Obtaining Fermentable Sugars from a Highly Productive Elm Clone Using Different Pretreatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Estimation of Wood Biomass Yield
2.3. Pretreatments
2.4. Enzymatic Hydrolysis
2.5. Analytical Methods
3. Results and Discussion
3.1. Estimation of Wood Biomass Yield
3.2. Composition Analysis of Raw Material and Pretreated Materials
3.2.1. Raw Material
3.2.2. Pretreated Materials
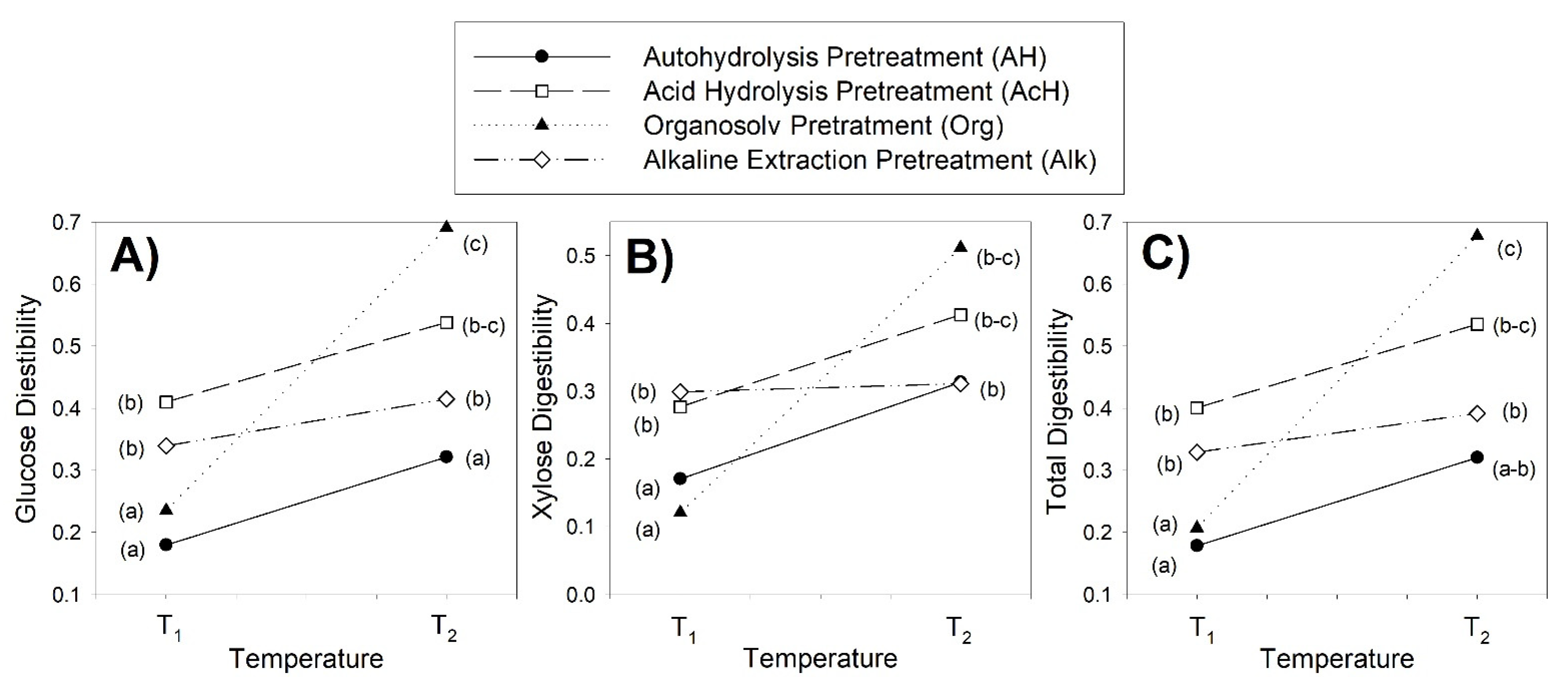
3.3. Production of Fermentable Sugars
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Bio-Based Chemicals Value Added Products from Biorefineries; IEA Bioenergy: Paris, France, 2012; Available online: https://www.ieabioenergy.com/wp-content/uploads/2013/10/Task-42-Biobased-Chemicals-value-added-products-from-biorefineries.pdf (accessed on 1 December 2020).
- Arevalo-Gallegos, A.; Ahmad, Z.; Asgher, M.; Parra-Saldivar, R.; Iqbal, H.M.N. Lignocellulose: A sustainable material to produce value-added products with a zero waste approach—A review. Int. J. Biol. Macromol. 2017, 99, 308–318. [Google Scholar] [CrossRef]
- Hall, J.P. Sustainable production of forest biomass for energy. For. Chron. 2002, 78, 391–396. [Google Scholar] [CrossRef]
- García-Morote, A.; López-Serrano, F.R.; Martínez-García, E.; Andres-Abellan, M.; Dadi, T.; Candel, D.; Rubio, E.; Lucas-Borja, M.E. Stem Biomass Production of Paulownia elongata x P. fortunei under Low Irrigation in a Semi-Arid Environment. Forests. 2014, 5, 2505–2520. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Wright, J.; Zalesny, R.S., Jr.; Nichols, E.G.; Hazel, D.W. Matching site-suitable poplars to rotation length for optimized productivity. For. Ecol. Manag. 2020, 457, 117670. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Martín, J.A.; Lopez, R.; Mutke, S.; Pinillos, F.; Gil, L. Influence of climate variables on resin yield and secretory structures in tapped Pinus pinaster Ait. in central Spain. Agric. For. Meteorol. 2015, 202, 83–93. [Google Scholar] [CrossRef]
- Fernández, M.; Alaejos, J.; Andivia, E.; Vázquez-Piqué, J.; Ruíz, F.; López, F.; Tapias, R. Eucalyptus x urograndis biomass production for energy purposes exposed to a Mediterranean climate under different irrigation and fertilisation regimes. Biomass Bioenergy 2018, 111, 22–30. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ibrahim, M.; Rashid, U.; Nawaz, M.; Ali, S.; Hussain, A.; Gull, M. Biomass production for bioenergy using marginal lands. Sustain. Prod. Consum. 2017, 9, 3–21. [Google Scholar] [CrossRef]
- Fernández, M.J.; Barro, R.; Pérez, J.; Ciria, P. Production and composition of biomass from short rotation coppice in marginal land: A 9-year study. Biomass Bioenergy 2020, 134, 105478. [Google Scholar] [CrossRef]
- IPCC. AR5 Synthesis Report: Climate Change 2014. Geneva (Switzerland). Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 1 December 2020).
- Martínez de Arano, I.; Muys, B.; Topi, C.; Pettenella, D.; Feliciano, D.; Rigilot, E.; Lefevre, F.; Prokofiva, I.; Labidi, J.; Carnus, J.-M.; et al. A Forest-Based Circular Bioeconomy for Southern Europe: Visions, Opportunities and Challenges; European Forest Institute: Joensuu, Finland, 2018; p. 124. Available online: https://espas.secure.europarl.europa.eu/orbis/sites/default/files/generated/document/en/EFI-Reflections%20on%20the%20bioeconomy%20-%20Synthesis%20Report%202018%20%28web%29_0.pdf (accessed on 1 December 2020).
- Wang, C.W. The Forests of China, with a Survey of Grassland and Dessert Vegetation; Harvard University: Cambridge, MA, USA, 1961. [Google Scholar] [CrossRef][Green Version]
- Martín, J.A.; Sobrino-Plata, J.; Rodríguez-Calcerrada, J.; Collada, C.; Gil, L. Breeding and scientific advances in the fight against Dutch elm disease—Will they allow the use of elms in forest restoration? New For. 2019, 50, 183–215. [Google Scholar] [CrossRef]
- Pérez-García, I. Evaluación de Ulmus Pumila L. y Populus Spp. Como Cultivos Energéticos en Corta Rotación. Ph.D. Thesis, E.T.S.I. Agrónomos. Universidad Politécnica de Madrid, Madrid, Spain, 2016. [Google Scholar] [CrossRef]
- Solla, A.; Martín, J.A.; Corral, P.; Gil, L. Seasonal changes in wood formation of Ulmus pumila and Ulmus minor and its relation with Dutch elm disease. New Phytol. 2005, 166, 1025–1034. [Google Scholar] [CrossRef]
- Martín, J.A.; Solla, A.; Venturas, M.; Collada, C.; Domínguez, J.; Miranda, E.; Fuentes, P.; Burón, M.; Iglesias, S.; Gil, L. Seven Ulmus minor clones tolerant to Ophiostoma novo-ulmi registered as forest reproductive material in Spain. iForest. 2015, 8, 172–180. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Sobrino-Plata, J.; Ormeño-Moncalvillo, S.; Gil, L.; Rodríguez-Calcerrada, J.; Martín, J.A. Endophyte inoculation enhances Ulmus minor resistance to Dutch elm disease. Fungal Ecol. 2015, 50, 101024. [Google Scholar] [CrossRef]
- Cogolludo-Agustín, M.A.; Agundez, D.; Gil, L. Identification of native and hybrid elms in Spain using isozyme gene markers. Heredity. 2000, 85, 157–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brännvall, E. Overview of pulp and paper processes. In Pulp and Paper Chemistry Technology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; De Gruyter: Berlin, Germany, 2009; pp. 1–12. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Carlos, D.; Dai, J.; Rojas, O.J.; Isogai, A.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Susmozas, A.; Martín-Sampedro, R.; Ibarra, D.; Eugenio, M.E.; Iglesias, R.; Manzanares, P.; Moreno, A.D. Process Strategies for the Transition of 1G to Advanced Bioethanol Production. Processes 2020, 8, 1310. [Google Scholar] [CrossRef]
- Alvira, P.; Ballesteros, M.; Negro, M.J. Progress on enzymatic saccharification technologies for biofuels production. In Biofuel Technologies; Gupta, V., Tuohy, M., Eds.; Springer: Berlin, Germany, 2013; pp. 145–169. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Rahikainen, J.L.; Johansson, L.S.; Marjamaa, K.; Laine, J.; Kruus, K.; Rojas, O.J. Preferential adsorption and activity of monocomponent cellulases on lignocellulose thin films with varying lignin content. Biomacromol 2013, 14, 1231–1239. [Google Scholar] [CrossRef]
- Rahikainen, J.L.; Martín-Sampedro, R.; Heikkinen, H.; Rovio, S.; Marjamaa, K.; Tamminen, T.; Rojas, O.J.; Kruus, K. Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on non-productive enzyme adsorption. Bioresour. Technol. 2013, 133, 270–278. [Google Scholar] [CrossRef]
- Alvira, P.; Pejó-Tomás, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Wyman, C.E. Handbook on Bioethanol: Production and Utilization; Taylor Francis: Washington, DC, USA, 1996; p. 417. [Google Scholar]
- Amiri, H.; Kamiri, K. Autohydrolysis: A promising pretreatment for the improvement of acetone, butanol, and ethanol production from woody materials. Chem. Eng. Sci. 2015, 137, 722–729. [Google Scholar] [CrossRef]
- Jiménez-López, L.; Eugenio, M.E.; Ibarra, D.; Darder, M.; Martín, J.A.; Martín-Sampedro, R. Cellulose Nanofibers from a Dutch Elm Disease-Resistant Ulmus minor Clone. Polymers 2020, 12, 2450. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; Fillat, U.; Martín, J.A.; Aranda, P.; Ruiz-Hitzky, E.; Ibarra, D.; Wicklein, B. Biorefinery of lignocellulosic biomass from an elm clone: Production of fermentable sugars and lignin-derived biochar for energy and environmental applications. Energy Technol. 2019, 7, 277–287. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pittermann, J. Drought experience and cavitation resistance in six shrubs from the Great Basin, Utah. Basic Appl. Ecol. 2000, 1, 31–41. [Google Scholar] [CrossRef]
- Ibarra, D.; Eugenio, M.E.; Cañellas, I.; Sixto, H.; Martín-Sampedro, R. Potential of different poplar clones for sugar production. Wood Sci. Technol. 2017, 51, 669–684. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory, NREL. Chem. Anal. Test. Lab. Anal. Proced. 2010. Available online: http://www.eere.energy.gov/biomass/analyticalprocedures.html (accessed on 23 February 2021).
- Das, A. The effect of size and competition on tree growth rate in old-growth coniferous forests. Can. J. Forest Res. 2012, 42, 1983–1995. [Google Scholar] [CrossRef]
- Pérez-Cruzado, C.; Sanchez-Ron, D.; Rodríguez-Soalleiro, R.; Hernández, M.J.; Sánchez-Martín, M.M.; Cañellas, I.; Sixto, H. Biomass production assessment from Populus spp. short-rotation irrigated crops in Spain. GCB Bioenergy 2014, 6, 312–326. [Google Scholar] [CrossRef]
- Noori, M.S.; Karimi, K. Detailed study of efficient ethanol production from Elmwood by alkali pretreatment. Biochem. Eng. J. 2016, 105, 197–204. [Google Scholar] [CrossRef]
- Pauly, M.; Keegstra, K. Plant cell wall polymers as precursors for biofuels. Curr. Opin. Plant Biol. 2010, 13, 305–312. [Google Scholar] [CrossRef]
- Ballesteros, M.; Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I. Ethanol from lignocellulosic materials by a simultaneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem. 2004, 39, 1843–1849. [Google Scholar] [CrossRef]
- Jiménez-López, L.; Martín-Sampedro, R.; Eugenio, M.E.; Santos, J.I.; Sixto, H.; Cañellas, I.; Ibarra, D. Co-Production of soluble sugars and lignin from short rotation white poplar and black locust crops. Wood Sci. Technol. 2020, 54, 1617–1643. [Google Scholar] [CrossRef]
- Moreno, A.D.; Olsson, L. Pretreatment of Lignocellulosic Feedstocks. In Extremophilic Enzymatic Processing of Lignocellulosic Feedstocks to Bioenergy; Sani, R., Krishnaraj, R., Eds.; Springer International Publishing AG: New York, NY, USA, 2017; pp. 31–52. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, Z.; Wang, D.D.; Laskar, M.S.; Swita, J.R.; Cort, J.R.; Yang, B. Characterization of lignin derived from water-only and dilute acid flowthrough pretreatment of poplar wood at elevated temperatures. Biotechnol. Biofuels 2015, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sampedro, R.; Santos, J.I.; Fillat, Ú.; Wicklein, B.; Eugenio, M.E.; Ibarra, D. Characterization of lignins from Populus alba L. generated as by-products in different transformation processes: Kraft pulping, organosolv and acid hydrolysis. Int. J. Biol. Macromol. 2019, 126, 18–29. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, J.; Wen, J.; Sun, G.; Sun, Y. Structural transformation of triploid of Populus tomentosa Carr. lignin during auto-catalyzed etanol organosolv pretreatment. Ind. Crops Prod. 2015, 76, 522–529. [Google Scholar] [CrossRef]
- Hage, R.E.; Brosse, N.; Sannigrahi, P.; Ragauskas. Effect of process severity on the chemical structure of mischanthus ethanol organosolv lignin. Polym. Degrad. Stab. 2010, 95, 997–1003. [Google Scholar] [CrossRef]
- Amiri, H.; Kamiri, K. Improvement of acetone, butanol, and ethanol production from woody biomass using organosolv pretreatment. Bioprocess Biosyst. Eng. 2015, 38, 1959–1972. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Zamani, A.; Amiri, H.; Horváth, I.S. Enhanced solid-state biogás production from lignocellulosic biomass by organosolv pretreatment. Biomed Res. Int. 2014, 2014, 350414. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Santos, J.I.; Fillat, Ú.; Eugenio, M.E.; Wicklein, B.; Jiménez-López, L.; Ibarra, D. Chemical and thermal analysis of lignin streams from Robinia pseudoacacia L. generated during organosolv and acid hydrolysis pre-treatments and subsequent enzymatic hydrolysis. Int. J. Biol. Macromol. 2019, 140, 311–322. [Google Scholar] [CrossRef]
- Santos, J.I.; Fillat, Ú.; Martín-Sampedro, R.; Eugenio, M.E.; Negro, M.J.; Ballesteros, I.; Rodríguez, A.; Ibarra, D. Evaluation of lignins from Side-Streams generated in an olive tree Pruning-Based biorefinery: Bioethanol production and alkaline pulping. Int. J. Biol. Macromol. 2017, 105, 238–251. [Google Scholar] [CrossRef]
- Foston, M.; Ragauskas, A.J. Changes in lignocellulosic supramolecular and ultrastructure during dilute acid pretreatment of Populus and switchgrass. Biomass Bioenergy 2010, 34, 1885–1895. [Google Scholar] [CrossRef]
- da Silva Morais, A.P.; Sansígolo, C.A.; de Oliveira Neto, M. Effects of autohydrolysis of Eucalyptus urograndis and Eucalyptus grandis on influence of chemical components and crystallinity index. Bioresour. Technol. 2016, 214, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, K.; Kabir, M.M.; Jeihanipour, K.; Karimi, K.; Taherzadeh, M.J. Alkaline pretreatment of spruce and birch to improve bioethanol and biogas production. Bioresources 2010, 5, 928–938. [Google Scholar]
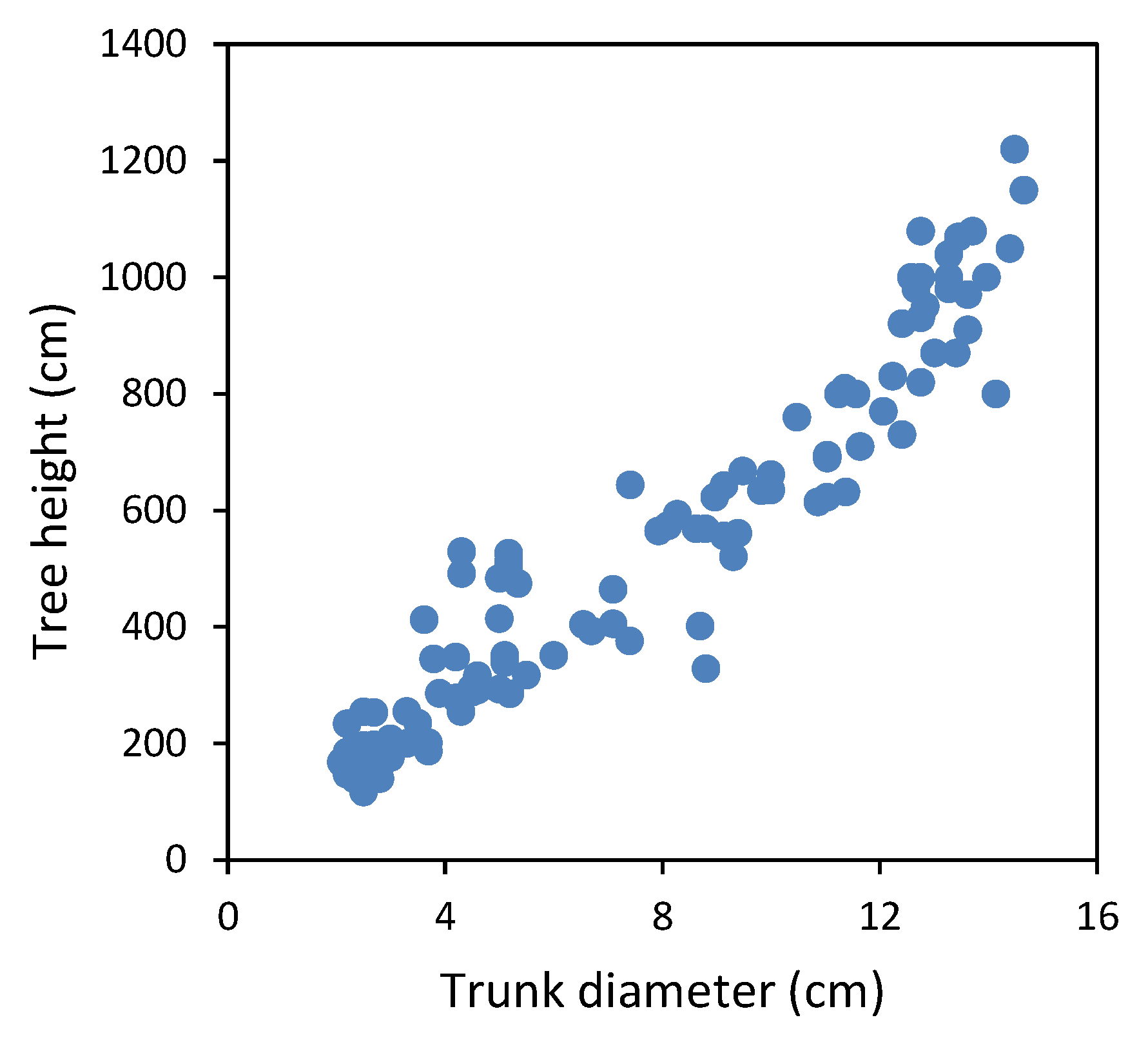

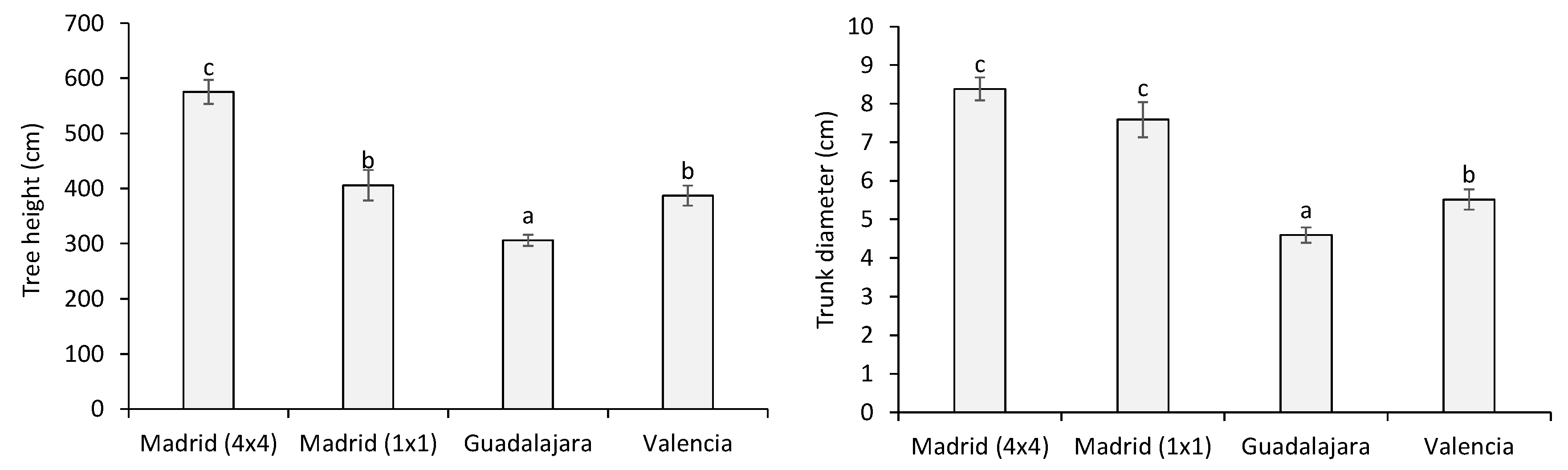



| Plot | Location in Spain | Climate (Altitude in m.a.s.l.) | T, P | N | Spacing (m) | Soil Type | Fertilization | Weed Management | Plant Propagation (Year) | Monitoring of h and d (Tree Age) |
|---|---|---|---|---|---|---|---|---|---|---|
| PH-C Ref | Puerta de Hierro, Madrid | Inland Mediterranean (597) | 14, 450 | 10 | 4 × 4 | Sandy | Slow release fertilizer in the year of planting | Anti-weed mesh | 2012 | 3 to 8 yr |
| PH-XXXVII | Puerta de Hierro, Madrid | Inland Mediterranean (597) | 14, 450 | 8 | 1 × 1 | Sandy | Slow release fertilizer in the year of planting | Clearing | 2013 | 2 to 4 yr |
| ES-VI | Guadalajara | Inland Mediterranean (685) | 13, 450 | 12 | 2 × 1.5 | Sandy-loam | None | Clearing | 2011 | 3 to 5 yr |
| AL-I | Alaquàs, Valencia | Coastal Mediterranean (42) | 17, 445 | 12 | 2 × 2 | Clay-loam | None | Clearing | 2013 | 2 and 4 yr |
| Pretreated Material Yield (%) | Acid Insoluble Lignin (%) | Acid Soluble Lignin (%) | Glucan (%) | Xylan (%) | Arabinan (%) | |
|---|---|---|---|---|---|---|
| Untreated | - | 18.0 ± 0.1 | 2.9 ± 0.1 | 43.6 ± 0.7 | 15.6 ±0.1 | 1.2 ± 0.0 |
| AH-160 | 71.5 | 27.2 ± 1.3 | 1.1 ± 0.1 | 58.6 ± 1.9 | 9.4 ± 0.3 | 0.1 ± 0.1 |
| AH-180 | 72.4 | 26.5 ± 1.1 | 1.1 ± 0.0 | 58.4 ± 1.7 | 7.0 ± 0.0 | 0.0 ± 0.0 |
| AcH-160 | 54.5 | 24.8 ± 0.2 | 0.9 ± 0.0 | 63.5 ± 0.2 | 4.7 ± 0.0 | 0.0 ± 0.0 |
| AcH-180 | 49.0 | 25.5 ± 0.2 | 0.5 ± 0.0 | 67.7 ± 0.4 | 1.6 ± 0.0 | 0.0 ± 0.0 |
| Org-160 | 76.0 | 19.9 ± 0.8 | 2.0 ± 0.3 | 52.5 ± 0.1 | 16.8 ± 0.2 | 0.0 ± 0.0 |
| Org-180 | 67.3 | 15.3 ± 0.8 | 0.6 ± 0.0 | 64.6 ± 0.6 | 5.1 ± 0.2 | 0.0 ± 0.0 |
| Alk-80 | 81.0 | 22.4 ± 0.6 | 2.0 ± 0.1 | 49.9 ± 0.4 | 17.1 ± 0.1 | 0.0 ± 0.0 |
| Alk-160 | 67.0 | 22.2 ± 0.5 | 2.0 ± 0.1 | 54.6 ± 0.4 | 15.5 ± 0.2 | 0.0 ± 0.0 |
| Total Lignin (%) | Glucan (%) | Xylan (%) | Arabinan (%) | |
|---|---|---|---|---|
| AH-160 | 3.4 | 3.8 | 56.8 | 92.2 |
| AH-180 | 4.8 | 2.9 | 67.2 | 100.0 |
| AcH-160 | 33.1 | 20.5 | 83.6 | 100.0 |
| AcH-180 | 39.2 | 23.8 | 95.0 | 100.0 |
| Org-160 | 20.3 | 8.5 | 17.8 | 100.0 |
| Org-180 | 48.9 | 0.2 | 77.9 | 100.0 |
| Alk-80 | 5.6 | 7.2 | 11.2 | 100.0 |
| Alk-160 | 22.6 | 15.9 | 33.1 | 100.0 |
| Glucose | Xylose | Arabinose | Acetic Acid | 5-HMF | Furfural | |
|---|---|---|---|---|---|---|
| AH-160 | 3.70 ± 0.3 (2.90) | 6.45 ± 0.6 (5.35) | 0.70 ± 0.1 (0.30) | 2.35 ± 0.3 (1.80) | 0.10 ± 0.0 | 0.05 ± 0.0 |
| AH-180 | 3.65 ± 0.3 (3.10) | 3.20 ± 0.6 (1.85) | 1.10 ± 0.0 (0.55) | 1.00 ± 0.2 (0.00) | 0.30 ± 0.1 | 0.40 ± 0.3 |
| AcH-160 | 3.45 ± 0.4 (0.40) | 5.55 ± 0.1 (0.50) | 0.90 ± 0.0 (0.00) | 1.85 ± 0.3 (0.10) | 0.30 ± 0.1 | 0.30 ± 0.4 |
| AcH-180 | 2.70 ± 0.0 (0.40) | 3.10 ± 0.3 (0.30) | 0.50 ± 0.1 (0.00) | 1.55 ± 0.3 (0.10) | 0.35 ± 0.1 | 0.90 ± 0.0 |
| Org-160 | 2.75 ± 0.3 (2.0) | 1.95 ± 0.4 (1.05) | 0.60 ± 0.0 (0.20) | 0.30 ± 0.3 (0.10) | 0.05 ± 0.0 | 0.02 ± 0.0 |
| Org-180 | 3.50 ± 0.3 (1.55) | 6.35 ± 0.1 (3.60) | 0.80 ± 0.2 (0.50) | 0.70 ± 0.3 (0.00) | 0.35 ± 0.1 | 0.35 ± 0.2 |
| Alk-80 | 0.90 ± 0.0 (0.55) | 0.75 ± 0.3 (0.25) | 0.30 ± 0.1 (0.25) | 2.30 ± 0.3 (0.10) | - | - |
| Alk-160 | 1.20 ± 0.5 (0.95) | 1.30 ± 0.3 (0.95) | 0.75 ± 0.0 (0.75) | 2.55 ± 0.3 (0.20) | - | - |
| Sugars (g/L) | Yield (%) | Digestibility (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| G | X | T | G | X | T | G | X | T | |
| Untreated | 2.80 | 0.38 | 3.18 | 5.3 | 0.7 | 6.0 | 12.2 | 4.7 | 10.2 |
| AH-160 | 5.55 | 0.84 | 6.39 | 7.5 | 1.1 | 8.7 | 18.0 | 17.0 | 17.9 |
| AH-180 | 9.88 | 1.16 | 11.04 | 13.6 | 1.6 | 15.2 | 32.1 | 31.3 | 32.1 |
| AcH-160 | 13.71 | 0.68 | 14.39 | 14.2 | 0.7 | 14.9 | 41.0 | 27.7 | 40.1 |
| AcH-180 | 19.17 | 0.35 | 19.52 | 17.9 | 0.3 | 18.2 | 53.8 | 41.3 | 53.5 |
| Org-160 | 6.49 | 1.07 | 7.56 | 9.4 | 1.5 | 10.9 | 23.5 | 12.1 | 20.7 |
| Org-180 | 23.48 | 1.38 | 24.86 | 30.0 | 1.8 | 31.8 | 69.0 | 51.2 | 67.7 |
| Alk-80 | 8.92 | 2.68 | 11.60 | 13.7 | 4.1 | 17.9 | 34.0 | 29.9 | 32.9 |
| Alk-160 | 11.93 | 2.54 | 14.47 | 15.2 | 3.2 | 18.4 | 41.5 | 31.1 | 39.2 |
| Total Sugars Yield Per Gram of Carbohydrates Contained in Raw Material (%) 1 | Total Sugars Yield Per Gram of Raw Material (%) 2 | Total Sugars Yield Per Hectare/Year (Mg DM/ha/Year) | ||||
|---|---|---|---|---|---|---|
| G | X | G | X | G | X | |
| Untreated | 5.3 | 0.7 | 2.3 | 0.1 | 0.21 (0.21) | 0.01 (0.01) |
| AH-160 | 20.3 | 63.4 | 8.8 | 9.9 | 0.30 (0.82) | 0.02 (0.92) |
| AH-180 | 26.1 | 32.2 | 11.4 | 5.0 | 0.55 (1.06) | 0.02 (0.46) |
| AcH-160 | 26.1 | 54.1 | 11.3 | 8.4 | 0.57 (1.05) | 0.01 (0.78) |
| AcH-180 | 27.1 | 30.3 | 11.8 | 4.7 | 0.72 (1.09) | 0.00 (0.43) |
| Org-160 | 18.8 | 20.4 | 8.2 | 3.2 | 0.37 (0.76) | 0.02 (0.29) |
| Org-180 | 42.0 | 62.8 | 18.3 | 9.8 | 1.22 (1.70) | 0.02 (0.91) |
| Alk-80 | 16.8 | 11.3 | 7.3 | 1.8 | 0.55 (0.67) | 0.06 (0.16) |
| Alk-160 | 19.3 | 15.8 | 8.4 | 2.5 | 0.62 (0.78) | 0.05 (0.23) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra, D.; Martín-Sampedro, R.; Jiménez-López, L.; Martín, J.A.; Díaz, M.J.; Eugenio, M.E. Obtaining Fermentable Sugars from a Highly Productive Elm Clone Using Different Pretreatments. Energies 2021, 14, 2415. https://doi.org/10.3390/en14092415
Ibarra D, Martín-Sampedro R, Jiménez-López L, Martín JA, Díaz MJ, Eugenio ME. Obtaining Fermentable Sugars from a Highly Productive Elm Clone Using Different Pretreatments. Energies. 2021; 14(9):2415. https://doi.org/10.3390/en14092415
Chicago/Turabian StyleIbarra, David, Raquel Martín-Sampedro, Laura Jiménez-López, Juan A. Martín, Manuel J. Díaz, and María E. Eugenio. 2021. "Obtaining Fermentable Sugars from a Highly Productive Elm Clone Using Different Pretreatments" Energies 14, no. 9: 2415. https://doi.org/10.3390/en14092415
APA StyleIbarra, D., Martín-Sampedro, R., Jiménez-López, L., Martín, J. A., Díaz, M. J., & Eugenio, M. E. (2021). Obtaining Fermentable Sugars from a Highly Productive Elm Clone Using Different Pretreatments. Energies, 14(9), 2415. https://doi.org/10.3390/en14092415







