Cardoon Hydrolysate Detoxification by Activated Carbon or Membranes System for Bioethanol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Chemicals
2.3. Raw Biomass and Pretreatment
2.4. Hydrolysate Detoxification by Activated Carbon
2.5. Hydrolysate Detoxification by Membrane Nanofiltration
2.6. Hydrolysates Concentration
2.7. Microbial Fermentations
2.7.1. Solid Culture Media
2.7.2. Liquid Culture Media
2.7.3. Pre-Inoculum and Inoculum Preparation
2.7.4. Fermentation Assays
- (i)
- Untreated hydrolysate;
- (ii)
- Synthetic culture medium YMP, pH 5.5, with the same sugar concentration as the detoxified hydrolysate;
- (iii)
- Hydrolysate detoxified by activated carbon and concentrated, pH 5.5;
- (iv)
- Hydrolysate detoxified by membranes and concentrated, pH 5.5.
- (i)
- Untreated hydrolysate;
- (ii)
- Synthetic LB medium, pH 7.0, with the same sugar concentration as the detoxified hydrolysate;
- (iii)
- Hydrolysate detoxified by activated carbon and concentrated, pH 7.0;
- (iv)
- Hydrolysate detoxified by membranes and concentrated, pH 7.0.
2.8. HPLC Analysis
2.9. Kinetic and Stoichiometric Parameters Calculation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of Detoxification Processes on the Composition of Cardoon Hydrolysates
3.1.1. Hydrolysate Composition
3.1.2. Detoxification of Cardoon Hydrolysate by Activated Carbon Adsorption
3.1.3. Detoxification of Cardoon Hydrolysate by Membrane Nanofiltration
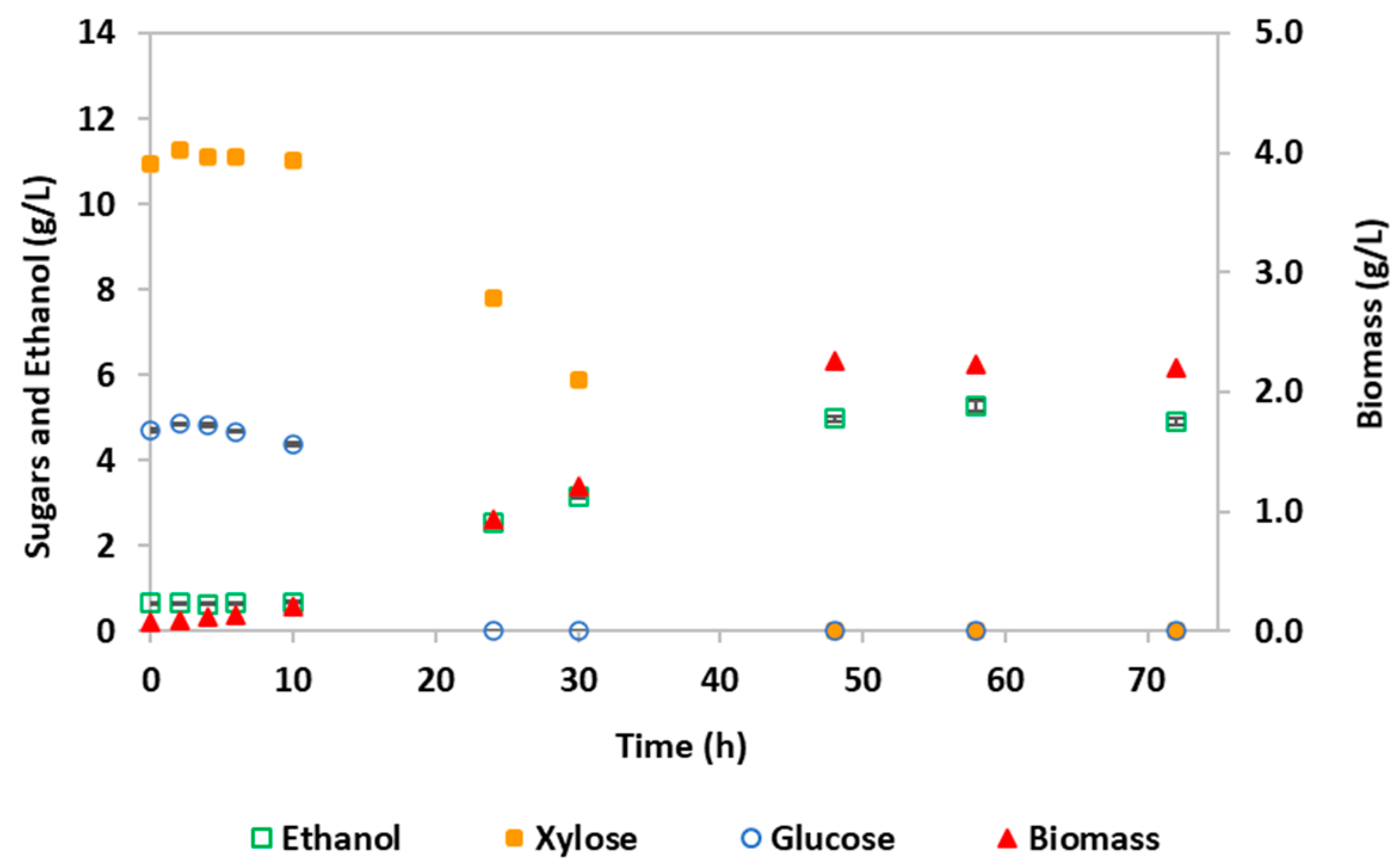
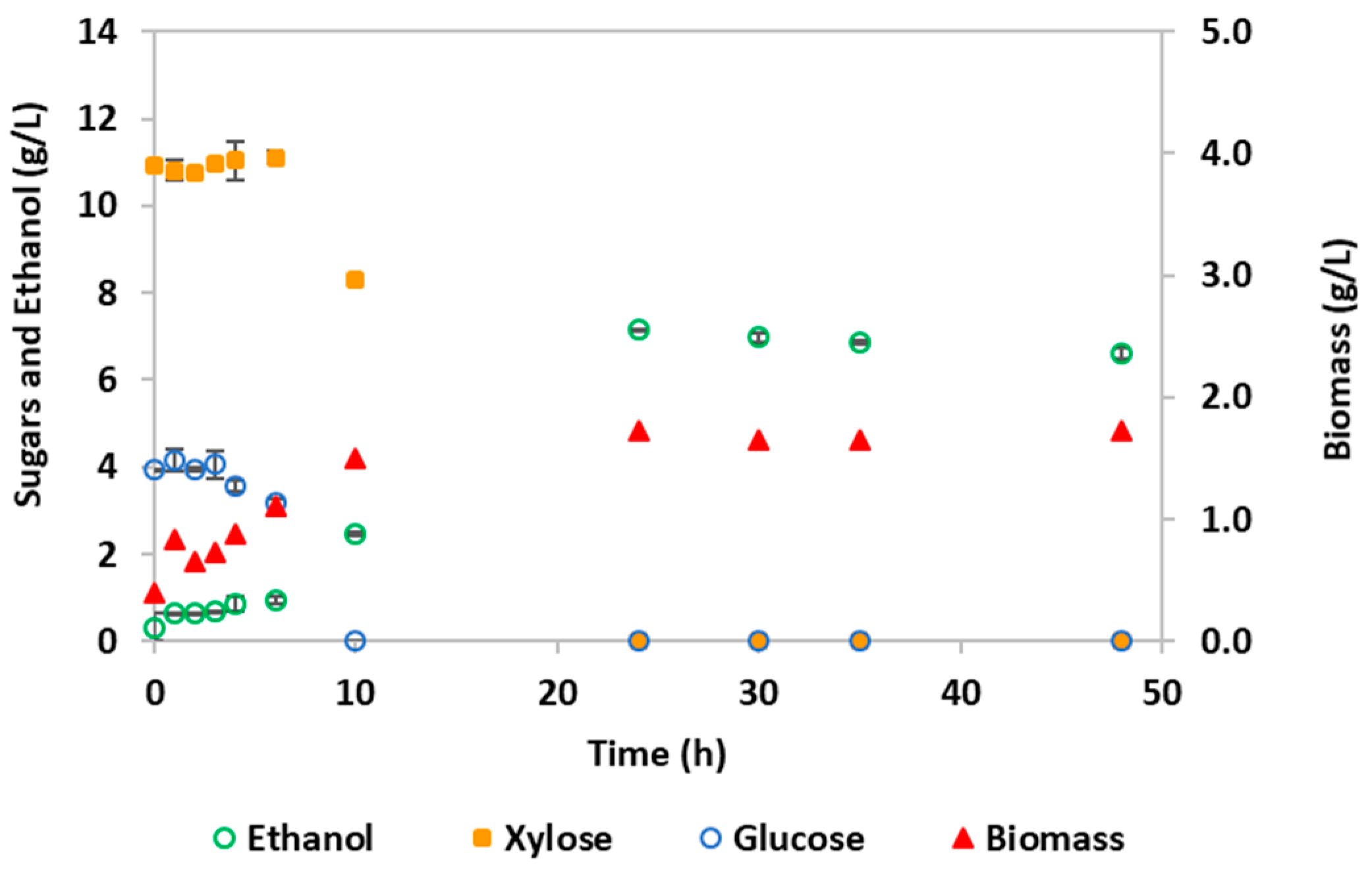
3.2. Bioethanol Production by S. stipitis Fermentation
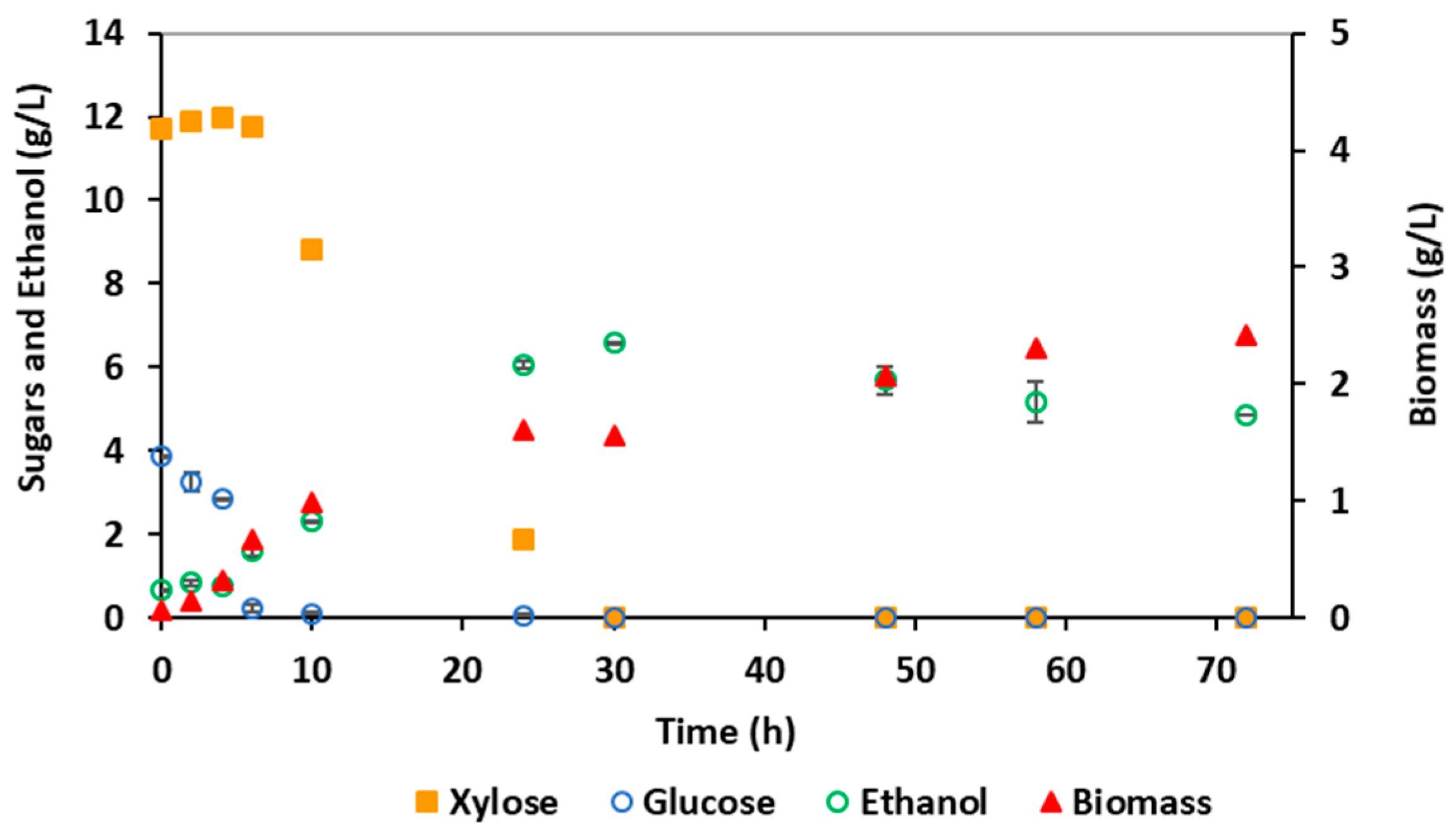
3.3. Bioethanol Production by E. coli MS04
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biofuels Barometer 2020—EurObserv’ER. Available online: https://www.eurobserv-er.org/biofuels-barometer-2020/ (accessed on 5 October 2021).
- Net Zero by 2050—Analysis-IEA. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 5 October 2021).
- Branco, R.H.R.; Serafim, L.S.; Xavier, A.M.R.B. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2018, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Vergel, O.A.; Cárdenas, D.; García-Contreras, R.; Mata, C. Bioethanol-Diesel Blends Used in Diesel Engines and Vehicles under Transient Operation. In Bioethanol Technologies; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Pereira, S.R.; Ivanuša, Š.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Biological treatment of eucalypt spent sulphite liquors: A way to boost the production of second generation bioethanol. Bioresour. Technol. 2012, 103, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Thamizhakaran Stanley, J.; Thanarasu, A.; Senthil Kumar, P.; Periyasamy, K.; Raghunandhakumar, S.; Periyaraman, P.; Devaraj, K.; Dhanasekaran, A.; Subramanian, S. Potential pre-treatment of lignocellulosic biomass for the enhancement of biomethane production through anaerobic digestion—A review. Fuel 2022, 318, 123593. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Sabio, E.; Ramiro, M.J. Preparation and Properties of Biodiesel from Cynara cardunculus L. Oil. Ind. Eng. Chem. Res. 1999, 38, 2927–2931. [Google Scholar] [CrossRef]
- Prussi, M.; O’Connell, A.; Lonza, L. Analysis of current aviation biofuel technical production potential in EU28. Biomass Bioenergy 2019, 130, 105371. [Google Scholar] [CrossRef]
- Pappalardo, H.D.; Toscano, V.; Puglia, G.D.; Genovese, C.; Raccuia, S.A. Cynara cardunculus L. as a Multipurpose Crop for Plant Secondary Metabolites Production in Marginal Stressed Lands. Front. Plant Sci. 2020, 11, 240. [Google Scholar] [CrossRef]
- Espada, J.J.; Villalobos, H.; Rodríguez, R. Environmental assessment of different technologies for bioethanol production from Cynara cardunculus: A Life Cycle Assessment study. Biomass Bioenergy 2021, 144, 105910. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Ferro, M.D.; Paulino, A.F.C.; Mendes, J.A.S.; Gravitis, J.; Evtuguin, D.V.; Xavier, A.M.R.B. Enzymatic saccharification and bioethanol production from Cynara cardunculus pretreated by steam explosion. Bioresour. Technol. 2015, 186, 309–315. [Google Scholar] [CrossRef]
- Amândio, M.S.T.; Rocha, J.M.S.; Serafim, L.S.; Xavier, A.M.R.B. Cellulosic Bioethanol from Industrial Eucalyptus globulus Bark Residues Using Kraft Pulping as a Pretreatment. Energies 2021, 14, 2185. [Google Scholar] [CrossRef]
- Pereira, S.R.; Sànchez i Nogué, V.; Frazão, C.J.R.; Serafim, L.S.; Gorwa-Grauslund, M.F.; Xavier, A.M.R.B. Adaptation of Scheffersomyces stipitis to hardwood spent sulfite liquor by evolutionary engineering. Biotechnol. Biofuels 2015, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Ruchala, J.; Kurylenko, O.O.; Dmytruk, K.V.; Sibirny, A.A. Construction of advanced producers of first- and second-generation ethanol in Saccharomyces cerevisiae and selected species of non-conventional yeasts (Scheffersomyces stipitis, Ogataea polymorpha). J. Ind. Microbiol. Biotechnol. 2020, 47, 109–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, B.A.; Jansen, T.; Volschenk, H.; Görgens, J.F.; Van Zyl, W.H.; Den Haan, R. Stress modulation as a means to improve yeasts for lignocellulose bioconversion. Appl. Microbiol. Biotechnol. 2021, 105, 4899–4918. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.M.R.B.; Correia, M.F.; Pereira, S.R.; Evtuguin, D.V. Second-generation bioethanol from eucalypt sulphite spent liquor. Bioresour. Technol. 2010, 101, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Weissgram, M.; Ters, T.; Weber, H.K.; Herwig, C. Investigating the potential of thermophilic species for ethanol production from industrial spent sulfite liquor. AIMS Energy 2015, 3, 592–611. [Google Scholar] [CrossRef]
- Sarawan, C.; Suinyuy, T.N.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Optimized activated charcoal detoxification of acid-pretreated lignocellulosic substrate and assessment for bioethanol production. Bioresour. Technol. 2019, 286, 121403. [Google Scholar] [CrossRef] [PubMed]
- Alves-Ferreira, J.; Carvalheiro, F.; Duarte, L.C.; Ferreira, A.R.P.; Martinez, A.; Pereira, H.; Fernandes, M.C. D-Lactic acid production from Cistus ladanifer residues: Co-fermentation of pentoses and hexoses by Escherichia coli JU15. Ind. Crops Prod. 2022, 177, 114519. [Google Scholar] [CrossRef]
- Arminda, M.; Josúe, C.; Cristina, D.; Fabiana, S.; Yolanda, M. Use of activated carbons for detoxification of a lignocellulosic hydrolysate: Statistical optimisation. J. Environ. Manag. 2021, 296, 113320. [Google Scholar] [CrossRef]
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for bioethanol production by pervaporation. Biotechnol. Biofuels 2021, 14, 10. [Google Scholar] [CrossRef]
- Pan, L.; He, M.; Wu, B.; Wang, Y.; Hu, G.; Ma, K. Simultaneous concentration and detoxification of lignocellulosic hydrolysates by novel membrane filtration system for bioethanol production. J. Clean. Prod. 2019, 227, 1185–1194. [Google Scholar] [CrossRef]
- Dos Santos, J.L.C.; Fernandes, M.C.; Lourenço, P.M.L.; Duarte, L.C.; Carvalheiro, F.; Crespo, J.G. Removal of inhibitory compounds from olive stone auto-hydrolysis liquors by nanofiltration. Desalin. Water Treat. 2011, 27, 90–96. [Google Scholar] [CrossRef]
- Fernández-Sandoval, M.T.; Huerta-Beristain, G.; Trujillo-Martinez, B.; Bustos, P.; González, V.; Bolivar, F.; Gosset, G.; Martinez, A. Laboratory metabolic evolution improves acetate tolerance and growth on acetate of ethanologenic Escherichia coli under non-aerated conditions in glucose-mineral medium. Appl. Microbiol. Biotechnol. 2012, 96, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.C.; Ferro, M.D.; Paulino, A.F.C.; Chaves, H.T.; Evtuguin, D.V.; Xavier, A.M.R.B. Comparative study on hydrolysis and bioethanol production from cardoon and rockrose pretreated by dilute acid hydrolysis. Ind. Crops Prod. 2018, 111, 633–641. [Google Scholar] [CrossRef]
- Brás, T.; Guerra, V.; Torrado, I.; Lourenço, P.; Carvalheiro, F.; Duarte, L.C.; Neves, L.A. Detoxification of hemicellulosic hydrolysates from extracted olive pomace by diananofiltration. Process Biochem. 2014, 49, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Tah, A.; Moss-Acosta, C.L.; Trujillo-Martinez, B.; Tiessen, A.; Lozoya-Gloria, E.; Orencio-Trejo, M.; Gosset, G.; Martinez, A. Non-severe thermochemical hydrolysis of stover from white corn and sequential enzymatic saccharification and fermentation to ethanol. Bioresour. Technol. 2015, 198, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Snedecor, G.; Cochran, W. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; ISBN 0813815614. [Google Scholar]
- Zhang, Y.; Li, M.; Wang, Y.; Ji, X.; Zhang, L.; Hou, L. Simultaneous concentration and detoxification of lignocellulosic hydrolyzates by vacuum membrane distillation coupled with adsorption. Bioresour. Technol. 2015, 197, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, I.; Ballesteros, M.; Manzanares, P.; Negro, M.J.; Oliva, J.M.; Sáez, F. Dilute sulfuric acid pretreatment of cardoon for ethanol production. Biochem. Eng. J. 2008, 42, 84–91. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Parajó, J.C.; Domínguez, H.; Domínguez, J.M. Xylitol production from Eucalyptus wood hydrolysates extracted with organic solvents. Process Biochem. 1997, 32, 599–604. [Google Scholar] [CrossRef]
- Hao, X.C.; Yang, X.S.; Wan, P.; Tian, S. Comparative proteomic analysis of a new adaptive Pichia Stipitis strain to furfural, a lignocellulosic inhibitory compound. Biotechnol. Biofuels 2013, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Günan Yücel, H.; Aksu, Z. Ethanol fermentation characteristics of Pichia stipitis yeast from sugar beet pulp hydrolysate: Use of new detoxification methods. Fuel 2015, 158, 793–799. [Google Scholar] [CrossRef]
- Farias, D.; Maugeri Filho, F. Co-culture strategy for improved 2G bioethanol production using a mixture of sugarcane molasses and bagasse hydrolysate as substrate. Biochem. Eng. J. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Fernández-Sandoval, M.T.; Galíndez-Mayer, J.; Bolívar, F.; Gosset, G.; Ramírez, O.T.; Martinez, A. Xylose-glucose co-fermentation to ethanol by Escherichia coli strain MS04 using single- and two-stage continuous cultures under micro-aerated conditions. Microb. Cell Fact. 2019, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Bren, A.; Park, J.O.; Towbin, B.D.; Dekel, E.; Rabinowitz, J.D.; Alon, U. Glucose becomes one of the worst carbon sources for E.coli on poor nitrogen sources due to suboptimal levels of cAMP. Sci. Rep. 2016, 6, 24834. [Google Scholar] [CrossRef] [PubMed]

| Hydrolysates Detoxified by Activated Carbon | Hydrolysates’ Composition (g/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| Glu 1 | Xyl | Ara | LV | AA | FA | Furfural | HMF | |
| Untreated hydrolysate | 2.57 | 11.48 | 1.02 | 0.26 | 4.48 | 3.32 | 2.19 | 0.04 |
| Detoxified at pH 5.5 | 2.36 | 9.68 | 0.47 | 0.22 | 3.27 | 2.71 | 0.05 | 0.01 |
| Detoxified at pH 7.0 | 2.29 | 9.28 | 0.53 | 0.20 | 3.92 | 3.25 | 0.00 | 0.00 |
| Detoxified at pH 5.5 and concentrated | 3.44 | 13.71 | 0.85 | 0.28 | 4.50 | 3.93 | 0.00 | 0.01 |
| Detoxified at pH 7.0 and concentrated | 3.27 | 13.37 | 0.75 | 0.56 | 5.79 | 4.79 | 0.00 | 0.01 |
| Hydrolysates Detoxified by Membrane Nanofiltration | Hydrolysates’ Composition (g/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| Glu 1 | Xyl | Ara | LV | AA | FA | Furfural | HMF | |
| Untreated hydrolysate | 2.47 | 11.81 | 0.59 | 0.26 | 3.73 | 0.386 | 2.02 | 1.411 |
| Detoxified by membrane | 2.47 | 8.13 | 0.59 | 0.04 | 0.04 | 0.00 | 0.10 | 0.01 |
| Detoxified by membrane and concentrated | 3.43 | 11.24 | 0.81 | 0.05 | 0.06 | 0.00 | 0.00 | 0.01 |
| Parameters 1 | Hydrolysate Detoxified by Membrane Nanofiltration | Synthetic YMP Medium | Significance 2 |
|---|---|---|---|
| YP/S (g/g) | 0.33 | 0.42 | NS |
| Etmax (g/L) | 5.28 (58 h) | 6.58 (30 h) | ** |
| CE (%) | 64.9 | 82.9 | NS |
| PEtmax (g/L·h) | 0.091 | 0.22 | *** |
| Parameters 1 | Hydrolysate Detoxified by Membrane Nanofiltration | Synthetic LB Medium | Significance 2 |
|---|---|---|---|
| YP/S (g/g) | 0.48 | 0.35 | NS |
| Etmax (g/L) | 7.16 (24 h) | 5.21 (24 h) | NS |
| CE (%) | 94.5 | 69.3 | NS |
| PEtmax (g/L·h) | 0.30 | 0.22 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, A.P.M.; Gonçalves, M.J.A.; Brás, T.; Pesce, G.R.; Xavier, A.M.R.B.; Fernandes, M.C. Cardoon Hydrolysate Detoxification by Activated Carbon or Membranes System for Bioethanol Production. Energies 2022, 15, 1993. https://doi.org/10.3390/en15061993
Tavares APM, Gonçalves MJA, Brás T, Pesce GR, Xavier AMRB, Fernandes MC. Cardoon Hydrolysate Detoxification by Activated Carbon or Membranes System for Bioethanol Production. Energies. 2022; 15(6):1993. https://doi.org/10.3390/en15061993
Chicago/Turabian StyleTavares, Ana P. M., Matthew J. A. Gonçalves, Teresa Brás, Gaetano R. Pesce, Ana M. R. B. Xavier, and Maria C. Fernandes. 2022. "Cardoon Hydrolysate Detoxification by Activated Carbon or Membranes System for Bioethanol Production" Energies 15, no. 6: 1993. https://doi.org/10.3390/en15061993
APA StyleTavares, A. P. M., Gonçalves, M. J. A., Brás, T., Pesce, G. R., Xavier, A. M. R. B., & Fernandes, M. C. (2022). Cardoon Hydrolysate Detoxification by Activated Carbon or Membranes System for Bioethanol Production. Energies, 15(6), 1993. https://doi.org/10.3390/en15061993









