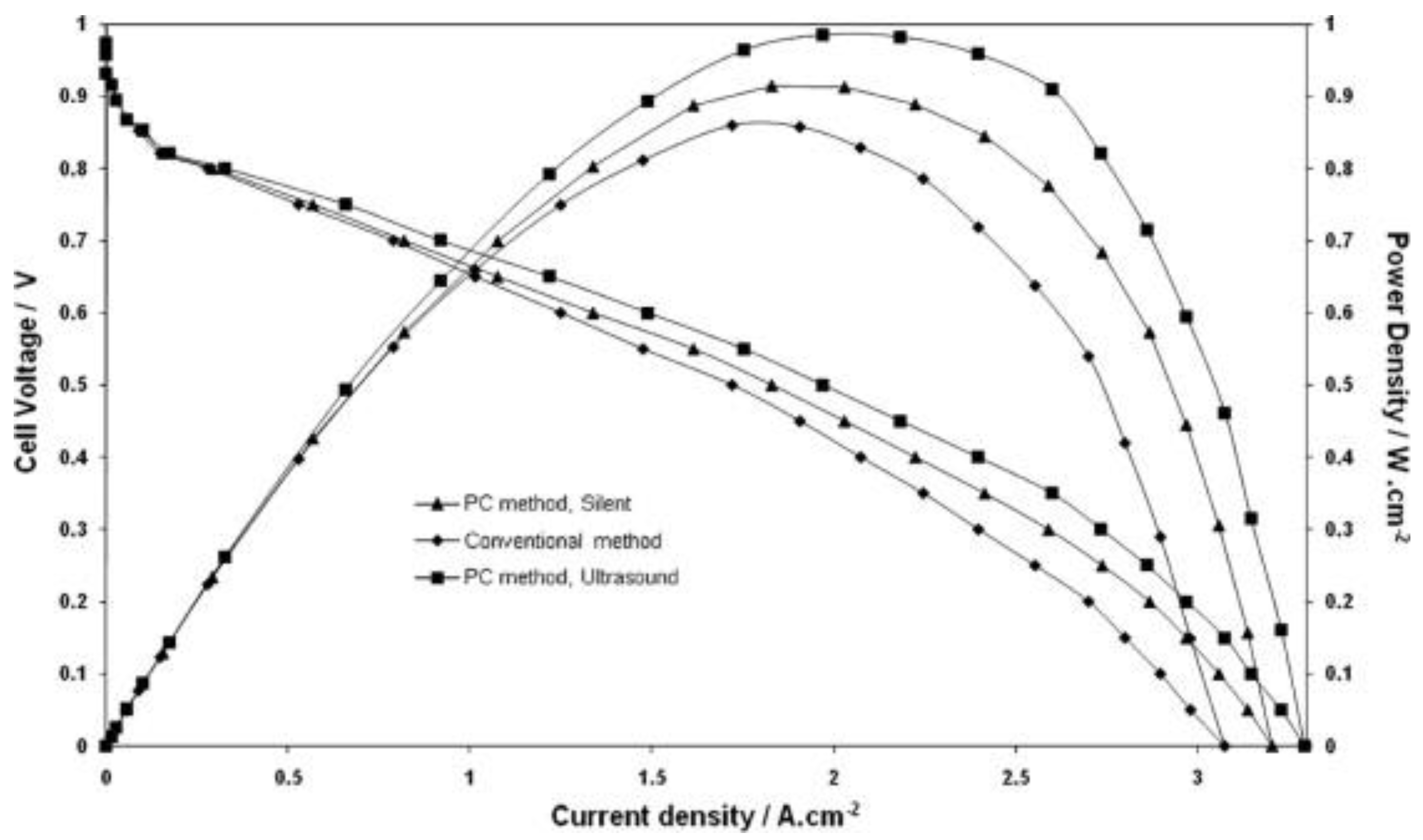
- Pollet, B.G. The use of power ultrasound for the production of PEMFC and PEMWE catalysts and low-Pt loading and high-performing electrodes. Catalysts 2019, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Foroughi, F.; Lamb, J.J.; Burheim, O.S.; Pollet, B.G. Sonochemical and sonoelectrochemical production of energy materials. Catalysts 2021, 11, 284. [Google Scholar] [CrossRef]
- Pollet, B.G. A novel method for preparing PEMFC electrodes by the ultrasonic and sonoelectrochemical techniques. Electrochem. Commun. 2009, 11, 1445–1448. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; Yan, H.; Zuo, X.; Cao, Z.; Li, H.; Shi, M.; Chen, H. A review of modified metal bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 8672–8701. [Google Scholar] [CrossRef]
- Meissner, R.; Irgang, M.; Eger, K.; Weidlich, P.; Dreyer, H. Graphite Moldings. U.S. Patent 5,736,076, 7 April 1998. [Google Scholar]
- San, F.G.B.; Okur, O. The effect of compression molding parameters on the electrical and physical properties of polymer composite bipolar plates. Int. J. Hydrogen Energy 2017, 42, 23054–23069. [Google Scholar]
- Kuan, Y.-D.; Ciou, C.-W.; Shen, M.-Y.; Wang, C.-K.; Fitriani, R.Z.; Lee, C.-Y. Bipolar plate design and fabrication using graphite reinforced composite laminate for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 16801–16814. [Google Scholar] [CrossRef]
- Yu, H.N.; Lim, J.W.; Kim, S.H.; Do Suh, J.; Ahn, B.K. Composite Separator for Polymer Electrolyte Membrane Fuel Cell and Method for Manufacturing the Same. U.S. Patent US9147889B2, 29 September 2015. [Google Scholar]
- Tran, T.D.; Huang, S.; Vu, D.H.; Duy, V.N. Effects of gas channel design on water management and on the performance of polymer electrolyte membrane fuel cells: A review. Int. J. Electrochem. Sci 2018, 13, 10480–10495. [Google Scholar] [CrossRef]
- Dundar, F.; Dur, E.; Mahabunphachai, S.; Koc, M. Corrosion resistance characteristics of stamped and hydroformed proton exchange membrane fuel cell metallic bipolar plates. J. Power Sources 2010, 195, 3546–3552. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, D.; Fu, H.; Huang, K. Investigation of stamping process of metallic bipolar plates in PEM fuel cell—Numerical simulation and experiments. Int. J. Hydrogen Energy 2014, 39, 13770–13776. [Google Scholar] [CrossRef]
- Khatir, F.A.; Elyasi, M.; Ghadikolaee, H.T.; Hosseinzadeh, M. Evaluation of effective parameters on stamping of metallic bipolar plates. Procedia Eng. 2017, 183, 322–329. [Google Scholar] [CrossRef]
- Bell, C.; Corney, J.; Zuelli, N. A state of the art review of hydroforming technology. Int. J. Mater. Form. 2020, 13, 789–828. [Google Scholar] [CrossRef] [Green Version]
- Mahabunphachai, S.; Cora, Ö.N.; Koç, M. Effect of manufacturing processes on formability and surface topography of proton exchange membrane fuel cell metallic bipolar plates. J. Power Sources 2010, 195, 5269–5277. [Google Scholar] [CrossRef]
- Peng, L.; Lai, X.; Hu, P.; Ni, J. Flow channel shape optimum design for hydroformed metal bipolar plate in PEM fuel cell. J. Power Sources 2008, 178, 223–230. [Google Scholar] [CrossRef]
- Osia, M.B.; Hosseinipour, S.J.; Bakhshi-Jooybari, M.; Gorgi, A. Forming metallic micro-feature bipolar plates for fuel cell using combined hydroforming and stamping processes. Iran. J. Energy Environ. 2013, 4, 91–98. [Google Scholar]
- Lee, J.; Kwon, H.; Rhee, M.; Im, Y.-T. Determination of forming limit of a structural aluminum tube in rubber pad bending. J. Mater. Processing Technol. 2003, 140, 487–493. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Yadav, D.; Bhu, I. Review of rubber based sheet hydro-forming processes. In Proceedings of the 5th International and 26th All India Manufacturing Technology, Design and Research Conference, Assam, India, 12–14 December 2014; pp. 1–5. [Google Scholar]
- Liu, Y.; Hua, L.; Lan, J.; Wei, X. Studies of the deformation styles of the rubber-pad forming process used for manufacturing metallic bipolar plates. J. Power Sources 2010, 195, 8177–8184. [Google Scholar] [CrossRef]
- Liu, Y.; Hua, L. Fabrication of metallic bipolar plate for proton exchange membrane fuel cells by rubber pad forming. J. Power Sources 2010, 195, 3529–3535. [Google Scholar] [CrossRef]
- Jin, C.K.; Jeong, M.G.; Kang, C.G. Fabrication of titanium bipolar plates by rubber forming and performance of single cell using TiN-coated titanium bipolar plates. Int. J. Hydrogen Energy 2014, 39, 21480–21488. [Google Scholar] [CrossRef]
- Elyasi, M.; Khatir, F.A.; Hosseinzadeh, M. Manufacturing metallic bipolar plate fuel cells through rubber pad forming process. Int. J. Adv. Manuf. Technol. 2017, 89, 3257–3269. [Google Scholar] [CrossRef]
- Son, C.-Y.; Jeon, Y.-P.; Kim, Y.-T.; Kang, C.-G. Evaluation of the formability of a bipolar plate manufactured from aluminum alloy Al 1050 using the rubber pad forming process. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2012, 226, 909–918. [Google Scholar] [CrossRef]
- Elyasi, M.; Ahmadi Khatir, F.; Hosseinzadeh, M. Investigation of die clearance in rubber pad forming of metallic bipolar plates. Mech. Eng. 2017, 1, 89–98. [Google Scholar]
- Chen, S.; Bourell, D.L.; Wood, K.L. Improvement of electrical conductivity of SLS PEM fuel cell bipolar plates. In Proceedings of the 2005 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 1–3 August 2005. [Google Scholar]
- Guo, N.; Leu, M.C. Effect of different graphite materials on the electrical conductivity and flexural strength of bipolar plates fabricated using selective laser sintering. Int. J. Hydrogen Energy 2012, 37, 3558–3566. [Google Scholar] [CrossRef]
- Chen, S.; Murphy, J.; Herlehy, J.; Bourell, D.L.; Wood, K.L. Development of SLS fuel cell current collectors. Rapid Prototyp. J. 2006, 12, 275–282. [Google Scholar] [CrossRef]
- Altan, T.; Miller, R.A. Design for forming and other near net shape manufacturing processes. CIRP Ann. 1990, 39, 609–620. [Google Scholar] [CrossRef]
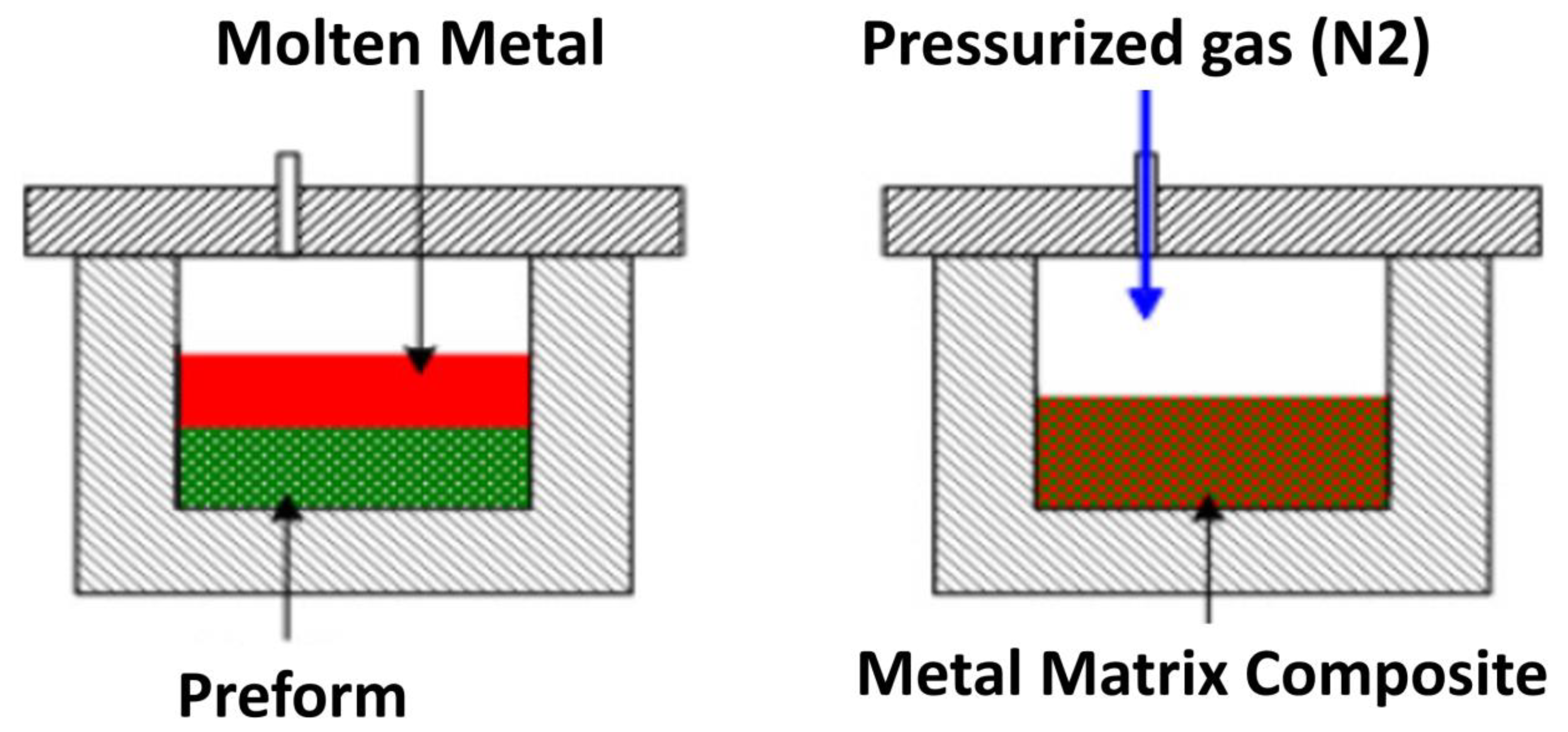
- Beroual, S.; Boumerzoug, Z.; Paillard, P.; Borjon-Piron, Y. Comparative study on the microstructures and hardness of the AlSi10. 6CuMg alloy produced by sand casting and high pressure die casting. Int. J. Cast Met. Res. 2019, 32, 191–212. [Google Scholar] [CrossRef]
- Garg, P.; Jamwal, A.; Kumar, D.; Sadasivuni, K.K.; Hussain, C.M.; Gupta, P. Advance research progresses in aluminium matrix composites: Manufacturing & applications. J. Mater. Res. Technol. 2019, 8, 4924–4939. [Google Scholar]
- Sahu, P.S.; Banchhor, R. Fabrication methods used to prepare Al metal matrix composites-A. Int. Res. J. Eng. Technol. 2016, 3, 123–132. [Google Scholar]
- Lim, J.W. Surface modifications of gasketless carbon composite bipolar plates for gas tightness of PEM fuel cells. Int. J. Hydrogen Energy 2013, 38, 12343–12352. [Google Scholar] [CrossRef]
- Available online: https://www.dbusiness.com/people/liquid-elastomer-molding-lemtm-technology-offers-innovative-solution-in-fuel-cell-development (accessed on 10 May 2022).



| Category | 6 kW Stack/5 kW System, $ | 12 kW Stack/10 kW System, $ | ||
|---|---|---|---|---|
| No. of units | 1000 | 50,000 | 1000 | 50,000 |
| MEA (PFSA 50 micrometer and PTFE reinforced) | 1473.3 | 281 | 1942.6 | 474 |
| Anode/cooling gasket (silicon rubber) | 37.9 | 24.0 | 47.9 | 30.4 |
| Cathode gasket (silicon rubber) | 22.7 | 11.6 | 28.6 | 14.6 |
| Anode bipolar plate (graphite composite) | 129.4 | 66.2 | 217.3 | 125.9 |
| Cathode bipolar plate (graphite composite) | 105.9 | 54.3 | 186.7 | 103.1 |
| End plates (A356 aluminum) | 39.2 | 17.5 | 48.0 | 21.6 |
| Assembly hardware | 45.0 | 40.2 | 45.0 | 40.2 |
| Assembly labor | 28.2 | 27.4 | 28.4 | 27.7 |
| Test and conditioning | 316.5 | 126.1 | 324.3 | 128.4 |
| Total | 2198 | 648 | 2869 | 966 |
| Nafion Commercial Name | Casting Procedure | Thickness (μm) | Ref. |
|---|---|---|---|
| Nafion® N1110 | Extrusion | 254 | [42] |
| Nafion® N117 | 183 | [39] | |
| Nafion® N115 | 127 | [40] | |
| Nafion® NR-212 | Dispersion cast | 50 | [41] |
| Nafion® NR-211 | 25 | [45] |
| ORR Catalysts (Cathode) | Reference | HOR Catalysts (Anode) | Reference |
|---|---|---|---|
| PtCo, PtNi, and PtCu | [63] | Pt/Ru0.7Ti0.3O2 | [64] |
| Pt-Ni-Ir/C | [65] | Pt-HxMoO3 | [66] |
| Pt–Pd/C | [67] | Pt-Fe/C | [68] |
| FexZr1−xO2−δ | [69] | Pt–Ru core-shell | [70] |
| Pt–Co nanowire | [71] | Pt/TiO2NCs-C | [72] |
| Pt/CeO2/C | [73] | PtMo/C | [74] |
| ZrO2 | [75] | Pt/Ti0.8Mo0.2O2–C | [76] |
| Category of Deposition Method | Techniques | Ref. |
|---|---|---|
| Applying the catalyst in solid state | Spraying | [184] |
| Decal method | ||
| Applying the catalyst as emulsion | Painting of ink | [184] |
| Screen painting | ||
| Vapor phase deposition of catalyst | Chemical vapor deposition | [185] |
| Magnetron sputtering | ||
| Electrode-assisted deposition of catalyst | Electro deposition | [186,187] |
| Electro-spraying | ||
| Applying the catalyst in a precursor state | Electron beam reduction | [187] |
| Impregnation reduction |
| Fabrication Method | Cost | Applications | Remarks |
|---|---|---|---|
| Liquid infiltration | Low/Medium | Used for the production of structural shapes, such as tubes and rods. | Uses filaments as reinforcement. |
| Squeeze casting | Medium | Used widely in the automotive industry to produce different components, such as pistons. | Generally suitable for any type of reinforcement and used for large-scale production. |
| Diffusion bonding | High | Used in the production of sheets, vane shafts, and blades’ structural parts. | Handles foils and sheets of matrix and filaments of reinforcing element. |
| Powder metallurgy | Medium | Mainly used for the production of small objects and especially round objects such as bolts. | Both matrix and reinforcement are used in powder form. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olabi, A.G.; Wilberforce, T.; Alanazi, A.; Vichare, P.; Sayed, E.T.; Maghrabie, H.M.; Elsaid, K.; Abdelkareem, M.A. Novel Trends in Proton Exchange Membrane Fuel Cells. Energies 2022, 15, 4949. https://doi.org/10.3390/en15144949
Olabi AG, Wilberforce T, Alanazi A, Vichare P, Sayed ET, Maghrabie HM, Elsaid K, Abdelkareem MA. Novel Trends in Proton Exchange Membrane Fuel Cells. Energies. 2022; 15(14):4949. https://doi.org/10.3390/en15144949
Chicago/Turabian StyleOlabi, Abdul Ghani, Tabbi Wilberforce, Abdulrahman Alanazi, Parag Vichare, Enas Taha Sayed, Hussein M. Maghrabie, Khaled Elsaid, and Mohammad Ali Abdelkareem. 2022. "Novel Trends in Proton Exchange Membrane Fuel Cells" Energies 15, no. 14: 4949. https://doi.org/10.3390/en15144949
APA StyleOlabi, A. G., Wilberforce, T., Alanazi, A., Vichare, P., Sayed, E. T., Maghrabie, H. M., Elsaid, K., & Abdelkareem, M. A. (2022). Novel Trends in Proton Exchange Membrane Fuel Cells. Energies, 15(14), 4949. https://doi.org/10.3390/en15144949









