Abstract
This study analyzed factors that influence the ignition delay characteristics of n-heptane/methane-blended fuel. The effects of chemical species, exhaust gas recirculation rate, compression ratio, cool/hot flames, and combustion chamber conditions (temperature, pressure, and O2 concentration) were determined and analyzed using CHEMKIN Pro. The experiment conditions for verification were 550–1000 K at 15 bar with 50% H2/50% CH4 fuel. The main combustion reactions were confirmed through reactivity analysis and sensitivity analysis on the ignition delay time. The ignition delay time at 14.7% O2 concentration was significantly higher than that at 21% O2 concentration by more than 30%. In addition, a higher ratio of methane in the blended fuel increased the ignition delay time as a result of methane dehydrogenation, delaying the ignition of heptane.
1. Introduction
Due to the depletion of fossil fuels and the environmental impacts, strong emission regulations such as EURO-6 and Tier-5 are implemented around the world. As a result, there is a growing interest in improving the efficiency of internal combustion engines and reducing their pollutant emissions [1]. An ongoing active area of study is homogeneous charge compression ignition (HCCI) engines, which combine the advantages of conventional gasoline engines (SI), diesel engines (CI), reactivity-controlled compression ignition (RCCI) engines, and partially pre-mixed combustion ignition (PPCI) engines [2,3]. In the HCCI system, a pre-mixture of air and fuel is formed in the combustion chamber during intake just like in the SI, while the piston compression and ignition combustion are performed similar to the CI. The combination of these technologies allows for engine operation in sparse conditions, achieving a high compression ratio (CR) by applying exhaust gas recirculation (EGR) to maintain low-temperature combustion (LTC), reducing carbon dioxide (CO2) emission by improving the thermal efficiency, and simultaneously decreasing the emission of NOx and particulate matters (PM) by forming a consistent mixture in the combustion chamber while preventing local hot spots during combustion [1,4]. However, the operation range is still limited at low loads and high loads. At low loads, a low temperature leads to incomplete combustion, causing an excessive discharge of carbon monoxide (CO) and hydrocarbons (HCs). At high loads, rapid ignition combustion increases the combustion pressure drastically, which results in engine knocking, damage, and noise.
Conventional diesel fuel is a mixture of various HCs with a high cetane number. Among the components, n-heptane with a high carbon number has a cetane number comparable to that of diesel. Hence, n-heptane is widely used as an alternative to diesel in experimental settings to effectively simulate two-stage ignition, cool flames, hot flames, and ignition delays. Also, many studies have been conducted on precise combustion control techniques to control the ignition delay by adjusting the fuel mixing ratio, pressure, and equivalence ratio [5,6]. ExxonMobil, a U.S. petrochemical company, recently predicted in its long-term energy outlook that oil and natural gas would remain the main sources of energy, accounting for 60% of the world’s energy supply by 2040. Particularly, the consumption of natural gas was expected to grow the fastest to meet 40% of the global energy demand. Mainly composed of methane, natural gas allows for an effective response to CO2 regulations, such as the Kyoto Protocol, with its lower emissions of PM and CO2 when compared to diesel fuel. Therefore, many researchers have been studying combustion characteristics such as ignition delay time, ignition temperature for operating pressure, burning velocity, etc., for alternative fuels [7,8,9,10,11,12,13,14,15,16,17,18].
Li et al. [7] studied the autoignition characteristics of gasoline/diesel/ethanol/PODE fuels. The effect of ethanol on chemical ignition delay is higher than that of physical ignition delay, and a higher EGR ratio also leads to a longer ignition delay time.
Kuszewski [8] conducted an experimental study of n-butanol-diesel blends to achieve low nitrogen oxides and smoke emissions from the diesel engines. The autoignition properties were tested by standard diesel fuel with oxygen compounds such as alcohols.
In the study of Wang et al. [9], ignition delay time gradually decreases with a high pressure and equivalence ratio which conditions show lower reactivity. The simulation results are in good agreement with experiment results. The gas-phase autoignition of methanol/diesel mixture was studied by Zhu et al. [10]. They observed the typical two-stage ignition of a mixture with an NTC response over the whole temperature regime. In terms of various fuels that have been studied, n-heptane/methane blends have also attracted focus. N-heptane has been used as a standard fuel in fundamental studies on fuel control methods, and methane recently gained attention as an alternative fuel. The high octane number of methane increases the ignition delay, providing sufficient time for the pre-mixing of fuel and air during engine operation and allowing for consistent pre-mixing [11]. However, the low cetane number of methane results in poor auto-ignition properties [12]. A reasonable way to meet the requirements of both SI and CI is mixing methane with hydrocarbon fuel having a high cetane number. There is an urgent need to verify the dual fuel system and analyze its combustion properties.
Yao et al. [5] simulated the ignition delay for n-heptane/methane blended fuel at different mixing ratios. They investigated the effects of three factors (oxygen partial pressure, specific heat, and chemical reaction) on ignition delay at 40 and 80% methane. They also carried out a comparative study through chemical reaction sensitivity analysis on the ignition delay, by designing reduced chemical mechanisms for methane (Hung mechanism) and for n-heptane (Curran mechanism). Aggarwal et al. [6] simulated the ignition delay time in n-heptane/methane-blended fuel under high-pressure conditions (30 and 55 atm) and equivalence ratios (Φ = 1 and 2). The methane ratios were 0, 20, 80, 95, and 100%. Their sensitivity analysis revealed that the heptyl (C7H15) and hydroxyl (OH) radicals played a significant role in ignition.
Our research team has been studying autoignition characteristics for n-heptane-based fuels such as n-heptane/hydrogen and n-heptane/ethanol with various operating conditions [13,14]. The EGR ratio, operating temperature, diluting ratio etc., have been studied with various fuels, and this study analyzed various factors affecting the chemical ignition of n-heptane/methane-blended fuel, including the combustion chamber environment (temperature, pressure, and O2 concentration), changes in OH radicals, and intermediate chemical species promoting oxidation reactions, cool/hot flames, and CR. No relevant information has been reported before. The goal here was to understand how the combustion reaction and ignition delay time of n-heptane/methane-blended fuel under low-temperature conditions are affected by the mixing ratio, heat production, concentration of intermediate chemical species, CR (2.5, 3.0, 3.5, 4.0, and 4.5), ignition delays by a cool/hot flame, and O2 concentration through EGR simulations.
2. Experimental Device and Method
2.1. Experimental Device
The rapid compression machine (RCM) is an ideal device for simulating pre-mixed gas (fuel, oxygen, and dilution gas) at a set temperature and pressure in a combustion chamber similar to the actual engine. The composition, CR, equivalence ratio, and compression temperature of the pre-mixed fuel can be adjusted. The combustion phenomenon at constant pressure may be measured and analyzed by varying the temperature [19,20]. Figure 1 illustrates in detail the RCM system consisting of two parts: a combustion chamber where the piston quickly compresses fuel for auto-ignition combustion, and an impact pneumatic cylinder (IPC) that operates the piston pneumatically. There is a pre-mixing chamber, a vacuum pump, and sensors and measurement equipment for acquiring experimental data. A piston compressed by air is installed inside a pneumatic cylinder where compression pressure is generated. The piston speed can be adjusted by the pressure inside a high-pressure air tank fed by an air compressor. The CR is adjusted using a pneumatic device that moves a rod connecting the combustion chamber and the pneumatic cylinder. The RCM used here is capable of very short compression times of less than 30 ms. To address the piston-slip phenomenon caused by the explosion, which may significantly affect the experimental results, a brake arm is used to fix the piston once it reaches the top dead center to prevent damage by the explosion. Table 1 provides detailed information on the RCM system.
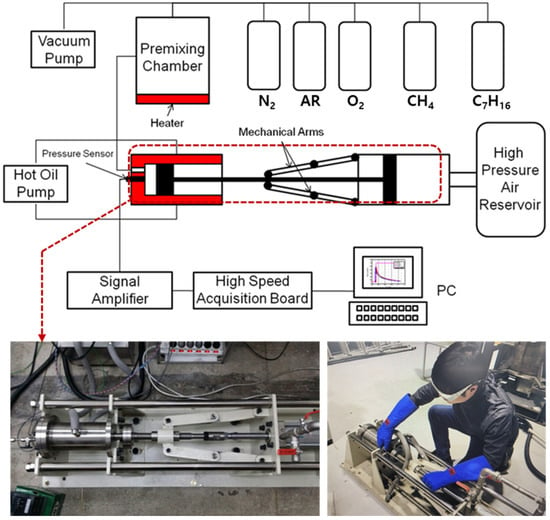
Figure 1.
Schematic of the rapid compression machine (RCM) system.

Table 1.
Specification of the RCM system.
2.2. Experimental Method and Conditions
This study used a blended fuel of n-heptane and methane with the mixing ratio of methane set at 100, 75, 50, and 25 mol.%. A uniform mixture was produced by vaporizing the blended fuel in the mixing chamber with magnetic stirrer, and the amount of injected gas and fuel was determined by calculating the absolute pressure and adjusted by measuring the partial pressure. A vacuum was created in the combustion chamber with a vacuum pump, and the fuel was injected into the pre-mixing chamber with a syringe. The experiment was conducted once the fuel tank, gas supply line, and combustion chamber reached a constant temperature of 293 ± 0.5 K. After a compression and explosion stroke were completed in the combustion chamber, the emissions were removed using the vacuum pump, and the experiment was repeated 6 to 10 times under the same conditions and an operating pressure of 8 bars to confirm reproducibility. The pressure changes inside the combustion chamber were measured by opening the solenoid valve, for the period from when the piston in IPC started operating to when the pressure value of the rear cylinder in IPC was opened following the explosion. The output signals of the pressure sensor (Kistler 6125b) and pressure transducer (Kistler 6041A) were amplified by the signal amplifier (Kistler 5018). The amplified analog output was acquired through a high-speed A/D board (Kistler 5018) at a sampling speed of 10,000 Hz and transformed with commercial software (LabVIEW 8.2). A K-type thermocouple was used to measure the temperature of the mixture supplied to the combustion chamber. The compression temperature (Tc) was calculated using Equation (1), assuming that compression inside the RCM was the adiabatic process of an ideal gas [21,22]:
where T0 and P0 are the initial temperature and pressure, Tc and Pc are the temperature and pressure of the mixture compressed at the top dead center, and is function of temperature corresponding to the ratio of specific heat of the mixture.
The ignition timing was analyzed by varying the ratio of methane from 100 to 25 mol.% in the blended fuel at low temperatures, as well as the O2 concentration (14.7–21%) and CR = 2.5–4.5 for EGR simulations. Table 2 provides the properties of the blended fuel used in the experiment.

Table 2.
Fuel properties.
3. Numerical Analysis
To verify the results of the ignition delay experiments conducted with the RCM, theoretical calculation was performed using the commercial analysis program CHEMKIN-PRO [23]. A closed homogeneous constant-volume combustion module was adopted to simulate the initial state at the temperature and pressure when the piston reached the TDC. A reaction model developed by Dooley et al. consisting of 1599 chemical species and 6633 chemical reactions was used for the n-heptane/methane-blended fuel [4]. The model was interpreted using a jet-stirred reactor, and it can be applied to a wide temperature range for low- and high-temperature reactions. In addition, the model is capable of calculating complex chemical reactions with a high carbon number. The detailed n-heptane reaction model proposed by Mehl et al. [24] was adopted for the calculations of n-heptane. The calculation was conducted at Φ = 1, Pc = 15 bar, Tc = 600–1000 K. The auto ignition characteristics for 0, 25, 50, 75, 100% of methane ratios in fuel mixture, 2.5, 3, 3.5, 4, and 4.5 of CR, and 0, 10, 20, and 30% of N2 dilution were considered.
The ignition delay time, an important factor in this study, is defined according to Figure 2. The Figure illustrates the time-dependent pressure and its change rate (dP/dt). The ignition delay (τ1) was measured from the end of the compression to the occurrence of a cool flame, and τ2 is the duration from the occurrence of the cool flame to the beginning of ignition. The total ignition delay time (τtot) is defined as τ1 + τ2, or the time interval from completing the compression stroke to the maximum value of dP/dt.
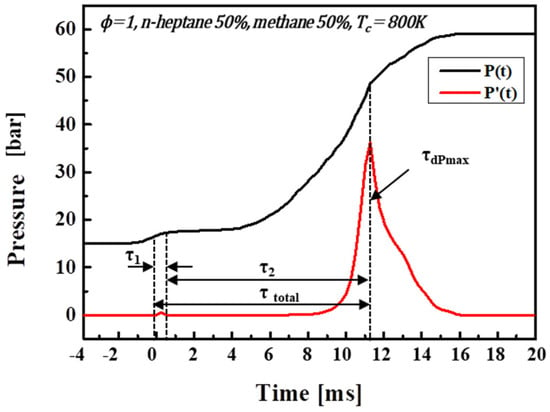
Figure 2.
Schematic of ignition delay time.
4. Results and Discussion
4.1. Reaction Pathways of Blended Fuel
Figure 3 shows the reaction pathways of pure n-heptane (left) and n-heptane/methane-blended fuel (right) [25,26,27,28]. The difference between the two systems is indicated by the red dotted box. Methane reacts with O2 to promote an oxidation reaction and produces formaldehyde (CH2O), using OH radicals formed from methane in the first oxidation reaction in the low-temperature range. Since formaldehyde is widely known as a stable molecule, the ignition delay time increases as the methane ratio in the blended fuel increases [29,30]. Also, in the n-heptane/methane mixture, methane reduces the production of H2O2 radicals at low temperatures and inhibits the production of active OH radicals that induce oxidation, thereby suppressing the low-temperature oxidation reactions. As n-heptane is highly reactive at low temperatures, the oxidation of mixed fuels can be promoted by a number of radicals produced by the reaction of n-heptane.
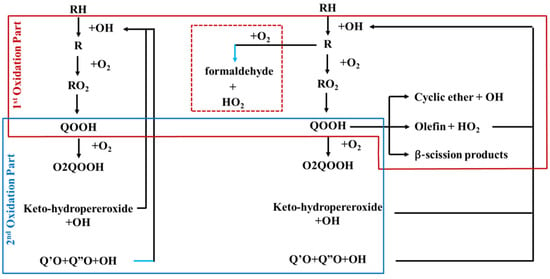
Figure 3.
Reaction pathways of pure n-heptane (left) and n-heptane/methane-blended fuels (right).
4.2. Chain Reactions
The following chain reactions R1–R5 are the main oxidation reactions [31]:
R1: RH → R + H (H-atom abstraction)
R2: R + O2 ↔ RO2
R3: RO2 ↔ QOOH
R4: QOOH + O2 ↔ O2QOOH
R5: O2QOOH → ketohydroperoxide + OH
Alkyl groups are formed from n-heptane after H atoms are separated in the early stage of low-temperature combustion, and they react with oxygen to form RO2 radicals. These radicals are converted to QOOH radicals through isomerization, which is the key reaction mechanism in low-temperature combustion. As the temperature increases, the strongly exothermic reaction R + O2 ⟶ RO2 is reversed to RO2 ⟶ R + O2. RO2 decomposes into cyclic ether + OH, conjugate olefin + HO2, in addition to the QOOH radicals. These chain reactions reduce the reactivity of the overall system, resulting in the development of a region with negative temperature coefficient (NTC) [32]. The NTC refers to the phenomenon that the ignition delay time of a fuel mixture increases with an increasing initial temperature within a certain temperature range [33,34,35,36,37]. The high-temperature combustion proceeds quite differently from the low-temperature one. In the high temperature range above 1000 K, the main chain reaction is H + O2 = O + OH, and the overall chemical decomposition reaction takes place in rapid combustion through β-scission of alkyl radicals [38]. As a result, OH radicals play an important role in the oxidation reaction and promote the reaction of n-heptane/methane-blended fuel.
4.3. Production of OH and H2O2 Radicals
For simplicity, in the following discussion we denote different blended fuels as xH(100−x)M, where x is the mol.% of n-heptane and (100−x) that of methane. For example, an equimolar mixture of n-heptane/methane is referred to as H50M50.
Figure 4 shows the timing for the formation of OH and H2O2 radicals. Due to their unpaired electron, OH radicals are high in energy and very reactive. The first step of n-heptane oxidation is dehydrogenation to form C7H15-1 to C7H15-4. The reaction of oxidized HO2 (HO2 + HO2 = H2O2 + O2) produces H2O2 radicals, which significantly affect the formation of OH radicals in the second oxidation reaction. In panel (a) for H75M25 and panel (b) for H25M75, OH and H2O2 radicals are formed in proportion to each other during the low-temperature oxidation process. As the methane ratio increases, the amount of H2O2 produced by the oxidation of n-heptane decreases, and the delay time increases. This is the main difference between the first oxidation time and the second oxidation time. The amounts of the formed products also vary significantly, which affects the temperature rise at the time of the first oxidation. The formation of H2O2 increases after the first ignition, but the extinction point depends on the methane ratio. When these radicals reach a certain level, the main ignition occurs [39,40].
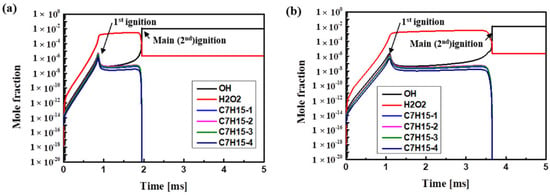
Figure 4.
Computed concentrations of various radical species (OH, H2O2, and C7H15) at the conditions of Φ = 1.0, Pc = 15 bar, CR = 3.5, O2 = 21% for (a) H75M25 and (b) H25M75.
Figure 5 shows the amount of H2O2 produced at various methane concentrations. The amount of H2O2 was similar when the methane concentration remained at 50% or less. As mentioned above, H2O2 production at low temperatures depends on the compression temperature Tc. As the methane ratio increases, the production time of H2O2 is delayed, which reduces the amount of generated H2O2. The main ignition is also delayed when the rate of H2O2 accumulation is slower, and the ignition temperature is reduced by 40–100 K, which delays the first ignition. It was confirmed that the total ignition delay time in the low-temperature region increased when the methane ratio in the blended fuel increased. This is consistent with previous studies [5,6,39], which showed that OH radicals produced at a higher methane ratio delay the ignition time. This is attributed to the H-abstraction reaction of methane, which consumes large amounts of OH radicals and oxygen, and the consequent lack of OH radicals and oxygen required for the oxidation of n-heptane is the major cause of the ignition delay. However, at high temperatures, OH radicals are rapidly promoted and decomposed by the production of H2O2. That is, ignition timing can be controlled with the mixing ratio of the blended fuel [5,6,39,40].
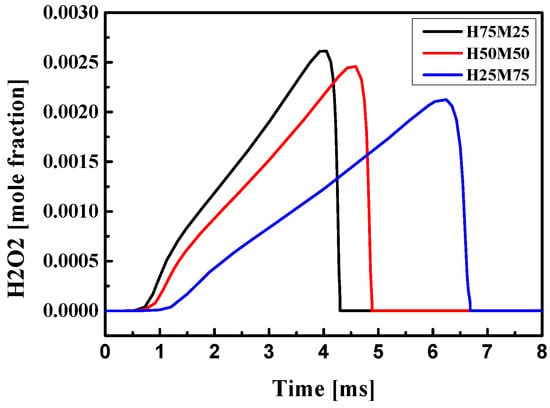
Figure 5.
Mole fraction of H2O2 for blended fuels at Tc = 900 K, Φ = 1.0, Pc = 15 bar, CR = 3.5, and O2 = 21%.
4.4. Analysis of Blended Fuel Reaction
4.4.1. Effect of Fuel Composition
Figure 6 shows the ignition delay time for n-heptane/methane-blended fuels at different compositions. Since the horizontal axis is the reciprocal temperature, the right-hand side represents the low-temperature region and vice versa. The methane ratio in the fuel was 0, 25, 50, 75%, and 100% while fixing the equivalence ratio at one, the compression pressure at 15 bar, and the O2 concentration at 21%. The ignition delay time was calculated while varying Tc from 600 to 1000 K in steps of 50 K. As Tc increased, the ignition delay time decreased. Even at a high methane ratio of 75%, an NTC region and the second ignition were observed, although this did not occur for 100% methane. Regardless of the mixing ratio, the ignition delay time decreased as Tc increased, which is the typical combustion characteristic of n-heptane. However, the ignition delay time started to increase or remained constant as the temperature increased near to the specific temperature of 800 K in the NTC region [39,40].
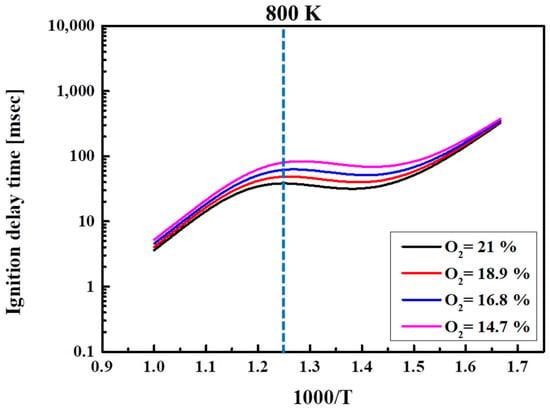
Figure 6.
Ignition delay times of blended fuels at Φ = 1.0, Pc = 15 bar, CR = 3.5, and O2 = 21%.
4.4.2. Temperature and Pressure Changes in the Combustion Chamber
Figure 7 shows the temperature distribution in the combustion chamber and the ignition delay time, as Tc is varied from 600 to 900 K and the CR from 2.5 to 4.5. For the combustion temperature, the combustion process was assumed to be adiabatic, whereby heat loss due to heat transfer only occurs through the boundary near the cylinder wall but not in the core.
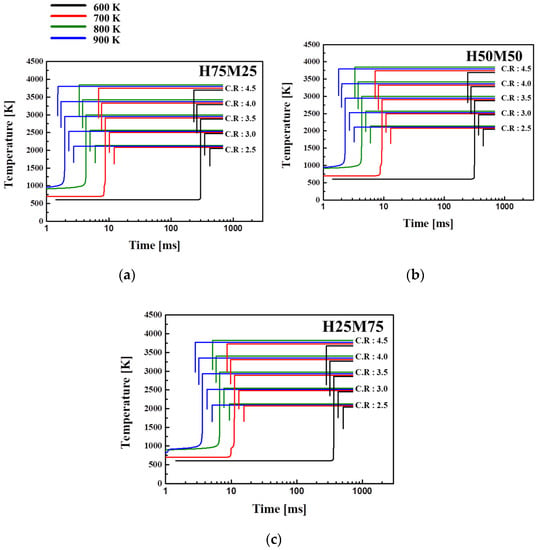
Figure 7.
Temperature dependence of ignition delay times for blended fuels at Φ = 1.0, Pc = 15 bar, and O2 = 21% in (a) H75M25, (b) H50M50, and (c) H25M75-blended fuels.
Tc was calculated using Equation (1), and the ignition delay time at Tc = 800 K was shorter than that at 900 K, which fell in the NTC region. Also, as the methane ratio increased and the initial Tc decreased, the ignition delay time increased [39,40,41]. Tc was the highest at 900–950 K for H75M25 and H25M75 and at 950–1000 K for H50M50. Regardless of the mixing ratio, the ignition delay time was the shortest at 800 K and the longest at 600 K. When the methane ratio increased, the pattern in temperature change was similar, but the ignition delay time changed. For H75M25 in (a), as the CR increased from 2.5 to 4.5, the combustion chamber temperature rose to a maximum of 3823 K, and the ignition delay time decreased from 2.84 to 1.5 ms or less. For H50M50 in (b), the combustion chamber temperature increased to a maximum of 3783 K, and the ignition delay time decreased from 3.5 to 1.7 ms or less. For H25M75 in (c), these values were 3774 K, 5.1 ms, and 2.8 ms, respectively. The ignition delay time decreased as a result of an increase in CR, and Tc and the compression pressure also increased. This reaction was attributed to the faster flame speed, because n-heptane promotes the combustion reaction of methane when the two components are blended. [40,41,42].
4.4.3. Concentration of Produced Chemical Species and Heat Production Analysis
Figure 8 compares heat production at the time of ignition depending on the concentration of the chemical species, when the methane ratio in the blended fuel is increased stepwise by 25%. For H75M25 in Figure 8a, n-heptane promotes the oxidation reaction of blended fuel and disappears quickly after the first ignition, but methane is oxidized and decomposed under the influence of n-heptane. This is because n-heptane promotes the oxidative decomposition of methane. The heat production was also found to increase rapidly. For H50M50 in Figure 8b, methane is consumed after n-heptane is consumed. For H25M75 in Figure 8c, n-heptane and methane are oxidized and consumed at the same time points during the first ignition and the main ignition. This was attributed to the delay of methane oxidative decomposition due to the lack of oxidizing agents for n-heptane. OH radicals are produced by low-temperature chain reactions of the blended fuel, as described above, and H2O2 radicals formed after the first ignition promote the production of OH radicals and disappear at the time of the main ignition. As the proportion of methane increases, the production of H2O2 decreases and its production period increases, resulting in a decrease in OH radicals and a longer time required for the main ignition to occur. Heat production was also confirmed to decrease as the methane ratio increased, and the cool flame took place at the first ignition. The amount of produced heat increased as Tc increased, but there was less heat production in the NTC region at 850 K. This is because the weak oxidation at low temperatures decreased the reactivity. Regarding fuel consumption, the decomposition and combustion of methane were slower than those of n-heptane due to the high octane number and auto-ignition temperature of methane. As a result, methane was combusted according to the promoted combustion pattern of n-heptane with a high cetane number.
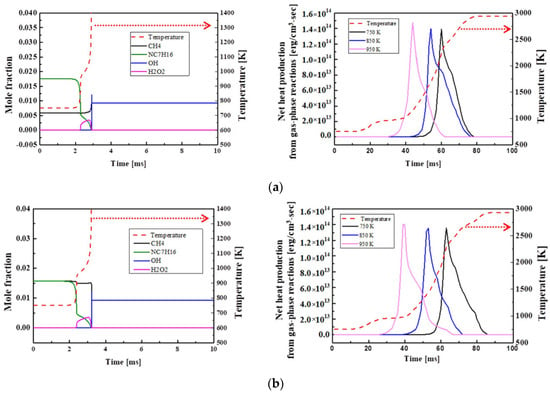
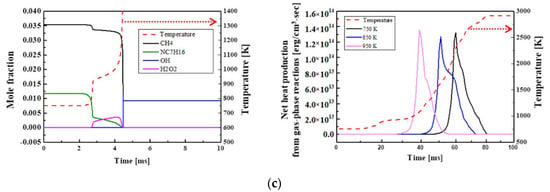
Figure 8.
Computed combustion temperature, net heat production, and the concentrations of fuel and other important species at Φ = 1.0, Pc = 15 bar, Tc = 750 K, CR = 3.5, and O2 = 21% for (a) H75M25, (b) H50M50, and (c) H25M75-blended fuels.
4.4.4. Analysis of Ignition Delays by Cold and Hot Flames
Figure 9 compares the delay times for the first ignition and the main ignition for each blended fuel composition at CR = 3.5. The first ignition time is exponentially decreased, and the second ignition delay time is exponentially increased d by increasing temperature. At a higher methane ratio and Tc = 700–850 K, the first ignition and the main ignition were delayed [41,42]. The main ignition delay time increased by 46% when methane ratio in the blended fuel increased from 25 to 75%, and the delay time increased by 87 and 83% at Tc = 800 and 850 K, respectively. Regardless of the fuel composition, only the main ignition occurred without the first ignition when Tc ≥ 900 K. This indicates that a higher methane ratio suppresses heptane ignition. The chain reaction for methane decomposition (CH4 + OH = CH3 + H2O) increases with the methane ratio, which in turn increases the consumption of OH radicals.
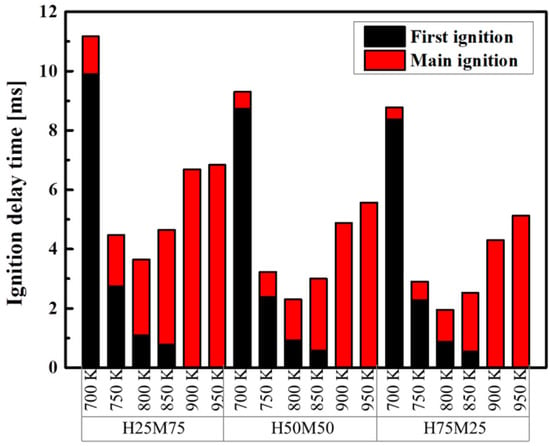
Figure 9.
Delay times for the 1st and main ignition of blended fuels at Φ = 1.0, Pc = 15 bar, CR = 3.5, and O2 = 21%.
This inhibits the ignition of n-heptane while delaying the ignition further. During the combustion process, a cool flame occurs at the first ignition, and a hot flame occurs at the main ignition. High-carbon fuels such as n-heptane allow a cool flame to occur prior to the ignition, which may affect the overall combustion reaction by isomerizing and decomposing fuel molecules [43]. The first and main ignition delay time can be controlled by the modulation of not only operating conditions but also the methane/n-heptane ratio. The sensitivity to first and main ignition delay time was greater for Tc when compared to the mixture ratio.
4.4.5. Effects of N2 Dilution Ratio
Prior to the LTC method, it is necessary to form a uniform pre-mixture in the cylinder to achieve simultaneous compression ignition at multiple points. LTC is the most common method to reduce NOx and PM by decreasing the oxygen concentration with a high EGR rate and lowering the combustion temperature [44,45]. Previous studies had used the following EGR rates and oxygen concentration ranges for LTC. NOx was reported to be reduced at 50–55% EGR (12–14% O2 conditions) but the amount of soot was significantly increased, while a smaller amount of NOx was emitted at 55–60% EGR (9–10% O2) and the amount of soot decreased drastically [46,47]. Low-temperature combustion was also found to be possible at an EGR of 60% or higher (<12% O2) in a naturally aspirated engine [48].
In the current study, the effect of O2 concentration was first analyzed before simulating the EGR rate. Figure 10 shows the ignition delay time at different O2 concentrations by N2 dilution for the n-heptane/methane-blended fuel. Tc was varied from 600 to 1000 K in steps of 50 K, and the temperature was shown in reverse order. The fuel composition was kept at H50M50. By reducing the O2 concentration in the atmosphere by 10, 20, and 30%, the oxygen concentration was controlled, respectively, at 18.9, 16.8, and 14.7%. According to the calculation results, the ignition delay time increased as the O2 concentration in the blended fuel decreased. In addition, the increase in ignition delay time was large at 800–900 K. This corresponds to the NTC region, where the production of OH radicals is insufficient at a lower O2 concentration and low temperatures, resulting in reduced oxidation reactions and an inhibited ignition.
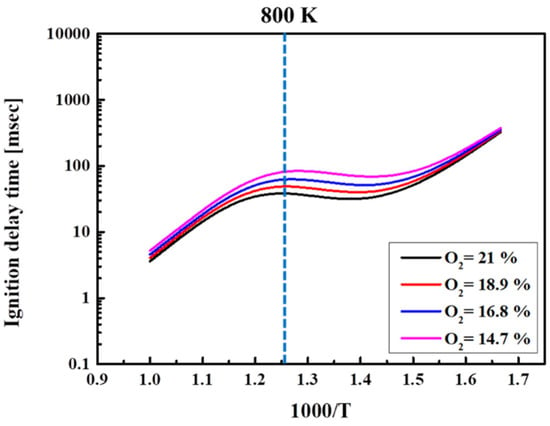
Figure 10.
Ignition time for different O2 mol.% in H50M50 at Φ = 1.0, Pc = 15 bar, and CR = 3.5.
Figure 11 illustrates the ignition delay times and the mole fractions of OH radicals, H2O2 radicals, and the fuel at different O2 concentration by applying EGR. Panel (a) shows the results at 16.8–21% O2, and panel (b) shows the results at 14.7% O2. When the O2 content was lowered from 21 to 18.9, 16.8, and 14.7%, the ignition delay time increased by 9.6, 29.5, and 59.9%, respectively. It was confirmed that the OH production rate decreased as the O2 content decreased. The analysis indicated that the temperature of the first ignition was 791 K at 21% O2 and lowered to 687 K at 14.7% O2. As a result, the delay times for the first ignition and the main ignition increased, in agreement with the analysis of chemical species that demonstrated lower concentrations of intermediate species and OH radials at lower O2 concentrations. Therefore, the time required for the main ignition, as well as the total ignition delay time, increased. This increase is explained by the fact that the inert gas N2 comprises 70–80% of the exhaust gas in the EGR simulation. N2 in the blended fuel introduced into the combustion chamber acts as a thermal load for the fuel inside. Therefore, it inhibits temperature increases in the combustion chamber and suppresses the production of intermediate chemical species. In addition, N2 gas itself has a high specific heat. These properties of N2 are responsible for the low temperature. The N2 ratio is an effective parameter to control ignition delay time and temperature.
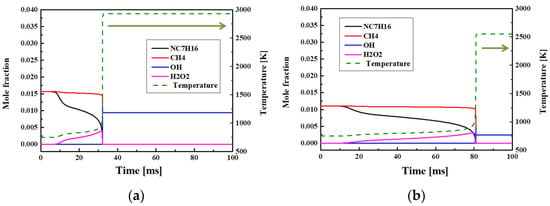
Figure 11.
Computed mole fractions of OH and H2O2 radicals and the temperature at (a) 21% O2 and (b) 14.7% O2 conditions. The other conditions were fixed at Φ = 1.0, Pc = 15 bar, Tc = 750 K, and CR = 3.5.
4.4.6. Changes in Compression Ratio
The engine output may be increased by increasing the air intake amount, the number of engine revolutions, or the CR. As CR increases, the blended gas is significantly compressed and the temperature of the mixture increases, thereby reducing the ignition delay time. Figure 12 shows the ignition delay time in H50M50 at different CR values. CR = 3.5 (solid line) corresponds to the experimental data, and data for the other CR values were from theoretical calculations (indicated by points). There is average error of 3% in all temperature ranges. As CR increased sequentially from 2.5 to 3.0, 3.5, 4.0, and 4.5, the compression pressure increased by 20% and the ignition delay time decreased by 25%, on average. The lower the Tc, the larger the change in the maximum compression pressure in the combustion chamber, and vice versa. In H75M25, the Tc value was lower, and the ignition delay time was longer due to the high methane content. The NTC region was observed near 800–950 K regardless of the CR. Also, at CR ≥ 4.5 and a pressure of 50 atm or higher, the NTC region became narrower as the CR and the pressure increased. The CR is an effective parameter to control ignition delay time in an especially low-temperature region.
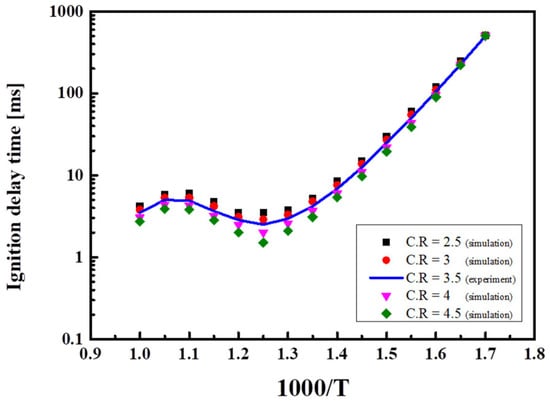
Figure 12.
Ignition delay times at various compression ratios (CR = 2.5–4.5) for blended fuels at Φ = 1.0, and O2 = 21%.
4.4.7. Reactivity Analysis of Ignition Delay Time
The fuel composition, O2 concentration, CR, and combustion chamber environment (temperature and pressure) all influence the ignition reaction of the blended fuel. In this experiment, H100 represents the ignition performance of 100% diesel, and M100 represents that of 100% gasoline. The ignition reaction in blended fuels containing 50% or less methane approximated the diesel ignition performance, and therefore the ignition reactivity of other blended fuels was expressed relative to H50M50. In Figure 13, we analyzed the ignition reactivity by setting the ignition delay time of H50M50 to one, and values below and above one are expressed as “+” and “−“, respectively. Figure 13a shows that the fuel composition significantly affects the ignition performance. In the oxidative decomposition of methane (CH4 + OH = CH3 + H2O, RO2, QOOH), since OH radicals cannot react with n-heptane (which dictates the combustion reaction at low temperatures) and are consumed by methane instead, the methane/n-heptane blended fuel is ultimately subjected to a strong inhibition in its oxidation reaction for the ignition of n-heptane. As the methane ratio increases, the ignition greatly decreases, and the ignition performance decreases. This indicates that the methane fuel is not compressed and is decomposed due to its high octane number and low cetane number. Figure 13b shows the ignition characteristics at different O2 concentrations from 21 (outside air) to 18.9, 16.8, and 14.7%. As the O2 concentration was reduced, the ignitability was reduced by 8 to 35% due to inhibited ignition. A previous study by Cho et al. [48] found that low-temperature combustion was possible under an EGR rate of 60% or higher (12% or less O2 condition). In the present experiment, the ignition performance at 14.7% O2 was 37.5% lower than that at 21% O2, meaning that a lower O2 concentration is correlated to poorer ignition performance. Figure 13c shows that the ignition performance is dependent on the CR, which was increased from 2.5 to 4.5 at intervals of 0.5. When CR increased from 3.5 to 4.5, the ignition performance improved by more than 35%, and inversely, when CR was decreased the ignition performance was reduced by 10% on average. Additionally, a higher CR improved the performance by 20–25% on average. This result is attributed to the faster flame speed, as n-heptane promotes the combustion reaction of methane when the two components are blended. At a higher CR, the ignition performance improved, and Tc and the compression pressure increased. A higher CR was found to increase the combustion temperature, and the ignition of the methane fuel was promoted in proportion to the combustion temperature.
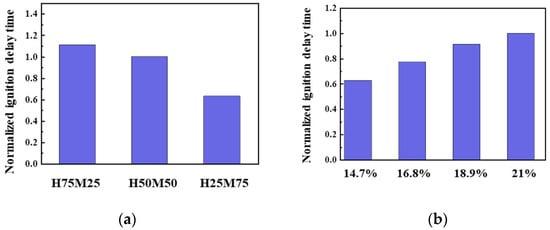
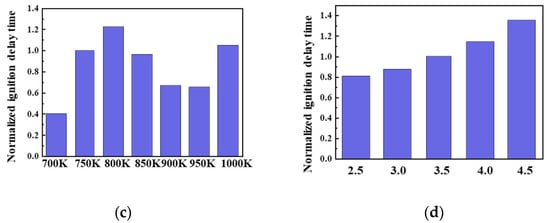
Figure 13.
Normalized ignition delay time in H50M50 at various (a) fuel compositions, (b) O2 concentrations, (c) CR values, and (d) Tc values at Φ = 1.0, Pc = 15 bar, TC = 750 K, and CR = 3.5.
Figure 13d shows the effect of Tc on the ignition performance. Tc has a large effect on chemical ignition. The graph compares the changes in ignitability within Tc = 700–1000 K, normalized against the value at Tc = 750 K. A higher Tc leads to a higher ignitability, and the NTC region was clearly observed at 850–950 K. Regarding the compression pressure, a lower Tc is correlated to a higher maximum compression pressure change in the combustion chamber, and vice versa. As the compression pressure increased, Tc increased more rapidly, and the ignitability improved.
4.4.8. Sensitivity Analysis
Figure 14 shows the results of sensitivity analysis to determine the main combustion reactions with high reactivity, in the case of H75M25 fuel under the conditions of a low temperature (850 K), 15 atm, and EGR (14.7% or 21% O2). Positive and negative sensitivity coefficients indicate reactions that inhibit and promote the ignition reactions, respectively. Particularly, CH4 + OH ⇔ CH3 + H2O is a typical chain propagation reaction that inhibits methane ignition at 21% O2. At 14.7% O2, most of the reactions inhibit ignition due to the lack of oxygen. The chain initiation step (CH3 + HO2 ⇔ CH4 + O2, the first oxidation reaction induced by methane dehydrogenation at low temperatures) and the decomposition reaction (CH2O + CH3 ⇔ HCO + CH4) were found to have significant effects on methane decomposition. For the n-heptane/methane-blended fuel, an NTC region was observed at 0–75% methane and the temperature range of 800–950 K. In this region, as Tc increases, the ignition delay time increases. OH radicals, which induce oxidation at 850 K, do not react with n-heptane. Instead, large amounts of OH and oxygen are consumed by methane dehydrogenation in a low temperature range, resulting in a shortage of them for the first oxidation reaction of heptane and strong inhibition of oxidation in the blended fuel. As a result, the reactivity is reduced, an NTC region is created, and thus the ignition delay time becomes longer. The HO2 and H2O2 species, which inhibit ignition through methane at 850 K, promote ignition at 1100 K, and the ignition delay time is shorter due to the explosive combustion of n-heptane species.
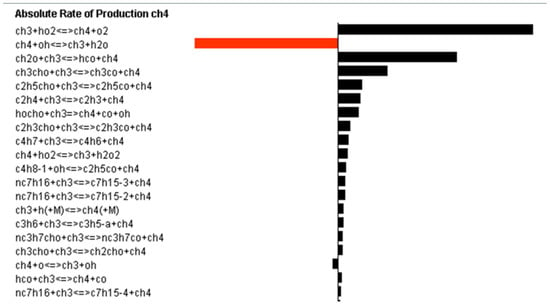
Figure 14.
Sensitivity analysis to identify factors that significantly affect the combustion of H75M25 at 14.7% or 21% O2.
5. Conclusions
An RCM system was employed to simulate the pre-mixed combustion technology. The autoignition characteristics of n-heptane/methane-blended fuel for various temperatures, CRs, and N2 dilution ratios were analyzed using simulations in this study.
- (1)
- Ignition reactivity is sensitive to the compression ratio and fuel composition. Even an increase of 0.5 in the compression ratio reduced the ignition delay time by more than 20%. An increased n-heptane ratio in the fuel significantly promoted ignition, whereas an increased methane ratio greatly inhibited it.
- (2)
- The ignition delay time was significantly longer (by more than 30%) at 14.7% O2 when compared to that at 21% O2 because a shortage of O2 inhibited the oxidation reaction.
- (3)
- The cool flame occurred in the range of Tc = 750–850 K therefore, the main ignition time was shorter when compared to that at Tc = 900–950 K, where only hot flames would occur. Therefore, oxidizing agents are important for achieving the low-temperature oxidation of fuel that is sensitive to temperature.
- (4)
- As the ratio of n-heptane in the blended fuel increased, the combustion reaction during the multi-point compression proceeded from a cool flame to NTC to a hot flame. The phenomena of second ignition and NTC were clearly observed as the characteristics of high-carbon fuel. This confirmed that the reaction of n-heptane was more dominant in the blended fuel.
- (5)
- The combustion performance similar to that of 100% n-heptane could be achieved when the ratio of methane was kept at less than 50%, because methane inhibits oxidation at low temperatures.
Author Contributions
Conceptualization, K.K. and C.O.; methodology, Y.P.; software, M.Y.; validation, K.K., C.O. and M.Y.; formal analysis, Y.P.; investigation, M.C.; resources, M.Y.; data curation, M.C.; writing—original draft preparation, M.Y.; writing—review and editing, Y.P.; visualization, Y.P.; supervision, G.C.; project administration, G.C.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Human Resources Development program (No. 20204030200030) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade, Industry and Energy and Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (20214000000140, Graduate School of Convergence for Clean Energy Integrated Power Generation).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhukov, V.P.; Sechenov, V.A.; Starikovskii, A.Y. Autoignition of n-decane at high pressure. Combust. Flame 2008, 153, 130–136. [Google Scholar] [CrossRef]
- An, H.; Chun, K.; Song, S. An experimental study of the effect of hydrogen additive on a gasoline HCCI combustion. Trans. KSME 2013, 12, 824–828. [Google Scholar]
- Sun, Y.; Reitz, R. Modeling Diesel Engine NOx and Soot Reduction with Optimized Two-Stage Combustion; SAE 2006-01-0027; Engine Research Center, University of Wisconsin-Madison: Madison, WI, USA, 2006. [Google Scholar]
- Dooley, S.; Won, S.H.; Chaos, M. A jet fuel surrogate formulated by real fuel properties. Combust. Flame 2010, 157, 2333–2339. [Google Scholar] [CrossRef]
- Yao, C.; Zang, R.; Wang, J.; Han, G. Simulation investigation on the ignition characteristics of n-heptane methane fuel blends. J. Tianjin Univ. Sci. Technol. 2015, 48, 119–125. [Google Scholar]
- Aggarwal, S.K.; Awomolo, O.; Akber, K. Ignition characteristics of heptane/hydrogen and heptane-methane fuel blends at elevated pressures. Int. J. Hydrog. Energy 2011, 36, 15392–15402. [Google Scholar]
- Li, B.; Yoo, K.; Wang, Z.; Boehman, A.L.; Wang, J. Experimental and Numerical Study on Autoignition Characteristics of the Gasoline/Diesel/Ethanol and Gasoline/Diesel/PODE/Ethanol Fuels. Energy Fuels 2019, 33, 11841–11849. [Google Scholar] [CrossRef]
- Kuszewski, H. Experimental study of the autoignition properties of n-butanol–diesel fuel blends at various ambient gas temperatures. Fuel 2019, 235, 1316–1326. [Google Scholar]
- Wang, S.; Liang, Y.; Zhu, J.; Raza, M.; Li, J.; Yu, L.; Qian, Y.; Lu, X. Experimental and modeling study of the autoignition for diesel and n-alcohol blends from ethanol to n-pentanol in shock tube and rapid compression machine. Combust. Flame 2021, 227, 296–308. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Raza, M.; Feng, Y.; Li, J.; Mao, Y.; Yu, L.; Qian, Y.; Lu, X. Autoignition behavior of methanol/diesel mixtures: Experiments and kinetic modeling. Combust. Flame 2021, 228, 1–12. [Google Scholar]
- Ribaucour, M.; Minetti, R.; Sochet, L.R. Autoignition of n-pentane and 1-pentene: Experimental data and kinetic modeling. Symp. Int. Combust. Proc. 1998, 27, 345–351. [Google Scholar] [CrossRef]
- Min, K.; Valco, D.J.; Oldani, A.; Kim, K.; Temme, J.; Kweon, C.B.M.; Lee, T. Autoignition of varied cetane number fuels at low temperatures. Proc. Combust. Inst. 2019, 37, 5003–5011. [Google Scholar] [CrossRef]
- Kang, K.; Shim, T.; Oh, C.; Choi, G.; Kim, D. Effects of fuel composition and exhaust gas recirculation on ignition delay characteristics of n-heptane/hydrogen blended fuel. J. Mech. Sci. Technol. 2017, 31, 1509–1516. [Google Scholar] [CrossRef]
- Kang, K.; Oh, C.; Shim, T.; Song, J.; Ryu, S.; Choi, G.; Kim, D. Effect of exhaust gas recirculation on ignition characteristics of n-heptane/ethanol blend fuels in rapid compression machine. J. Mech. Sci. Technol. 2017, 31, 3619–3625. [Google Scholar] [CrossRef]
- Malewicki, T.; Brezinsky, K. Experimental and modeling study on the pyrolysis and oxidation of n-decane and n-dodecane. Proc. Combust. Inst. 2013, 34, 361–368. [Google Scholar] [CrossRef]
- Brandes, D.H.; Frobese, M.; Mitu, M. Safety characteristics of ethanol/automotive petrol mixtures. Oil Gas Eur. Mag. 2006, 32, 199–202. [Google Scholar]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Influence of Initial Pressure and Vessel’s Geometry on Deflagration of Stoichiometric Methane–Air Mixture in Small-Scale Closed Vessels. Energy Fuels 2020, 34, 3828–3835. [Google Scholar] [CrossRef]
- Mitu, M.; Razus, D.; Schroeder, V. Laminar Burning Velocities of Hydrogen-Blended Methane–Air and Natural Gas–Air Mixtures, Calculated from the Early Stage of p(t) Records in a Spherical Vessel. Energies 2021, 14, 7556. [Google Scholar] [CrossRef]
- Lee, D.Y. Autoignition Measurements and Modeling in a Rapid Compression Machine. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1997. [Google Scholar]
- Weber, B.W.; Kumar, K.; Zhang, Y.; Sung, C.-J. Autoignition of n-butanol at elevated pressure and low-to-intermediate temperature. Combust. Flame 2011, 158, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, Z.; Wei, L.; Zhang, J.; Law, C.K. Experimental and modeling study on ignition delays of lean mixtures of methane, hydrogen, oxygen, and argon at elevated pressures. Combust. Flame 2012, 159, 918–931. [Google Scholar] [CrossRef]
- Zang, Y.; Kumar, K.; Sung, C.-J. Autoignition of blends of n-butanol and n-heptane in a rapid compression machine. In Proceedings of the 49th AIAA Aerospace Science Meeting Including the New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 4–7 January 2011; p. 92. [Google Scholar]
- CHEMKIN-PRO, Release 15083; Reaction Design: San Diego, CA, USA, 2009.
- Mehl, M.; Pitz, W.J.; Westbrook, C.K.; Curran, H.J. Kinetic modeling of gasoline surrogate components and mixture under engine conditions. Proc. Combust. Inst. 2011, 33, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Tropin, D.A.; Fedorov, A.V. Physicomathematical Modeling of Detonation Suppression by Inert Particles in Methane–Oxygen and Methane–Hydrogen–Oxygen Mixtures. Combust. Explos. Shock 2014, 50, 542–546. [Google Scholar] [CrossRef]
- Cui, X. Modelling research on oxidation of CH4 & C7H16 from small to large scale of temperature at high pressures. IOP Conf. Ser. Mater. Sci. Eng. 2020, 715, 012017. [Google Scholar]
- Zhang, P.; Ji, W.; He, T.; He, X.; Wang, Z.; Yang, B.; Law, C.K. First-stage ignition delay in the negative temperature coefficient behavior: Experiment and simulation. Combust. Flame 2016, 167, 14–23. [Google Scholar] [CrossRef]
- Luong, M.B.; Yu, G.H.; Lu, T.; Chung, S.H.; Yoo, C.S. Direct numerical simulations of ignition of a lean n-heptane/air mixture with temperature and composition inhomogeneities relevant to HCCI and SCCI combustion. Combust. Flame 2015, 162, 4566–4585. [Google Scholar] [CrossRef] [Green Version]
- Karim, G.A.; Ito, K.; Abraham, M.; Jensen, L. An Examination of the Role of Formaldehyde in the Ignition Processes of a Dual Fuel Engine. SAE Int. J. Fuels Lubr. 1991, 100, 975–982. [Google Scholar]
- Thorsen, L.; Nordhjort, C.; Hashemi, H.; Pang, K.M.; Glarborg, P. aluation of a Semiglobal Approach for Modeling Methane/n-Heptane Dual-Fuel Ignition. Energy Fuels 2021, 35, 14042–14050. [Google Scholar] [CrossRef]
- Zhang, J.; Niu, S.; Zhang, Y.; Tang, C.; Jiang, X.; Hu, E.; Huang, Z. Experimental and modeling study of the auto-ignition of n-heptane/n-butanol mixture. Combust. Flame 2013, 160, 31–39. [Google Scholar] [CrossRef]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A comprehensive modeling study of n-heptane oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Deng, S.; Zhao, P.; Zhu, D.; Law, C.K. NTC-affected ignition and low-temperature flames in nonpremixed DME/air counterflow. Combust. Flame 2014, 161, 1993–1997. [Google Scholar] [CrossRef]
- Wenkai, L.; Law, C.K. Theory of first-stage ignition delay in hydrocarbon NTC chemistry. Combust. Flame 2018, 188, 162–169. [Google Scholar]
- Zhao, P.; Law, C.K. The role of global and detailed kinetics in the first-stage ignition delay in NTC-affected phenomena. Combust. Flame 2013, 160, 2352–2358. [Google Scholar] [CrossRef]
- Ji, W.; Zhao, P.; Zhang, P.; Ren, Z.; He, X.; Law, C.K. On the crossover temperature and lower turnover state in the NTC regime. Combust. Flame 2017, 36, 343–353. [Google Scholar]
- Liu, W.; Qi, Y.; Zhang, R.; Wang, Z. Flame propagation and auto-ignition behavior of iso-octane across the negative temperature coefficient (NTC) region on a rapid compression machine. Combust. Flame 2022, 235, 111688. [Google Scholar] [CrossRef]
- Ratkiewicz, A.; Truong, T.N. Kinetics of the C–C bond beta scission reactions in alkyl radicals. J. Phys. Chem. A 2012, 116, 6643–6654. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, Z.; Li, G.; Wan, Q.; Xu, L.; Fan, S. Experimental and kinetic studies of ignition processes of the methane–n-heptane mixtures. Fuel 2019, 235, 522–529. [Google Scholar] [CrossRef]
- Gong, Z.; Feng, L.; Wei, L.; Qu, W.; Li, L. Shock tube and kinetic study on ignition characteristics of lean methane/n-heptane mixtures at low and elevated pressures. Energy 2020, 197, 117242. [Google Scholar] [CrossRef]
- Wei, H.; Qi, J.; Zhou, L.; Zhao, W.; Shu, G. Ignition Characteristics of Methane/n-Heptane Fuel Blends under Engine-like Conditions. Energy Fuels 2018, 32, 6264–6277. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Liu, H.; Ye, Y.; Wang, H.; Dong, J.; Liu, B.; Yao, M. Kinetic Study of the Ignition Process of Methane/n-Heptane Fuel Blends under High-Pressure Direct-Injection Natural Gas Engine Conditions. Energy Fuels 2020, 34, 14796–14813. [Google Scholar] [CrossRef]
- Naidja, A.; Krishna, C.R.; Butcher, T.; Mahajan, D. Cool flame partial oxidation and its role in combustion and reforming of fuels for fuel cell systems. Prog. Energy Combust. Sci. 2003, 29, 155–191. [Google Scholar] [CrossRef]
- Shi, L.; Xiao, W.; Li, M.; Lou, L.; Deng, K. Research on the effects of injection strategy on LTC combustion based on two-stage fuel injection. Energy 2017, 121, 21–31. [Google Scholar] [CrossRef]
- Putrasari, Y.; Lim, O. A Review of Gasoline Compression Ignition: A Promising Technology Potentially Fueled with Mixtures of Gasoline and Biodiesel to Meet Future Engine Efficiency and Emission Targets. Energies 2019, 12, 238. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Mulenga, M.C.; Reader, G.T.; Wang, M.; Ting, D.S.-K.; Tjong, J. Biodiesel engine performance and emissions in low temperature combustion. Fuel 2008, 87, 714–722. [Google Scholar] [CrossRef]
- Ogawa, H.; Li, T.; Miyamoto, N. Characteristics of low temperature and low oxygen diesel combustion with ultra-high exhaust gas recirculation. Int. J. Engine Res. 2007, 8, 365–378. [Google Scholar] [CrossRef]
- Cho, S.; Oh, K.-C.; Lee, C.B. Characteristics of low temperature combustion in single cylinder engine by high EGR rate. Trans. KSAE 2009, 17, 79–85. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).