1. Introduction
Faced with growing energy shortages, environmental pollution, ecological deterioration, the development of clean and efficient new energy is the only way to solve the above problems. Hydrogen has become the most promising secondary energy of the 21st century for its excellent advantages such as broad source, no polluting product, high combustion efficiency, and reproducible ability. It has important strategic significance for solving the problems of energy shortage and environmental pollution puzzling all over the world [
1,
2,
3,
4]. With the deepening of research, hydrogen energy has been used in aviation and spaceflight, clean utilization of fossil energy, fuel cell vehicles, national defense and the military industry, mass energy storage, and other fields [
5]. However, a hydrogen energy system is a large and complex engineering system, including production, storage, and utilization of hydrogen. In order to promote the practicality of the hydrogen energy economy, science researchers and energy policy experts from all over the world have carried out a lot of systematic research. The development of hydrogen energy is gaining more and more attention from the international community [
6].
In the development of hydrogen energy, the density of hydrogen is low at normal temperatures and pressures. How to realize safe and efficient storage and transportation of hydrogen is one of the key problems for the large-scale application of hydrogen [
7]. According to different storage methods, it can be divided into chemical hydrogen storage and physical hydrogen storage [
8]. Chemical hydrogen storage is realized by hydrogen compound, which is generated through chemical reaction or chemical changes [
9,
10], including metal hydride, coordination hydride, and organic liquid hydride. Physical hydrogen storage is a traditional way to store hydrogen, including liquid and high-pressure hydrogen storage; liquid hydrogen storage is to store the liquefied low-temperature hydrogen (about 20 K) in the tank, which often adopts a high-vacuum adiabatic low-pressure tank as strict heat insulation is required in order to minimize the vaporization of liquid hydrogen storage. High-pressure hydrogen storage is to directly store the high-pressure gaseous hydrogen in a tank, which usually requires the pressure of tens of MPa in order to get a high volume density for hydrogen storage [
11].
By comparing the above hydrogen storage methods, chemical hydrogen storage is far from meeting the requirements of a vehicle system because of material costs, control of actual reaction temperatures, the pressure of absorbing and releasing hydrogen, and the weight of materials. Liquid hydrogen storage has the disadvantages of high energy consumption and complex equipment because it requires more than 30% consumption of liquid hydrogen for hydrogen storage energy and the adiabatic layer further increases the weight of the tank. Thus, it is not a good prospect for application, though it has high-quality density. High-pressure storage is a common method of vehicle-mounted hydrogen storage at present because of its fast speed of absorbing and releasing hydrogen, simple equipment structure, and low energy consumption of compression in comparison to other hydrogen storage methods [
12]. There are methods for storing the amount of hydrogen fuel in different forms, as shown in
Table 1 [
13,
14,
15,
16,
17].
The key technology for compressed hydrogen for widespread use is the tank. The requirements for material used in the tank are safety, reliability, and cost-effectiveness [
2]. According to the division in ANSI HGV 2 Tanks [
18], high-pressure hydrogen storage tanks are divided into four basic types: type I, all-metal tank; type II, hoop-wrapped tank with the inner container; type III, materials (liner, composite shell); type IV, carbon fiber-reinforced polymer. Type I and type II are heavier, which make it difficult to get high hydrogen storage density, and are suitable for hydrogen-filling stations, but not for a vehicle-mounted tank. Type III and type IV adopt light and high-intensity fibers, which not only reduces the mass of the hydrogen tank, but also bears higher pressure; thus, it is widely used as vehicle-mounted hydrogen storage tank.
Table 2 shows the four different types of hydrogen storage tanks (Type I to Type IV) [
13,
19,
20,
21].
During the cyclic experiment of hydrogen gas, the change of temperature is significant as the temperature of hydrogen rises sharply during filling because of compression of hydrogen, conversion of kinetic energy into internal energy, and negative Joule–Thomson effect [
22]. In the high-pressure filling process, the internal pressure of the tank increases, and thermal stress may occur due to the temperature rise of hydrogen. Therefore, it is of practical significance to study the temperature rise and the amount of charging of hydrogen for hydrogen safety.
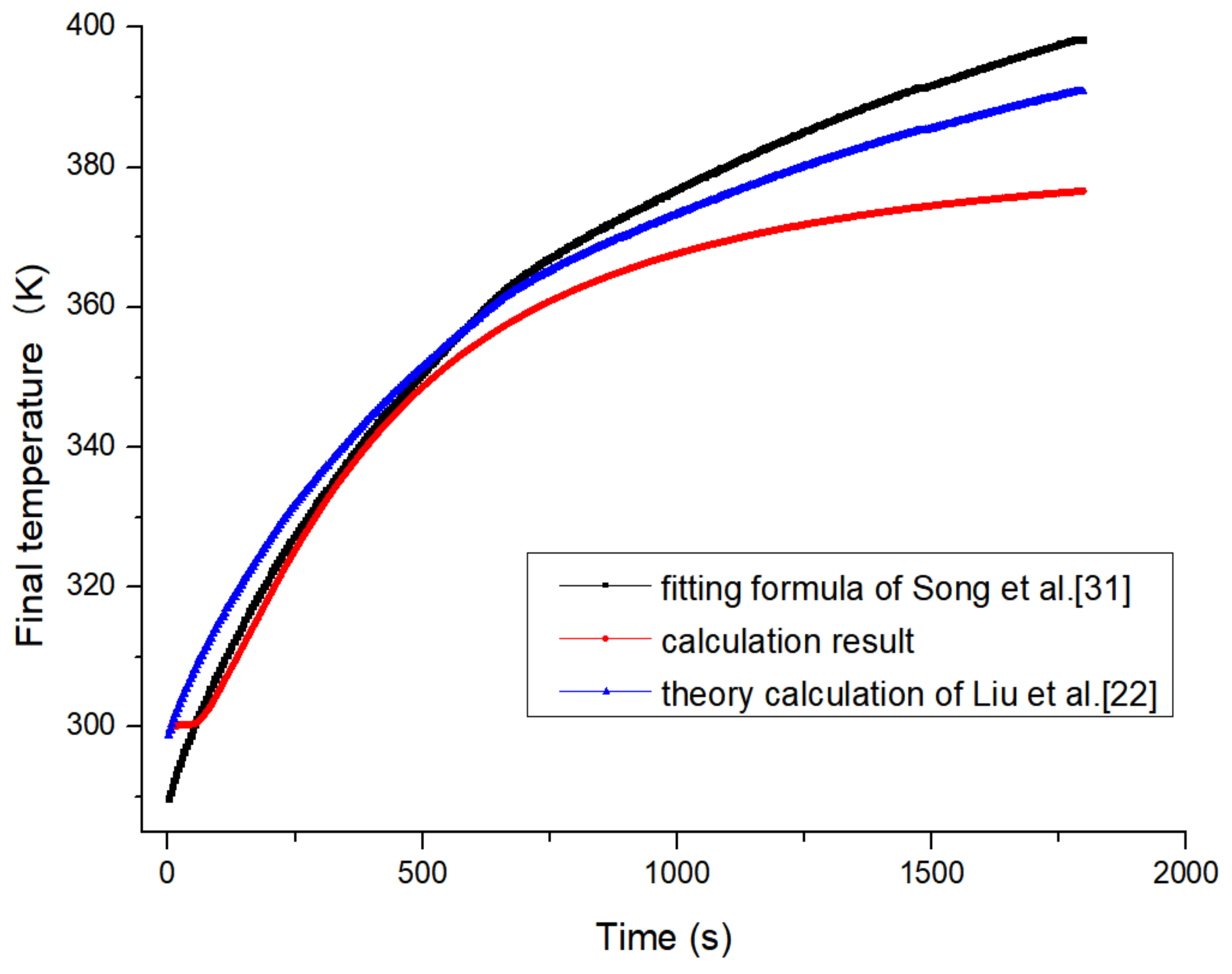
In the research on temperature rise during fast filling, many domestic and overseas research institutions and scholars have conducted research from the perspectives of theoretical and simulation. Previous research studies have paved the way for advancements in the filling of hydrogen gas. Theoretical study is based on a simplified model, which combines mass and energy law, real gas equations and receives the expression of temperature and pressure after filling. At the thermodynamic model of the reference [
22], it takes the internal energy and kinetic energy of hydrogen in the tank into account, and neglects the heat transferred by internal hydrogen to the environment as the filling time is short. This assumes that the hydrogen’s initial temperature is the same as the temperature of hydrogen within the tank before the filling process. The result is that temperature rise can be calculated accurately by using relevant parameters in the process of theoretical analysis.
Hosseini et al. [
23] studied the effect of the initial conditions on temperature rise based on energy analysis, and found that higher initial pressure resulted in smaller final temperature rise. Yang et al. [
24] conducted model analysis on the fast-filling of hydrogen by taking advantage of ideal and real gas in adiabatic and isothermal conditions. The result shows that the filling time under adiabatic and isothermal conditions is the lower and upper limit for a given final target pressure. The reference [
25] established a thermodynamic model based on the energy equation, which can analyze and obtain the filling’s mass and final temperature after filling. Monde et al. [
26] developed a theoretical model to study the effects of the heat transfer coefficient on the final temperature. The results showed that the use of an appropriate constant heat transfer coefficient calculated final temperature is feasible. Yong Zhi et al. [
27] developed a two-dimensional axisymmetric model, and the effects of the boost mode and supercharged the hydrogen tank temperature rise rate during the rapid inflation by the model. The expression of temperature change can be clearly understood by theoretical study, which concludes that the temperature rise of hydrogen mainly depends on filling parameters. Besides, the geometric parameters and types of tanks may affect the temperature and mass after filling. The various theoretical analyses of temperature rise are shown in
Table 3.
In order to study the relationship between temperature and parameters, some experiments have studied temperature rise in the compression tank. Hirotain et al. [
32] have been carried out fast-filling tests of 34-L and 74-L type-III tanks with a working pressure of 35 MPa, 65-L type-IV tanks, and 41-L type-III tanks, and 31-L type-IV tanks with a working pressure of 70 MPa. The results showed that pressure mode (constant speed, first fast and then slow, first slow and then fast, stepped pressure increase) has no obvious effect on the temperature rise of type III tanks, but had a significant effect on type IV tanks. Modnde et al. [
33,
34,
35] studied the change of convective heat transfer coefficient through the fast-filling test, and conducted a three-stage fast-filling test, indicating that three-stage filling was one of the effective ways to address excessive temperature rise. Dicken et al. [
36,
37] conducted fast-filling experiments on 35 MPa, 74 L type III gas tanks with different initial pressures and different pressure rise rates. Since 63 thermocouples were installed in the test tank, detailed distribution of the temperature in the tank was obtained. Zheng Jinyang et al. [
38] developed a 70 MPa fast-filling temperature rise test device, conducted the first 70 MPa fast charging experiment in China, and analyzed the influence of the gas tank structure on the temperature rise. Since the actual fast-filling process will be affected by the specific hydrogenation system settings of the hydrogen refueling station and the manufacturing process attributes of the hydrogen storage bottle itself, the existing fast-filling temperature of the analysis results is weak, so the technical standardization of detailed and universal quantitative analysis is being further promoted.
To sum up, since the actual fast-filling process will be affected by the specific hydrogenation system settings of the hydrogen refueling station and the manufacturing process attributes of the hydrogen storage bottle itself, the existing fast-filling temperature of the analysis results is weak, so the technical standardization of detailed and universal quantitative analysis is being further promoted. At present, there are relatively few studies on 70–100 MPa high-pressure filling experiments. The setting of fast-filling conditions such as the filling control scheme of the fast-filling experiment of the hydrogen tank lacks sufficient experiments, numerical and theoretical analysis as demonstration; for the temperature rise mechanism of the fast-filling process, energy consumption optimization and complex and changeable filling conditions. The effects of temperature rise and other aspects are still in the accumulation stage and have not been fully understood; in addition, because the hydrogen storage tank and its hydrogen filling system are a complex system, and because the standardization process of the system is still in the research stage, only some qualitative analysis can be given, but a quantitative analysis with wide applicability cannot be provided at this time.
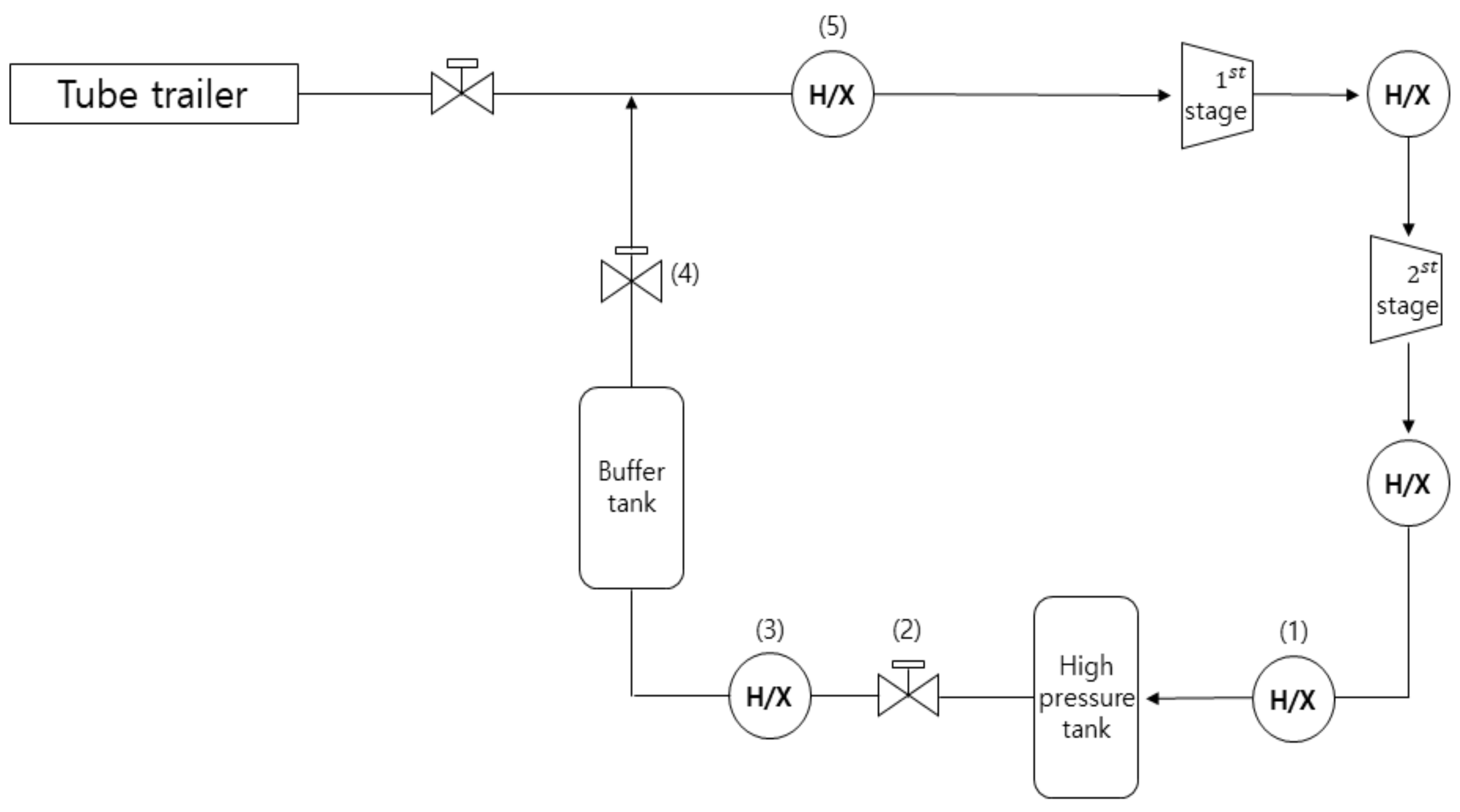
In this paper, we have two main extensions based on previous work reviews. First, we described an experiment involving hydrogen gas in a large storage tank and obtained the data of the pressure sensor during the fast-filling process. A theoretical model for the final temperature and mass of hydrogen gas during filling processes was developed, and analysis of the hydrogen temperature change, the amount of filling of hydrogen, the pressure reducing valve, and the required capacity of the heat exchanger for cooling the high-pressure tank through theoretical and numerical methods. Second, the real gas equation of state and thermodynamic properties were calculated by the National Institute of Standards and Technology (NIST) database: REFPROP 9.5. Final hydrogen mass and final temperature from the theoretical adiabatic condition are estimated based on the mass and energy balance method. Theoretical formulae fit the experimental/simulated data very well. The results of the study will be very useful for future research and development on hydrogen energy and high-pressure hydrogen stations.
5. Conclusions
In current hydrogen filling systems, hydrogen is compressed and stored at high pressure in a tank through a compressor. In this process, the internal pressure of the tank increases, and thermal stress may occur due to the temperature rise of the hydrogen. In order to secure the reliability of the high-pressure tank, it is important to study and predict the temperature rise of hydrogen gas. In this paper, the change in the hydrogen’s temperature in the tank according to the pressure rise during the process of filling the high-pressure tank in an 82 MPa hydrogen filling system. As such, the final temperature, the amount of filling of hydrogen gas, the pressure-reducing valve, and the change and the performance of the heat exchanger for cooling high-temperature hydrogen were analyzed by theoretical and numerical methods.
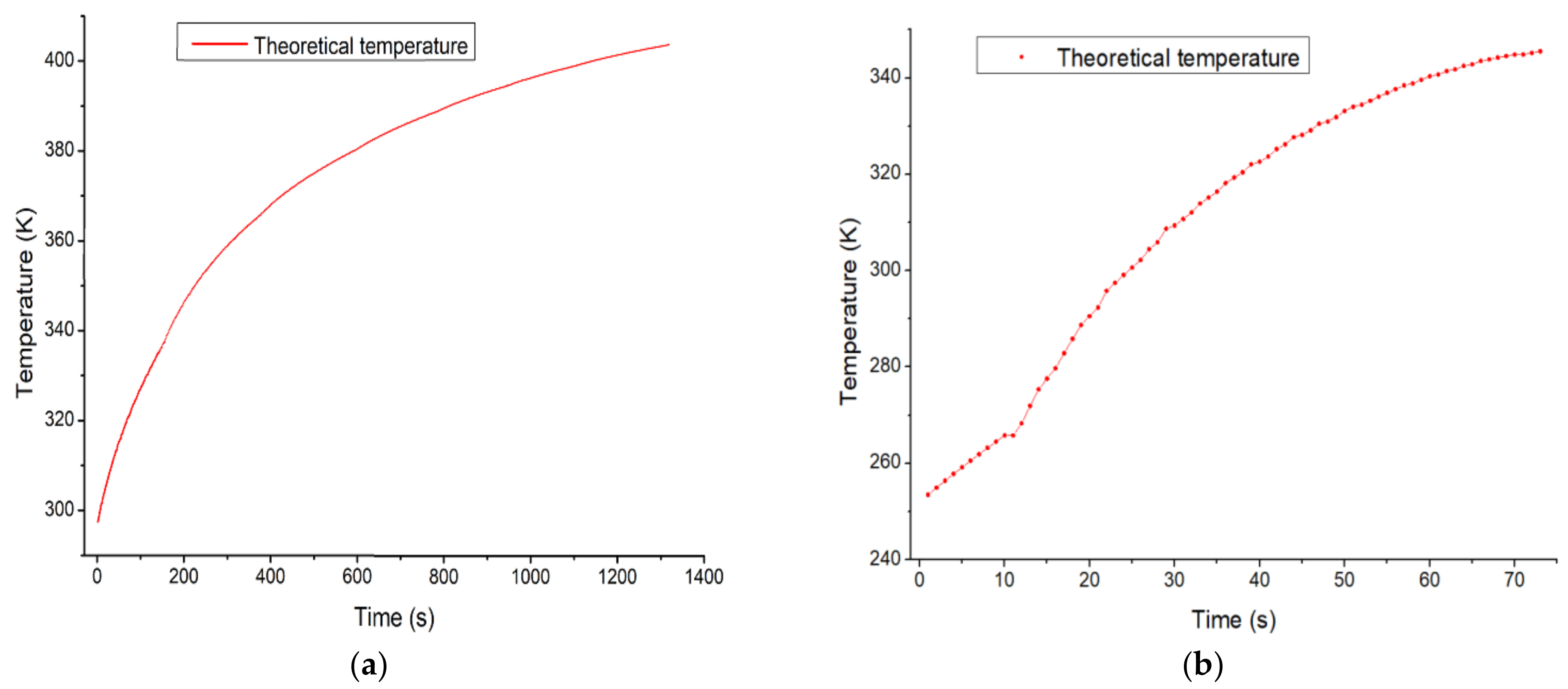
As a result of the study, the following conclusions were drawn. First, as a result of the theoretical approach, the internal temperature of the high-pressure tank was calculated to increase by 436.09 K and 376.55 K on average in the first cycle and the second cycle. Second, the hydrogen filling amount in the high-pressure tank and the buffer tank increased by 11.58 kg and 3.49 kg, respectively, in the first cycle, and increased by 9.44 kg and 1.61 kg, respectively, in the second cycle. The amount of increased temperature decreased as the cycle continued.
In this paper, the thermal insulation condition was assumed during the charging process, and the heat transfer condition to the surroundings after charging was completed and was analyzed considering only conduction and convection. Considering the abnormal heat transfer process, not the adiabatic condition, and the radiant heat transfer, the time at which thermal equilibrium takes place will be different. In the future, we will consider the above to more accurately determine the temperature and thermal equilibrium. The works of this paper will be useful for system design and operation in industrial sites that require a hydrogen compression system.