Electrified Hydrogen Production from Methane for PEM Fuel Cells Feeding: A Review
Abstract
:1. Introduction
- Inefficient heat transfer. The heat for the reaction must first be transferred by radiation from the burning fuel to the outer walls of the reactor tube. Then, from the external wall, it must reach the inside of the pipe by conduction and finally reach the surface of the catalyst. Furthermore, generally the supports used for the catalysts are made of alumina, and therefore are poor thermal conductors.
- The maintenance costs of the reactor are relatively high. The high temperatures required stress the materials of which the pipes are made. Typically, a tube replacement is required after an average of 5 years of operation. Additionally, the costs for these interventions are very high, and can reach millions of dollars [12].
- Large quantities of methane (natural gas) are consumed in providing the heat energy for the reforming reaction. For example, a typical 1100 ton d−1 ammonia plant requires approximately 400,000 m3 d−1 of natural gas only for the heating stage. While natural gas is a relatively cheap fuel today, it is a nonrenewable resource and may not remain cheap in the future.
- To supply thermal energy to the reforming reaction, it is necessary to burn large quantities of methane gas. Even though it is a relatively cheap fuel, it is nonetheless a fossil fuel and therefore not renewable.
2. Microwaves
2.1. MW-Assisted Reforming
- Steam reforming of methane, SRM, Equation (1), developed for the first time by Sabatier and Senderens in 1902 [51];
- Dry reforming of methane, DRM, Equation (2), which was studied for the first time by Fischer and Tropsch in 1928 over cobalt and nickel catalysts [54], and then optimized in 1948 by Reitmeier et al. [55], in whose studies the presence of steam into DRM feeding stream resulted in suppressing coke formation;
- Partial oxidation of methane, POM, Equation (3), studied for the first time in 1949 by Lewis et al. [56], whose studies were performed by reforming methane through oxidizing it with oxygen using supported copper oxide catalyst.
- (i)
- The first step, considered as the rate-determining one, is the methane activation. The energy needed for the rupture of C-Hx or CHx-Hx bonds is governed by the surface properties. Each partially dissociated CHx species is adsorbed on the catalyst surface, which sees also the presence of active carbonaceous species, C*.
- (ii)
- The second step is the activation of coreactants (H2O, CO2, or O2), whose absorption and dissociation on the catalyst surface or metal–support interface results in the formation of active oxygen species.
- (iii)
- The third step is the formation of the O-H group: the reaction between COx and H radicals or surface O and H radicals allows the formation of surface hydroxyl (OH-) groups. The interaction of the adsorbed CHx species and the O-H groups results in the formation of CHxO intermediates, which then decompose to CO and H2. The catalyst–support interface normally serves as an active site for CHxO formation.
- (iv)
- The fourth step is the oxidation and desorption of intermediates: the oxygen species on the metal catalyst surface may react with a surface group (CHx group, CHxO or COsurface). Consequently, the formation of CO through the dissociation of CHxO and COsurface or the Boudouard reaction (Equation (5)) may be obtained. Other side reactions affecting the overall reforming performance and product selectivity may occur, including reverse water gas shift (Equation (6)), and CH4 decomposition shift (Equation (7)) [66].
2.2. MW Reactors
2.2.1. Fluid–Fluid Systems
2.2.2. Fluid–Solid Systems
2.3. Remarks
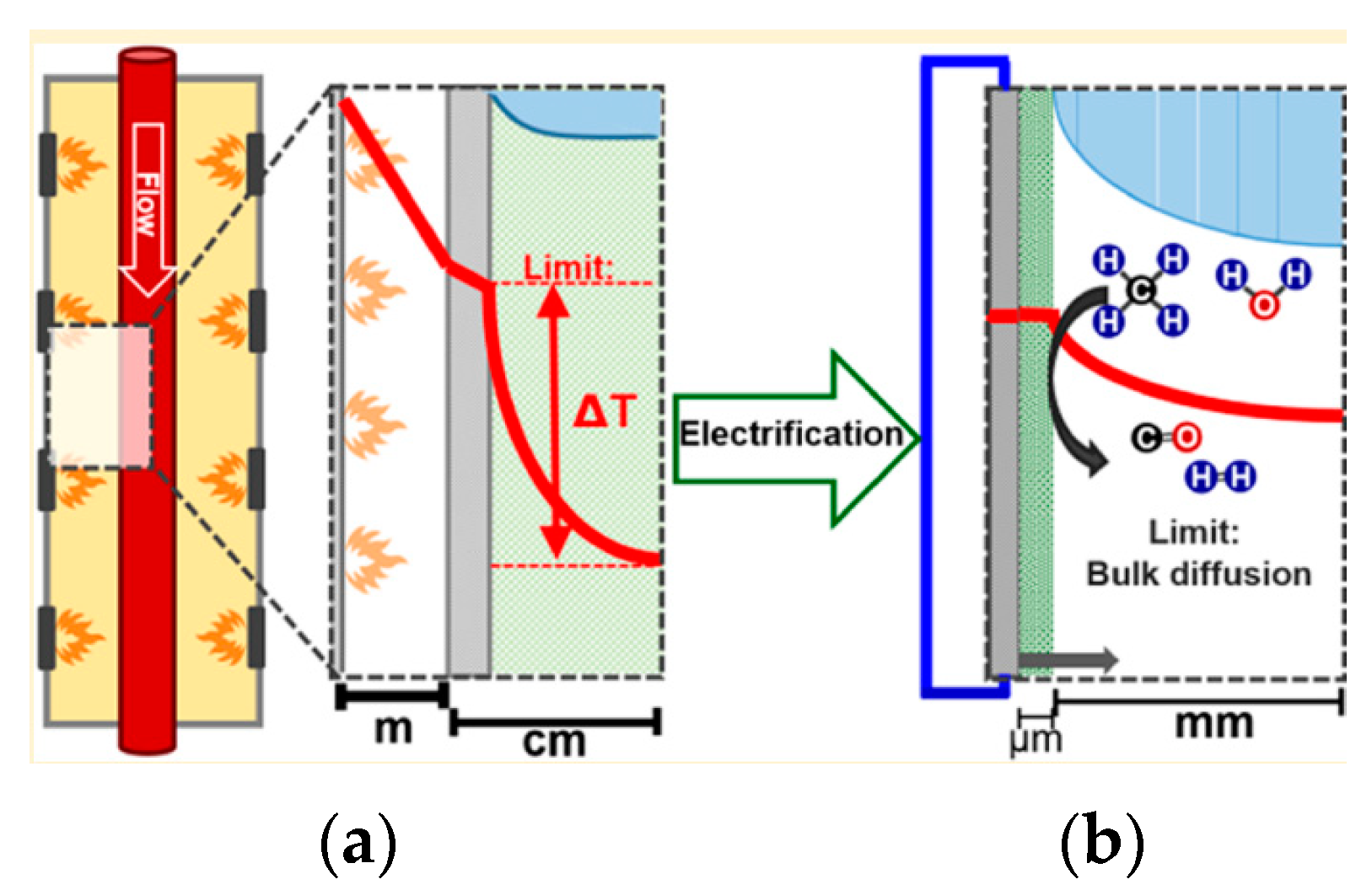
3. Electrical Resistive (Joule) Heating
3.1. Reactor Configuration for Resistive (Joule) Heating
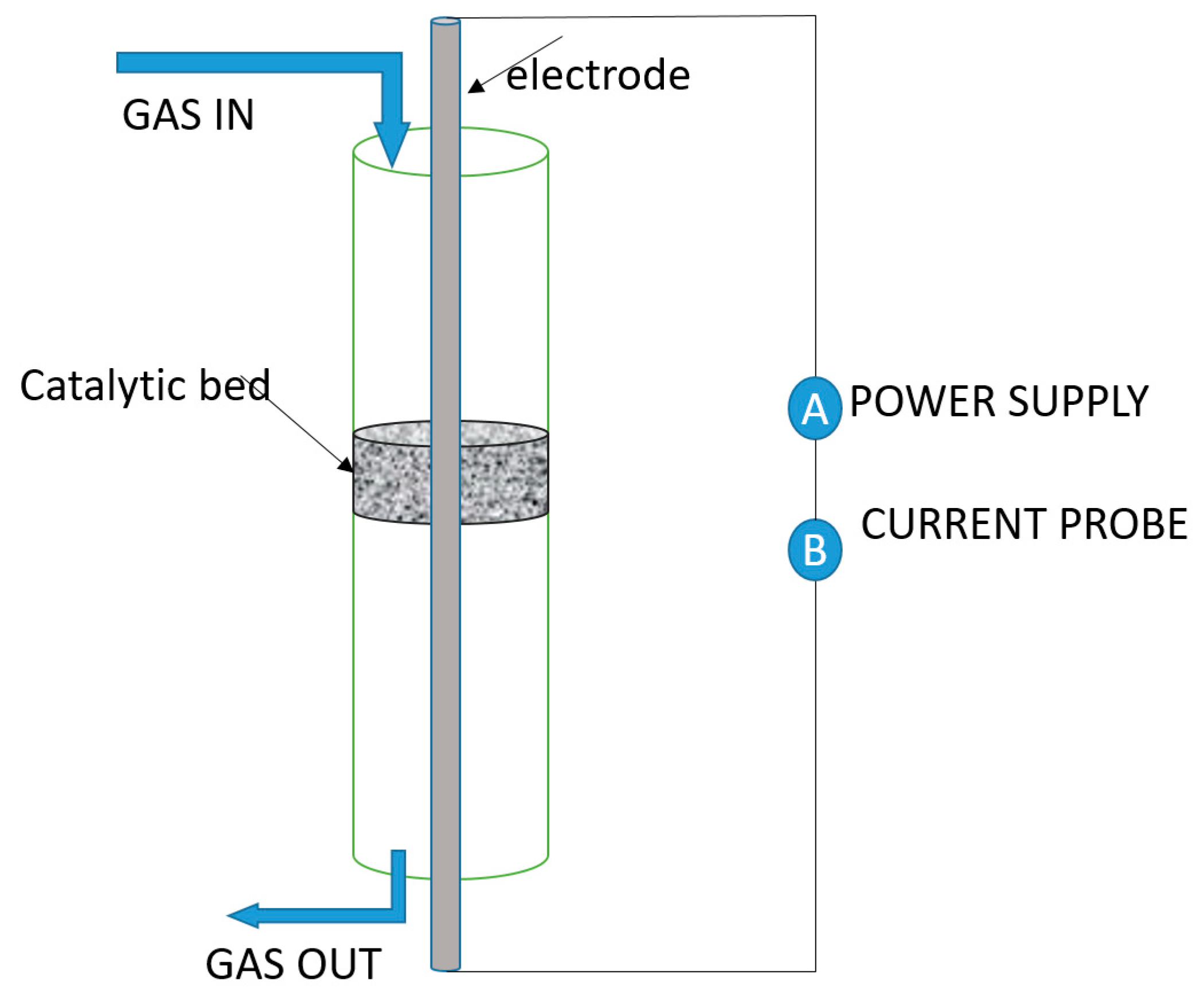
3.1.1. Parallel Wire (PW)
3.1.2. Parallel Tube Reactor (PTR)
3.2. Remarks
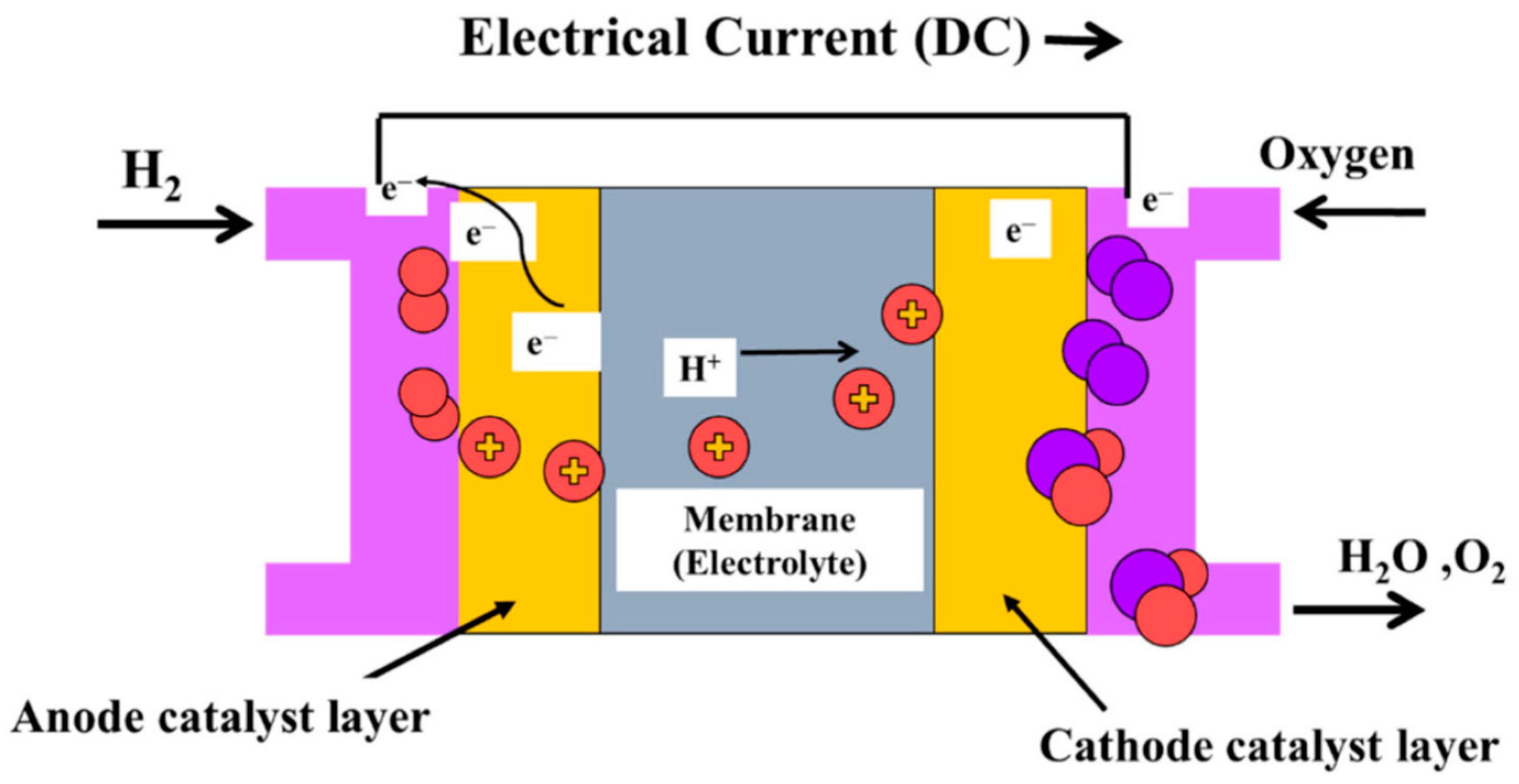
4. PEM Fuel Cells
4.1. Proton Exchange Membrane Fuel Cells
4.1.1. Pt-Based
4.1.2. PGM-Free-Based
4.1.3. Remarks
- -
- The first is to improve the platinum-based catalyst performance by mainly acting on the catalytic formulation using Pt-M alloys or by acting on the catalyst support. The studies have shown that high electrochemical active surface areas and strong interactions between platinum nanoparticles with the catalytic support and/or a second metal can enhance ORR performance, improving, at the same time, the catalyst durability.
- -
- The second is to eliminate the platinum dependence using PGM-free-based catalysts. Although great strides have been made in this field, thanks to very promising metals such as Fe and Co, these catalysts are still not able to meet the required performance and durability standards. However, the studies performed on this type of catalyst have led to very interesting results, making their use very promising for PEMFC applications.
5. Conclusions
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CA | Carbon aerogel |
| CB | Carbon black |
| CE | Conversion efficiency |
| DC | Direct current |
| DOE | Department of Energy |
| DRM | Dry reforming of methane |
| Ein | Electrical energy input to the catalyst in kW |
| ECSA | Electrochemical active surface areas |
| ER | Electrical energy |
| FM | Inlet methane flow in mL min−1 |
| HOPC | Hierarchically ordered porous carbon |
| HOR | Hydrogen oxidation reaction |
| HPNC | Hierarchically porous N-doped carbon |
| MA | Mass activity |
| MOF | Metal organic framework |
| MW | Microwave |
| N | Molar ratio of H2 produced to CH4 converted |
| ORR | Oxygen reduction reaction |
| P | Peak power density |
| PECNTs | Partially exfoliated carbon nanotubes |
| PEMFCs | Proton Exchange Membrane Fuel Cells |
| PGM | Platinum group metal |
| POM | Partial oxidation of methane |
| PTR | Parallel tube reactor |
| PW | Parallel wire |
| rEGO | Reduced electrochemically exfoliated graphene oxide |
| SA | Surface activity |
| SAS | Single atom sites |
| SiC | Silicon carbide |
| SRM | Steam reforming of methane |
| ZIF | Zeolitic imidazolate framework |
References
- Stankiewicz, A.I.; Nigar, H. Beyond electrolysis: Old challenges and new concepts of electricity-driven chemical reactors. React. Chem. Eng. 2020, 5, 1005–1016. [Google Scholar] [CrossRef]
- Sapountzi, F.; Tsampas, M.; Fredriksson, H.; Gracia, J.; Niemantsverdriet, H. Hydrogen from electrochemical reforming of C1–C3 alcohols using proton conducting membranes. Int. J. Hydrogen Energy 2017, 42, 10762–10774. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2017, 5, 1700464. [Google Scholar] [CrossRef] [PubMed]
- Jhong, H.-R.; Ma, S.; Kenis, P.J. Electrochemical conversion of CO2 to useful chemicals: Current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2013, 2, 191–199. [Google Scholar] [CrossRef]
- Sequeira, C.A.C.; Santos, D.M.F. Electrochemical routes for industrial synthesis. J. Braz. Chem. Soc. 2009, 20, 387–406. [Google Scholar] [CrossRef]
- Ding, Q.; Song, B.; Xu, P.; Jin, S. Efficient Electrocatalytic and Photoelectrochemical Hydrogen Generation Using MoS2 and Related Compounds. Chem 2016, 1, 699–726. [Google Scholar] [CrossRef] [Green Version]
- Iervolino, G.; Tantis, I.; Sygellou, L.; Vaiano, V.; Sannino, D.; Lianos, P. Photocurrent increase by metal modification of Fe2O3 photoanodes and its effect on photoelectrocatalytic hydrogen production by degradation of organic substances. Appl. Surf. Sci. 2017, 400, 176–183. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Rizzo, L. Visible light active Fe-doped TiO2 for the oxidation of arsenite to arsenate in drinking water. Chem. Eng. Trans. 2018, 70, 1573–1578. [Google Scholar]
- Iervolino, G.; Vaiano, V.; Pepe, G.; Campiglia, P.; Palma, V. Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process. Catalysts 2020, 10, 888. [Google Scholar] [CrossRef]
- Palma, V.; Cortese, M.; Renda, S.; Ruocco, C.; Martino, M.; Meloni, E. A Review about the Recent Advances in Selected NonThermal Plasma Assisted Solid–Gas Phase Chemical Processes. Nanomaterials 2020, 10, 1596. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, R.; Zhou, R.; Liu, B.; Zhang, T.; Xian, Y.; Cullen, P.J.; Lu, X.; Ostrikov, K. Sustainable ammonia production by non-thermal plasmas: Status, mechanisms, and opportunities. Chem. Eng. J. 2021, 421, 129544. [Google Scholar] [CrossRef]
- Spagnolo, D.; Cornett, L.; Chuang, K. Direct electro-steam reforming: A novel catalytic approach. Int. J. Hydrogen Energy 1992, 17, 839–846. [Google Scholar] [CrossRef]
- López, E.; Divins, N.J.; Anzola, A.; Schbib, S.; Borio, D.; Llorca, J. Ethanol steam reforming for hydrogen generation over structured catalysts. Int. J. Hydrogen Energy 2013, 38, 4418–4428. [Google Scholar] [CrossRef]
- Ricca, A.; Palma, V.; Martino, M.; Meloni, E. Innovative catalyst design for methane steam reforming intensification. Fuel 2017, 198, 175–182. [Google Scholar] [CrossRef]
- Renda, S.; Cortese, M.; Iervolino, G.; Martino, M.; Meloni, E.; Palma, V. Electrically driven SiC-based structured catalysts for intensified reforming processes. Catal. Today 2022, 383, 31–43. [Google Scholar] [CrossRef]
- Balzarotti, R.; Ambrosetti, M.; Beretta, A.; Groppi, G.; Tronconi, E. Investigation of packed conductive foams as a novel reactor configuration for methane steam reforming. Chem. Eng. J. 2019, 391, 123494. [Google Scholar] [CrossRef]
- Sanz, O.; Velasco, I.; Reyero, I.; Legorburu, I.; Arzamendi, G.; Gandía, L.M.; Montes, M. Effect of the thermal conductivity of metallic monoliths on methanol steam reforming. Catal. Today 2016, 273, 131–139. [Google Scholar] [CrossRef]
- Goyal, H.; Chen, T.-Y.; Chen, W.; Vlachos, D.G. A review of microwave-assisted process intensified multiphase reactors. Chem. Eng. J. 2021, 430, 133183. [Google Scholar] [CrossRef]
- Newman, J.; Bonino, C.A.; Trainham, J.A. The energy future. Ann. Rev. Chem. Biomol. Eng. 2018, 9, 153–174. [Google Scholar] [CrossRef]
- Jones, D.A.; Lelyveld, T.P.; Mavrofidis, S.W.; Kingman, S.W.; Miles, N.J. Microwave heating applications in environmental engineering—A review. Resour. Conserv. Recycl. 2001, 34, 75–90. [Google Scholar] [CrossRef]
- Hu, J.; Wildfire, C.; Stiegman, A.E.; Dagle, R.A.; Shekhawat, D.; Abdelsayed, V.; Bai, X.; Tian, H.; Bogle, M.B.; Hsu, C.; et al. Microwave-driven heterogeneous catalysis for activation of dinitrogen to ammonia under atmospheric pressure. Chem. Eng. J. 2020, 397, 125388. [Google Scholar] [CrossRef]
- Luque, R.; Menendez, J.A.; Arenillas, A.; Cot, J. Microwave-assisted pyrolysis of biomass feedstocks: The way forward? Energy Environ. Sci. 2012, 5, 5481–5488. [Google Scholar] [CrossRef]
- Julian, I.; Ramirez, H.; Hueso, J.L.; Mallada, R.; Santamaria, J. Non-oxidative methane conversion in microwave-assisted structured reactors. Chem. Eng. J. 2019, 377, 119764. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Song, Z.; Chen, H.; Zhao, X.; Sun, J.; Mao, Y.; Wang, X.; Wang, W. Fe/HZSM-5 synergizes with biomass pyrolysis carbon to reform CH4–CO2 to syngas in microwave field. Int. J. Hydrogen Energy 2022, 47, 11153–11163. [Google Scholar] [CrossRef]
- De Bruyn, M.; Budarin, V.L.; Sturm, G.S.J.; Stefanidis, G.D.; Radoiu, M.; Stankiewicz, A.; Macquarrie, D.J. Subtle Microwave-Induced Overheating Effects in an Industrial Demethylation Reaction and Their Direct Use in the Development of an Innovative Microwave Reactor. J. Am. Chem. Soc. 2017, 139, 5431–5436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horikoshi, S.; Osawa, A.; Sakamoto, S.; Serpone, N. Control of microwave-generated hot spots. Part V. Mechanisms of hot-spot generation and aggregation of catalyst in a microwave-assisted reaction in toluene catalyzed by Pd-loaded AC particulates. Appl. Catal. A Gen. 2013, 460–461, 52–60. [Google Scholar] [CrossRef]
- Musho, T.; Wildfire, C.; Houlihan, N.M.; Sabolsky, E.M.; Shekhawat, D. Study of Cu2O particle morphology on microwave field enhancement. Mater. Chem. Phys. 2018, 216, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Gutmann, B.; Kappe, C.O. Characterization of Microwave-Induced Electric Discharge Phenomena in Metal-Solvent Mixtures. ChemistryOpen 2012, 1, 39–48. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Abe, M.; Serpone, N. On the Generation of Hot-Spots by Microwave Electric and Magnetic Fields and Their Impact on a Microwave-Assisted Heterogeneous Reaction in the Presence of Metallic Pd Nanoparticles on an Activated Carbon Support. J. Phys. Chem. C 2011, 115, 23030–23035. [Google Scholar] [CrossRef]
- Horikoshi, S.; Suttisawat, Y.; Osawa, A.; Takayama, C.; Chen, X.; Yang, S.; Sakai, H.; Abe, M.; Serpone, N. Organic syntheses by microwave selective heating of novel metal/CMC catalysts–The Suzuki-Miyaura coupling reaction in toluene and the dehydrogenation of tetralin in solvent-free media. J. Catal. 2012, 289, 266–271. [Google Scholar] [CrossRef]
- Horikoshi, S.; Osawa, A.; Sakamoto, S.; Serpone, N. Control of microwave generated hot spots. Part IV. Control of hot spots on a heterogeneous microwaveabsorber catalyst surface by a hybrid internal/external heating method. Chem. Eng. Process. Process Intensif. 2013, 69, 52–56. [Google Scholar] [CrossRef]
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Apparent equilibrium shifts and hot-spot formation for catalytic reactions induced by microwave dielectric heating. Chem. Commun. 1999, 11, 975–976. [Google Scholar] [CrossRef] [Green Version]
- Horikoshi, S.; Kamata, M.; Mitani, T.; Serpone, N. Control of Microwave-Generated Hot Spots. 6. Generation of Hot Spots in Dispersed Catalyst Particulates and Factors That Affect Catalyzed Organic Syntheses in Heterogeneous Media. Ind. Eng. Chem. Res. 2014, 53, 14941–14947. [Google Scholar] [CrossRef]
- Suttisawat, Y.; Horikoshi, S.; Sakai, H.; Abe, M. Hydrogen production from tetralin over microwave-accelerated Pt-supported activated carbon. Int. J. Hydrogen Energy 2010, 35, 6179–6183. [Google Scholar] [CrossRef]
- Suttisawat, Y.; Horikoshi, S.; Sakai, H.; Rangsunvigit, P.; Abe, M. Enhanced conversion of tetralin dehydrogenation under microwave heating: Effects of temperature variation. Fuel Process. Technol. 2011, 95, 27–32. [Google Scholar] [CrossRef]
- Horikoshi, S.; Kamata, M.; Sumi, T.; Serpone, N. Selective heating of Pd/AC catalyst in heterogeneous systems for the microwave-assisted continuous hydrogen evolution from organic hydrides: Temperature distribution in the fixed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 12029–12037. [Google Scholar] [CrossRef]
- Manno, R.; Sebastian, V.; Mallada, R.; Santamaria, J.S. 110th Anniversary: Nucleation of Ag Nanoparticles in Helical Microfluidic Reactor. Comparison between Microwave and Conventional Heating. Ind. Eng. Chem. Res. 2019, 58, 12702–12711. [Google Scholar] [CrossRef]
- Ramirez, A.; Hueso, J.L.; Abian, M.; Alzueta, M.U.; Mallada, R.; Santamaria, J. Escaping undesired gas-phase chemistry: Microwave-driven selectivity enhancement in heterogeneous catalytic reactors. Sci. Adv. 2019, 5, eaau9000. [Google Scholar] [CrossRef] [Green Version]
- Meloni, E.; Martino, M.; Ricca, A.; Palma, V. Ultracompact methane steam reforming reactor based on microwaves susceptible structured catalysts for distributed hydrogen production. Int. J. Hydrogen Energy 2020, 46, 13729–13747. [Google Scholar] [CrossRef]
- Ramirez, A.; Hueso, J.L.; Mallada, R.; Santamaria, J. In situ temperature measurements in microwave-heated gas-solid catalytic systems. Detection of hot spots and solid-fluid temperature gradients in the ethylene epoxidation reaction. Chem. Eng. J. 2017, 316, 50–60. [Google Scholar] [CrossRef]
- Ramirez, A.; Hueso, J.L.; Mallada, R.; Santamaria, J. Microwave-activated structured reactors to maximize propylene selectivity in the oxidative dehydrogenation of propane. Chem. Eng. J. 2020, 393, 124746. [Google Scholar] [CrossRef]
- Suttisawat, Y.; Sakai, H.; Abe, M.; Rangsunvigit, P.; Horikoshi, S. Microwave effect in the dehydrogenation of tetralin and decalin with a fixed-bed reactor. Int. J. Hydrogen Energy 2012, 37, 3242–3250. [Google Scholar] [CrossRef]
- Haneishi, N.; Tsubaki, S.; Abe, E.; Maitani, M.M.; Suzuki, E.-I.; Fujii, S.; Fukushima, J.; Takizawa, H.; Wada, Y. Enhancement of Fixed-bed Flow Reactions under Microwave Irradiation by Local Heating at the Vicinal Contact Points of Catalyst Particles. Sci. Rep. 2019, 9, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durka, T.; Stefanidis, G.D.; Van Gerven, T.; Stankiewicz, A.I. Microwave-activated methanol steam reforming for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 12843–12852. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Pullumbi, P.; Brandani, F.; Palma, V. Intensification of TSA processes using a microwave-assisted regeneration step. Chem. Eng. Process. Process Intensif. 2020, 160, 108291. [Google Scholar] [CrossRef]
- Ergan, B.T.; Bayramoğlu, M.; Özcan, S. Emulsion polymerization of styrene under continuous microwave irradiation. Eur. Polym. J. 2015, 69, 374–384. [Google Scholar] [CrossRef]
- Ricciardi, L.; Verboom, W.; Lange, J.; Huskens, J. Reactive Extraction Enhanced by Synergic Microwave Heating: Furfural Yield Boost in Biphasic Systems. ChemSusChem 2020, 13, 3589–3593. [Google Scholar] [CrossRef]
- Motasemi, F.; Gerber, A.G. Multicomponent conjugate heat and mass transfer in biomass materials during microwave pyrolysis for biofuel production. Fuel 2018, 211, 649–660. [Google Scholar] [CrossRef]
- Aguilera, A.F.; Tolvanen, P.; Eränen, K.; Wärnå, J.; Leveneur, S.; Marchant, T.; Salmi, T. Kinetic modelling of Prileschajew epoxidation of oleic acid under conventional heating and microwave irradiation. Chem. Eng. Sci. 2019, 199, 426–438. [Google Scholar] [CrossRef]
- Aguilera, A.F.; Tolvanen, P.; Wärnå, J.; Leveneur, S.; Salmi, T. Kinetics and reactor modelling of fatty acid epoxidation in the presence of heterogeneous catalyst. Chem. Eng. J. 2019, 375, 121936. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Xiouras, C.; Stefanidis, G.D.; Li, X.; Gao, X. Fundamentals and applications of microwave heating to chemicals separation processes. Renew. Sustain. Energy Rev. 2019, 114, 109316. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and Heterogeneous Catalysis: A Review on Selected Catalytic Processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef] [Green Version]
- Enger, B.C.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Fisher, F.; Tropsch, H. Conversion of methane into hydrogen and carbon monoxide. Brennst.-Chem. 1928, 9, 39–46. [Google Scholar]
- Reitmeier, R.E.; Atwood, K.; Bennett, H.; Baugh, H. Production of synthetic gas-reaction of light hydrocarbons with steam and carbon dioxide. Ind. Eng. Chem. 1948, 40, 620–626. [Google Scholar] [CrossRef]
- Lewis, W.K.; Gilliland, E.R.; Reed, W.A. Reaction of methane with copper oxide in a fluidized bed. Ind. Eng. Chem. 1949, 41, 1227–1237. [Google Scholar] [CrossRef]
- Li, F.; Ao, M.; Pham, G.H.; Jin, Y.; Nguyen, M.H.; Alawi, N.M.; Tade, M.O.; Liu, S. A novel UiO-66 encapsulated 12-silicotungstic acid catalyst for dimethyl ether synthesis from syngas. Catal. Today 2020, 355, 3–9. [Google Scholar] [CrossRef]
- de García, I.D.; Stankiewicz, A.; Nigar, H. Syngas production via microwave-assisted dry reforming of methane. Catal. Today 2020, 362, 72–80. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Etminan, A.; Sadrnezhaad, S.K. A two step Microwave-assisted coke resistant mesoporous Ni-Co catalyst for methane steam reforming. Fuel 2022, 317, 122411. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.-M.; Barkschat, A.; Rodemerck, U. Stable low-temperature dry reforming of methane over mesoporous La2O3-ZrO2 supported Ni catalyst. Appl. Catal. B Environ. 2012, 113, 19–30. [Google Scholar] [CrossRef]
- Baudouin, D.; Rodemerck, U.; Krumeich, F.; Mallmann, A.D.; Szeto, K.C.; Ménard, H.; Veyre, L.; Candy, J.-P.; Webb, P.B.; Thieuleux, C.; et al. Particle size effect in the low temperature reforming of methane by carbon dioxide on silica-supported Ni nanoparticles. J. Catal. 2013, 297, 27–34. [Google Scholar] [CrossRef]
- Matsumura, Y.; Nakamori, T. Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal. A Gen. 2004, 258, 107–114. [Google Scholar] [CrossRef]
- An, Y.; Dou, J.; Tian, L.; Zhao, X.; Yu, J. Role of microwave during microwave-assisted catalytic reforming of guaiacol, syringolbio-oil as model compounds. J. Anal. Appl. Pyrolysis 2021, 158, 105290. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Sunarso, J.; Li, C.; Pham, G.H.; Phan, C.; Liu, S. Microwave-assisted catalytic methane reforming: A review. Appl. Catal. A Gen. 2020, 599, 117620. [Google Scholar] [CrossRef]
- Díaz-Ortiz, Á.; Prieto, P.; de la Hoz, A. A critical overview on the effect of microwave irradiation in organic synthesis. Chem. Rec. 2019, 19, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Zhou, J.; Ou, Y.; Luo, Y.; You, Z. Microwave selective effect: A new approach towards oxygen inhibition removal for highly-effective NO decomposition by Microwave catalysis over BaMnxMg1−xO3 mixed oxides at low temperature under excess oxygen. Chem. Commun. 2015, 51, 4073–4076. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Q.; Peng, K.; Chen, F.; Han, X.; Wang, X.; Zhou, J. Development of MgCo2O4–BaCO3 composites as microwave catalysts for the highly effective direct decomposition of NO under excess O2 at a low temperature. Catal. Sci. Technol. 2019, 9, 4276–4285. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Zazo, J.A.; Casas, J.A. Microwave-assisted catalytic wet peroxide oxidation: Energy optimization. Sep. Purif. Technol. 2019, 215, 62–69. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Dsouza, K.; Vashaee, D. Field induced decrystallization of silicon: Evidence of a microwave non-thermal effect. Appl. Phys. Lett. 2018, 112, 093103. [Google Scholar] [CrossRef]
- Einaga, H.; Nasu, Y.; Oda, M.; Saito, H. Catalytic performances of perovskite oxides for CO oxidation under microwave irradiation. Chem. Eng. J. 2016, 283, 97–104. [Google Scholar] [CrossRef]
- Stuerga, D.A.C.; Gaillard, P. Microwave Athermal Effects in Chemistry: A Myth’s Autopsy: Part II: Orienting effects and thermodynamic consequences of electric field. J. Microw. Power Electromagn. Energy 1996, 31, 101–113. [Google Scholar] [CrossRef]
- Stuerga, D.A.C.; Gaillard, P. Microwave Athermal Effects in Chemistry: A Myth’s Autopsy: Part I: Historical background and fundamentals of wave-matter interaction. J. Microw. Power Electromagn. Energy 1996, 31, 87–100. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, J.; Su, Z.; Ou, Y.; You, Z. Microwave catalytic effect: A new exact reason for microwave-driven heterogeneous gas-phase catalytic reactions. Catal. Sci. Technol. 2015, 6, 698–702. [Google Scholar] [CrossRef]
- Bai, X.; Robinson, B.; Killmer, C.; Wang, Y.; Li, L.; Hu, J. Microwave catalytic reactor for upgrading stranded shale gas to aromatics. Fuel 2019, 243, 485–492. [Google Scholar] [CrossRef]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-Specific Enhancement of the Carbon–Carbon Dioxide (Boudouard) Reaction. J. Phys. Chem. C 2013, 117, 26871–26880. [Google Scholar] [CrossRef]
- Bai, X.; Muley, P.D.; Musho, T.; Abdelsayed, V.; Robinson, B.; Caiola, A.; Shekhawat, D.; Jiang, C.; Hu, J. A combined experimental and modeling study of Microwave-assisted methane dehydroaromatization process. Chem. Eng. J. 2022, 433, 134445. [Google Scholar] [CrossRef]
- Goyal, H.; Mehdad, A.; Lobo, R.F.; Stefanidis, G.; Vlachos, D.G. Scaleup of a Single-Mode Microwave Reactor. Ind. Eng. Chem. Res. 2019, 59, 2516–2523. [Google Scholar] [CrossRef]
- Sturm, G.S.J.; Verweij, M.D.; Van Gerven, T.; Stankiewicz, A.I.; Stefanidis, G. On the effect of resonant microwave fields on temperature distribution in time and space. Int. J. Heat Mass Transf. 2012, 55, 3800–3811. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Baker-Fales, M.; Vlachos, D.G. Operation and Optimization of Microwave-Heated Continuous-Flow Microfluidics. Ind. Eng. Chem. Res. 2020, 59, 10418–10427. [Google Scholar] [CrossRef]
- Malhotra, A.; Chen, W.; Goyal, H.; Plaza-Gonzalez, P.J.; Julian, I.; Catala-Civera, J.M.; Vlachos, D.G. Temperature Homogeneity under Selective and Localized Microwave Heating in Structured Flow Reactors. Ind. Eng. Chem. Res. 2021, 60, 6835–6847. [Google Scholar] [CrossRef]
- Robinson, J.; Kingman, S.; Irvine, D.; Licence, P.; Smith, A.; Dimitrakis, G.; Obermayer, D.; Kappe, C.O. Electromagnetic simulations of microwave heating experiments using reaction vessels made out of silicon carbide. Phys. Chem. Chem. Phys. 2010, 12, 10793–10800. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Moral, L.M.; Navarrete, A.; Sturm, G.; Link, G.; Rueda, M.; Stefanidis, G.; Martín, Á. Release of hydrogen from nanoconfined hydrides by application of microwaves. J. Power Sources 2017, 353, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Nigar, H.; Sturm, G.S.J.; Garcia-Baños, B.; Peñaranda-Foix, F.L.; Catalá-Civera, J.M.; Mallada, R.; Stankiewicz, A.; Santamaría, J. Numerical analysis of microwave heating cavity: Combining electromagnetic energy, heat transfer and fluid dynamics for a NaY zeolite fixed-bed. Appl. Therm. Eng. 2019, 155, 226–238. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, L.; Gong, S.S. Recent developments in microwave-assisted polymerization with a focus on ring-opening polymerization. Green Chem. 2007, 9, 303–314. [Google Scholar] [CrossRef]
- Ebner, C.; Bodner, T.; Stelzer, F.; Wiesbrock, F. One Decade of Microwave-Assisted Polymerizations: Quo vadis? Macromol. Rapid Commun. 2011, 32, 254–288. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schubert, U.S. Microwave-Assisted Polymer Synthesis: Recent Developments in a Rapidly Expanding Field of Research. Macromol. Rapid Commun. 2007, 28, 368–386. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Caratzoulas, S.; Davis, M.E.; Gorte, R.J.; Gounder, R.; Lobo, R.F.; Nikolakis, V.; Sandler, S.I.; Snyder, M.A.; Tsapatsis, M.; Vlachos, D.G. Challenges of and Insights into Acid-Catalyzed Transformations of Sugars. J. Phys. Chem. C 2014, 118, 22815–22833. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-Y.; Cheng, Z.; Desir, P.; Saha, B.; Vlachos, D.G. Fast microflow kinetics and acid catalyst deactivation in glucose conversion to 5-hydroxymethylfurfural. React. Chem. Eng. 2020, 6, 152–164. [Google Scholar] [CrossRef]
- Palma, V.; Martino, M.; Meloni, E.; Ricca, A. Novel structured catalysts configuration for intensification of steam reforming of methane. Int. J. Hydrogen Energy 2017, 42, 1629–1638. [Google Scholar] [CrossRef]
- Bianchi, E.; Heidig, T.; Visconti, C.G.; Groppi, G.; Freund, H.; Tronconi, E. An appraisal of the heat transfer properties of metallic open-cell foams for strongly exo-/endo-thermic catalytic processes in tubular reactors. Chem. Eng. J. 2012, 198–199, 512–528. [Google Scholar] [CrossRef]
- Ramírez, A.; Hueso, J.L.; Mallada, R.; Santamaría, J. Ethylene epoxidation in microwave heated structured reactors. Catal. Today 2016, 273, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Palma, V.; Ricca, A.; Martino, M.; Meloni, E. Innovative structured catalytic systems for methane steam reforming intensification. Chem. Eng. Process. Process Intensif. 2017, 120, 207–215. [Google Scholar] [CrossRef]
- Zhang, H.; Geerlings, H.; Lin, J.; Chin, W.S. Rapid microwave hydrogen release from MgH2 and other hydrides. Int. J. Hydrogen Energy 2011, 36, 7580–7586. [Google Scholar] [CrossRef]
- Mao, Y.; Yang, S.; Xue, C.; Zhang, M.; Wang, W.; Song, Z.; Zhao, X.; Sun, J. Rapid degradation of malachite green by CoFe2O4–SiC foam under microwave radiation. R. Soc. Open Sci. 2018, 5, 180085. [Google Scholar] [CrossRef] [Green Version]
- Gangurde, L.S.; Sturm, G.S.J.; Valero-Romero, M.J.; Mallada, R.; Santamaria, J.; Stankiewicza, A.I.; Stefanidis, G.D. Synthesis, characterization, and application of ruthenium-doped SrTiO3 perovskite catalysts for microwave-assisted methane dry reforming. Chem. Eng. Process. Process Intensif. 2018, 127, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Marin, C.M.; Popczun, E.J.; Nguyen-Phan, T.-D.; De Tafen, N.; Alfonso, D.; Waluyo, I.; Hunt, A.; Kauffman, D.R. Designing perovskite catalysts for controlled active-site exsolution in the microwave dry reforming of methane. Appl. Catal. B Environ. 2020, 284, 119711. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Pham, G.H.; Tade, M.; Phan, C.; Vagnoni, R.; Liu, S. Microwave-Assisted Dry and Bi-reforming of Methane over M–Mo/TiO2 (M = Co, Cu) Bimetallic Catalysts. Energy Fuels 2020, 34, 7284–7294. [Google Scholar] [CrossRef]
- Li, R.; Xu, W.; Deng, J.; Zhou, J. Coke-Resistant Ni−Co/ZrO2−CaO-Based Microwave Catalyst for Highly Effective Dry Reforming of Methane by Microwave Catalysis. Ind. Eng. Chem. Res. 2021, 60, 17458–17468. [Google Scholar] [CrossRef]
- Sharifvaghefi, S.; Shirani, B.; Eic, M.; Zheng, Y. Application of Microwave in Hydrogen Production from Methane Dry Reforming: Comparison between the Conventional and Microwave-Assisted Catalytic Reforming on Improving the Energy Efficiency. Catalysts 2019, 9, 618. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Malhotra, A.; Yu, K.; Zheng, W.; Plaza-Gonzalez, P.J.; Catala-Civera, J.M.; Santamaria, J.; Vlachos, D.G. Intensified microwave-assisted heterogeneous catalytic reactors for sustainable chemical manufacturing. Chem. Eng. J. 2021, 420, 130476. [Google Scholar] [CrossRef]
- Wismann, S.T.; Engbæk, J.S.; Vendelbo, S.B.; Eriksen, W.L.; Frandsen, C.; Mortensen, P.M.; Chorkendorff, I. Electrified Methane Reforming: Understanding the Dynamic Interplay. Ind. Eng. Chem. Res. 2019, 58, 23380–23388. [Google Scholar] [CrossRef]
- Balakotaiah, V.; Ratnakar, R.R. Modular reactors with electrical resistance heating for hydrocarbon cracking and other endothermic reactions. AIChE J. 2021, 68, e17542. [Google Scholar] [CrossRef]
- Yabe, T.; Mitarai, K.; Oshima, K.; Ogo, S.; Sekine, Y. Low-temperature dry reforming of methane to produce syngas in an electric field over La-doped Ni/ZrO2 catalysts. Fuel Process. Technol. 2017, 158, 96–103. [Google Scholar] [CrossRef]
- Rieks, M.; Bellinghausen, R.; Kockmann, N.; Mleczko, L. Experimental study of methane dry reforming in an electrically heated reactor. Int. J. Hydrogen Energy 2015, 40, 15940–15951. [Google Scholar] [CrossRef]
- Atwood, K.; Knight, C.B. Relbrming kinetics and catalysis. In Fertilizer Science and Technology; Slack, A.V., James, G.R., Eds.; Marcel Dekker: New York, NY, USA, 1973; Volume 2. [Google Scholar]
- Sekine, Y.; Haraguchi, M.; Matsukata, M.; Kikuchi, E. Low temperature steam reforming of methane over metal catalyst supported on CexZr1−xO2 in an electric field. Catal. Today 2011, 171, 116–125. [Google Scholar] [CrossRef]
- Froment, G.; Bischoff, K.; Wilde, J. Transport Processes with Reactions Catalyzed by Solids. In Chemical Reactor Analysis and Design, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1990; pp. 148–149. [Google Scholar]
- Ratnakar, R.R.; Dadi, R.K.; Balakotaiah, V. Multi-scale reduced order models for transient simulation of multi-layered monolith reactors. Chem. Eng. J. 2018, 352, 293–305. [Google Scholar] [CrossRef]
- Sarkar, B.; Ratnakar, R.R.; Balakotaiah, V. Multi-scale coarse-grained continuum models for bifurcation and transient analysis of coupled homogeneous-catalytic reactions in monoliths. Chem. Eng. J. 2020, 407, 126500. [Google Scholar] [CrossRef]
- Olabi, A.; Wilberforce, T.; Abdelkareem, M.A. Fuel cell application in the automotive industry and future perspective. Energy 2020, 214, 118955. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.-Y.; Han, M.; Yong, R.-Y.; Sun, D.; Chen, J. Review on current research of materials, fabrication and application for bipolar plate in proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2019, 45, 29832–29847. [Google Scholar] [CrossRef]
- Treccani, Il Portale Del Sapere. Available online: https://www.treccani.it/enciclopedia/celle-a-combustibile_%28Enciclopedia-della-Scienza-e-della-Tecnica%29/ (accessed on 16 December 2021).
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A Critical Review on the Use of Ionic Liquids in Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Tzelepis, S.; Kavadias, K.A.; Marnellos, G.E.; Xydis, G. A review study on proton exchange membrane fuel cell electrochemical performance focusing on anode and cathode catalyst layer modelling at macroscopic level. Renew. Sustain. Energy Rev. 2021, 151, 111543. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Y.; Liu, A.; Zhang, Z.; Lv, Q.; Liu, B. Current progress and performance improvement of Pt/C catalysts for fuel cells. J. Mater. Chem. A 2020, 8, 24284–24306. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef] [Green Version]
- Pourrahmani, H.; Shakeri, H.; Van Herle, J. Thermoelectric Generator as the Waste Heat Recovery Unit of Proton Exchange Membrane Fuel Cell: A Numerical Study. Energies 2022, 15, 3018. [Google Scholar] [CrossRef]
- Huang, L.; Zaman, S.; Tian, X.; Wang, Z.; Fang, W.; Xia, B.Y. Advanced Platinum-Based Oxygen Reduction Electrocatalysts for Fuel Cells. Accounts Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Spasov, D.D.; Ivanova, N.A.; Pushkarev, A.S.; Pushkareva, I.V.; Presnyakova, N.N.; Chumakov, R.G.; Presnyakov, M.Y.; Grigoriev, S.A.; Fateev, V.N. On the Influence of Composition and Structure of Carbon-Supported Pt-SnO2 Hetero-Clusters onto Their Electrocatalytic Activity and Durability in PEMFC. Catalysts 2019, 9, 803. [Google Scholar] [CrossRef] [Green Version]
- Garapati, M.S.; Sundara, R. Highly efficient and ORR active platinum-scandium alloy-partially exfoliated carbon nanotubes electrocatalyst for Proton Exchange Membrane Fuel Cell. Int. J. Hydrogen Energy 2019, 44, 10951–10963. [Google Scholar] [CrossRef]
- MoghadamEsfahani, R.A.; Vankova, S.K.; Easton, E.B.; Ebralidze, I.; Specchia, S. A hybrid Pt/NbO/CNTs catalyst with high activity and durability for oxygen reduction reaction in PEMFC. Renew. Energy 2020, 154, 913–924. [Google Scholar] [CrossRef]
- Basumatarya, P.; Konwarb, D.; Yoon, Y.S. Conductivity-tailored PtNi/MoS2 3D nanoflower catalyst via Sc doping as a hybrid anode for a variety of hydrocarbon fuels in proton exchange membrane fuel cells. Appl. Catal. B Environ. 2020, 267, 118724. [Google Scholar] [CrossRef]
- Lin, R.; Che, L.; Shen, D.; Cai, X. High durability of Pt-Ni-Ir/C ternary catalyst of PEMFC by stepwise reduction synthesis. Electrochim. Acta 2019, 330, 135251. [Google Scholar] [CrossRef]
- Zhu, F.; Luo, L.; Wu, A.; Wang, C.; Cheng, X.; Shen, S.; Ke, C.; Yang, H.; Zhang, J. Improving the High-Current-Density Performance of PEMFC through Much Enhanced Utilization of Platinum Electrocatalysts on Carbon. ACS Appl. Mater. Interfaces 2020, 12, 26076–26083. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.-I.; Jo, S.G.; Hong, N.E.; Ye, B.; Lee, S.; Dow, H.S.; Lee, D.H.; Lee, J.W. Enhanced activity and durability of Pt nanoparticles supported on reduced graphene oxide for oxygen reduction catalysts of proton exchange membrane fuel cells. Catal. Today 2019, 352, 10–17. [Google Scholar] [CrossRef]
- Esfahani, R.A.M.; Easton, E.B. Exceptionally durable Pt/TOMS catalysts for fuel cells. Appl. Catal. B Environ. 2020, 268, 118743. [Google Scholar] [CrossRef]
- Xu, C.; Fan, C.; Zhang, X.; Chen, H.; Liu, X.; Fu, Z.; Wang, R.; Hong, T.; Cheng, J. MXene (Ti3C2Tx) and Carbon Nanotube Hybrid-Supported Platinum Catalysts for the High-Performance Oxygen Reduction Reaction in PEMFC. ACS Appl. Mater. Interfaces 2020, 12, 19539–19546. [Google Scholar] [CrossRef]
- Wang, R.; Chang, Z.; Fang, Z.; Xiao, T.; Zhu, Z.; Ye, B.; Xu, C.; Cheng, J. Pt nanowire/Ti3C2Tx-CNT hybrids catalysts for the high performance oxygen reduction reaction for high temperature PEMFC. Int. J. Hydrogen Energy 2020, 45, 28190–28195. [Google Scholar] [CrossRef]
- Wei, L.; Shi, J.; Cheng, G.; Lu, L.; Xu, H.; Li, Y. Pt/TiN–TiO2 catalyst preparation and its performance in oxygen reduction reaction. J. Power Sources 2020, 454, 227934. [Google Scholar] [CrossRef]
- Devrim, Y.; Arıca, E.D. Investigation of the effect of graphitized carbon nanotube catalyst support for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2019, 45, 3609–3617. [Google Scholar] [CrossRef]
- Chu, T.; Xie, M.; Yang, D.; Ming, P.; Li, B.; Zhang, C. Highly active and durable carbon support Pt-rare earth catalyst for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2020, 45, 27291–27298. [Google Scholar] [CrossRef]
- Tetteh, E.B.; Lee, H.-Y.; Shin, C.-H.; Kim, S.-H.; Ham, H.C.; Tran, T.-N.; Jang, J.-H.; Yoo, S.J.; Yu, J.-S. New PtMg Alloy with Durable Electrocatalytic Performance for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell. ACS Energy Lett. 2020, 5, 1601–1609. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Q.; Rui, Z.; Li, J.; Liu, J. High-Loading Pt–Co/C Catalyst with Enhanced Durability toward the Oxygen Reduction Reaction through Surface Au Modification. ACS Appl. Mater. Interfaces 2020, 12, 30381–30389. [Google Scholar] [CrossRef]
- Lin, R.; Zheng, T.; Chen, L.; Wang, H.; Cai, X.; Sun, Y.; Hao, Z. Anchored Pt-Co Nanoparticles on Honeycombed Graphene as Highly Durable Catalysts for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2021, 13, 34397–34409. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Perez-Page, M.; Chen, J.; Rodriguez, R.G.; Cai, R.; Haigh, S.J.; Holmes, S.M. A structured catalyst support combining electrochemically exfoliated graphene oxide and carbon black for enhanced performance and durability in low-temperature hydrogen fuel cells. Energy 2021, 226, 120318. [Google Scholar] [CrossRef]
- Islam, J.; Kim, S.-K.; Kim, K.-H.; Lee, E.; Park, G.-G. Enhanced durability of Pt/C catalyst by coating carbon black with silica for oxygen reduction reaction. Int. J. Hydrogen Energy 2020, 46, 1133–1143. [Google Scholar] [CrossRef]
- Gu, K.; Kim, E.J.; Sharma, S.; Sharma, P.R.; Bliznakov, S.; Hsiao, B.S.; Rafailovich, M.H. Mesoporous carbon aerogel with tunable porosity as the catalyst support for enhanced proton-exchange membrane fuel cell performance. Mater. Today Energy 2020, 19, 100560. [Google Scholar] [CrossRef]
- Eren, E.O.; Ozkan, N.; Devrim, Y. Polybenzimidazole-modified carbon nanotubes as a support material for platinum-based high-temperature proton exchange membrane fuel cell electrocatalysts. Int. J. Hydrogen Energy 2020, 46, 29556–29567. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Du, L.; Zhang, J.; Chiang, F.-K.; Wen, Y.; Wang, X.; Wu, Y.; Chen, N.; Sun, S. PGM-Free Fe/N/C and Ultralow Loading Pt/C Hybrid Cathode Catalysts with Enhanced Stability and Activity in PEM Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 13739–13749. [Google Scholar] [CrossRef] [PubMed]
- Osmieri, L.; Park, J.; Cullen, D.A.; Zelenay, P.; Myers, D.J.; Neyerlin, K.C. Status and challenges for the application of platinum group metal-free catalysts in proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 2021, 25, 100627. [Google Scholar] [CrossRef]
- Zhan, Y.; Zeng, H.; Xie, F.; Zhang, H.; Zhang, W.; Jin, Y.; Zhang, Y.; Chen, J.; Meng, H. Templated growth of Fe/N/C catalyst on hierarchically porous carbon for oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2019, 431, 31–39. [Google Scholar] [CrossRef]
- Wan, X.; Liu, X.; Li, Y.; Yu, R.; Zheng, L.; Yan, W.; Wang, H.; Xu, M.; Shui, J. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2019, 2, 259–268. [Google Scholar] [CrossRef]
- Zhang, H.; Chung, H.T.; Cullen, D.A.; Wagner, S.; Kramm, U.I.; More, K.L.; Zelenay, P.; Wu, G. High-performance fuel cell cathodes exclusively containing atomically dispersed iron active sites. Energy Environ. Sci. 2019, 12, 2548–2558. [Google Scholar] [CrossRef]
- Qiao, M.; Wang, Y.; Wang, Q.; Hu, G.; Mamat, X.; Zhang, S.; Wang, S. Hierarchically Ordered Porous Carbon with Atomically Dispersed FeN 4 for Ultraefficient Oxygen Reduction Reaction in Proton-Exchange Membrane Fuel Cells. Angew. Chem. 2019, 132, 2710–2716. [Google Scholar] [CrossRef]
- He, Y.; Hwang, S.; Cullen, D.A.; Uddin, M.A.; Langhorst, L.; Li, B.; Karakalos, S.; Kropf, A.J.; Wegener, E.C.; Sokolowski, J.; et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy. Energy Environ. Sci. 2018, 12, 250–260. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Zheng, T.; Cuello, N.C.; Goenaga, G.; Zawodzinski, T.A.; Tian, H.; Wright, J.T.; Meulenberg, R.W.; Wang, X.; et al. CrN-Encapsulated Hollow Cr-N-C Capsules Boosting Oxygen Reduction Catalysis in PEMFC. CCS Chem. 2021, 3, 208–218. [Google Scholar] [CrossRef]
- Xie, X.; He, C.; Li, B.; He, Y.; Cullen, D.A.; Wegener, E.C.; Kropf, A.J.; Martinez, U.; Cheng, Y.; Engelhard, M.H.; et al. Performance enhancement and degradation mechanism identification of a single-atom Co–N–C catalyst for proton exchange membrane fuel cells. Nat. Catal. 2020, 3, 1044–1054. [Google Scholar] [CrossRef]
- He, Y.; Guo, H.; Hwang, S.; Yang, X.; He, Z.; Braaten, J.; Karakalos, S.; Shan, W.; Wang, M.; Zhou, H.; et al. Single Cobalt Sites Dispersed in Hierarchically Porous Nanofiber Networks for Durable and High-Power PGM-Free Cathodes in Fuel Cells. Adv. Mater. 2020, 32, 2003577. [Google Scholar] [CrossRef]
- Miao, Z.; Xia, Y.; Liang, J.; Xie, L.; Chen, S.; Li, S.; Wang, H.; Hu, S.; Han, J.; Li, Q. Constructing Co–N–C Catalyst via a Double Crosslinking Hydrogel Strategy for Enhanced Oxygen Reduction Catalysis in Fuel Cells. Small 2021, 17, 2100735. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, X.; Yang, F.; Li, B.; Stracensky, T.; Karakalos, S.; Mukerjee, S.; Jia, Q.; Su, D.; Wang, G.; et al. Atomically Dispersed MnN4 Catalysts via Environmentally Benign Aqueous Synthesis for Oxygen Reduction: Mechanistic Understanding of Activity and Stability Improvements. ACS Catal. 2020, 10, 10523–10534. [Google Scholar] [CrossRef]
- Luo, F.; Roy, A.; Silvioli, L.; Cullen, D.A.; Zitolo, A.; Sougrati, M.T.; Oguz, I.C.; Mineva, T.; Teschner, D.; Wagner, S.; et al. P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction. Nat. Mater. 2020, 19, 1215–1223. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhang, G.; Du, L.; Yang, L.; Markiewicz, M.; Choi, J.-Y.; Chenitz, R.; Sun, S. SiO2-Fe/N/C catalyst with enhanced mass transport in PEM fuel cells. Appl. Catal. B Environ. 2019, 264, 118523. [Google Scholar] [CrossRef]
- Uddin, A.; Dunsmore, L.; Zhang, H.; Hu, L.; Wu, G.; Litster, S. High Power Density Platinum Group Metal-free Cathodes for Polymer Electrolyte Fuel Cells. ACS Appl. Mater. Interfaces 2019, 12, 2216–2224. [Google Scholar] [CrossRef]
- Xu, J.; Liang, G.; Chen, D.; Li, Z.; Zhang, H.; Chen, J.; Xie, F.; Jin, Y.; Wang, N.; Meng, H. Iron and nitrogen doped carbon derived from ferrocene and ZIF-8 as proton exchange membrane fuel cell cathode catalyst. Appl. Surf. Sci. 2021, 573, 151607. [Google Scholar] [CrossRef]
- Liu, S.; Meyer, Q.; Li, Y.; Zhao, T.; Su, Z.; Ching, K.; Zhao, C. Fe–N–C/Fe nanoparticle composite catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. Chem. Commun. 2022, 58, 2323–2326. [Google Scholar] [CrossRef]
- Xie, X.; Shang, L.; Xiong, X.; Shi, R.; Zhang, T. Fe Single-Atom Catalysts on MOF-5 Derived Carbon for Efficient Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Adv. Energy Mater. 2021, 12, 2102688. [Google Scholar] [CrossRef]
- Zhu, W.; Pei, Y.; Douglin, J.C.; Zhang, J.; Zhao, H.; Xue, J.; Wang, Q.; Li, R.; Qin, Y.; Yin, Y.; et al. Multi-scale study on bifunctional Co/Fe–N–C cathode catalyst layers with high active site density for the oxygen reduction reaction. Appl. Catal. B Environ. 2021, 299, 120656. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Wu, X.; Tang, C.; Yang, S.-Z.; Su, P.; Thomsen, L.; Zhao, F.; Lu, S.; Liu, J.; et al. A template-free method to synthesis high density iron single atoms anchored on carbon nanotubes for high temperature polymer electrolyte membrane fuel cells. Nano Energy 2020, 80, 105534. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, B.; Baik, C.; Kim, T.-Y.; Pak, C. Multifunctional Ir–Ru alloy catalysts for reversal-tolerant anodes of polymer electrolyte membrane fuel cells. J. Mater. Sci. Technol. 2020, 60, 105–112. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, C.; Liu, X.; Wang, X.; Zhou, F.; Wang, J.; Hu, Y.; Zhao, Y.; Yao, T.; Yang, L.-M.; et al. Single Atomic Cerium Sites with a High Coordination Number for Efficient Oxygen Reduction in Proton-Exchange Membrane Fuel Cells. ACS Catal. 2021, 11, 3923–3929. [Google Scholar] [CrossRef]





| Mechanism | Principle | Pro | Cons |
|---|---|---|---|
| Ohmic or Joule | electric current passing through a resistive conductor produces heat ð, a conductive structured support (foam, monolith) is used on which the catalyst is deposited as thin film |
|
|
| Induction heating | rapidly alternating magnetic field either generates eddy currents in conducting materials, resulting in the Joule heating of those materials, or generates heat in ferro-/ferrimagnetic materials by the magnetic hysteresis losses |
|
|
| Microwave/RF heating | rapidly alternating electric field of the microwave generates heat by moving dipolar molecules or ions in liquids, or by getting absorbed in the so-called “dielectric lossy” solid nonmagnetic materials | industrial experience for fine chemicals production relatively simple technology | effective with liquids, less with gas reactions; main challenge with heterogeneous catalysts to achieve uniform heating; proposed solutions difficult to scale-up |
| Process | Catalyst | MW Input | Operating Condition | XCH4; XCO2 | Energy Consumption kWh Nm−3H2 | Reference |
|---|---|---|---|---|---|---|
| DRM | Ni/Al2O3 | P = 45–60 W | CO2/CH4 = 1 T = 800 °C WHSV = 11,000 mL g−1 h−1 | XCH4 = 90% XCO2 = 90% | - | [58] |
| DRM | Pt/C | P = 45–60 W | CO2/CH4 = 1 T = 800 °C WHSV = 11,000 mL g−1 h−1 | XCH4 = 90% XCO2 = 90% | - | [58] |
| DRM | Ni/Al2O3-SiC | P = 45–60 W | CO2/CH4 = 1 T = 800 °C WHSV = 11,000 mL g−1 h−1 | XCH4 = 90% XCO2 = 90% | - | [58] |
| DRM | Ni/SiC | P = 45–60 W | CO2/CH4 = 1 T = 800 °C WHSV = 11,000 mL g−1 h−1 | XCH4 = 90% XCO2 = 90% | - | [58] |
| DRM | 7Ru/SrTiO3 | P = 36.99 kW | CO2/CH4 = 1 T = 940 °C. | XCH4 = 99.5% XCO2 = 94% | 18.58 | [98] |
| DRM | Fe/HZSM-5 | P = 700 W | CO2/CH4 = 1 WHSV = 2.4 L h−1 g−1 | XCH4 = 63.03% XCO2 = 91.27% | - | [24] |
| DRM | LaxSr2−xCoO4-Mn | P = 140 W | CO2/CH4 = 1 WHSV = 10 L h−1 g−1 | XCH4 = 80% XCO2 = 80% | 3.98 | [99] |
| DRM | Co-Mo/TiO2 | P = 100 W | CO2/CH4 = 1 WHSV = 10 L h−1 g−1 | XCH4 = 81% XCO2 = 86% | - | [100] |
| DRM | Cu-Mo/TiO2 | P = 100 W | CO2/CH4 = 1 WHSV = 10 L h−1 g−1 | XCH4 = 76% XCO2 = 62% | - | [100] |
| DRM | Ni-Co/ZrO2-CaO + SiC | - | CO2/CH4 = 1 WHSV = 10 L h−1 g−1 T = 800 °C | XCH4 = 97.1% XCO2 = 99.2% | - | [101] |
| DRM | 10Ni/AC | - | CO2/CH4 = 1 WHSV = 33 L h−1 g−1 T = 650 °C | XCH4 = 48% XCO2 = 51% | - | [102] |
| DRM | Ni/MgO/AC | - | CO2/CH4 = 1 WHSV = 33 L h−1 g−1 T = 650 °C | XCH4 = 82% XCO2 = 85% | - | [102] |
| SRM | 15%Ni/CeO2-Al2O3 on a SiC monolith | P = 800 W @ GHSV = 3300 h−1 P = 1000 W @ GHSV = 5000 h−1 | GHSV = 3300 and 5000 h−1 T = 550–950 °C P = 1 bar S/C = 3 | CH4 equilibrium conversion T = 800 °C—GHSV = 3300 h−1 T = 850 °C—GHSV = 5000 h−1 | 3.8 | [39] |
| Process | Heating Element | Catalyst | Electric Parameters | Operating Condition | XCH4; YH2 | Energy Consumption kWh Nm−3H2 | Reference |
|---|---|---|---|---|---|---|---|
| DRM | SiC heating element (Joule Effect) | Ni/Al2O3 | P = 218 W | CO2/CH4 = 1 T = 790 °C. | XCH4 = 85% YH2 = 82% | 5.1 | [15] |
| SRM | SiC heating element (Joule Effect) | Ni/Al2O3 | P = 220 W | CH4/H2O = 3 T = 790 °C. | XCH4 = 80% YH2 = 80% | 4.8 | [15] |
| DRM | Stainless electrode (Joule Effect) | Ni/La-ZrO2 | I = 12 mA V = 0.4 kV P = 5 W | T = 576 K CH4/CO2 = 1 | XCH4 30.2% YH2 = - | 18 | [106] |
| SRM | Stainless electrode rode (Joule Effect) | Ni/CeZrO2 Ce/Zr = 3 | I = 3 mA EPC = 1.29 W | T = 190 °C H2O/CH4 = 2 | XCH4 12% YH2 = - | 3.98 | [109] |
| SRM | Stainless electrode rode (Joule Effect) | Ni/CeZrO2 Ce/Zr = 1 | I = 3 mA EPC = 1.53 W | 3.21 | [109] | ||
| SRM | Stainless electrode rode (Joule Effect) | Ni/CeZrO2 Ce/Zr = 0.3 | I = 3 mA EPC = 2.54 W | 3.75 | [109] |
| Catalyst | P [W cmPt−2] | MA [A mgPt−1] | SA [mA cmPt−2] | ECSA [m2 gPt−1] | Ref |
|---|---|---|---|---|---|
| Pt/10wt%SnO2/C | 0.25 (40 °C) | - | - | 57 | [124] |
| Pt3Sc/PECNTs | 0.76 (60 °C) | 0.0803 | - | 102.1 | [125] |
| Pt/NbO/CNTs | 0.772 (80 °C) | 0.057 | - | 81.62 | [126] |
| PtSc0.5Ni/MoS2@graphene | 0.0517 (50 °C) | 3.579 | 4.1 | 86.27 | [127] |
| Pt-Ni-Ir/C Pt:Ni:Ir = 2:2:1 | 0.4 (80 °C) | 0.0004 | - | 85 | [128] |
| Pt/VC | 0.9 (80 °C) | 0.096 | 56.4 | [129] | |
| Pt/rGO | - | - | 10.5 | 0.51 | [130] |
| Pt/Ti3O5Mo0.2Si0.4 | 0.97 (80 °C) | - | 2.35 | 89.6 | [131] |
| Pt/CNT-Ti3C2Tx (1:1) | 0.175 (60 °C) | 0.163 | 0.26 | 63 | [132] |
| Pt NWs/Ti3C2Tx-CNT | 0.182 (180 °C) | 0.32 | 0.21 | 63.59 | [133] |
| Pt/TiN–TiO2 | 0.392 (70 °C) | - | - | 73 | [134] |
| Pt/GCNT | 0.38 (160 °C) | - | - | 59 | [135] |
| Pt-YOx/C | - | 0.108 | 0.204 | 52.82 | [136] |
| PtxMg/C | 1.275 (80 °C) | 1.09 | 2.67 | 40 | [137] |
| Au−Pt−Co/C-0.015 | - | 0.386 | 0.535 | 72.2 | [138] |
| Pt-Co/3D rHPGO | - | 0.167 | 0.31 | 53.1 | [139] |
| Pt/rEGO2-CB3 | 0.537 (60 °C) | 0.006416 | 4.682 | 41.73 | [140] |
| Pt/CB-SiO220%, | - | 0.26 | 0.933 | 29.27 | [141] |
| Pt/CA-200 | 0.642 (80 °C) | - | - | 187.9 | [142] |
| Pt-PBI/MWCNT | 0.047 (180 °C) | - | - | 43 | [143] |
| Catalyst | P [W cm−2] | MA [A mg−1] | SA [mA cm−2] | ECSA [m2 g−1] | Ref |
|---|---|---|---|---|---|
| MgO@Phen-Fe-800-3/1 | 0.63 (70 °C) | - | - | - | [146] |
| TPI@Z8(SiO2)-650-C | 1.18 (80 °C) | - | - | - | [147] |
| 1.5Fe-ZIF | 0.67 (80 °C) | - | - | 556 | [148] |
| FeN4/HOPC-c-1000 | 0.69 (80 °C) | - | - | - | [149] |
| Co–N–C@F127 | 0.87 (80 °C) | - | - | - | [150] |
| CrN@H-Cr-Nx-C | 0.533 (80 °C) | - | - | - | [151] |
| Co/N/C | 0.64 (80 °C) | - | - | - | [152] |
| Co–N–PCNF | 0.71 (80 °C) | - | - | - | [153] |
| P(AA–AM)(5-1)/Co/N/C | 0.66 (80 °C) | - | - | - | [154] |
| Mn-N-C-HCl-800/1100 | 0.6 (80 °C) | - | - | - | [155] |
| Sn/N/C | - | 0.0009 | - | - | [156] |
| SiO2-Fe/N/C | >0.3 (80 °C) | - | - | - | [157] |
| Fe/N/C 100 nm; I/C = 0.6 | 0.610 (94 °C) | 0.001 | - | - | [158] |
| FeNC-1:30 Ferrocene:ZIF-8 = 1/30 | 0.602 (70 °C) | - | - | - | [159] |
| Fe/N/C-300 | 1.08 (80 °C) | - | - | - | [160] |
| Fe SAC-MOF-5 | 0.84 (80 °C) | - | - | - | [161] |
| 0.14Co0.01Fe–CB | 0.465 (80 °C) | - | - | - | [162] |
| FeSa/HP | 0.266 (240 °C) | - | - | - | [163] |
| IrRu2/C | - | - | 7.69 | - | [164] |
| Ce SAS/HPNC | 0.525 (80 °C) | - | - | - | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, E.; Iervolino, G.; Ruocco, C.; Renda, S.; Festa, G.; Martino, M.; Palma, V. Electrified Hydrogen Production from Methane for PEM Fuel Cells Feeding: A Review. Energies 2022, 15, 3588. https://doi.org/10.3390/en15103588
Meloni E, Iervolino G, Ruocco C, Renda S, Festa G, Martino M, Palma V. Electrified Hydrogen Production from Methane for PEM Fuel Cells Feeding: A Review. Energies. 2022; 15(10):3588. https://doi.org/10.3390/en15103588
Chicago/Turabian StyleMeloni, Eugenio, Giuseppina Iervolino, Concetta Ruocco, Simona Renda, Giovanni Festa, Marco Martino, and Vincenzo Palma. 2022. "Electrified Hydrogen Production from Methane for PEM Fuel Cells Feeding: A Review" Energies 15, no. 10: 3588. https://doi.org/10.3390/en15103588
APA StyleMeloni, E., Iervolino, G., Ruocco, C., Renda, S., Festa, G., Martino, M., & Palma, V. (2022). Electrified Hydrogen Production from Methane for PEM Fuel Cells Feeding: A Review. Energies, 15(10), 3588. https://doi.org/10.3390/en15103588