Are Wetlands as an Integrated Bioremediation System Applicable for the Treatment of Wastewater from Underground Coal Gasification Processes?
Abstract
1. Introduction
2. Bibliometric Analysis
3. Underground Coal Gasification
3.1. Recent Worldwide Experiments
3.2. Experiments from Central Mining Institute (GIG)
3.3. Characterisation of Wastewater from Underground Coal Gasification Process: Experience from GIG
4. Bioremediation Process: Current Challenges and Trends
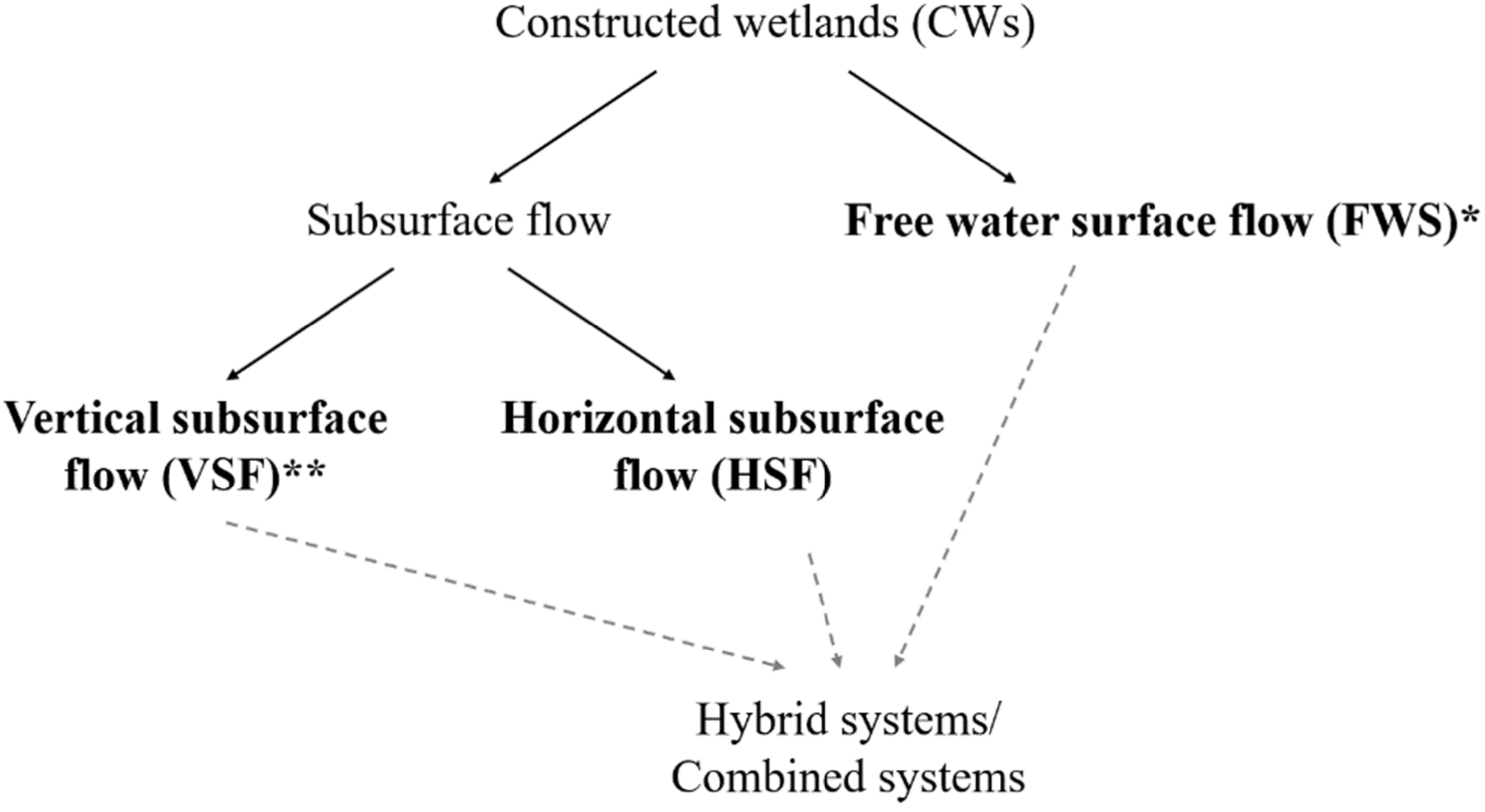
5. Wetlands as Natural and Engineering Systems to Clean up Industrial Wastewater
| Pollutants | Type of Wastewater | Type of Wetlands and the Plant Species Used | Removal Rate/ Comment | References |
|---|---|---|---|---|
| Heavy metals (Mn, Cd, Zn and Pb), arsenic (As) | Mining (the pilot-scale experiment) | The combination of adsorption (modified iron-ore drainage sludge) and HSF CWs with Phragmites australis | The average removals during four months of operation was as follows:Mn—96,9%, Cd—79,6%, Zn—52,9%, Pb—38,7% and As—96,9% | [76] |
| Heavy metals (Fe, Mn, Al, Co, Ni and Cr), sulphate (900–1500 mg L−1) | Synthetic acid mine drainage (laboratory-scale experiment) | HSF-CWs planted with Typha latifolia and organic-rich substrate (cow manure and bamboo chips) | After the 6-month metal removal efficiency: Cr—99.7%, Ni—97.8%, Co—93.7%, Fe—91.6% and Al—59.7%. Microbial sulphate reduction 44–75%. | [77] |
| Heavy metals (Mn, Cu, Co, Cr and Cd) | Coke plant effluents | Natural wetland. Dominant emergent plants: Colocasia esculenta, Scirpus grossus and Typha latifolia | Natural wetlands seems to be efficient in removal of selected heavy metals from coke-oven effluent | [78] |
| Heavy metals, phenol | Post-methanated distillery effluent (PMDE) | FWS CWs in India planted with Typha angustata L. | Removal: Cd (34–62), Cr (36–58), Cu (33–54), Fe (33–52), Mn (36–83), Ni (36–59), Pb (33–60), Zn (32–54), phenol—93.75% after 7 days of free water surface flow treatment | [79] |
| Ammonium, iron and traces of organic compounds | Coke plant effluents(pilot-scale) | HSF-CWs with two-stage gravel bed planted with Phragmites australis | Nitrogen removal efficiency (54–94%). Removal of COD from 35 to 52% of inlet concentrations | [80] |
| Heavy metals (Cu, Ni, Pb and Zn), cyanides (CN−) and sulphates (SO42−) | Synthetic electroplating wastewater (laboratory-scale experiment in columns) | Up-flow VSF-CWs (lactates as carbon source, peat or gravel as medium). Some columns planted with Phragmites australis | Maximum removal: Cu, Ni, Zn, CN− > 90%; Pb > 70% SO42− > 10%. Insignificant effect of vegetation | [81] |
| Phenol, m-cresol, methyl tertiary-butyl ether (MTBE), benzene | Contaminated groundwater (the pilot-scale experiment) | Three separate HSF CWs. Two of them with Phragmites australis and one without vegetation | Surface load removal rates (SRR; mg m−2 d−1) were as follows: MTBE—20, m-cresol—80, benzene—335, phenol—620. The presence of Phragmites australis significant improved the contaminant removal performance. | [82] |
| Phenol, ammonium andnitrogen | Domestic wastewater spiked with phenol (laboratory-scale experiment) | HSF CWs planted with Typha latifolia with different substrate | CWs with Typha latifolia were able to remove phenol completely (C0 = 500 mg L−1) after 36 days with rice husk in substrate and by 60% in gravel in substrate. Planted wetland units performed better than the unplanted ones. | [83] |
| Phenol, organics (COD), thiocyanate and ammonium nitrogen | Synthetic wastewater (laboratory-scale experiment) | HSF-CWs planted with Typha angustifolia | Efficiency removals (operation time period—158 days): Phenol- 99%, COD—93%, ammonia nitrogen—17–30%. Alkalinity improved thiocyanate removal to 91%. | [84] |
| BTEX | Groundwater from the former refinery site(pilot-scale system consisted of four subsurface flow treatment cells equipped with aeration). | HSF-CWs. Types of plants: Salix, Phragmites, Scirpus, Juncus and Cornus | Removal after one year operating: benzene—80%, total BTEX- 88% | [85] |
| PAHs (naphthalene; mixture of phenanthrene and pyrene) | A laboratory-scale experiment investigating the effects of PAHs on plant growth and development. | Hydroponics/pot experiment.Species: Baumea juncea, Baumea articulata, Schoenoplectus Validus and Juncus subsecundus | The effect of PAHs on plant growth in CWs may be species-specific and can depend on the type of PAHs and the substrate | [86] |
| PAHs | A laboratory-scale experiment | VSF-CW planted with Iris pseudacorus | The reduction of phenanthrene using biochar-loading copper ions (Cu-BC) was about 94% (HRT lasted 3 days) | [87] |
| Hydrocarbons and cyanides | FWS-CWs. Shallow basins, initially planted with: Typha latifolia and Schoenoplectus tabernaemontani, subsequently converted to Ceratophyllum demersum and Potamageton spp. | Removal after 7 days detention: total cyanide—56%, free cyanide—88%, Gasoline, diesel—~67% | [88] |
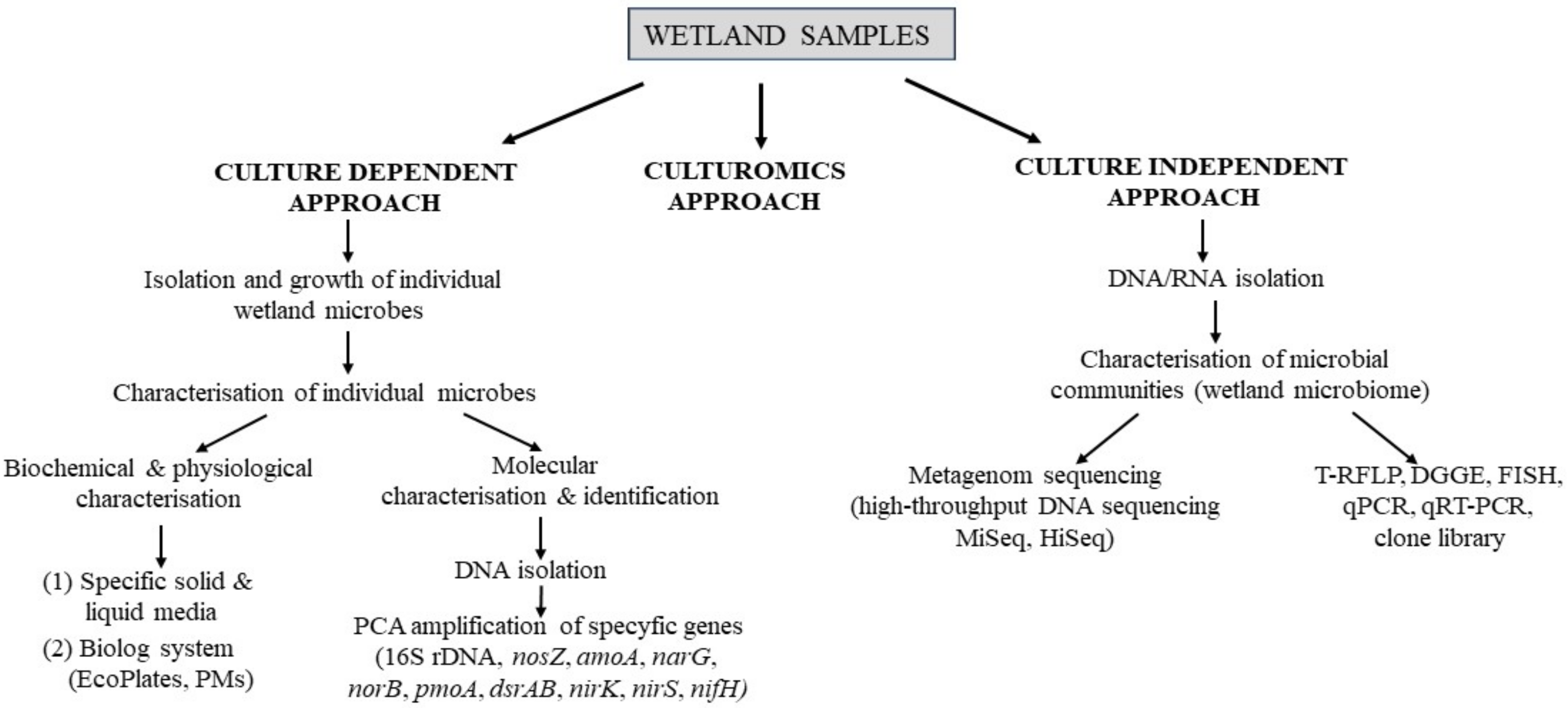
6. Role of Microorganisms in Wetlands
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edgar, T.F.; Humenick, M.J.; Kaiser, W.R.; Charbeneau, R.J. Environmental Effects of in situ Gasification of Texas Lignite. Project Summary. US EPA; Final Report; Texas University: Austin, TX, USA, 1981. [Google Scholar]
- Burton, E.; Friedmann, J.; Upadhye, R. Best Practices in Underground Coal Gasification; Lawrence Livermore National Laboratory: Livermore, CA, USA, 2006. [Google Scholar]
- Liu, S.; Li, J.; Mei, M.; Dong, D. Groundwater pollution from underground coal gasification. J. China Univ. Min. Technol. 2007, 17, 0467–0472. [Google Scholar] [CrossRef]
- Perkins, G. Underground coal gasification Part I: Field demonstrations and process performance. Prog. Energy Combust. Sci. 2018, 67, 158–187. [Google Scholar] [CrossRef]
- Xu, B.; Yang, M.; Xing, B.; Su, F.; Chen, L.; Wang, F.; Zhang, Y.; Yi, G. Removal of pollutants from aqueous solutions by coals and residual cokes obtained from simulated underground coal gasification experiments. Fuel 2021, 292, 120292. [Google Scholar] [CrossRef]
- Grabowski, J.; Korczak, K.; Tokarz, A. Aquatic risk assessment based on the results of research on mine waters as a part of a pilot underground coal gasification process. Process Saf. Environ. Prot. 2021, 148, 548–558. [Google Scholar] [CrossRef]
- Fergusson, L. A study of underground coal gasification (UCG) wastewater and sludge. Int. J. Environ. Res. 2015, 9, 777–784. [Google Scholar]
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Hussain, C.M.; Gaurav, G.K.; Kumar, K.S. Environmental and health impacts of contaminants of emerging concerns: Recent treatment challenges and approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef]
- Reed, S.C. Constructed wetlands for wastewater treatment. BioCycle 1991, 32, 44–49. [Google Scholar]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Couch, G.R. Underground Coal Gasification, IEA Clean Coal Centre; International Energy Agency: London, UK, 2009. [Google Scholar]
- Wiatowski, M.; Stańczyk, K.; Świądrowski, J.; Kapusta, K.; Cybulski, K.; Krause, E.; Grabowski, J.; Rogut, N.; Howaniec, N.; Smolinski, A. Semi-technical underground coal gasification (UCG) using the shaft method in Experimental Mine “Barbara”. Fuel 2012, 99, 170–179. [Google Scholar] [CrossRef]
- Wiatowski, M.; Kapusta, K.; Świądrowski, J.; Cybulski, K.; Ludwik-Pardała, M.; Grabowski, J.; Stańczyk, K. Technological aspects of underground coal gasification in the Experimental ”Barbara” Mine. Fuel 2015, 159, 454–462. [Google Scholar] [CrossRef]
- Mocek, P.; Pieszczek, M.; Świądrowski, J.; Kapusta, K.; Wiatowski, M.; Stańczyk, K. Pilot-scale underground coal gasification (UCG) experiment in an operating mine “Wieczorek” in Poland. Energy 2016, 111, 313–321. [Google Scholar] [CrossRef]
- Smoliński, A.; Stańczyk, K.; Kapusta, K.; Howaniec, N. Chemometric study of the ex situ underground coal gasification wastewater experimental data. Water Air Soil Pollut. 2012, 223, 5745–5758. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kapusta, K.; Stańczyk, K. Pollution of water during underground coal gasification of hard coal and lignite. Fuel 2011, 90, 1927–1934. [Google Scholar] [CrossRef]
- Kapusta, K. Effect of lignite properties on its suitability for the implementation of underground coal gasification (UCG) in selected deposits. Energies 2021, 14, 5816. [Google Scholar] [CrossRef]
- Kapusta, K.; Stańczyk, K.; Wiatowski, M. Comparison of the contaminants in the wastewater produced in the ex situ underground ortho and meta-lignite gasification. Water Air Soil Pollut. 2019, 230, 200. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Research and Innovation; Bălăcescu, S.; Chitescu, I.; Kapusta, K.; Dobrin, M.; Wollenweber, J.; Stanczyk, K.; Burnete, D.; Wassing, B.; Roser, J.; et al. Enhanced Coal Exploitation through Underground coal Gasification in European Lignite Mines (COAL2GAS): Final Report, Publications Office. 2019. Available online: https://data.europa.eu/doi/10.2777/608299 (accessed on 13 June 2022).
- Wiatowski, M.; Kapusta, K.; Nowak, J.; Szyja, M.; Basa, W. An ex situ underground coal gasification experiment with a siderite interlayer: Course of the process, production gas, temperatures and energy efficiency. Int. J. Coal Sci. Technol. 2021, 8, 1447–1460. [Google Scholar] [CrossRef]
- Pankiewicz-Sperka, M.; Kapusta, K.; Basa, W.; Stolecka, K. Characteristics of water contaminants from underground coal gasification (UCG) process—effect of coal properties and gasification pressure. Energies 2021, 14, 6533. [Google Scholar] [CrossRef]
- Sobolewski, A.; Ściążko, M. Best Available Techniques BAT. Guidelines for the coke industry, Publishing House of the Institute for Chemical Processing of Coal, Zabrze. 2006. Available online: http://www.ekoportal.gov.pl/fileadmin/Ekoportal/Pozwolenia_zintegrowane/poradniki_branzowe/15._Najlepsze_Dostepne_Techniki__BAT__wytyczne_dla_branzy_koksowniczej.pdf (accessed on 13 June 2022).
- Osmólski, J. Wastewater treatment at Koksownia PRZYJAŹŃ S.A. in Dąbrowa Górnicza [Oczyszczanie ścieków w Koksowni PRZYJAŹŃ S.A. w Dąbrowie Górniczej]. Forum Eksploatatora 2013, 3, 44–49. [Google Scholar]
- Smoliński, A.; Stańczyk, K.; Kapusta, K.; Howaniec, N. Analysis of the organic contaminants in the condensate produced in the in situ underground coal gasification process. Water Sci. Technol. 2013, 67, 644–650. [Google Scholar] [CrossRef]
- Kapusta, K.; Stańczyk, K.; Wiatowski, W.; Chećko, J. Environmental aspects of a field-scale underground coal gasification trial in a shallow coal seam at the Experimental Mine Barbara in Poland. Fuel 2013, 113, 196–208. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Prakash, J.; Awasthi, G. Microbial bioremediation: An advanced approach for waste management. Int. J. Eng. Technol. Sc. Res. 2016, 3, 50–62. [Google Scholar]
- Jain, P.K.; Bajpai, V. Biotechnology of bioremediation—A review. Int. J. Environ. Sci. 2012, 3, 535–549. [Google Scholar]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D.G. A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, S.J.; Jamal, Y. Hybrid anaerobic-aerobic biological treatment for real textile wastewater. J. Water Process. Eng. 2019, 29, 100804. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 1–18. [Google Scholar] [CrossRef]
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, biostimulation and bioaugmention: A review. Int. J. Environ. Bioremed. Biodegrad. 2015, 3, 28–39. [Google Scholar]
- Mohajeri, L.; Zahed, M.A.; Aziz, H.A.; Isa, M.H. Assessment of bioaugmentation and biostimulation efficiencies for petroleum contaminated sediments. Environ. Energy Econ. Int. Res. 2016, 1, 91–99. [Google Scholar]
- Paniagua-Michel, J.; Fathepure, B.Z. Microbial consortia and biodegradation of petroleum hydrocarbons in marine environments. In Microbial Action on Hydrocarbons; Kumar, V., Kumar, M., Prasad, R., Eds.; Springer: Singapore, 2018; pp. 1–20. [Google Scholar]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Development of an efficient bacterial consortium for the potential remediation of hydrocarbons from contaminated sites. Front. Microbiol. 2016, 7, 1092. [Google Scholar] [CrossRef]
- Dvorak, P.; Nikel, P.I.; Damborsky, J.; de Lorenzo, V. Bioremediation 3.0: Engineering pollutant-removing bacteria in the times of systemic biology. Biotechnol. Adv. 2017, 35, 845–866. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Banerjee, A.; Roy, A.; Dutta, S.; Mondal, S. Bioremediation of hydrocarbon—A review. Intern. J. Ad. Res. 2016, 4, 1303–1313. [Google Scholar] [CrossRef]
- Aissaoui, S.; Ouled-Haddar, H.; Sifour, M.; Beggah, C.; Benhamada, F. Biological removal of the mixed pharmaceuticals: Diclofenac, ibuprofen, and sulfamethoxazole using a bacterial consortium. Iran J. Biotechnol. 2017, 15, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Parte, S.G.; Mohekar, A.D.; Kharat, A.S. Microbial degradation of pesticide: A review. Afr. J. Microbiol. Res. 2017, 11, 992–1012. [Google Scholar]
- Rana, R.S.; Singh, P.; Kandari, V.; Singh, R.; Dobhal, R.; Gupta, S. A review on characterization and bioremediation of pharmaceutical industries’ wastewater: An Indian perspective. Appl. Water Sci. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3- phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Jariyal, M.; Jindal, V.; Mandal, K.; Gupta, V.K.; Singh, B. Bioremediation of organophosphorus pesticide phorate in soil by microbial consortia. Ecotoxicol. Environ. Saf. 2018, 159, 310–316. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; Lopez-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Gupta, R.; Sati, B.; Gupta, A. Treatment and recycling of wastewater from pharmaceutical industry. In Advances in Biological Treatment of Industrial Waste Water and Their Recycling for a Sustainable Future; Singh, R.L., Singh, R.P., Eds.; Springer: Singapore, 2019; pp. 267–302. [Google Scholar]
- Shah, A.; Shah, M. Characterization and bioremediation of wastewater: A review exploring bioremediation as a sustainable technique for pharmaceutical wastewater. Groundw. Sustain. Dev. 2020, 11, 100383. [Google Scholar] [CrossRef]
- Meena, M.; Sonigra, P.; Yadav, G. Biological-based methods for the removal of volatile organic compounds (VOCs) and heavy metals. Environ. Sci. Pollut. Res. 2021, 28, 2485–2508. [Google Scholar] [CrossRef]
- Meena, M.; Sonigra, P.; Yadav, G.; Barupal, T. Removal of emerging contaminants through microbial processes. In Wastewater Treatment Techniques: An Introduction; Shah, M.P., Ed.; Springer: Singapore, 2021; pp. 161–182. [Google Scholar]
- Santos, M.; Melo, V.F.; Serrat, B.M.; Bonfleur, E.; Araújo, E.M.; Cherobim, V.F. Hybrid technologies for remediation of highly Pb contaminated soil: Sewage sludge application and phytoremediation. Int. J. Phytoremediat. 2021, 23, 328–335. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, R.P. Advances in Biological Treatment of Industrial Wastewater and Their Recycling for a Sustainable Future; Springer: Singapore, 2019; p. 225. [Google Scholar]
- Thorslund, J.; Jarsjo, J.; Jaramillo, F.; Jawitz, J.W.; Manzoni, S.; Basu, N.B.; Chalov, S.R.; Cohen, M.J.; Creed, I.F.; Goldenberg, R.; et al. Wetlands as large-scale nature-based solutions: Status and challenges for research, engineering and management. Ecol. Eng. 2017, 108, 489–497. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Manickam, P. Phycoremediation of Industrial Wastewater: Challenges and Prospects. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 99–123. [Google Scholar]
- Radziff, S.B.M.; Ahmad, S.A.; Shaharuddin, N.A.; Merican, F.; Kok, Y.-Y.; Zulkharnain, A.; Gomez-Fuentes, C.; Wong, C.-Y. Potential application of algae in biodegradation of phenol: A review and bibliometric study. Plants 2021, 10, 2677. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Kumar, M.; Vivekanand, V.; Pareek, N. Photoautotrophic Microorganisms and Bioremediation of Industrial Effluents: Current Status and Future Prospects. 3 Biotech. 2017, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Touliabah, H.E.-S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A review of microalgae and cyanobacteria-based biodegradation of organic pollutants. Molecules 2022, 27, 1141. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—A review. Water 2020, 13, 27. [Google Scholar] [CrossRef]
- Hassan, I.; Chowdhury, S.R.; Prihartato, P.K.; Razzak, S.A. Wastewater treatment using constructed wetland: Current trends and future potential. Processes 2021, 9, 1917. [Google Scholar] [CrossRef]
- Nagda, A.; Meena, M.; Shah, M.P. Bioremediation of industrial effluents: A synergistic approach. J. Basic Microbiol. 2021, 3, 1–20. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa, K.M. Contribution of microbes in the renovation of wetlands. In Restoration of Wetlands Ecosystem: A Trajectory towards a Sustainable Environment; Upadhyay, A.K., Ed.; Springer Nature: Singapore, 2020; pp. 101–124. [Google Scholar]
- Vymazal, J.; Zhao, Y.; Mander, Ü. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Olejnik, D.; Wojciechowski, K. The conception of constructed wetland for dyes removal in water solutions. Chemik 2012, 66, 611–614. [Google Scholar]
- Metcalfe, C.D.; Nagabhatla, N.; Fitzgerald, S.K. Multifunctional wetlands: Pollution abatement by natural and constructed wetlands. In Multifunctional Wetlands; Springer: Cham, Switzerland, 2018; pp. 1–14. [Google Scholar]
- Rana, V.; Maiti, S.K. Municipal and Industrial Wastewater Treatment Using Constructed Wetlands. In Phytoremediation. Concepts and Strategies in Plant Sciences; Shmaefsky, B., Ed.; Springer: Cham, Switzerland, 2020; pp. 329–367. [Google Scholar] [CrossRef]
- Masi, F.; Rizzo, A.; Regelsberger, M. The role of constructed wetlands in a new circular economy, resource oriented, and ecosystem services paradigm. J. Environ. Manag. 2018, 216, 275–284. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Phragmites australis–A preliminary study of soil-oxidizing sites and internal gas transport pathways. New Phytol. 1988, 108, 373–382. [Google Scholar] [CrossRef]
- Gajewska, M.; Obarska-Pempkowiak, H.; Wojciechowska, E. Hydrophytic Treatment of Water and Wastewater [Hydrofitowe Oczyszczanie Wód i Scieków]. WN PWN, Warszawa. 2010. Available online: https://cf2-taniaksiazka.statiki.pl/images/files/42F/@9788301164201_33,119986.pdf (accessed on 13 June 2022). (In Polish).
- Maiti, D.; Ansari, I.; Rather, M.A.; Deepa, A. Comprehensive review on wastewater discharged from the coal-related industries—Characteristics and treatment strategies. Water Sci. Technol. 2019, 79, 2023–2035. [Google Scholar] [CrossRef] [PubMed]
- Adyel, T.M.; Oldham, C.E.; Hipsey, M.R. Stormwater nutrient attenuation in a constructed wetland with alternating surface and subsurface flow pathways: Event to annual dynamics. Water Res. 2016, 107, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kuschk, P.; Brix, H.; Vymazal, J.; Dong, R. Development of constructed wetlands in performance in tensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Res. 2014, 57, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Constructed wetlands for treatment of industrial wastewaters: A review. Ecol. Eng. 2014, 73, 724–751. [Google Scholar] [CrossRef]
- Dąbrowski, W.; Karolinczak, B.; Gajewska, M.; Wojciechowska, E. Application of subsurface vertical flow constructed wetlands to reject water treatment in dairy wastewater treatment plant. Environ. Technol. 2017, 38, 175–182. [Google Scholar] [CrossRef]
- Ghermandi, A.; Bixo, D.; Thoeye, C. The role of free water surface constructed wetlands as polishing step in municipal wastewater reclamation and reuse. Sci. Total Environ. 2007, 380, 247–258. [Google Scholar] [CrossRef]
- Vymazal, J. The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: A review of a recent development. Water Res. 2013, 47, 4795–4811. [Google Scholar] [CrossRef]
- Sandoval, L.; Zamora-Castro, S.A.; Vidal-Álvarez, M.; Marín-Muñiz, J.L. Role of wetland plants and use of ornamental flowering plants in constructed wetlands for wastewater treatment: A review. Appl. Sci. 2019, 9, 685. [Google Scholar] [CrossRef]
- Vymazal, J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol. Eng. 2009, 35, 1–17. [Google Scholar] [CrossRef]
- Stefanakis, A.; Akratos, C.S.; Tsihrintzis, V.A. Vertical Flow Constructed Wetlands: Eco-Engineering Systems for Wastewater and Sludge Treatment; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-404612-2. [Google Scholar]
- Nguyen, H.T.; Nguyen, B.Q.; Duong, T.T.; Bui, A.T.; Nguyen, H.T.; Cao, H.T.; Kim, K.W. Pilot-scale removal of arsenic and heavy metals from mining wastewater using adsorption combined with constructed wetland. Minerals 2019, 9, 379. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, S. Performance of organic substrate amended constructed wetland treating acid mine drainage (AMD) of North-Eastern India. J. Hazard. Mater. 2020, 397, 122719. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.; Maiti, S.K. Metal accumulation strategies of emergent plants in natural wetland ecosystems contaminated with coke-oven effluent. Bull. Environ. Contam. Toxicol. 2018, 101, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Yadav, S.; Bharagava, R.N.; Murthy, R.C. Bacterial pretreatment enhances removal of heavy metals during treatment of post-methanated distillery effluent by Typha angustata L. J. Environ. Manag. 2008, 88, 1016–1024. [Google Scholar] [CrossRef]
- Jardinier, N.; Blake, G.; Mauchamp, A.; Merlin, G. Design and performance of experimental constructed wetlands treating coke plant effluents. Water Sci. Technol. 2001, 44, 485–491. [Google Scholar] [CrossRef]
- Sochacki, A.; Surmacz-Gorska, J.; Faure, O.; Guy, B. Polishing of synthetic electroplating wastewater in microcosm upflow constructed wetlands: Effect of operating conditions. Chem. Eng. J. 2014, 237, 250–258. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Seeger, E.; Dorer, C.; Sinke, A.; Thullner, M. Performance of pilot-scale horizontal subsurface flow constructed wetlands treating groundwater contaminated with phenols and petroleum derivatives. Ecol. Eng. 2016, 95, 514–526. [Google Scholar] [CrossRef]
- Tee, H.C.; Seng, C.E.; Noor, A.M.; Lim, P.E. Performance comparison of constructed wetlands with gravel-and rice husk-based media for phenol and nitrogen removal. Sci. Total Environ. 2009, 407, 3563–3571. [Google Scholar] [CrossRef]
- Benny, C.; Chakraborty, S. Continuous removals of phenol, organics, thiocyanate and nitrogen in horizontal subsurface flow constructed wetland. J. Water Process Eng. 2020, 33, 101099. [Google Scholar] [CrossRef]
- Bedessem, M.E.; Ferro, A.M.; Hiegel, T. Pilot-scale constructed wetlands for petroleum-contaminated groundwater. Water Environ. Res. 2007, 79, 581–586. [Google Scholar] [CrossRef]
- Zhang, Z.; Rengel, Z.; Meney, K. Polynuclear aromatic hydrocarbons (PAHs) differentially influence growth of various emergent wetland species. J. Hazard. Mater. 2010, 182, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, J.; Xie, H.; Hu, Z.; Liang, S.; Ngo, H.H.; Zhao, C. Intensive removal of PAHs in constructed wetland filled with copper biochar. Ecotoxicol. Environ. Saf. 2020, 205, 111028. [Google Scholar] [CrossRef] [PubMed]
- Gessner, T.P.; Kadlec, R.H.; Reaves, R.P. Wetland remediation of cyanide and hydrocarbons. Ecol. Eng. 2005, 25, 457–469. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kastner, A.; Bederski, O.; Muller, R.A.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Ad. 2003, 22, 93–117. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Zhang, J.; Ngo, H.H.; Guo, W.; Liang, S.; Fan, J.; Lu, S.; Wu, H. Optimizations on supply and distribution of dissolved oxygen in constructed wetlands: A review. Bioresour. Technol. 2016, 214, 797–805. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Xie, H.; Yang, Z. Constructed wetlands: A review on the role of radial oxygen loss in the rizosphere by macrophytes. Water 2018, 10, 678. [Google Scholar] [CrossRef]
- Alam, M.S.; Jia, Z. Inhibition of methane oxidation by nitrogenous fertilizers in a paddy soil. Front. Microbiol. 2012, 3, 246. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Dedysh, S.N. Microbiology of wetlands. Front. Microbiol. 2012, 4, 79. [Google Scholar]
- Irvine, I.C.; Vivanco, L.; Bentley, P.N.; Martiny, J.B.H. The effect of nitrogen enrichment on c (1)-cycling microorganisms and methane flux in salt marsh sediments. Front. Microbiol. 2012, 3, 90. [Google Scholar] [CrossRef]
- Kolb, S.; Horn, M.A. Microbial CH(4) and N(2)O consumption in acidic wetlands. Front. Microbiol. 2012, 3, 78. [Google Scholar] [CrossRef]
- Lamers, L.P.; Van Diggelen, J.M.; Op Den Camp, H.J.; Visser, E.J.; Lucassen, E.C.; Vile, M.A.; Jetten, M.S.; Smolders, A.J.; Roelofs, J.G. Microbial transformations of nitrogen, sulfur, and iron dictate vegetation composition in wetlands: A review. Front. Microbiol. 2012, 3, 156. [Google Scholar] [CrossRef] [PubMed]
- Lovell, C.R.; Davis, D.A. Specificity of salt marsh diazotrophs for vegetation zone sand plant hosts: Results from a North American marsh. Front. Microbiol. 2012, 3, 84. [Google Scholar] [CrossRef] [PubMed]
- Pester, M.; Knorr, K.-H.; Friedrich, M.W.; Wagner, M.; Loy, A. Sulfate-reducing microorganisms in wetlands—Fameless actors in carbon cycling and climate change. Front. Microbiol. 2012, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.D.; Smemo, K.A.; McLaughlin, J.W.; Basilico, N. Peatland microbial communities and decomposition processes in the James Bay lowlands, Canada. Front. Microbiol. 2012, 3, 70. [Google Scholar] [CrossRef]
- Putkinen, A.; Larmola, T.; Tuomivirta, T.; Siljanen, H.M.P.; Bodrossy, L.; Tuittila, E.S. Water dispersal of methanotrophic bacteria maintains functional methane oxidation in sphagnum mosses. Front. Microbiol. 2012, 3, 15. [Google Scholar] [CrossRef]
- Chandra, R. Advances in Biodegradation and Bioremediation of Industrial Waste, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Umar, A.; Zafar, A.; Wali, H.; Siddique, M.P.; Qazi, M.A.; Naeem, A.H.; Malik, Z.A.; Safia, A. Low-cost production and application of lipopeptide for bioremediation and plant growth by Bacillus subtilis SNW3. AMB Express 2021, 11, 165. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome significance of plant beneficial, plant pathogenic and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Backer, R.J.; Rokem, S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sc. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.; del Carmen Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Arya, P.; Ravindra, I. Metagenomics based approach to reveal the secrets of unculturable microbial diversity from aquatic environment. In Recent Advancements in Microbial Diversity; De Mandal, S., Bhatt, P., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2020; pp. 537–558. [Google Scholar] [CrossRef]
- Mellado, M.; Vera, J. Microorganisms that participate in biochemical cycles in wetlands. Can. J. Microbiol. 2021, 67, 771–788. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Sharma, K. Microbial biodiversity and bioremediation assessment through omics approaches. Front. Environ. Chem. 2020, 1, 570326. [Google Scholar] [CrossRef]
- De Mandal, S.; Laskar, F.; Panda, A.K.; Mishra, R. Microbial diversity and functional potentail in wetland ecosystems. In Recent Advancements in Microbial Diversity, 1st ed.; De Mandal, S., Bhatt, P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 289–314. [Google Scholar]
- Salomo, A.; Munch, C.; Roske, I. Evaluation of the metabolic diversity of microbial communities in four different filter layers of a constructed wetland with vertical flow by BiologTM analysis. Water Res. 2009, 43, 4569–4578. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, P.; Shirin, S. Impact of microbial activity on the performance of planted and unplanted wetland at laboratory scale. Water Pract. Technol. 2021, 16, 472–489. [Google Scholar] [CrossRef]
- Xu, Q.L.; Cai, X.J.; Fu, L.; Hu, Y. Space distribution of bacterial communities and substrate enzymes in vertical flow constructed wetlands. App. Ecol. Environ. Res. 2020, 18, 959–971. [Google Scholar] [CrossRef]
- Xiang, W.; Xiao, X.; Xue, J. Purification effect and microorganisms diversity in an Acorus calamus constructed wetland on petroleum-containing wastewater. Environ. Poll. Bioavail. 2020, 32, 19–25. [Google Scholar] [CrossRef]
- Ma, X.; Song, X.; Li, X.; Fu, S.; Li, M.; Liu, Y. Characterization of microbial communities in pilot-scale constructed wetlands with Salicornia for treatment of marine aquaculture effluents. Archaea 2018, 2018, 7819840. [Google Scholar] [CrossRef]
- Lv, X.; Yu, J.; Fu, Y.; Ma, B.; Qu, F.; Ning, K.; Wu, H. A Meta-Analysis of the Bacterial and Archaeal Diversity Observed in Wetland Soils. Sci. World J. 2014, 2014, 437684. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, P.; Dai, W.; Zheng, X.; He, S.; Zhao, M. Pathways regulating nitrogen removal in constructed ditch wetlands: Effects of different inflow ratios and artificial aeration. Environ. Sci. Pollut. Res. Int. 2020, 27, 42571–42581. [Google Scholar] [CrossRef]
- Mai, W.; Chen, J.; Liu, H.; Liang, J.; Tang, J.; Wei, Y. Advances in studies on microbiota involved in nitrogen removal processes and their applications in wastewater treatment. Front. Microbiol. 2021, 12, 746293. [Google Scholar] [CrossRef]
- Li, J.; Tabassum, S. Simultaneous removal of ammonia nitrogen and sulfide by coupled anammox and sulfur autotrophic denitrification process from industrial wastewater. Clean. Eng. Technol. 2022, 8, 100469. [Google Scholar] [CrossRef]
- Billah, M.M.; Bhuiyan, M.K.A.; Islam, M.A.; Das, J.; Hoque, A.T.M. Salt marsh restoration: An overview of techniques and success indicators. Environ. Sci. Pollut. Res. 2022, 29, 15347–15363. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Bai, R.; Zhang, Y.; Zhao, B.; Xiao, Y. Application of metagenomics to biological wastewater treatment. Sci. Total Environ. 2022, 807, 150737. [Google Scholar] [CrossRef] [PubMed]
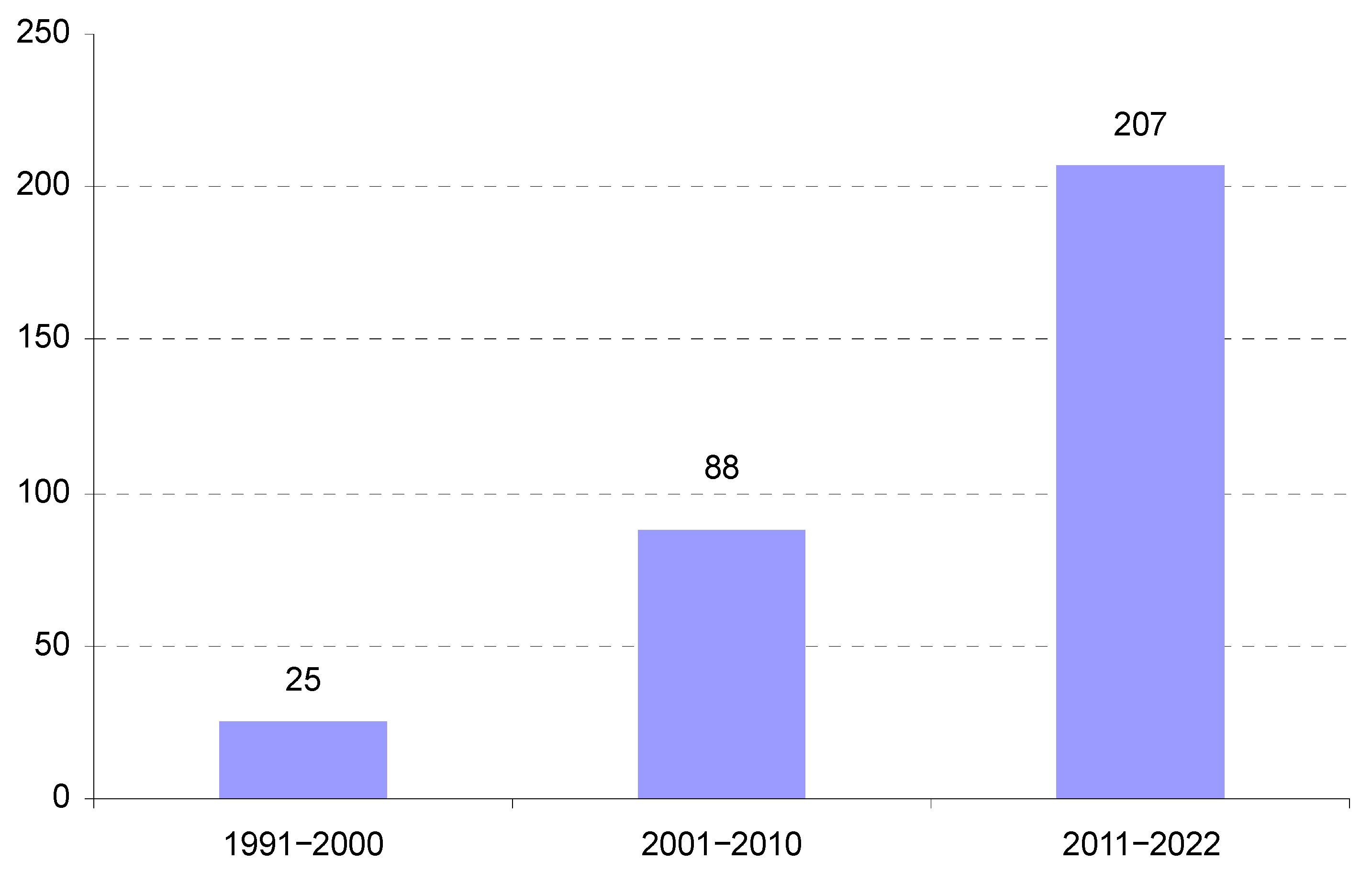
| Criterium | Phrase | Number of Publications Identified |
|---|---|---|
| 1 Title, abstract, keywords | “Constructed wetlands” and “Industrial wastewater” | 603 |
| 2 Document type and language | Article, Review, English | 462 |
| 3 Subject area | Environmental Science, Agriculture and Biological Sciences, Chemistry, Engineering, Chemical Engineering, Multidisciplinary, Immunology and Microbiology | 321 |
| Country | UCG Site | Startup Year | Coal Type/Seam Depth and Average Thickness [m] | Gasifying Agent |
|---|---|---|---|---|
| Australia | Chinchilla G1 | 2000 | subbituminous/132/10 | air |
| Chinchilla G3 | 2007 | subbituminous/132/10 | air | |
| Chinchilla G4 | 2009 | subbituminous/132/10 | air | |
| Chinchilla G5 | 2011 | subbituminous/132/5.5 | air, oxygen/steam | |
| Bloodwood Creek P1 | 2009 | subbituminous/200/9 | air, oxygen/steam | |
| Bloodwood Creek P2 | 2011 | subbituminous/200/9 | air | |
| Canada | Swan Hills | 2009–2011 | high volatile bituminous/1400/4.5 | oxygen/steam |
| China | Xinwen | 2000 | high volatile coal/100/1.8 | air/steam |
| Feichang | 2001 | bituminous/90/1.5 | air | |
| Xiyang | 2001 | anthracite/190/6 | air/steam |
| Origin of Coal | Type of Coal | Type of Experiment/ Installation Pressure * | Gasifying Agent | Experiment Duration [h] | Coal Gasified [kg] | Wastewater Produced [kg] | Wastewater Outflow [kg/kg Gasified Coal] | References |
|---|---|---|---|---|---|---|---|---|
| Experimental mine “Barbara” (I) (Poland) | subbituminous | in situ | oxygen/air | 355 | 21,980 | 14,810 | 0.67 | [12] |
| Experimental mine “Barbara” (II) (Poland) | subbituminous | in situ | oxygen/steam | 142 | 5364 | 2960 | 0.55 | [13] |
| Hard coal mine “Wieczorek” (Poland) | subbituminous | in situ | air/oxygen/CO2 | 60 days | 230,500 | d.n.a. | d.n.a. | [14] |
| Hard coal mine “Bobrek” (Poland) | bituminous | ex situ | oxygen | 48 | 176.9 | 46.0 | 0.26 | [15] |
| Hard coal mine “Ziemowit” (Poland) | subbituminous | ex situ | oxygen | 48 | 164.2 | 130 | 0.79 | [15] |
| Brown coal mine “Bełchatów” (Poland) | lignite | ex situ | oxygen | 50 | 970.0 | 480 | 0.49 | [16] |
| Hard coal mine “Bielszowice” (Poland) | bituminous | ex situ | oxygen/air/steam | 73 | 145.0 | 79.6 | 0.55 | [16] |
| Premogovnik Velenje (Slovenia) | meta-lignite | ex situ | oxygen | 120 | 730.0 | d.n.a. | d.n.a. | [17] |
| Premogovnik Velenje (Slovenia) | meta-lignite | ex situ/3.5 MPa | oxygen | 72 | 591.0 | d.n.a. | d.n.a. | [18,19] |
| Coal mine Oltenia (Romania) | ortho-lignite | ex situ | oxygen/steam | 96 | 790.0 | d.n.a. | d.n.a. | [17] |
| Coal mine Oltenia (Romania) | ortho-lignite | ex situ/1 MPa | oxygen | 72 | 585.0 | d.n.a. | d.n.a. | [18,19] |
| Hard coal mine “Piast” (Poland) | subbituminous | ex situ | oxygen | 72 | 2300 | 521 | 0.23 | [20] |
| Coal mine “Six Feet” (UK) | semi-anthracite | ex situ/2 MPa | oxygen/steam | 96 | 436.1 | 46.5 | 0.11 | [21] |
| Coal mine “Six Feet” (UK) | semi-anthracite | ex situ/4 MPa | oxygen/steam | 96 | 455.5 | 38.6 | 0.08 | [21] |
| Hard coal mine “Wesoła” (Poland) | bituminous | ex situ/2 MPa | oxygen/steam | 96 | 504 | 67.3 | 0.13 | [21] |
| Hard coal mine “Wesoła” (Poland) | bituminous | ex situ/4 MPa | oxygen/steam | 96 | 530.2 | 55.2 | 0.10 | [21] |
| Parameter/ Compound * | Unit | Coal Type and Origin (Installation Pressure) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SB Barbara I (atm) [12] | SB Wieczorek (atm) [14] | B Bielszowice (atm) [16] | L Bełchatów (atm) [16] | SA Six Feet (2 MPa) [21] | SA Six Feet (4 MPa) [21] | B Wesoła (2 MPa) [21] | B Wesoła (4 MPa) [21] | ML Velenje (atm) [17] | ML Velenje (3.5 MPa) [18,19] | OL Oltenia (atm) [17] | OL Oltenia (1 MPa) [18,19] | ||
| pH | - | 6.3 | 7.3 | 7.8 | 5.4 | 6.4 | 5.2 | 5.3 | 4.9 | 7.3 | 6.0 | 7.7 | 5.1 |
| Conductivity | µS/cm | 14,425 | 57,400 | 19,200 | 8638.0 | 1228.4 | 253.38 | 942.00 | 1006.7 | 2478.0 | 1770.0 | 3155.0 | 5253.0 |
| CODCr | mg/L O2 | 4308 | 5330 | n.d. | n.d. | 151.6 | 48.63 | 322.7 | 185.9 | 5060 | 691.0 | 2010 | 4177 |
| BOD5 | mg/L O2 | 2228 | 2840 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 4373 | 300.0 | 1048 | 2105 |
| Ammonia nitrogen | mg/L N | 1950 | 7800 | 3300 | 1225 | 160.1 | 11.68 | 96.41 | 95.74 | 280.0 | 189.0 | 463.0 | 778.0 |
| Chlorides | mg/L | 1660 | 18,000 | n.d. | n.d. | 11.15 | 11.68 | 29.18 | 45.94 | n.d. | n.d. | n.d. | n.d. |
| Cyanides | mg/L | 1.26 | 3.90 | 5.69 | <0.5 | 1.11 | 1.43 | 1.70 | 0.87 | 1.31 | 0.70 | 1.01 | 3.00 |
| Sulphates | mg/L | 3220 | 980.0 | 651.0 | 1014 | 33.51 | 47.66 | 42.86 | 52.97 | 45.70 | 105.0 | 44.30 | 204.0 |
| Mn | mg/L | 4.91 | n.d. | n.d. | n.d. | 0.017 | 0.021 | 0.018 | 0.012 | 0.010 | 0.13 | 0.050 | 0.34 |
| Fe | mg/L | 650 | n.d. | 10.6 | 325 | 0.823 | 0.284 | 0.131 | 0.245 | 0.050 | 2.49 | 0.020 | 21.98 |
| Sb | mg/L | <0.05 | n.d. | n.d. | n.d. | 0.036 | 0.12 | 0.064 | 0.013 | 0.030 | 0.030 | 0.030 | 0.070 |
| As | mg/L | 2.93 | n.d. | n.d. | n.d. | 0.036 | <0.02 | <0.01 | <0.01 | 0.040 | <0.005 | 0.21 | 0.16 |
| B | mg/L | 6.5 | n.d. | 3.1 | 0.18 | 0.072 | 0.056 | 0.13 | 0.25 | 0.21 | 0.58 | 0.040 | 0.48 |
| Cr | mg/L | 0.51 | 7.3 | 0.022 | <0.005 | 0.013 | 0.012 | 0.010 | 0.006 | <0.005 | 0.17 | <0.005 | 1.8 |
| Zn | mg/L | 3.53 | 0.570 | 1.15 | 10.8 | 0.021 | 0.499 | 0.320 | 0.200 | 0.060 | 0.180 | 0.080 | 0.300 |
| Al | mg/L | 17.7 | n.d. | n.d. | n.d. | 0.031 | 0.046 | 0.029 | 0.023 | 0.060 | 0.220 | <0.01 | 1.41 |
| Cd | mg/L | <0.02 | n.d. | n.d. | <0.002 | <0.0005 | 0.001 | <0.0005 | <0.0005 | <0.001 | 0.001 | <0.001 | 0.002 |
| Co | mg/L | 0.031 | n.d. | n.d. | n.d. | 0.004 | 0.003 | <0.003 | <0.003 | <0.005 | 0.010 | <0.005 | 0.043 |
| Cu | mg/L | <0.01 | 0.062 | 0.065 | <0.01 | 0.005 | 0.010 | 0.009 | 0.002 | 0.010 | <0.005 | 0.010 | <0.005 |
| Mo | mg/L | 0.133 | n.d. | 0.140 | <0.01 | 0.005 | <0.005 | 0.026 | <0.005 | <0.01 | 0.020 | <0.01 | 0.011 |
| Ni | mg/L | 0.243 | 1.16 | 0.029 | <0.01 | 0.098 | 0.312 | 0.051 | 0.027 | 0.050 | 1.16 | 0.010 | 2.83 |
| Pb | mg/L | 0.044 | 0.035 | <0.02 | <0.005 | <0.005 | 0.064 | 0.046 | 0.060 | 0.010 | 0.040 | <0.01 | 0.28 |
| Hg | mg/L | <0.005 | n.d. | n.d. | n.d. | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | 0.003 |
| Se | mg/L | 0.14 | n.d. | n.d. | n.d. | 0.016 | 0.017 | 0.036 | 0.027 | 0.040 | <0.01 | 0.030 | 0.066 |
| Ti | mg/L | 0.52 | n.d. | 0.050 | <0.005 | <0.0005 | 0.001 | 0.001 | <0.0005 | <0.003 | 0.004 | <0.003 | 0.055 |
| Total phenol | mg/L | 484 | 820 ** | 3090 | 247 | 29.7 | 2.14 | 49.5 | 29.2 | 733 | 17.0 | 246 | 201 |
| TOC | mg/L | 616.0 | 1500 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2400 | 167.0 | 882.5 | 1250 |
| Total BTEX incl.: | µg/L | 55.80 | 414.0 | 790.0 | 106.0 | 5483 | 1497 | 2514 | 1354 | 1994 | 804.0 | 1784 | 1562 |
| Benzene | µg/L | 51.10 | n.d. | 504.0 | 96.00 | 4156 | 1341 | 2197 | 1059 | 1189 | 512.3 | 1190 | 1072 |
| Toluene | µg/L | 3.730 | n.d. | 140.0 | 7.000 | n.d. | n.d. | n.d. | n.d. | 356.3 | 175.2 | 277.0 | 236.3 |
| Ethylbenzene | µg/L | 0.700 | n.d. | 22.00 | 0.500 | n.d. | n.d. | n.d. | n.d. | 238.7 | 24.40 | 263.5 | 209.8 |
| Xylene | µg/L | 1.820 | n.d. | 124.0 | 2.500 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total PAH | µg/L | 1912 | 399.0 | 1887 | 1066 | 1658 | 362.0 | 1090 | 407.2 | n.d. | n.d. | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgulat, J.; Ponikiewska, K.; Jałowiecki, Ł.; Strugała-Wilczek, A.; Płaza, G. Are Wetlands as an Integrated Bioremediation System Applicable for the Treatment of Wastewater from Underground Coal Gasification Processes? Energies 2022, 15, 4419. https://doi.org/10.3390/en15124419
Borgulat J, Ponikiewska K, Jałowiecki Ł, Strugała-Wilczek A, Płaza G. Are Wetlands as an Integrated Bioremediation System Applicable for the Treatment of Wastewater from Underground Coal Gasification Processes? Energies. 2022; 15(12):4419. https://doi.org/10.3390/en15124419
Chicago/Turabian StyleBorgulat, Jacek, Katarzyna Ponikiewska, Łukasz Jałowiecki, Aleksandra Strugała-Wilczek, and Grażyna Płaza. 2022. "Are Wetlands as an Integrated Bioremediation System Applicable for the Treatment of Wastewater from Underground Coal Gasification Processes?" Energies 15, no. 12: 4419. https://doi.org/10.3390/en15124419
APA StyleBorgulat, J., Ponikiewska, K., Jałowiecki, Ł., Strugała-Wilczek, A., & Płaza, G. (2022). Are Wetlands as an Integrated Bioremediation System Applicable for the Treatment of Wastewater from Underground Coal Gasification Processes? Energies, 15(12), 4419. https://doi.org/10.3390/en15124419






