A Comparative Review of Lead-Acid, Lithium-Ion and Ultra-Capacitor Technologies and Their Degradation Mechanisms
Abstract
1. Introduction
2. Overview of Energy Storage Devices
2.1. Battery Technology
2.1.1. Lead Acid Battery Technologies
2.1.2. Lithium-Ion Battery Technologies
2.2. Ultra-Capacitor Technology
2.3. Separator Technology
3. Degradation of Energy Storage Devices
3.1. Lead Acid Battery Technology
3.2. Lithium-Ion Battery Technology
3.3. Ultra-Capacitor Technology
3.4. Separator Based ESD Degradation
3.5. Methods in Combatting/Reducing Degradation
3.5.1. Battery Management System
3.5.2. Charge Controller
3.5.3. Hybrid Energy Storage System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawder, M.T.; Suthar, B.; Northrop, P.W.C.; De, S.; Hoff, C.M.; Leitermann, O.; Crow, M.L.; Santhanagopalan, S.; Subramanian, V.R. Battery Energy Storage System (BESS) and Battery Management System (BMS) for Grid-Scale Applications. Proc. IEEE 2014, 102, 1014–1030. [Google Scholar] [CrossRef]
- Cagnano, A.; De Tuglie, E.; Gibilisco, P. Assessment and Control of Microgrid Impacts on Distribution Networks by Using Experimental Tests. IEEE Trans. Ind. Appl. 2019, 55, 7157–7164. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Ball, M.; Weeda, M. The Hydrogen Economy—Vision or Reality? In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 237–266. [Google Scholar]
- Zsiborács, H.; Baranyai, N.H.; Vincze, A.; Zentkó, L.; Birkner, Z.; Máté, K.; Pintér, G. Intermittent Renewable Energy Sources: The Role of Energy Storage in the European Power System of 2040. Electronics 2019, 8, 729. [Google Scholar] [CrossRef]
- Asiaban, S.; Kayedpour, N.; Samani, A.E.; Bozalakov, D.; De Kooning, J.D.M.; Crevecoeur, G.; Vandevelde, L. Wind and Solar Intermittency and the Associated Integration Challenges: A Comprehensive Review Including the Status in the Belgian Power System. Energies 2021, 14, 2630. [Google Scholar] [CrossRef]
- Neill, S.P.; Hashemi, M.R. Tidal Energy. In Fundamentals of Ocean Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–81. [Google Scholar]
- Suchet, D.; Jeantet, A.; Elghozi, T.; Jehl, Z. Defining and Quantifying Intermittency in the Power Sector. Energies 2020, 13, 3366. [Google Scholar] [CrossRef]
- Notton, G.; Nivet, M.-L.; Voyant, C.; Paoli, C.; Darras, C.; Motte, F.; Fouilloy, A. Intermittent and Stochastic Character of Renewable Energy Sources: Consequences, Cost of Intermittence and Benefit of Forecasting. Renew. Sustain. Energy Rev. 2018, 87, 96–105. [Google Scholar] [CrossRef]
- Platt, G.; Paevere, P.; Higgins, A.; Grozev, G. Electric Vehicles. In Distributed Generation and Its Implications for the Utility Industry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 335–355. [Google Scholar]
- Hasanuzzaman, M.; Kumar, L. Energy Supply. In Energy for Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–104. [Google Scholar]
- Abdi, H.; Mohammadi-ivatloo, B.; Javadi, S.; Khodaei, A.R.; Dehnavi, E. Energy Storage Systems. In Distributed Generation Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 333–368. [Google Scholar]
- Aktaş, A.; Kirçiçek, Y. Hybrid Energy Storage and Innovative Storage Technologies. In Solar Hybrid Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 139–152. [Google Scholar]
- Zafirakis, D.P. Overview of Energy Storage Technologies for Renewable Energy Systems. In Stand-Alone and Hybrid Wind Energy Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 29–80. [Google Scholar]
- Poullikkas, A. A Comparative Overview of Large-Scale Battery Systems for Electricity Storage. Renew. Sustain. Energy Rev. 2013, 27, 778–788. [Google Scholar] [CrossRef]
- Panchabikesan, K.; Mastani Joybari, M.; Haghighat, F.; Eicker, U.; Ramalingam, V. Analogy between Thermal, Mechanical, and Electrical Energy Storage Systems. In Encyclopedia of Energy Storage; Elsevier: Amsterdam, The Netherlands, 2022; pp. 315–328. [Google Scholar]
- Ding, K.; Zhi, J. Wind Power Peak-Valley Regulation and Frequency Control Technology. In Large-Scale Wind Power Grid Integration; Elsevier: Amsterdam, The Netherlands, 2016; pp. 211–232. [Google Scholar]
- Abdin, Z.; Khalilpour, K.R. Single and Polystorage Technologies for Renewable-Based Hybrid Energy Systems. In Polygeneration with Polystorage for Chemical and Energy Hubs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–131. [Google Scholar]
- Semadeni, M. Storage of Energy, Overview. In Encyclopedia of Energy; Elsevier: Amsterdam, The Netherlands, 2004; pp. 719–738. [Google Scholar]
- Stadler, I.; Sterner, M. Urban Energy Storage and Sector Coupling. In Urban Energy Transition; Elsevier: Amsterdam, The Netherlands, 2018; pp. 225–244. [Google Scholar]
- Jana, A.; Paul, R.; Roy, A.K. Architectural Design and Promises of Carbon Materials for Energy Conversion and Storage: In Laboratory and Industry. In Carbon Based Nanomaterials for Advanced Thermal and Electrochemical Energy Storage and Conversion; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–61. [Google Scholar]
- Revankar, S.T. Chemical Energy Storage. In Storage and Hybridization of Nuclear Energy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–227. [Google Scholar]
- Divya, K.C.; Østergaard, J. Battery Energy Storage Technology for Power Systems—An Overview. Electr. Power Syst. Res. 2009, 79, 511–520. [Google Scholar] [CrossRef]
- Townsend, A.; Jiya, I.N.; Martinson, C.; Bessarabov, D.; Gouws, R. A Comprehensive Review of Energy Sources for Unmanned Aerial Vehicles, Their Shortfalls and Opportunities for Improvements. Heliyon 2020, 6, 1–22. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and Fuel Cells for Emerging Electric Vehicle Markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Srinivasan, S. Fuel Cells; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-25116-5. [Google Scholar] [CrossRef]
- Williamson, S.S.; Cassani, P.A.; Lukic, S.; Blunier, B. Energy Storage. In Power Electronics Handbook; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1331–1356. [Google Scholar]
- Qi, Z.; Koenig, G.M. Review Article: Flow Battery Systems with Solid Electroactive Materials. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2017, 35, 040801. [Google Scholar] [CrossRef]
- Guan, W.; Huang, X. A Modular Active Balancing Circuit for Redox Flow Battery Applied in Energy Storage System. IEEE Access 2021, 9, 127548–127558. [Google Scholar] [CrossRef]
- Motapon, S.N.; Lachance, E.; Dessaint, L.-A.; Al-Haddad, K. A Generic Cycle Life Model for Lithium-Ion Batteries Based on Fatigue Theory and Equivalent Cycle Counting. IEEE Open J. Ind. Electron. Soc. 2020, 1, 207–217. [Google Scholar] [CrossRef]
- Zhang, D.; Dey, S.; Perez, H.E.; Moura, S.J. Real-Time Capacity Estimation of Lithium-Ion Batteries Utilizing Thermal Dynamics. IEEE Trans. Control Syst. Technol. 2020, 28, 992–1000. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A Review on Lithium-Ion Battery Ageing Mechanisms and Estimations for Automotive Applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, J.; Zhang, F. An Overview of Different Approaches for Battery Lifetime Prediction. IOP Conf. Ser. Mater. Sci. Eng. 2017, 199, 012134. [Google Scholar] [CrossRef]
- Sedlakova, V.; Sikula, J.; Majzner, J.; Sedlak, P.; Kuparowitz, T.; Buergler, B.; Vasina, P. Supercapacitor Degradation Assesment by Power Cycling and Calendar Life Tests. Metrol. Meas. Syst. 2016, 23, 345–358. [Google Scholar] [CrossRef]
- Mendis, N.; Muttaqi, K.M.; Perera, S. Management of Low- and High-Frequency Power Components in Demand-Generation Fluctuations of a DFIG-Based Wind-Dominated RAPS System Using Hybrid Energy Storage. IEEE Trans. Ind. Appl. 2014, 50, 2258–2268. [Google Scholar] [CrossRef]
- Sathishkumar, R.; Kollimalla, S.K.; Mishra, M.K. Dynamic Energy Management of Micro Grids Using Battery Super Capacitor Combined Storage. In Proceedings of the 2012 Annual IEEE India Conference (INDICON), Kochi, India, 7–9 December 2012; IEEE: New York, NY, USA, 2012; pp. 1078–1083. [Google Scholar]
- Basic, H.; Pandzic, H.; Miletic, M.; Pavic, I. Experimental Testing and Evaluation of Lithium-Ion Battery Cells for a Special-Purpose Electric Vacuum Sweeper Vehicle. IEEE Access 2020, 8, 216308–216319. [Google Scholar] [CrossRef]
- McKeon, B.B.; Furukawa, J.; Fenstermacher, S. Advanced Lead–Acid Batteries and the Development of Grid-Scale Energy Storage Systems. Proc. IEEE 2014, 102, 951–963. [Google Scholar] [CrossRef]
- Al-Haj Hussein, A.; Batarseh, I. A Review of Charging Algorithms for Nickel and Lithium Battery Chargers. IEEE Trans. Veh. Technol. 2011, 60, 830–838. [Google Scholar] [CrossRef]
- da Cunha, A.B.; de Almeida, B.R.; da Silva, D.C. Remaining Capacity Measurement and Analysis of Alkaline Batteries for Wireless Sensor Nodes. IEEE Trans. Instrum. Meas. 2009, 58, 1816–1822. [Google Scholar] [CrossRef]
- Kupsch, C.; Weik, D.; Feierabend, L.; Nauber, R.; Buttner, L.; Czarske, J. Vector Flow Imaging of a Highly Laden Suspension in a Zinc-Air Flow Battery Model. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 761–771. [Google Scholar] [CrossRef]
- Al-Humaid, Y.M.; Khan, K.A.; Abdulgalil, M.A.; Khalid, M. Two-Stage Stochastic Optimization of Sodium-Sulfur Energy Storage Technology in Hybrid Renewable Power Systems. IEEE Access 2021, 9, 162962–162972. [Google Scholar] [CrossRef]
- Aktaş, A.; Kirçiçek, Y. Solar Hybrid Systems and Energy Storage Systems. In Solar Hybrid Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 87–125. [Google Scholar]
- Battery University BU-201: How Does the Lead Acid Battery Work? Available online: https://batteryuniversity.com/article/bu-201-how-does-the-lead-acid-battery-work (accessed on 22 April 2022).
- Dhundhara, S.; Verma, Y.P.; Williams, A. Techno-Economic Analysis of the Lithium-Ion and Lead-Acid Battery in Microgrid Systems. Energy Convers. Manag. 2018, 177, 122–142. [Google Scholar] [CrossRef]
- Jaiswal, A. Lithium-Ion Battery Based Renewable Energy Solution for off-Grid Electricity: A Techno-Economic Analysis. Renew. Sustain. Energy Rev. 2017, 72, 922–934. [Google Scholar] [CrossRef]
- Eldeeb, H.H.; Elsayed, A.T.; Lashway, C.R.; Mohammed, O. Hybrid Energy Storage Sizing and Power Splitting Optimization for Plug-In Electric Vehicles. IEEE Trans. Ind. Appl. 2019, 55, 2252–2262. [Google Scholar] [CrossRef]
- Diaz, J.; Martin-Ramos, J.A.; Pernia, A.M.; Nuno, F.; Linera, F.F. Intelligent and Universal Fast Charger for Ni-Cd and Ni-MH Batteries in Portable Applications. IEEE Trans. Ind. Electron. 2004, 51, 857–863. [Google Scholar] [CrossRef]
- Buchmann, I. Batteries in a Portable World: A Handbook on Rechargeable Batteries for Non-Engineers, 4th ed.; CADEX: Richmond, BC, Canada, 2017; ISBN 978-0968211847. [Google Scholar]
- Lim, M.B.; Lambert, T.N.; Chalamala, B.R. Rechargeable Alkaline Zinc–Manganese Oxide Batteries for Grid Storage: Mechanisms, Challenges and Developments. Mater. Sci. Eng. R Rep. 2021, 143, 100593. [Google Scholar] [CrossRef]
- De Angelis, V.; Yadav, G.; Huang, J.; Couzis, A.; Banerjee, S. Rechargeable Zn-MnO2 Batteries for Utility Load Management and Renewable Integration. In Proceedings of the 2018 International Symposium on Power Electronics, Electrical Drives, Automation and Motion (SPEEDAM), Amalfi, Italy, 20 June 2018; IEEE: New York, NY, USA, 2018; pp. 50–54. [Google Scholar]
- Salkind, A.J.; Klein, M. Batteries, Alkaline Secondary Cells. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Metal-Air Batteries—A Review. Energies 2021, 14, 7373. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery Technologies for Grid-Level Large-Scale Electrical Energy Storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef]
- Chawla, N.; Safa, M. Sodium Batteries: A Review on Sodium-Sulfur and Sodium-Air Batteries. Electronics 2019, 8, 1201. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in Electrical Energy Storage System: A Critical Review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Doetsch, C.; Pohlig, A. The Use of Flow Batteries in Storing Electricity for National Grids. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 263–277. [Google Scholar]
- Zhang, D.; Liu, Q.; Li, Y. Design of Flow Battery. In Reactor and Process Design in Sustainable Energy Technology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 61–97. [Google Scholar]
- Chalamala, B.R.; Soundappan, T.; Fisher, G.R.; Anstey, M.R.; Viswanathan, V.V.; Perry, M.L. Redox Flow Batteries: An Engineering Perspective. Proc. IEEE 2014, 102, 976–999. [Google Scholar] [CrossRef]
- Ferrari, J. Energy Storage and Conversion. In Electric Utility Resource Planning; Elsevier: Amsterdam, The Netherlands, 2021; pp. 73–107. [Google Scholar]
- Nguyen, T.A.; Crow, M.L.; Elmore, A.C. Optimal Sizing of a Vanadium Redox Battery System for Microgrid Systems. IEEE Trans. Sustain. Energy 2015, 6, 729–737. [Google Scholar] [CrossRef]
- Bahramirad, S.; Reder, W.; Khodaei, A. Reliability-Constrained Optimal Sizing of Energy Storage System in a Microgrid. IEEE Trans. Smart Grid 2012, 3, 2056–2062. [Google Scholar] [CrossRef]
- Brady, R.N. Internal Combustion (Gasoline and Diesel) Engines. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Mahapatra, M.K.; Singh, P. Fuel Cells. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 511–547. [Google Scholar]
- Bharadwaj, S.R.; Varma, S.; Wani, B.N. Electroceramics for Fuel Cells, Batteries and Sensors. In Functional Materials; Elsevier: Amsterdam, The Netherlands, 2012; pp. 639–674. [Google Scholar]
- Campanari, S.; Guandalini, G. Fuel Cells: Opportunities and Challenges. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–358. [Google Scholar]
- Pollet, B.G.; Staffell, I.; Shang, J.L.; Molkov, V. Fuel-Cell (Hydrogen) Electric Hybrid Vehicles. In Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 685–735. [Google Scholar]
- Dincer, I.; Siddiqui, O. Fundamentals. In Ammonia Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–32. [Google Scholar]
- Coralli, A.; Sarruf, B.J.M.; de Miranda, P.E.V.; Osmieri, L.; Specchia, S.; Minh, N.Q. Fuel Cells. In Science and Engineering of Hydrogen-Based Energy Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–122. [Google Scholar]
- Hebling, C.; Rochlitz, L.; Aicher, T. Micro-Fuel Cells. In Comprehensive Microsystems; Elsevier: Amsterdam, The Netherlands, 2008; pp. 613–634. [Google Scholar]
- Thangavelautham, J. Degradation in PEM Fuel Cells and Mitigation Strategies Using System Design and Control. In Proton Exchange Membrane Fuel Cell; InTech: London, UK, 2018. [Google Scholar]
- Surabattula, Y.; Balaji, R.; Rajalakshmi, N.; Prakash, K.A. First and Second Law of Thermodynamics—Analysis for Fuel Cells. In Encyclopedia of Energy Storage; Elsevier: Amsterdam, The Netherlands, 2022; pp. 307–314. [Google Scholar]
- Popov, S.P.; Baldynov, O.A. Evaluation of Energy Efficiency of the Long Distance Energy Transport Systems for Renewable Energy. E3S Web Conf. 2019, 114, 02003. [Google Scholar] [CrossRef]
- Behling, N.H. Fuel Cells and the Challenges Ahead. In Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2013; pp. 7–36. [Google Scholar]
- Russel Rhodes Explosive Lessons in Hydrogen Saftey. Available online: https://www.nasa.gov/pdf/513855main_ASK_41s_explosive.pdf (accessed on 22 June 2022).
- Coddet, P.; Pera, M.-C.; Candusso, D.; Hissel, D. Study of Proton Exchange Membrane Fuel Cell Safety Procedures in Case of Emergency Shutdown. In Proceedings of the 2007 IEEE International Symposium on Industrial Electronics, Vigo, Spain, 4–7 June 2007; IEEE: New York, NY, USA, 2007; pp. 725–730. [Google Scholar]
- Fathima, A.H.; Palanisamy, K. Renewable Systems. In Hybrid-Renewable Energy Systems in Microgrids; Elsevier: Amsterdam, The Netherlands, 2018; pp. 147–164. [Google Scholar]
- Yoomak, S.; Ngaopitakkul, A. Feasibility Analysis of Different Energy Storage Systems for Solar Road Lighting Systems. IEEE Access 2019, 7, 101992–102001. [Google Scholar] [CrossRef]
- Akinyele, D.; Belikov, J.; Levron, Y. Battery Storage Technologies for Electrical Applications: Impact in Stand-Alone Photovoltaic Systems. Energies 2017, 10, 1760. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002; Available online: https://en.wikipedia.org/wiki/Special:BookSources/0-07-135978-8 (accessed on 4 April 2022).
- Schwimmbeck, S.; Schroer, P.; Buchner, Q.; Herzog, H.-G. Modeling the Dynamic Behavior of 12V AGM Batteries and Its Degradation. In Proceedings of the 2019 IEEE Vehicle Power and Propulsion Conference (VPPC), Hanoi, Vietnam, 14–17 October 2019; IEEE: New York, NY, USA, 2019; pp. 1–6. [Google Scholar]
- May, G.J.; Davidson, A.; Monahov, B. Lead Batteries for Utility Energy Storage: A Review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Satpathy, R.; Pamuru, V. Off-Grid Solar Photovoltaic Systems. In Solar PV Power; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–315. [Google Scholar]
- Pascoe, P.E.; Anbuky, A.H. VRLA Battery Discharge Reserve Time Estimation. IEEE Trans. Power Electron. 2004, 19, 1515–1522. [Google Scholar] [CrossRef]
- Torabi, F.; Ahmadi, P. Lead–Acid Batteries. In Simulation of Battery Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–215. [Google Scholar]
- Jie, W.; Hua, L.; Peijie, C.; Deyu, Q.; Shan, L. Design of Energy Storage System Using Retired Valve Regulated Lead Acid (VRLA) Batteries in Substations. In Proceedings of the 2019 IEEE Conference on Energy Conversion (CENCON), Yogyakarta, Indonesia, 16–17 October 2019; IEEE: New York, NY, USA, 2019; pp. 132–136. [Google Scholar]
- Spiers, D. Batteries in PV Systems. In McEvoy’s Handbook of Photovoltaics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 789–843. [Google Scholar]
- Bonduelle, G.; Muneret, X. VRLA Batteries in Telecom Application: AGM or Gel? In Proceedings of the TELESCON 2000 Third International Telecommunications Energy Special Conference (IEEE Cat. No.00EX424), Dresden, Germany, 10 May 2000; VDE-Verlag: Berlin, Germany; pp. 75–79. [Google Scholar]
- Mahlia, T.M.I.; Saktisahdan, T.J.; Jannifar, A.; Hasan, M.H.; Matseelar, H.S.C. A Review of Available Methods and Development on Energy Storage; Technology Update. Renew. Sustain. Energy Rev. 2014, 33, 532–545. [Google Scholar] [CrossRef]
- Trojan J200-RE Deep Cycle Flooded/Advanced Lead Acid Battery. Available online: http://www.trojanbattery.com/pdf/J200-RE_Trojan_Data_Sheets.pdf (accessed on 18 May 2022).
- Components, R. FG20201 Lead Acid Battery-12 V, 2 Ah. Available online: https://za.rs-online.com/web/p/lead-acid-batteries/8431308/ (accessed on 18 May 2020).
- Renogy Deep Cycle AGM Battery 12 V 200 Ah. Available online: https://www.renogy.com/content/RNG-BATT-AGM12-200/AGM200-Datasheet.pdf (accessed on 18 May 2022).
- Kuang, X.; Li, X.; Li, S.; Xiang, J.; Wang, X. Silicon Nanoparticles Within the Carbonized SU-8 Cages as A Micro Lithium-Ion Battery Anode. J. Microelectromechanical Syst. 2018, 27, 201–209. [Google Scholar] [CrossRef]
- Turgeman, M.; Wineman-Fisher, V.; Malchik, F.; Saha, A.; Bergman, G.; Gavriel, B.; Penki, T.R.; Nimkar, A.; Baranauskaite, V.; Aviv, H.; et al. A Cost-Effective Water-in-Salt Electrolyte Enables Highly Stable Operation of a 2.15-V Aqueous Lithium-Ion Battery. Cell Rep. Phys. Sci. 2022, 3, 100688. [Google Scholar] [CrossRef]
- Xiong, R.; Tian, J.; Mu, H.; Wang, C. A Systematic Model-Based Degradation Behavior Recognition and Health Monitoring Method for Lithium-Ion Batteries. Appl. Energy 2017, 207, 372–383. [Google Scholar] [CrossRef]
- Daud, M.Z.; Mohamed, A.; Hannan, M.A. A Novel Coordinated Control Strategy Considering Power Smoothing for a Hybrid Photovoltaic/Battery Energy Storage System. J. Cent. South Univ. 2016, 23, 394–404. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Kim, S.; Kim, Y. Fluorination of Free Lithium Residues on the Surface of Lithium Nickel Cobalt Aluminum Oxide Cathode Materials for Lithium Ion Batteries. Mater. Des. 2016, 100, 175–179. [Google Scholar] [CrossRef]
- Cui, Y.; Mahmoud, M.M.; Rohde, M.; Ziebert, C.; Seifert, H.J. Thermal and Ionic Conductivity Studies of Lithium Aluminum Germanium Phosphate Solid-State Electrolyte. Solid State Ion. 2016, 289, 125–132. [Google Scholar] [CrossRef]
- Zhao, C.; Yin, H.; Ma, C. Quantitative Evaluation of LiFePO4 Battery Cycle Life Improvement Using Ultracapacitors. IEEE Trans. Power Electron. 2016, 31, 3989–3993. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Recham, N.; Armand, M.; Chotard, J.-N.; Barpanda, P.; Walker, W.; Dupont, L. Hunting for Better Li-Based Electrode Materials via Low Temperature Inorganic Synthesis. Chem. Mater. 2010, 22, 724–739. [Google Scholar] [CrossRef]
- Kennedy, B.; Patterson, D.; Camilleri, S. Use of Lithium-Ion Batteries in Electric Vehicles. J. Power Sources 2000, 90, 156–162. [Google Scholar] [CrossRef]
- Omar, N.; Verbrugge, B.; Mulder, G.; Van den Bossche, P.; Van Mierlo, J.; Daowd, M.; Dhaens, M.; Pauwels, S. Evaluation of Performance Characteristics of Various Lithium-Ion Batteries for Use in BEV Application. In Proceedings of the 2010 IEEE Vehicle Power and Propulsion Conference, Lille, France, 1–3 September 2010; IEEE: New York, NY, USA, 2010; pp. 1–6. [Google Scholar]
- Meng, J.; Luo, G.; Gao, F. Lithium Polymer Battery State-of-Charge Estimation Based on Adaptive Unscented Kalman Filter and Support Vector Machine. IEEE Trans. Power Electron. 2016, 31, 2226–2238. [Google Scholar] [CrossRef]
- Kim, T.; Qiao, W.; Qu, L. Power Electronics-Enabled Self-X Multicell Batteries: A Design Toward Smart Batteries. IEEE Trans. Power Electron. 2012, 27, 4723–4733. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Zhao, Y.; Yang, K.; Gao, F.; Li, X. Influence of Cooling Mode on the Electrochemical Properties of Li4Ti5O12 Anode Materials for Lithium-Ion Batteries. Ionics 2016, 22, 789–795. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Jia, X.; Xia, B. The Characterization of Lithium Titanate Microspheres Synthesized by a Hydrothermal Method. J. Chem. 2013, 2013, 497654. [Google Scholar] [CrossRef]
- Llinas, J.P.; Fairbrother, A.; Borin Barin, G.; Shi, W.; Lee, K.; Wu, S.; Yong Choi, B.; Braganza, R.; Lear, J.; Kau, N.; et al. Short-Channel Field-Effect Transistors with 9-Atom and 13-Atom Wide Graphene Nanoribbons. Nat. Commun. 2017, 8, 633. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Chen, Y.; Bao, J.; Zhou, X.; Xie, Z.; Wei, J.; Zhou, Z. The First Introduction of Graphene to Rechargeable Li-CO2 Batteries. Angew. Chem. 2015, 127, 6650–6653. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, C.S.; Hwang, J.-Y.; Kim, S.-J.; Maglia, F.; Lamp, P.; Myung, S.-T.; Sun, Y.-K. High-Energy-Density Lithium-Ion Battery Using a Carbon-Nanotube–Si Composite Anode and a Compositionally Graded Li[Ni0.85Co0.05Mn0.10]O2 Cathode. Energy Environ. Sci. 2016, 9, 2152–2158. [Google Scholar] [CrossRef]
- Quartarone, E.; Dall’Asta, V.; Resmini, A.; Tealdi, C.; Tredici, I.G.; Tamburini, U.A.; Mustarelli, P. Graphite-Coated ZnO Nanosheets as High-Capacity, Highly Stable, and Binder-Free Anodes for Lithium-Ion Batteries. J. Power Sources 2016, 320, 314–321. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, J.; Ng, C.B.; Jia, C.; Wang, Q. A Redox Flow Lithium Battery Based on the Redox Targeting Reactions between LiFePO4 and Iodide. Energy Environ. Sci. 2016, 9, 917–921. [Google Scholar] [CrossRef]
- Gong, H.; Xue, H.; Wang, T.; He, J. In-Situ Synthesis of Monodisperse Micro-Nanospherical LiFePO4/Carbon Cathode Composites for Lithium-Ion Batteries. J. Power Sources 2016, 318, 220–227. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Thomas, J.O.; Whittingham, M.S. Science and Applications of Mixed Conductors for Lithium Batteries. MRS Bull. 2000, 25, 39–46. [Google Scholar] [CrossRef]
- Omar, N.; Monem, M.A.; Firouz, Y.; Salminen, J.; Smekens, J.; Hegazy, O.; Gaulous, H.; Mulder, G.; Van den Bossche, P.; Coosemans, T.; et al. Lithium Iron Phosphate Based Battery–Assessment of the Aging Parameters and Development of Cycle Life Model. Appl. Energy 2014, 113, 1575–1585. [Google Scholar] [CrossRef]
- Häggström, F.; Delsing, J. IoT Energy Storage-A Forecast. Energy Harvest. Syst. 2018, 5, 43–51. [Google Scholar] [CrossRef]
- Bueno, P.R. Nanoscale Origins of Super-Capacitance Phenomena. J. Power Sources 2019, 414, 420–434. [Google Scholar] [CrossRef]
- Gupta, S.S.; Islam, M.R.; Pradeep, T. Capacitive Deionization (CDI): An Alternative Cost-Efficient Desalination Technique. In Advances in Water Purification Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–202. [Google Scholar]
- Shafiei, N.; Nasrollahzadeh, M.; Hegde, G. Biopolymer-Based (Nano)Materials for Supercapacitor Applications. In Biopolymer-Based Metal Nanoparticle Chemistry for Sustainable Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 609–671. [Google Scholar]
- Grahame, D.C. The Electrical Double Layer and the Theory of Electrocapillarity. Chem. Rev. 1947, 41, 441–501. [Google Scholar] [CrossRef]
- Ellenbogen, J.C. Supercapacitors: A Brief Overview; MITRE: McLean, VA, USA, 2006. [Google Scholar]
- Helmholtz, H. Ueber Einige Gesetze Der Vertheilung Elektrischer Ströme in Körperlichen Leitern Mit Anwendung Auf Die Thierisch-Elektrischen Versuche. Ann. Der Phys. Und Chem. 1853, 165, 211–233. [Google Scholar] [CrossRef]
- Frackowiak, E.; Jurewicz, K.; Delpeux, S.; Béguin, F. Nanotubular Materials for Supercapacitors. J. Power Sources 2001, 97–98, 822–825. [Google Scholar] [CrossRef]
- Conway, B.E.; Birss, V.; Wojtowicz, J. The Role and Utilization of Pseudocapacitance for Energy Storage by Supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “Supercapacitor” to “Battery” Behavior in Electrochemical Energy Storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Simon, P.; Burke, A. Nanostructured Carbons: Double-Layer Capacitance and More. Electrochem. Soc. Interface 2008, 17, 38–43. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.P.; Shao, Z. Intercalation Pseudocapacitance in Electrochemical Energy Storage: Recent Advances in Fundamental Understanding and Materials Development. Mater. Today Adv. 2020, 7, 100072. [Google Scholar] [CrossRef]
- Yoong, M.; Gan, Y.; Gan, G.; Leong, C.; Phuan, Z.; Cheah, B.; Chew, K. Studies of Regenerative Braking in Electric Vehicle. In Proceedings of the 2010 IEEE Conference on Sustainable Utilization and Development in Engineering and Technology, Kuala Lumpur, Malaysia, 20–21 November 2010; IEEE: New York, NY, USA, 2010; pp. 40–45. [Google Scholar]
- Camara, M.B.; Gualous, H.; Gustin, F.; Berthon, A. Design and New Control of DC/DC Converters to Share Energy between Supercapacitors and Batteries in Hybrid Vehicles. IEEE Trans. Veh. Technol. 2008, 57, 2721–2735. [Google Scholar] [CrossRef]
- Worsley, M.A.; Baumann, T.F. Carbon Aerogels. In Handbook of Sol-Gel Science and Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–36. [Google Scholar]
- Najib, S.; Erdem, E. Current Progress Achieved in Novel Materials for Supercapacitor Electrodes: Mini Review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef]
- Lazarov, V.; Francois, B.; Kanchev, H.; Zarkov, Z.; Stoyanov, L. Application of Supercapacitors in Hybrid Systems. Proc. Tech. Univ.-Sofia 2010, 60, 299–310. [Google Scholar]
- Jagadale, A.; Zhou, X.; Xiong, R.; Dubal, D.P.; Xu, J.; Yang, S. Lithium Ion Capacitors (LICs): Development of the Materials. Energy Storage Mater. 2019, 19, 314–329. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal Oxide-Based Supercapacitors: Progress and Prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef]
- Rani, J.; Thangavel, R.; Oh, S.-I.; Lee, Y.; Jang, J.-H. An Ultra-High-Energy Density Supercapacitor; Fabrication Based on Thiol-Functionalized Graphene Oxide Scrolls. Nanomaterials 2019, 9, 148. [Google Scholar] [CrossRef]
- Ge, Y.; Xie, X.; Roscher, J.; Holze, R.; Qu, Q. How to Measure and Report the Capacity of Electrochemical Double Layers, Supercapacitors, and Their Electrode Materials. J. Solid State Electrochem. 2020, 24, 3215–3230. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, J.; Dong, L.; Chen, D.; Zhong, S.; Liang, Y.; Liu, Y.; Ji, Y.; Wu, X.; Kong, Q.; et al. A Single-Layer Composite Separator with 3D-Reinforced Microstructure for Practical High-Temperature Lithium Ion Batteries. Small 2022, 18, 2107664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. Identifying Rate Limitation and a Guide to Design of Fast-charging Li-ion Battery. InfoMat 2020, 2, 942–949. [Google Scholar] [CrossRef]
- Lu, J.; Wu, T.; Amine, K. State-of-the-Art Characterization Techniques for Advanced Lithium-Ion Batteries. Nat. Energy 2017, 2, 17011. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Zhan, H. Advanced Separators for Lithium-Ion Batteries. IOP Conf. Ser. Earth Environ. Sci. 2022, 1011, 012009. [Google Scholar] [CrossRef]
- Boateng, B.; Zhang, X.; Zhen, C.; Chen, D.; Han, Y.; Feng, C.; Chen, N.; He, W. Recent Advances in Separator Engineering for Effective Dendrite Suppression of Li-metal Anodes. Nano Sel. 2021, 2, 993–1010. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-Safety Separators for Lithium-Ion Batteries and Sodium-Ion Batteries: Advances and Perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Lizundia, E.; Costa, C.M.; Alves, R.; Lanceros-Méndez, S. Cellulose and Its Derivatives for Lithium Ion Battery Separators: A Review on the Processing Methods and Properties. Carbohydr. Polym. Technol. Appl. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Liu, L.; Hsu, H.; Lu, L.; Wang, L.; He, X.; Ouyang, M. Investigating the Relationship between Internal Short Circuit and Thermal Runaway of Lithium-Ion Batteries under Thermal Abuse Condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Oh, Y.-S.; Jung, G.Y.; Kim, J.-H.; Kim, J.-H.; Kim, S.H.; Kwak, S.K.; Lee, S.-Y. Janus-Faced, Dual-Conductive/Chemically Active Battery Separator Membranes. Adv. Funct. Mater. 2016, 26, 7074–7083. [Google Scholar] [CrossRef]
- Xie, Y.; Zou, H.; Xiang, H.; Xia, R.; Liang, D.; Shi, P.; Dai, S.; Wang, H. Enhancement on the Wettability of Lithium Battery Separator toward Nonaqueous Electrolytes. J. Memb. Sci. 2016, 503, 25–30. [Google Scholar] [CrossRef]
- Nishio, K. PRIMARY BATTERIES–NONAQUEOUS SYSTEMS|Lithium Primary: Overview. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 68–75. [Google Scholar]
- Li, A.; Yuen, A.C.Y.; Wang, W.; De Cachinho Cordeiro, I.M.; Wang, C.; Chen, T.B.Y.; Zhang, J.; Chan, Q.N.; Yeoh, G.H. A Review on Lithium-Ion Battery Separators towards Enhanced Safety Performances and Modelling Approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A Review of Recent Developments in Membrane Separators for Rechargeable Lithium-Ion Batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Prout, L. Aspects of Lead/Acid Battery Technology 7. Separators. J. Power Sources 1993, 46, 117–138. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, X. Recent Advances in Lithium-Ion Battery Separators with Enhanced Safety. In 60 Years of the Loeb-Sourirajan Membrane; Elsevier: Amsterdam, The Netherlands, 2022; pp. 269–304. [Google Scholar]
- Huang, X. Separator Technologies for Lithium-Ion Batteries. J. Solid State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Tahalyani, J.; Akhtar, M.J.; Cherusseri, J.; Kar, K.K. Characteristics of Capacitor: Fundamental Aspects. In Handbook of Nanocomposite Supercapacitor Materials; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–51. [Google Scholar]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and Opportunities towards Fast-Charging Battery Materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent Advances on Separator Membranes for Lithium-Ion Battery Applications: From Porous Membranes to Solid Electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. A Highly Thermostable Ceramic-Grafted Microporous Polyethylene Separator for Safer Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 24119–24126. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and Performance Evaluation of Lithium-Ion Battery Separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef]
- Zhang, S.S. A Review on the Separators of Liquid Electrolyte Li-Ion Batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Song, J.; Ryou, M.-H.; Son, B.; Lee, J.-N.; Lee, D.J.; Lee, Y.M.; Choi, J.W.; Park, J.-K. Co-Polyimide-Coated Polyethylene Separators for Enhanced Thermal Stability of Lithium Ion Batteries. Electrochim. Acta 2012, 85, 524–530. [Google Scholar] [CrossRef]
- Zhang, X.; Sahraei, E.; Wang, K. Li-Ion Battery Separators, Mechanical Integrity and Failure Mechanisms Leading to Soft and Hard Internal Shorts. Sci. Rep. 2016, 6, 32578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B.; et al. Sustainable, Heat-Resistant and Flame-Retardant Cellulose-Based Composite Separator for High-Performance Lithium Ion Battery. Sci. Rep. 2015, 4, 3935. [Google Scholar] [CrossRef]
- Xu, Q.; Cheong, Y.-K.; He, S.-Q.; Tiwari, V.; Liu, J.; Wang, Y.; Raja, S.N.; Li, J.; Guan, Y.; Li, W. Suppression of Spinal Connexin 43 Expression Attenuates Mechanical Hypersensitivity in Rats after an L5 Spinal Nerve Injury. Neurosci. Lett. 2014, 566, 194–199. [Google Scholar] [CrossRef][Green Version]
- Xu, Q.; Kong, Q.; Liu, Z.; Zhang, J.; Wang, X.; Liu, R.; Yue, L.; Cui, G. Polydopamine-Coated Cellulose Microfibrillated Membrane as High Performance Lithium-Ion Battery Separator. RSC Adv. 2014, 4, 7845. [Google Scholar] [CrossRef]
- Liu, A.; Walther, A.; Ikkala, O.; Belova, L.; Berglund, L.A. Clay Nanopaper with Tough Cellulose Nanofiber Matrix for Fire Retardancy and Gas Barrier Functions. Biomacromolecules 2011, 12, 633–641. [Google Scholar] [CrossRef]
- Waqas, M.; Ali, S.; Feng, C.; Chen, D.; Han, J.; He, W. Recent Development in Separators for High-Temperature Lithium-Ion Batteries. Small 2019, 15, 1901689. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, F.; Li, S.; Zhang, Y.; Tian, Z.; Xu, Q.; Xin, S.; Guo, Y. Advances of Polymer Binders for silicon-based Anodes in High Energy Density lithium-ion Batteries. InfoMat 2021, 3, 460–501. [Google Scholar] [CrossRef]
- Heidari, A.A.; Mahdavi, H.; Karami, M. Recent Advances in Polyolefin-Based Separators for Li-Ion Battery Applications: A Review. Iran. J. Polym. Sci. Technol. 2022, 34, 423–442. [Google Scholar] [CrossRef]
- Li, J.; Dai, L.; Wang, Z.; Wang, H.; Xie, L.; Chen, J.; Yan, C.; Yuan, H.; Wang, H.; Chen, C. Cellulose Nanofiber Separator for Suppressing Shuttle Effect and Li Dendrite Formation in Lithium-Sulfur Batteries. J. Energy Chem. 2022, 67, 736–744. [Google Scholar] [CrossRef]
- Thiangtham, S.; Saito, N.; Manuspiya, H. Asymmetric Porous and Highly Hydrophilic Sulfonated Cellulose/Biomembrane Functioning as a Separator in a Lithium-Ion Battery. ACS Appl. Energy Mater. 2022, 5, 6206–6218. [Google Scholar] [CrossRef]
- Hendricks, C.; Williard, N.; Mathew, S.; Pecht, M. A Failure Modes, Mechanisms, and Effects Analysis (FMMEA) of Lithium-Ion Batteries. J. Power Sources 2015, 297, 113–120. [Google Scholar] [CrossRef]
- McEvoy, A.; Markvart, T.; Castaner, L. Practical Handbook of Photovoltaics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780123859341. [Google Scholar]
- Ruetschi, P. Aging Mechanisms and Service Life of Lead–Acid Batteries. J. Power Sources 2004, 127, 33–44. [Google Scholar] [CrossRef]
- Sun, Y.-H.; Jou, H.-L.; Wu, J.-C. Aging Estimation Method for Lead-Acid Battery. IEEE Trans. Energy Convers. 2011, 26, 264–271. [Google Scholar] [CrossRef]
- Coleman, M.; Hurley, W.G.; Lee, C.K. An Improved Battery Characterization Method Using a Two-Pulse Load Test. IEEE Trans. Energy Convers. 2008, 23, 708–713. [Google Scholar] [CrossRef]
- Gates Energy Products Sealed Lead Cells and Batteries. In Rechargeable Batteries Applications Handbook; Elsevier: Amsterdam, The Netherlands, 1998; pp. 153–235. ISBN 9780080515939.
- Badeda, J.; Huck, M.; Sauer, D.U.; Kabzinski, J.; Wirth, J. Basics of Lead–Acid Battery Modelling and Simulation. In Lead-Acid Batteries for Future Automobiles; Elsevier: Amsterdam, The Netherlands, 2017; pp. 463–507. [Google Scholar]
- Catherino, H.A.; Feres, F.F.; Trinidad, F. Sulfation in Lead–Acid Batteries. J. Power Sources 2004, 129, 113–120. [Google Scholar] [CrossRef]
- Apǎteanu, L.; Hollenkamp, A.F.; Koop, M.J. Electrolyte Stratification in Lead/Acid Batteries: Effect of Grid Antimony and Relationship to Capacity Loss. J. Power Sources 1993, 46, 239–250. [Google Scholar] [CrossRef]
- Pavlov, D. H2SO4 Electrolyte—An Active Material in the Lead–Acid Cell. In Lead-Acid Batteries: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 133–167. [Google Scholar]
- Lin, C.; Tang, A.; Mu, H.; Wang, W.; Wang, C. Aging Mechanisms of Electrode Materials in Lithium-Ion Batteries for Electric Vehicles. J. Chem. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Reniers, J.M.; Mulder, G.; Howey, D.A. Review and Performance Comparison of Mechanical-Chemical Degradation Models for Lithium-Ion Batteries. J. Electrochem. Soc. 2019, 166, A3189–A3200. [Google Scholar] [CrossRef]
- Waldmann, T.; Wilka, M.; Kasper, M.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. Temperature Dependent Ageing Mechanisms in Lithium-Ion Batteries–A Post-Mortem Study. J. Power Sources 2014, 262, 129–135. [Google Scholar] [CrossRef]
- Bensebaa, F. Clean Energy. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 279–383. [Google Scholar]
- Millange, F.; Férey, G.; Morcrette, M.; Serre, C.; Doublet, M.-L.; Grenèche, J.-M.; Tarascon, J.-M. Towards the Reactivity of MIL-53 or FeIII(OH)0.8F0.2[O2C-C6H4-CO2] versus Lithium. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; pp. 2037–2041. [Google Scholar]
- Ren, D.; Feng, X.; Lu, L.; He, X.; Ouyang, M. Overcharge Behaviors and Failure Mechanism of Lithium-Ion Batteries under Different Test Conditions. Appl. Energy 2019, 250, 323–332. [Google Scholar] [CrossRef]
- Fedorova, A.A.; Anishchenko, D.V.; Beletskii, E.V.; Kalnin, A.Y.; Levin, O.V. Modeling of the Overcharge Behavior of Lithium-Ion Battery Cells Protected by a Voltage-Switchable Resistive Polymer Layer. J. Power Sources 2021, 510, 230392. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation Diagnostics for Lithium Ion Cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Sreenarayanan, B.; Tan, D.H.S.; Bai, S.; Li, W.; Bao, W.; Meng, Y.S. Quantification of Lithium Inventory Loss in Micro Silicon Anode via Titration-Gas Chromatography. J. Power Sources 2022, 531, 231327. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium Ion Battery Degradation: What You Need to Know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode Degradation in Lithium-Ion Batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef]
- Lin, X.; Khosravinia, K.; Hu, X.; Li, J.; Lu, W. Lithium Plating Mechanism, Detection, and Mitigation in Lithium-Ion Batteries. Prog. Energy Combust. Sci. 2021, 87, 100953. [Google Scholar] [CrossRef]
- Kreczanik, P.; Venet, P.; Hijazi, A.; Clerc, G. Study of Supercapacitor Aging and Lifetime Estimation According to Voltage, Temperature, and RMS Current. IEEE Trans. Ind. Electron. 2014, 61, 4895–4902. [Google Scholar] [CrossRef]
- Murray, D.B.; Hayes, J.G. Cycle Testing of Supercapacitors for Long-Life Robust Applications. IEEE Trans. Power Electron. 2015, 30, 2505–2516. [Google Scholar] [CrossRef]
- Rizoug, N.; Bartholomeus, P.; Le Moigne, P. Study of the Ageing Process of a Supercapacitor Module Using Direct Method of Characterization. IEEE Trans. Energy Convers. 2012, 27, 220–228. [Google Scholar] [CrossRef]
- Bohlen, O.; Kowal, J.; Sauer, D.U. Ageing Behaviour of Electrochemical Double Layer Capacitors. J. Power Sources 2007, 172, 468–475. [Google Scholar] [CrossRef]
- Gualous, H.; Louahlia, H.; Gallay, R. Supercapacitor Characterization and Thermal Modelling With Reversible and Irreversible Heat Effect. IEEE Trans. Power Electron. 2011, 26, 3402–3409. [Google Scholar] [CrossRef]
- German, R.; Sari, A.; Briat, O.; Vinassa, J.-M.; Venet, P. Impact of Voltage Resets on Supercapacitors Aging. IEEE Trans. Ind. Electron. 2016, 63, 7703–7711. [Google Scholar] [CrossRef]
- German, R.; Sari, A.; Venet, P.; Ayadi, M.; Briat, O.; Vinassa, J.M. Prediction of Supercapacitors Floating Ageing with Surface Electrode Interface Based Ageing Law. Microelectron. Reliab. 2014, 54, 1813–1817. [Google Scholar] [CrossRef]
- Azaïs, P.; Duclaux, L.; Florian, P.; Massiot, D.; Lillo-Rodenas, M.-A.; Linares-Solano, A.; Peres, J.-P.; Jehoulet, C.; Béguin, F. Causes of Supercapacitors Ageing in Organic Electrolyte. J. Power Sources 2007, 171, 1046–1053. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Liao, H.; Lyu, C.; Zhou, Y.; Jiao, Y.; Li, H.; Hu, C.; Peng, J. A Temperature-Suppression Charging Strategy for Supercapacitor Stack With Lifetime Maximization. IEEE Trans. Ind. Appl. 2019, 55, 6173–6183. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A.L. Electrochemical Sensors, a Bright Future in the Fabrication of Portable Kits in Analytical Systems. Chem. Rec. 2020, 20, 682–692. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent Advances in Removal Techniques of Cr(VI) Toxic Ion from Aqueous Solution: A Comprehensive Review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Fu, L.; Xie, K.; Wu, D.; Wang, A.; Zhang, H.; Ji, Z. Electrochemical Determination of Vanillin in Food Samples by Using Pyrolyzed Graphitic Carbon Nitride. Mater. Chem. Phys. 2020, 242, 122462. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; Zhang, M.; Zheng, Y.; Wu, M.; Lan, Z.; Pu, J.; Zhang, H.; Chen, F.; Su, W.; et al. Electrochemical Sex Determination of Dioecious Plants Using Polydopamine-Functionalized Graphene Sheets. Front. Chem. 2020, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Xue, N.; Xie, J.; Xu, R.; Lei, C. Separator Aging and Performance Degradation Caused by Battery Expansion: Cyclic Compression Test Simulation of Polypropylene Separator. J. Electrochem. Soc. 2021, 168, 030506. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Sahraei, E. Degradation of Battery Separators under Charge–Discharge Cycles. RSC Adv. 2017, 7, 56099–56107. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Y.; Sun, T.; Qi, Y.; Li, X. Probing the Roles of Polymeric Separators in Lithium-Ion Battery Capacity Fade at Elevated Temperatures. J. Electrochem. Soc. 2014, 161, A1241–A1246. [Google Scholar] [CrossRef]
- Yan, S.; Xiao, X.; Huang, X.; Li, X.; Qi, Y. Unveiling the Environment-Dependent Mechanical Properties of Porous Polypropylene Separators. Polymer 2014, 55, 6282–6292. [Google Scholar] [CrossRef]
- Lithium-Secondary Cell. In Electrochemical Power Sources: Fundamentals, Systems, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–266.
- Rahimi-Eichi, H.; Ojha, U.; Baronti, F.; Chow, M.-Y. Battery Management System: An Overview of Its Application in the Smart Grid and Electric Vehicles. IEEE Ind. Electron. Mag. 2013, 7, 4–16. [Google Scholar] [CrossRef]
- Faisal, M.; Hannan, M.A.; Ker, P.J.; Hossain Lipu, M.S.; Uddin, M.N. Fuzzy-Based Charging–Discharging Controller for Lithium-Ion Battery in Microgrid Applications. IEEE Trans. Ind. Appl. 2021, 57, 4187–4195. [Google Scholar] [CrossRef]
- Verbrugge, M.W. Adaptive Characterization and Modeling of Electrochemical Energy Storage Devices for Hybrid Electric Vehicle Applications. In Modeling and Numerical Simulations; Springer: Berlin/Heidelberg, Germany, 2008; pp. 417–524. [Google Scholar]
- Verbrugge, M.; Koch, B. Generalized Recursive Algorithm for Adaptive Multiparameter Regression. J. Electrochem. Soc. 2006, 153, A187. [Google Scholar] [CrossRef]
- Verbrugge, M.W.; Conell, R.S. Electrochemical and Thermal Characterization of Battery Modules Commensurate with Electric Vehicle Integration. J. Electrochem. Soc. 2002, 149, A45. [Google Scholar] [CrossRef]
- Wang, B.; Xu, J.; Cao, B.; Ning, B. Adaptive Mode Switch Strategy Based on Simulated Annealing Optimization of a Multi-Mode Hybrid Energy Storage System for Electric Vehicles. Appl. Energy 2017, 194, 596–608. [Google Scholar] [CrossRef]
- Guo, M.; Sikha, G.; White, R.E. Single-Particle Model for a Lithium-Ion Cell: Thermal Behavior. J. Electrochem. Soc. 2011, 158, A122. [Google Scholar] [CrossRef]
- Arora, S.; Abkenar, A.T.; Jayasinghe, S.G.; Tammi, K. Battery Management System: Charge Balancing and Temperature Control. In Heavy-Duty Electric Vehicles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 173–203. [Google Scholar]
- Wang, P.; Zhu, C. Summary of Lead-Acid Battery Management System. IOP Conf. Ser. Earth Environ. Sci. 2020, 440, 022014. [Google Scholar] [CrossRef]
- Vezzini, A. Lithium-Ion Battery Management. In Lithium-Ion Batteries; Elsevier: Amsterdam, The Netherlands, 2014; pp. 345–360. [Google Scholar]
- Qazi, S. Portable Standalone PV Systems for Disaster Relief and Remote Areas. In Standalone Photovoltaic (PV) Systems for Disaster Relief and Remote Areas; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–138. [Google Scholar]
- Murtaza, A.F.; Chiaberge, M.; Spertino, F.; Ahmad, J.; Ciocia, A. A Direct PWM Voltage Controller of MPPT & Sizing of DC Loads for Photovoltaic System. IEEE Trans. Energy Convers. 2018, 33, 991–1001. [Google Scholar] [CrossRef]
- Antonov, I.; Kanchev, H.; Hinov, N. Study of PWM Solar Charge Controller Operation Modes in Autonomous DC System. In Proceedings of the 2019 II International Conference on High Technology for Sustainable Development (HiTech), Sofia, Bulgaria, 10–11 October 2019; IEEE: New York, NY, USA, 2019; pp. 1–4. [Google Scholar]
- Acharya, P.S.; Aithal, P.S. A Comparative Study of MPPT and PWM Solar Charge Controllers and Their Integrated System. J. Phys. Conf. Ser. 2020, 1712, 012023. [Google Scholar] [CrossRef]
- Rezoug, M.R.; Chenni, R.; Taibi, D. A New Approach for Optimizing Management of a Real Time Solar Charger Using the Firebase Platform Under Android. J. Low Power Electron. Appl. 2019, 9, 23. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Samanta, H.; Banerjee, N.; Saha, H. Development and Validation of a Real Time Flow Control Integrated MPPT Charger for Solar PV Applications of Vanadium Redox Flow Battery. Energy Convers. Manag. 2018, 171, 1449–1462. [Google Scholar] [CrossRef]
- Monden, Y.; Mizutani, M.; Yamazaki, S.; Kobayashi, T. Charging and Discharging Control of a Hybrid Battery Energy Storage System Using Different Battery Types in Order to Avoid Degradation. In Proceedings of the 2021 IEEE International Future Energy Electronics Conference (IFEEC), Taipei, Taiwan, 16 November 2021; IEEE: New York, NY, USA, 2021; pp. 1–6. [Google Scholar]
- Garcia, P.; Fernandez, L.M.; Garcia, C.A.; Jurado, F. Energy Management System of Fuel-Cell-Battery Hybrid Tramway. IEEE Trans. Ind. Electron. 2010, 57, 4013–4023. [Google Scholar] [CrossRef]
- Shen, J.; Khaligh, A. A Supervisory Energy Management Control Strategy in a Battery/Ultracapacitor Hybrid Energy Storage System. IEEE Trans. Transp. Electrif. 2015, 1, 223–231. [Google Scholar] [CrossRef]
- Carter, R.; Cruden, A.; Hall, P.J. Optimizing for Efficiency or Battery Life in a Battery/Supercapacitor Electric Vehicle. IEEE Trans. Veh. Technol. 2012, 61, 1526–1533. [Google Scholar] [CrossRef]
- Santucci, A.; Sorniotti, A.; Lekakou, C. Power Split Strategies for Hybrid Energy Storage Systems for Vehicular Applications. J. Power Sources 2014, 258, 395–407. [Google Scholar] [CrossRef]
- Townsend, A.; Martinson, C.; Gouws, R.; Bessarabov, D. Effect of Supercapacitors on the Operation of an Air-Cooled Hydrogen Fuel Cell. Heliyon 2021, 7, e06569. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, R.; Member, S.; Lin, C.; Shen, W. Model Predictive Control Based Real-Time Energy Management for a Hybrid Energy Storage System. CSEE J. Power Energy Syst. 2020, 7, 862–874. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of Energy Storage Systems for Electric Vehicle Applications: Issues and Challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Ren, G.; Ma, G.; Cong, N. Review of Electrical Energy Storage System for Vehicular Applications. Renew. Sustain. Energy Rev. 2015, 41, 225–236. [Google Scholar] [CrossRef]
- Song, Z.; Hofmann, H.; Li, J.; Han, X.; Ouyang, M. Optimization for a Hybrid Energy Storage System in Electric Vehicles Using Dynamic Programing Approach. Appl. Energy 2015, 139, 151–162. [Google Scholar] [CrossRef]
- Ramoul, J.; Chemali, E.; Dorn-Gomba, L.; Emadi, A. A Neural Network Energy Management Controller Applied to a Hybrid Energy Storage System Using Multi-Source Inverter. In Proceedings of the 2018 IEEE Energy Conversion Congress and Exposition (ECCE), Portland, OR, USA, 23–27 September 2018; IEEE: New York, NY, USA, 2018; pp. 2741–2747. [Google Scholar]
- Xiong, R.; Cao, J.; Yu, Q. Reinforcement Learning-Based Real-Time Power Management for Hybrid Energy Storage System in the Plug-in Hybrid Electric Vehicle. Appl. Energy 2018, 211, 538–548. [Google Scholar] [CrossRef]
- Salmasi, F.R. Control Strategies for Hybrid Electric Vehicles: Evolution, Classification, Comparison, and Future Trends. IEEE Trans. Veh. Technol. 2007, 56, 2393–2404. [Google Scholar] [CrossRef]
- Xiong, R.; Chen, H.; Wang, C.; Sun, F. Towards a Smarter Hybrid Energy Storage System Based on Battery and Ultracapacitor—A Critical Review on Topology and Energy Management. J. Clean. Prod. 2018, 202, 1228–1240. [Google Scholar] [CrossRef]
- Trovão, J.P.; Pereirinha, P.G.; Jorge, H.M.; Antunes, C.H. A Multi-Level Energy Management System for Multi-Source Electric Vehicles–An Integrated Rule-Based Meta-Heuristic Approach. Appl. Energy 2013, 105, 304–318. [Google Scholar] [CrossRef]
- Schouten, N.J.; Salman, M.A.; Kheir, N.A. Energy Management Strategies for Parallel Hybrid Vehicles Using Fuzzy Logic. Control Eng. Pract. 2003, 11, 171–177. [Google Scholar] [CrossRef]
- Zandi, M.; Payman, A.; Martin, J.-P.; Pierfederici, S.; Davat, B.; Meibody-Tabar, F. Energy Management of a Fuel Cell/Supercapacitor/Battery Power Source for Electric Vehicular Applications. IEEE Trans. Veh. Technol. 2011, 60, 433–443. [Google Scholar] [CrossRef]
- Hung, Y.-H.; Wu, C.-H. An Integrated Optimization Approach for a Hybrid Energy System in Electric Vehicles. Appl. Energy 2012, 98, 479–490. [Google Scholar] [CrossRef]
- Song, Z.; Hofmann, H.; Li, J.; Hou, J.; Han, X.; Ouyang, M. Energy Management Strategies Comparison for Electric Vehicles with Hybrid Energy Storage System. Appl. Energy 2014, 134, 321–331. [Google Scholar] [CrossRef]
- Hredzak, B.; Agelidis, V.G.; Jang, M. A Model Predictive Control System for a Hybrid Battery-Ultracapacitor Power Source. IEEE Trans. Power Electron. 2014, 29, 1469–1479. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, R.; Sun, F. Model Predictive Control for Power Management in a Plug-in Hybrid Electric Vehicle with a Hybrid Energy Storage System. Appl. Energy 2017, 185, 1654–1662. [Google Scholar] [CrossRef]
- Sun, C.; Hu, X.; Moura, S.J.; Sun, F. Velocity Predictors for Predictive Energy Management in Hybrid Electric Vehicles. IEEE Trans. Control Syst. Technol. 2015, 23, 1197–1204. [Google Scholar] [CrossRef]
- Song, K.S.; Park, S.-J.; Kang, F.-S. Internal Parameter Estimation of Lithium-Ion Battery Using AC Ripple With DC Offset Wave in Low and High Frequencies. IEEE Access 2021, 9, 76083–76096. [Google Scholar] [CrossRef]
- Moral, C.G.; Laborda, D.F.; Alonso, L.S.; Guerrero, J.M.; Fernandez, D.; Rivas Pereda, C.; Reigosa, D.D. Battery Internal Resistance Estimation Using a Battery Balancing System Based on Switched Capacitors. IEEE Trans. Ind. Appl. 2020, 56, 5363–5374. [Google Scholar] [CrossRef]
| Battery Technology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LA | NiMH | LI | NiCd | LiPo | Zn-O2 | NaS | VFB | FC | ||
| Nominal cell voltage | V | 2.1 | 1.2 | 3.6–3.85 | 1.2 | 2.7–3 | 1.45–1.65 | 1.78–2.208 | 1.15–1.55 | 0.6–0.7 |
| Energy density | Wh/kg | 30–40 | 60–120 | 100–265 | 40–60 | 100–265 | 442 | 240 | 25 | 1500 |
| Power density | W/kg | 180 | 250–1000 | 250–340 | 150 | 245–430 | 100 | 230 | 100 | 400 |
| Cycle life | Cycles | <1000 | 180–2000 | 400–1200 | 2000 | 500 | 100 | 4500 | >10,000 | ~9000 * |
| Charge/discharge efficiency | % | 50–95 | 66–92 | 80–90 | 70–90 | 90 | 60–70 | 87 | 70–80 | 40–60 |
| Self-discharge rate | % | 3–20 | 13.9–70.6 | 0.35–2.5 | 10 | 0.3 | 0.17 | 2 | ~0 | 0 |
| DoD | % | 50 | 100 | 80 | 60–80 | 80 | 60–65 | 100 | 100 | 100 |
| Cost | USD/Wh | 0.69750 | 0.8546 | 0.9361 | 2.6778 | 2.3095 | 0.3095 | 0.5 | 5.7 | 0.02 |
| TRL | 9 | 9 | 9 | 9 | 9 | 9 | 7 | 9 | 9 | |
| Energy Density (Wh/kg) | Power Density (W/kg) | Cycles | Cost * (USD/kWh) | Cost per Cycle (USD/kWh/Cycle) | |
|---|---|---|---|---|---|
| Flooded | 34.29 | 68.57 | 350 | 55.56 | 0.16 |
| Deep cycle | 40 | 52.80 | 500 | 186.72 | 0.37 |
| AGM | 41.38 | 153.97 | 600 | 142.86 | 0.24 |
| Gel | 35.82 | 125.37 | 750 | 168.06 | 0.22 |
| Energy Density (Wh/kg) | Power Density (W/kg) | Safety/Thermal Runaway (°C) | Maximum Discharge/Charge C-Rate | Cycles | Cost * (USD/kWh) | Cost per Cycle | |
|---|---|---|---|---|---|---|---|
| LCO | 150–200 | 50–100 | 150 | 1/1 | 500–1000 | 385 | 0.39–0.77 |
| LMO | 100–150 | 250–400 | 250 | 10/1 | 300–700 | 400 | 0.57–1.33 |
| NMC | 150–220 | 100–150 | 210 | 2/1 | 1000–2000 | 420 | 0.21–0.42 |
| LFP | 90–160 | 200–1200 | 270 | 25/2 | >2000 | 580 | 0.29 |
| LTO | 50–80 | 3000–5100 | 280 | 10/10 | >5000 | 1005 | 0.14–0.34 |
| Energy Density (Wh/kg) | Power Density (W/kg) | Cycle Durability | Operating Temperature (°C) | Cost * (USD/Wh) | Cost per Cycle | TRL | |
|---|---|---|---|---|---|---|---|
| EDL | 0.9–2.5 | 900–10,000 | >1000 k | −40–+70 | 219.80 | 0.00022 | 9 |
| EP | 1–10 | 500–7000 | >100 k | −20–+70 | N/A ** | N/A ** | 4 |
| HC | 5–55 | 250–5000 | >20 k | −20–+70 | 103.90 | 0.00519 | 9 |
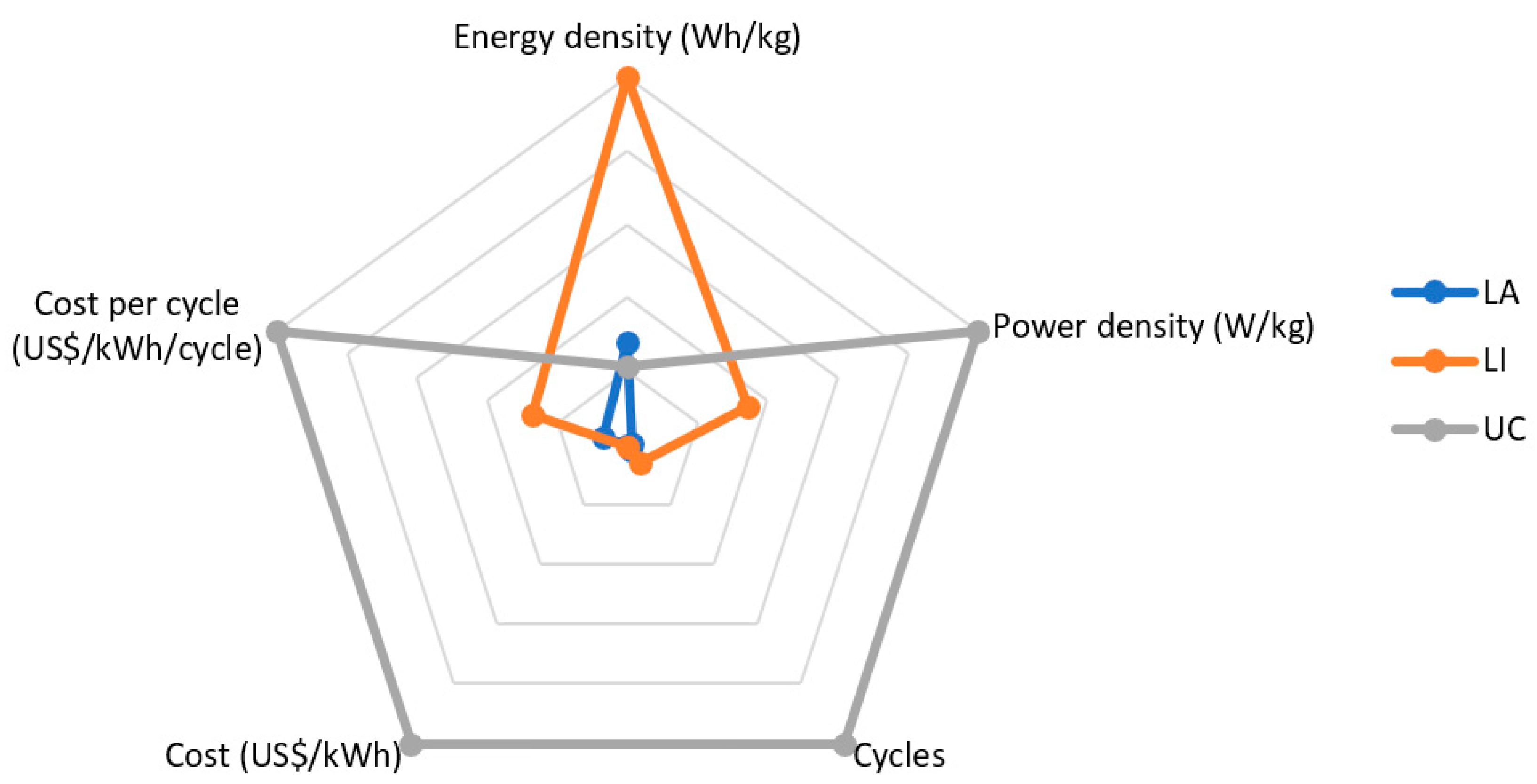
| LA | LI | UC | |
|---|---|---|---|
| Energy density (Wh/kg) | 35–40 | 50–220 | 2.5–55 |
| Power density (W/kg) | 69–154 | 50–5100 | 5000–10,000 |
| Cycle life | 800 | 3000 | >50,000 |
| Self-discharge rate (%pm) | <3 | <2 | >54 * |
| Operating temperature (℃) | −40–+60 | −50–+85 | −40–+70 |
| Cost (USD **/kWh) | 55–168 | 385–1005 | 103 k–220 k |
| Cost per cycle | 0.07–0.32 | 0.14–1.13 | 0.22–5.19 |
| Type | Description | Consequence |
|---|---|---|
| Over-discharge | When the battery is discharged lower than the recommended DoD voltage. As the battery discharges lead sulfation accumulates on the surface of the electrodes; if over-discharged this sulfation crystalizes. Also leads to overexposure of the electrodes. | Crystal sulfation formation and electrode corrosion Effective surface area of the electrode is reduced, power density, overall capacity and cycle life of the battery is reduced. Extreme case—crystal sulfation will occupy the majority of the battery and the battery will be rendered useless; electrode corrosion will lead to collection of active material at the bottom of the battery which can potentially lead to a short circuit between the electrodes. |
| Over-charge | When the battery is left to charge for extended periods of time after reaching full charge status. For both FLA and SLA, heat leads to an increase in current transfer rate, which increases the chances of overcharging. The process of recharging this battery releases a lot of heat which is exacerbated when continued indefinitely. | Excessive heat— Leads to mechanical damage (warping of collector plates, shutdown of separator); evaporates the water in the electrolyte, increases acidity of the electrolyte and exposure of the electrodes; both increase the rate of corrosion, decrease the effective surface area, capacity, power density and service life of the battery. Extreme case—the evaporated hydrogen and oxygen cannot escape (larger risk in SLA) which poses a highly combustible and explosive hazard. |
| Crystal sulfation | When battery remains in extended state of discharge (partially/fully). Soft lead sulfation formed during discharge becomes hard crystals that cannot be broken down. | Effective surface area is reduced— Reduces power density, capacity and cycle life of the battery; Can also lead to damage of the separator (to be discussed under separator section of this article). Extreme case—crystal sulfation will occupy the majority of the battery and the battery will be rendered useless. |
| Stratification | Acid molecules in electrolyte gather towards the bottom of the battery. When a battery is stored discharged (partially/fully), the acid molecules separate from the water molecules. | Causes current flow predominantly in the acidic area increasing corrosion/wear of the electrodes. |
| Water loss | Any action that leads to loss of the liquid electrolyte or water element of the electrolyte. Mainly attributed to heat or leakage of the electrolyte. Heat is attributed to excessive environmental temperatures, high charge/discharge current or short circuits. | Decrease in volume of electrolyte— exposes the electrodes, increases electrolyte acidity and electrode corrosion, decreases effective surface area, power density and cycle life. |
| Short-circuit | When the electrodes are electrically connected and allow conduction between them. Caused by ineffective separator (poor battery assembly, defective, rough electrodes), collection of active material at the bottom of the battery (due to damage of the electrode from stratification or other causes), presence of conductive materials inside the battery (during assembly or maintenance of FLA batteries) or warping of the collector plates due to excessive heat. | Excessive heat generation— reduction in water concentration of the electrolyte, increase in acidity, increased corrosion of the electrode, decrease in overall battery capacity and subsequently, of the service life thereof. |
| Type | Description | Consequence |
|---|---|---|
| Over-discharge | When the battery is discharged lower than the recommended DoD voltage. Over discharge leads to over-deintercalation of the LIs in the anode. | Leads to decomposition of the solid electrolyte interface (SEI) and generates CO2 gasses; recharge allows for new SEI film formation with a different morphology that degrades the electrochemical charge transfer process and increases the internal resistance; Leads to oxidization of the copper collector plates—higher internal resistance and lower capacity; also leads to power losses; Lithium intercalation process causes the electrode structure to expand and contract, forming fine cracks in the structure. This effect is exacerbated when over-deintercalation occurs and leads to increased degradation rate of the electrodes and thus decreases the service life of the battery. |
| Over-charge | When the battery is left on charge for extended periods of time after reaching full charge status. Leads to excessive heat and eventually thermal runaway. | Thermal runaway causes the anode to overheat and the cathode to release oxygen—poses a potential fire risk; the electrolyte is usually of a flammable substance; this all leads to a potential fire hazard. Excessive heat can also lead to partial shutdown of the separator (explained later in this article), which increases internal resistance, decreases capacity and charge/discharge rate and subsequently decreases service life of the battery. |
| High charge/discharge rate | When the battery is either charged or discharged at a rate higher than recommended. | This leads to excessive heat, LLI and lithium plating, all of which decrease the capacity of the battery permanently and can lead to potential fire hazards. |
| Loss of lithium inventory (LLI) | Loss of usable LIs. Caused by parasitic reactions and continuous SEI growth. | Decrease in LI leads to lower levels of intercalation and less movement of electrons and thus lower energy density. |
| Loss of active material (LAM) | Structural and mechanical degradation—breakdown of graphite molecular structure, corrosion of copper collector plates. Insertion or intercalation of LIs into the molecular gaps of the graphite. Subsequent insertion (and removal) leads to the breakdown of the graphite structure. | Can trigger a sudden rapid capacity loss, capacity and power fade as result. Quantity of molecular gaps reduces; less lithium can be intercalated; reduces the energy density. |
| Ohmic resistance increase | Increase in electronic and ionic resistance of a cell. Due to LLI and LAM. | Increases self-discharge—thus decreasing energy density. Also decreases power density due to resistance of power release. |
| Lithium plating or dendrite growth | Lithium deposits onto the anode instead of intercalating during a charge. If the charge current is too high, faster reactions than what can occur are required; if the operating temperature is too low, reaction rate is too slow—both lead to lithium accumulation on the surface of the anode. | Leads to short circuiting between the electrodes, excessive heat and fire hazards, LLI and LAM. |
| Type | Description | Consequence |
|---|---|---|
| Electrochemical reactions | The operating reactions between the electrodes and electrolytes produces solids and gases | Increases internal pressure—leads to electrode cracks; packaging elongation and damages collectors; Blocks pores of electrode—reduces reactive surface area; Blocks separator—disturbs circulation of the ions |
| Voltage resets | Periodically discharging the UC to a lower voltage than that which is used during operation | Reorganizes the charge distribution within the electrode pores which exposes new aging zones and leads to a significant increase in aging |
| Uneven charge distribution | Uneven charge current distribution amongst cells due to individual cell degradation levels | Uneven charging of cells leads to overcharging and overheating of certain cells and thus increased degradation of those cells |
| Overcharging | When too high voltage is applied to the UC for a period of time | Pressure build up occurs inside the UC due to electrolyte decomposition and increased temperature |
| Consequence | Description | Cause |
|---|---|---|
| Shrinkage | Separator shrinks smaller than required size—creates an electrical conduction path between the electrodes—short circuits, service life degradation, higher internal resistance and possible thermal runaway | Excessive operating temperature— High charge/discharge rate; Overcharging |
| Shutdown effect | Leads to melting/partial melting of the separator pores decreasing the ion conductivity and uniform current distribution. Reduces service life of ESD, reduces charge/discharge rate, can lead to premature failure of ESD | Excessive operating temperature — High charge/discharge rate; Overcharging |
| Piercing | Decreases integrity of the separator, creates an electrical conduction path between the electrodes—short circuit, reduces service life of ESD | Growth formations on the electrodes— High charge/discharge rate |
| Stress effect | Cyclic compression and expansion of the separator—leads to a decrease in the integrity of the separator, can cause shrinkage and wrinkles in the separator | Electrode expansion during charge/discharge— Cyclic effect Frequent charge/discharge cycles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Townsend, A.; Gouws, R. A Comparative Review of Lead-Acid, Lithium-Ion and Ultra-Capacitor Technologies and Their Degradation Mechanisms. Energies 2022, 15, 4930. https://doi.org/10.3390/en15134930
Townsend A, Gouws R. A Comparative Review of Lead-Acid, Lithium-Ion and Ultra-Capacitor Technologies and Their Degradation Mechanisms. Energies. 2022; 15(13):4930. https://doi.org/10.3390/en15134930
Chicago/Turabian StyleTownsend, Ashleigh, and Rupert Gouws. 2022. "A Comparative Review of Lead-Acid, Lithium-Ion and Ultra-Capacitor Technologies and Their Degradation Mechanisms" Energies 15, no. 13: 4930. https://doi.org/10.3390/en15134930
APA StyleTownsend, A., & Gouws, R. (2022). A Comparative Review of Lead-Acid, Lithium-Ion and Ultra-Capacitor Technologies and Their Degradation Mechanisms. Energies, 15(13), 4930. https://doi.org/10.3390/en15134930







