Abstract
The pulp and paper industry faces an emerging challenge for valorising wastes and side-streams generated according to the biorefinery concept. Eucalyptus globulus bark, an abundant industrial residue in the Portuguese pulp and paper sector, has a high potential to be converted into biobased products instead of being burned. This work aimed to evaluate the ethanol production from E. globulus bark previously submitted to kraft pulping through separate hydrolysis and fermentation (SHF) configuration. Fed-batch enzymatic hydrolysis provided a concentrated hydrolysate with 161.6 g·L−1 of cellulosic sugars. S. cerevisiae and Ethanol Red® strains demonstrated a very good fermentation performance, despite a negligible xylose consumption. S. passalidarum, a yeast known for its capability to consume pentoses, was studied in a simultaneous co-culture with Ethanol Red®. However, bioethanol production was not improved. The best fermentation performance was achieved by Ethanol Red®, which provided a maximum ethanol concentration near 50 g·L−1 and fermentation efficiency of 80%. Concluding, kraft pulp from E. globulus bark showed a high potential to be converted into cellulosic bioethanol, being susceptible to implementing an integrated biorefinery on the pulp and paper industrial plants.
1. Introduction
Over the last few decades, governments and energy authorities worldwide have been pushing for a transition from fossil fuels to a circular bio-based economy [1]. Accordingly, regulations on greenhouse gas emissions and the burning of fossil fuels have been implemented, imposing biofuels production goals and contributing to environmental sustainability [2]. The European Union established a Renewable Energy Directive II, which would define a target of 3.6% of transportation fuels derived from advanced biofuels in 2030, including bioethanol, biodiesel, biobutanol, biomethanol, among others. Biofuels production is being incentivized progressively to support their future utilization [3,4].
Currently, biomass is recognized as one of the most promising renewable resources with high potential for replacing fossil fuels and their derived products due to its low cost, sustainability, and worldwide availability when it does not compete with the food chain [5,6].
Overall, pulp and paper production are among the largest industries and, undoubtedly, the major consumer of woody biomass [7,8]. This sector is crucial for the socio-economic development of Portugal, a country characterized by a high forest area [9]. Eucalyptus globulus is the most widely used wood source in the Portuguese pulp and paper sector, which accounted for an area of 812,000 ha in 2016 [9,10,11]. The main competitive advantages of this species include its fast-growing rate and great adaptability to soils and climates. Moreover, it has high cellulose coupled with low lignin content, which results in a high pulping yield [2,10,11,12,13].
The circular economy model has redefined economic and industrial practices by promoting sustainable waste management through recycling, reuse, extended life cycle, and residues as raw materials to produce value-added products [5,14,15]. Therefore, the pulp and paper industry has started to move its processes towards upgrading the wastes, sub-products, and side-streams generated during all the process stages [11,16,17,18]. During the wood handling step, the bark is removed from the tree logs since its presence negatively impacts the yield of the pulping process, compromising the quality of the pulp, leading to extended cooking times and higher bleaching chemical requirements [19,20,21]. The stem is composed of about 11–15% of bark (dry weight basis), which results in the wide availability of this industrial residue [10,22]. According to Neiva et al. [22], about 0.5 Mton of E. globulus bark was generated in Portugal in 2017. Considering the low economic value of bark coupled with its chemical complexity, this industrial residue is usually burned for energy and steam generation or even just left in the forest for soil nutrition [10,19]. Nevertheless, to foster sustainability, some other potential routes for upgrading bark into value-added products are welcome within the circular economy model. The production of cellulosic sugars is one of the target alternatives, which can feed the promising sugar-based platform for biochemicals and biofuels, namely bioethanol [10,11,23]. The absence of bioethanol production for transport fuel in Portugal is another driving force and motivation for focusing on this valorization route [24]. For full exploitation of this raw material, it could be considered the separation of the polyphenolic fraction before converting the carbohydrates and according to the literature, this extraction can be performed without compromising the polysaccharides fraction in future research. The polyphenolic fraction has potential application in cosmetics, pharmaceutical, and food industries since it exhibits anti-inflammatory, antioxidant, antimicrobial, and antibacterial properties [11,12,25,26,27]. The fractionation of the main components of bark for further valorisation is one of the basic principles of the biorefinery approach [28,29].
The conversion of E. globulus bark into cellulosic sugars involves two main stages: pretreatment and hydrolysis. For this purpose, the kraft process can be considered as a chemical pretreatment since it is a well-known efficient delignification technology [30]. The primary purpose of enzymatic hydrolysis is to convert carbohydrates fraction into simple fermentable sugars using a specific combination of enzymes already available as a commercial consortium of enzymes. For bioethanol production, an alcoholic fermentation step is required to convert sugars through the metabolic activity of ethanologenic microorganisms [7].
Implementing this industrial residue’s valorization pathway into the pulp and paper mill plant contributes to the promising approach of an integrated biorefinery. This would increase revenues, expand the portfolio of products, and boost the market opportunities supporting the circular economy model [7,31,32,33]. Moreover, this sector already has industrial facilities and logistics well-established, which is a competitive advantage [11,34].
The present work aims at evaluating the E. globulus bark kraft pulp fed-batch hydrolysate’s fermentability using some ethanolic yeasts through a separate hydrolysis and fermentation (SHF) configuration. For this purpose, the hydrolysate rich in cellulosic sugars obtained through a fed-batch strategy was submitted to a set of experiments on Erlenmeyer flasks with S. cerevisiae and Ethanol Red®. Then, since S. passalidarum was already reported as a strain naturally able to ferment pentoses besides hexoses, the simultaneous co-culture of Ethanol Red® and S. passalidarum was assessed aiming to maximize the consumption of all sugars present in the hydrolysate, namely xylose. Finally, the fermentation ability of S. passalidarum was evaluated in both hydrolysate and synthetic media. Despite all the research conducted regarding bioethanol production, to our knowledge, this is the first time that E. globulus bark previously submitted to a kraft pulping process is converted into bioethanol through an SHF configuration. In general, there is a scarcity of works published related to the valorization of eucalyptus bark for bioethanol production. Furthermore, it is also the first time a co-culture strategy involving both Ethanol Red® and S. passalidarum strains is reported. The results of this research could contribute to evaluating the feasibility of converting pulp and paper mills into biorefineries, taking advantage of kraft pulping as a pretreatment method. This conversion route gives a more sustainable bark application present in this sector in a considerable amount.
2. Materials and Methods
2.1. Kraft Pulp
Unbleached kraft pulp from Eucalyptus globulus bark was kindly provided by The Navigator Company and RAIZ—Instituto de Investigação da Floresta e do Papel (Eixo, Portugal). The chemical composition of the raw material, namely its carbohydrates and lignin contents, was determined according to NREL standard protocols.
2.2. Enzymatic Hydrolysis
Enzymatic hydrolysis assays were performed under fed-batch operational mode using a commercial cellulases consortium kindly provided by The Navigator Company and RAIZ—Instituto de Investigação da Floresta e do Papel. The filter paper unit (FPU) activity of this commercial mixture was determined as 168.7 FPU·mL−1, whose analysis was based on the NREL standard procedure. An enzymatic load of 25 FPU gcarbohydrates−1 was selected for this work.
The experiments were conducted for 24 h, with a stirring rate of 150 rpm, at 50 °C, using 0.05 M sodium citrate buffer, maintaining the pH between 4.5 and 5.5 (adjusted through the addition of H2SO4 2M and NaOH 2M solutions). A fed-batch approach was followed with an initial solids loading of 11% (w/v) based on the total working volume (3 L), where 3% fresh solids were fed consecutively every 2 h until achieving a final concentration of 20% (w/v). The commercial enzymatic mixture was fed all at once initially. All the hydrolysates were mixed for homogenization, centrifuged for 1 h at 5000 rpm and 4 °C (Megafuge 16R, Thermo Scientific, Osterode am Harz, Germany) and, finally, sterilized by autoclaving at 121 °C for 20 min (Uniclave 88, AJC, Cacém, Portugal). The resulting hydrolysate was analysed by HPLC and kept frozen.
2.3. Microorganisms
Saccharomyces cerevisiae PYCC 5246 (ATCC 24860) described as able to consume xylose was gently supplied by Portuguese Yeast Culture. Ethanol Red® was kindly provided by Leaf by Lesaffre Advanced Fermentations (Marcq-en-Baroeul, France). Spathaspora passalidarum CBS 10155 (NRRL Y-27907) was purchased at the Westerdijk Fungal Biodiversity Institute (Utrecht, The Netherlands). These strains were grown at 28 °C and maintained at 4 °C on Petri dishes with solid yeast medium (YM) composed of 10 g·L−1 glucose, 5 g·L−1 peptone, 3 g·L−1 malt extract, 3 g·L−1 yeast extract, and 20 g·L−1 agar.
2.4. Pre-Inoculum and Inoculum
Pre-inoculum was prepared by transferring a colony from a maintenance YM Petri dish to 10 mL liquid YM (similar to solid YM, except agar) and was incubated for 24 h at 28 °C and 180 rpm. The inoculum was prepared by transferring the exact volume of pre-inoculum to fresh liquid YM, which guarantees an OD620 nm of about 0.400. The inoculum was incubated at 28 °C and 180 rpm for 14 h. These procedures were carried out in duplicate.
2.5. Fermentation
Fermentation assays were carried out in duplicate using 250 mL Erlenmeyer flasks with a working volume of 100 mL, incubated at 28 °C and 180 rpm. Fermentation media was composed by 85% (v/v) hydrolysate, 10% (v/v) inoculum, and 5% (v/v) of supplementation composed by 2.0 g·L−1 (NH4)2HPO4, 1.0 g·L−1 (NH4)2SO4, 0.5 g·L−1 MgSO4·7H2O, and 2.5 g·L−1 yeast extract. Sampling was made during the operational time of assays.
2.6. Analytical Methods
The pH of the samples was measured using an electrode InPro 3030/200 (Mettler Toledo, Columbus, OH, USA) connected to a benchtop meter sensION+ MM340 (Hach, Loveland, CO, USA).
Biomass was monitored by measuring optical density at 620 nm (UVmini-1240, Shimadzu, Tokyo, Japan) and converted into concentration using a calibration curve.
High-performance liquid chromatography (HPLC) was used to quantify glucose, xylose, ethanol, and glycerol. After properly diluted centrifuged and filtered for 8 min at 8000 rpm (Eppendorf, Hamburg, Germany), samples were injected by autosampler L-2200 (MiniSprin centrifuge, Hitachi, Ltd., Chiyoda, Japan) on a Rezex ROA-Organic Acid H+ (8%) 300 × 7.8 mm ion-exchange column (Phenomenex, Torrance, CA, USA) at 65 °C (oven Gecko 2000, CIL Cluzeau, Sainte-Foy-la-Grande, France) and detected by a refraction index detector L-2490 (Hitachi, Chiyoda, Japan). The injection volume was 10 µL and the eluent used was H2SO4 0.005 N, with a flow rate of 0.500 mL·min−1 (pump L-2130, Hitachi). A standard calibration curve was used for the determination of metabolites concentration.
2.7. Calculations
The theoretical glucose concentration ([Glucose]theoretical) corresponds to the mass of glucose if the cellulose present in the kraft pulp would be fully hydrolyzed in glucose. It was calculated from the mass of cellulose present in the pulp () and from the maximum theoretical hydrolysis yield of glucose () (Equation (1)). Similarly, theoretical xylose concentration ([Xylose]theoretical) was calculated considering the mass of hemicelluloses () and the maximum theoretical yield of xylose () (Equation (2)):
The hydrolysis yield (Yhydrolysis), was calculated using Equation (3) by the ratio between the sum of glucose ([Glucose]hydrolysate) and xylose ([Xylose]hydrolysate) concentrations evaluated in the hydrolysate and the sum of respective theoretical concentrations ([Glucose]theoretical + [Xylose]theoretical) on the kraft pulp predicted by their chemical composition:
The maximum specific growth rate (h−1) corresponded to the linear regression slope of plotting the natural logarithm of biomass concentration versus time during the exponential phase.
The volumetric rate of glucose consumption (g·L−1·h−1) was calculated from the module of the slope of the linear regression of glucose concentration over time.
The volumetric ethanol productivity (Prodvol) was calculated based on Equation (4), considering the differences (∆) from the beginning of the fermentation until the maximum ethanol concentration was reached:
The ethanol yield (Yethanol/substrate) was calculated according to Equation (5), considering glucose and xylose as substrates and the maximum ethanol concentration achieved. Glucose, xylose, and ethanol concentration differences were calculated in the previously referred time period:
The conversion efficiency of the fermentation was calculated by Equation (6), considering the maximum theoretical ethanol yield of 0.511 g·g−1 [33]:
3. Results and Discussion
3.1. Chemical Characterization of the Bark Kraft Pulp
The major components of E. globulus kraft pulp were cellulose (79.8 ± 3.8 wt %) and hemicelluloses (15.5 ± 0.6 wt %), representing a fraction of carbohydrates up to 90 wt %. Total lignin content was only about 2.6 wt % since the industrial kraft process is an efficient chemical delignification process to obtain polysaccharides fibers for pulp and paper final products.
3.2. Enzymatic Hydrolysis
Enzymatic hydrolysis using a fed-batch strategy enabled a high solids loading (20%), which resulted in the maximum concentration of cellulosic sugars of 161.6 g·L−1. However, a relatively low hydrolysis yield (66.8%) was achieved, which can be related to some technical issues, namely operation reaction time, mass and heat transfer phenomena, potential inhibition of the enzymatic activity due to the high sugars concentration, accumulation of inhibitory compounds, among others [35,36,37,38]. In the future, a more extended saccharification period should be applied to improve the process efficiency, as already found by Gomes et al. [29].
3.3. Fermentation to Bioethanol
3.3.1. Fermentation by S. cerevisiae
The fermentation of hydrolysate (from fed-batch saccharification) by S. cerevisiae was assayed in Erlenmeyer flasks and profiles of biomass, glucose, xylose, and ethanol are presented in Figure 1. Two complementary experiments were carried out, attempting to assess the complete concentration profiles of the experiment. This fermentation was carried out using a working volume of 100 mL at 180 rpm and 28 °C.
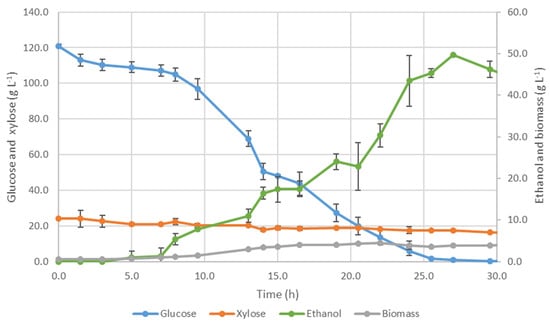
Figure 1.
Evolution of glucose, xylose, ethanol and biomass concentrations for S. cerevisiae fermentation (100 mL, 180 rpm and 28 °C).
The lag phase lasted ca. 5 h, when glucose consumption for biomass growth and ethanol production started to be detected. Biomass concentration increased exponentially with a specific growth rate of 0.151 ± 0.012 h−1. Most of the glucose was consumed during this period, with a volumetric consumption rate of 5.99 ± 0.38 g·L−1·h−1 (calculated between 8 h and 25.5 h).
Even using a S. cerevisiae strain described as able to consume a slight amount of xylose [39], only a slight decrease was noticed. However, most of the xylose remained in the medium at the end of fermentation and its contribution to ethanol production was not remarked. The maximum ethanol concentration, 49.7 ± 0.1 g·L−1, was achieved after 27 h of fermentation, which corresponds to a conversion efficiency of 76.7 ± 1.1% and a productivity of 1.84 ± 0.00 g·L−1·h−1. It was noticed synchrony between the maximum ethanol concentration and glucose depletion at 27 h of fermentation.
As already described for alcoholic fermentation, some residual glycerol was produced, achieving a maximum concentration of 2.27 ± 0.14 g·L−1 at the end of the assay. The most relevant parameters that influence glycerol formation include the yeast strain and environmental factors, namely pH, temperature, oxygen, and composition of the fermentation medium [40,41,42].
Regarding pH, a slight decrease from 4.79 to 4.24 was observed during the assay. The carbon dioxide released during the process is in balance with carbonic acid when in an aqueous solution, which is the main responsible for the decrease of the pH [43,44]. This drop-in pH was not as pronounced as that observed by Branco et al. [39] with kraft wood pulps hydrolysate. Besides the difference in hydrolysates raw material, also the hydrolysate content in the fermentation medium was different: 85% (v/v) in the present work, which represents an increase of 20% (v/v) compared to that performed by Branco et al. [39]. A more concentrated bark kraft pulp hydrolysate could intensify a buffer capacity, improving yeast performance.
Some authors have been evaluating kraft pulping as a potential pretreatment for lignocellulosic feedstocks for bioethanol and, in Table 1, a comparison of S. cerevisiae fermentation parameters using different raw materials is presented.
Monrroy et al. [45] studied the effect of several kraft cooking conditions, namely time, temperature, and active alkali charge on the fermentation yield. The authors demonstrated that the highest severity of kraft pulping allowed to reach the highest lignin removal of about 78%. Ethanol was produced with concentrations ranging from 30 to 38 g·L−1, corresponding to conversion efficiencies between 65% and 78% (wood basis). Accordingly, these authors proved that the kraft process was a promising pretreatment for E. globulus wood bioethanol production through simultaneous saccharification and fermentation (SSF) strategy [45]. Similarly, Wistara et al. [46] concluded that the bioethanol production from bleached kraft Jabon (Anthocephalus cadamba Miq) wood pulp is highly dependent on lignin content, achieving a significantly higher performance for a degree of delignification above 90% (corresponding to a Klason lignin content of 1.5% or even lower) [46].
The fermentability of hydrolysates derived from seven kraft pulps (two poplar species, aspen, beech, birch, bleached pine pulp, and unbleached pine pulp) was evaluated by Buzała and co-workers [47] in a 15 L bioreactor using an SHF configuration. The authors achieved yields from 0.11 to 0.20 g·gdry weight wood−1 and found that the discrepancy in ethanol production was due to the presence of inhibitory compounds, botanical origin, and residual lignin content from the feedstocks [47].
Recently, Branco et al. [39] proved that the unbleached kraft pulp of E. globulus using an SHF configuration showed a high performance regarding ethanol production by S. cerevisiae ATCC 24860. At Erlenmeyer-scale, a maximum ethanol concentration of 19.81 ± 0.15 g·L−1 and a productivity of 2.01 ± 0.01 g·L−1·h−1 were produced, corresponding to a conversion efficiency of 88.3 ± 1.7% [39]. It was found that the present work showed a delay in glucose exhaustion and maximum ethanol concentration (27 h instead of 8.9 h) comparatively to the assay performed by Branco and co-workers [39]. Probably, this delay is due to the about 3-fold higher initial glucose concentration achieved through a fed-batch approach followed in the enzymatic hydrolysis step. Accordingly, it was clear that the E. globulus bark showed slightly lower productivity and conversion efficiency than the work carried out by Branco et al. [39] using good quality wood. In general, the chemical composition of bark diverges from wood regarding its lower concentration of carbohydrates and higher content of ash and extractives [10,48]. Consequently, the mass of ethanol that potentially can be produced from a dry metric tonne of bark is lower than that from wood [48].
Similarly, ethanol production in SSF operation mode from unbleached kraft pulp by S. cerevisiae ATCC 26602 and K. marxianus NCYC 1426 strains was studied by Mendes et al. [49]. According to the reported results, the fed-batch SSF process at Erlenmeyer scale using S. cerevisiae allowed obtaining a significantly high ethanol concentration (55.3 ± 0.3 g L−1). The maximum ethanol concentration was achieved after 79 h, which corresponds to the productivity of 0.70 g·L−1 h−1. The conversion efficiency was 64.9% of the theoretical value [49]. Although a slightly higher ethanol concentration was reached, lower productivity and conversion efficiency were obtained compared with the present work.
Edgardo et al. [50] also studied the SSF process using isolated S. cerevisiae IR2-9a, which demonstrated the ability to work at temperatures above 35 °C. This thermotolerant strain’s performance was confirmed by subjecting bleached kraft pulp obtained from Pinus radiata chips to the SSF process, which resulted in ethanol concentration and conversion efficiency of 28 g L−1 and 62%, respectively [50].
Guigou et al. [34] subjected sawdust from Eucalyptus grandis to several combined pretreatments. An autohydrolysis (170 °C for 40 min, severity factor of 3.66) followed by kraft pulping (90 min and 3.4% active alkali) achieved the best enzymatic hydrolysis efficiency of 95 ± 2%. Regarding the fermentation stage using S. cerevisiae PE-2 through a pre-saccharification and fermentation (PS-SSF) strategy, this pretreated raw material reached a maximum ethanol concentration of 57 g·L−1, which corresponds to 81% of the theoretical yield and a productivity of 1.20 g·L−1·h−1. By solubilizing more than 95% of the original lignin content, Kraft pulping contributed significantly to improving the performance of the fermentation process [34]. These results were similar (both in ethanol concentration and conversion efficiency) to those obtained in the present work with E. globulus bark without using the additional step of autohydrolysis.

Table 1.
Summary table with the fermentation parameters of some studies involving the fermentation of kraft pulps from various raw materials using S. cerevisiae strain.
Table 1.
Summary table with the fermentation parameters of some studies involving the fermentation of kraft pulps from various raw materials using S. cerevisiae strain.
| Feedstock | Yeast Strain | Operation Conditions | Conf | [EtOH]max (g·L−1) | Prod (g·L−1·h−1) | Yethanol/substrate | Ref. |
|---|---|---|---|---|---|---|---|
| E. globulus unbleached kraft pulp | S. cerevisiae ATCC 24860 | 28 c 150 rpm 100 mL | SHF | 19.81 ± 0.15 | 2.01 ± 0.01 | 88.3 ± 1.7% theoretical | [39] |
| E. globulus kraft pulp | S. cerevisiae ATCC 26602 | 38 °C 150 rpm 100 mL | Fed-batch SSF | 55.3 ± 0.3 | 0.70 | 64.9% theoretical | [49] |
| E. globulus kraft pulp | S. cerevisiae IR2T9 | 40 °C 150 rpm 96 h | SSF | 30.0–38.0 | N.S. | 0.168–0.202 g gdry wood−1 | [45] |
| Jabon bleached kraft pulp | S. cerevisiae | 40 °C 72 h | SSF | N.S. | N.S. | 2.89–16.39% (v/wd.w. pulp) | [46] |
| Seven kraft pulps | S. cerevisiae | 25 °C 48 h 15 L | SHF | N.S. | N.S. | 0.11–0.20 g gd.w. wood−1 | [47] |
| E. grandis sawdust | S. cerevisiae PE-2 | 35 °C 150 rpm 100 mL 48 h | PS-SSF | 57.0 | 1.20 | 81% theoretical | [34] |
| Pinus radiata chips bleached kraft pulp | S. cerevisiae IR2-9a | 40 °C 150 rpm 72 h | SSF | 28 | 0.39 | 62% theoretical | [50] |
| E. globulus unbleached kraft pulp | S. cerevisiae D5A | N.S. | SSF | 5.67 | 0.032 | 0.04227 g gdry wood−1 | [51] |
| Industrial E. globulus bark unbleached kraft pulp | S. cerevisiae ATCC 24860 | 28 °C 150 rpm 100 mL | SHF | 49.7 ± 0.1 | 1.84 ± 0.00 | 76.7 ± 1.1% theoretical | This work |
“Conf” stands for configuration; “N.S.” stands for not specified.
3.3.2. Fermentation by Ethanol Red®
Ethanol Red® is an industrial yeast strain designed for high sugar and ethanol tolerance, high resistance to temperature, and notable fermentation ability [52,53]. Kossatz et al. [52] already reported the high ethanol tolerance, up to 90 g·L−1, of this strain. An Erlenmeyer flask fermentation assay with Ethanol Red® (Figure 2) was carried out to evaluate its capability to ferment the hydrolysate from E. globulus bark kraft pulp. Two complementary experiments were performed, attempting to assess the complete concentration profiles of the experiment.
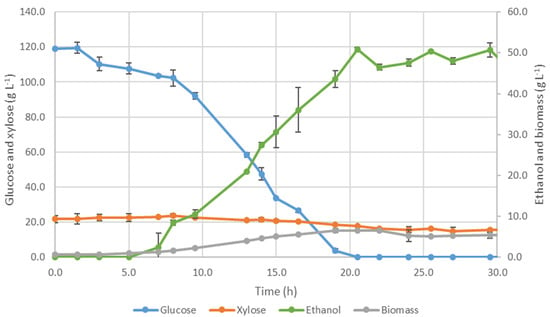
Figure 2.
Evolution of glucose, xylose, ethanol, and biomass concentrations for Ethanol Red® fermentation (100 mL, 180 rpm, and 28 °C).
At the beginning of the assay, the fermentation media was composed of 119.0 g·L−1 of glucose and 21.7 g·L−1 of xylose. The cell adaptation period took approximately 5 h, followed by glucose consumption for growth and ethanol production. Biomass concentration increased exponentially with a specific growth rate of 0.196 ± 0.009 h−1, indicating faster growth than S. cerevisiae in the previous assay. The evolution of biomass and ethanol concentrations followed a similar trend.
The glucose consumption was fast, following the biomass growth, and was depleted at 21 h. A slight decrease in pH was observed alongside the experiment from 4.77 to 4.15. The volumetric ethanol rate was 9.19 ± 0.30 g·L−1·h−1 (calculated from 8 h to 19 h), representing a significant improvement compared to the previous S. cerevisiae assay (5.99 ± 0.38 g·L−1·h−1). Regarding xylose, a reduced amount was consumed without contributing to ethanol production. The Ethanol Red® strain proved to be more efficient and robust than S. cerevisiae with a slight decrease in glycerol formation (2.1 ± 0.1 g·L−1).
The maximum ethanol concentration of 50.8 ± 0.5 g·L−1 was achieved after 20.5 h, corresponding to the productivity of 2.48 ± 0.02 g·L−1·h−1 and 81.0 ± 0.6% of the theoretical ethanol yield, based on consumed sugars. Although this strain was designed for starch-based raw materials [54], Ethanol Red® demonstrated successful performance in this assay, achieving a significant improvement regarding the productivity of the process compared to the previous strain. Furthermore, this range of concentration is higher than the minimum value required for the ethanol distillation (>40 g·L−1), allowing the production process to be considered with economic feasibility [35,36].
Works regarding the conversion of E. globulus bark into bioethanol are scarce. Recently, Gomes et al. [29] pretreated E. globulus bark through hydrothermal pretreatment. Two different strategies were used for bioethanol production from the autohydrolysed solid: SSF and PS-SSF. At 15% of solids loading, both approaches without supplementation obtained a similar fermentation performance. An ethanol concentration of around 25 g·L−1 was achieved, which corresponds to a conversion efficiency of 55%. However, comparing both configurations, it was found that SSF was doubled in terms of productivity [29]. The highest ethanol production, about 38 g·L−1, was achieved using the PS-SSF approach with a solids loading of 17.5%, an extended pre-saccharification period of 48 h, and using a supplementation solution. The conversion efficiency and productivity corresponded to 73% and 0.52 g·L−1·h−1, respectively. Compared to the present work, the authors added a high concentration of peptone and yeast extract, 20 g·L−1 and 10 g·L−1, respectively, which might hinder the economic viability of the process on a commercial scale. The yeast extract concentration was about 4-fold higher than the one utilized in the present work even though the productivity was nearly four times lower [29]. These results are summarized in Table S1 (Supplementary Materials).
Some other studies focused on the fermentation performance of bark derived from other wood species. Kemppainen et al. [26] studied the fermentability of the spruce bark subjected to three different pretreatments: steam explosion (SE), hot water extraction (HWE), and sequential HWE and SE. HWE and the sequential method led to a similar hydrolysis yield of about 60%. The sugar mixture obtained after HWE and enzymatic hydrolysis was fed to a fermenter, with a working volume of 1.5 L and a consistency of 15%, resulting in 18.3 g·L−1 of ethanol and conversion efficiency of 62.3% [26]. The use of mixtures of woodchips with different spruce bark ratios as the substrate for bioethanol production using Ethanol Red® was investigated by Frankó and colleagues [55]. These mixtures were subjected to SO2-catalysed steam pretreatment carried out at 210 °C for 5 min. The liquid fractions were subjected to both process configurations, SHF and SSF. Regardless of the bark content, SSF proved to be more efficient, considering the overall efficiency of the process. The range of ethanol concentrations reported for the SSF process varied from 20.9 g·L−1 to 45.8 g·L−1 for the proportion of bark from 100% to 0%, respectively. Therefore, an increase in the bark content negatively affected the ethanol conversion efficiency, probably due to a decreased hydrolysis capacity since the recovery of sugars in the liquid fraction decreased [55].
3.3.3. Fermentation Using Simultaneous Co-Culture of Ethanol Red® and S. passalidarum
According to the previous results, xylose was almost not consumed by the tested yeast and did not contribute to ethanol production. Therefore, aiming to ferment all the sugars present in the hydrolysate, a simultaneous co-culture strategy using Ethanol Red® and S. passalidarum was followed (Figure 3). S. passalidarum is a native xylose-fermenting yeast, recognized as a promising candidate for the fermentation of bioethanol from lignocellulosic hydrolysates [56,57].
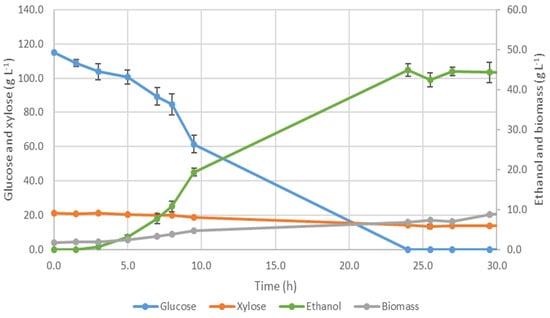
Figure 3.
Evolution of glucose, xylose, ethanol and biomass concentrations for co-culture of Ethanol Red® and S. passalidarum fermentation (100 mL, 180 rpm and 28 °C).
After 10 h of fermentation, around 60 g·L−1 of glucose was still present in the medium, being depleted before 24 h.
Regarding xylose, only 4 g·L−1 between 10 h and 24 h of the fermentation were consumed. However, it did not improve ethanol production since the maximum ethanol concentration of 46.4 ± 1.7 g·L−1 was detected at 24 h, corresponding to a productivity of 1.93 ± 0.07 g·L−1·h−1 and conversion efficiency of 78.8 ± 3.2%. This performance was slightly worse than the observed in the previous assay where Ethanol Red®® was cultivated alone. Bonan et al. [58] referred catabolic repression and Farias et al. [59] reported xylose-fermenting yeasts as following the diauxic growth mechanism where the presence of glucose represses the enzymatic system of xylose assimilation preventing its consumption before glucose exhaustion. They also reported that the growth of S. passalidarum is much slower than S. cerevisiae and justified their lower productivity on simultaneous co-culture with high ethanol concentration when glucose was exhausted [58,59]. Based on these results we can conclude that in our work S. passalidarum certainly has started to consume glucose to grow and when the exhaustion of glucose occurred as there was already a high ethanol concentration (46.4 ± 1.7 g·L−1), inhibition of S. passalidarum prevented xylose fermentation [57].
A sequential co-culture could be an alternative to minimize this repression of xylose consumption. However, it is important to note that for a successful approach, it would be necessary to apply an additional step to recover the ethanol due to the low tolerance of xylose-fermenting yeast [57]. This strategy has been proposed for the sequential co-culture of S. cerevisiae and S. stipitis, the most recurrent combination [60]. Furthermore, it might be recommended to adjust the time at which the fermentation medium is inoculated to be possible to identify the moment in which glucose exhaustion occurs.
No studies were found in the literature using a simultaneous co-culture of both Ethanol Red® and S. passalidarum strains. As previously mentioned, Farias and Filho [59] carried out a co-culture experiment using S. cerevisiae and S. passalidarum Y-207907 strains for bioethanol production from a hemicellulosic hydrolysate. The sugarcane bagasse hydrolysate was mainly composed of xylose (94.39 g·L−1), glucose (12.22 g·L−1), arabinose (8.01 g·L−1), cellobiose (2.12 g·L−1), and acetic acid (5.47 g·L−1). The hydrolysate was supplemented with molasses to a 50:50 (w/w) ratio to reduce the inhibition caused by the high acetic acid concentration. Besides, molasses simultaneously supplemented the culture medium and provided a higher buffering potential. Moreover, yeast extract, malt extract, mineral, and trace elements were added to the fermentation medium. Despite a considerable reduction in the xylose uptake rate was observed (compared to monocultures), the co-culture combination led to the complete exhaustion of reducing sugars. This fact could be related to the competition for oxygen and the faster production of ethanol by S. cerevisiae. The increase of initial sugars concentration from 50 to 100 g·L−1 resulted in a minimum increment of about 6 g·L−1 on ethanol production. A maximum ethanol concentration of 30.2 g·L−1 and a productivity of 4.44 g·L−1·h−1 were reached for an initial sugars concentration of 100 g·L−1. In terms of conversion efficiency, these values correspond to 61.0%, representing a reduction of about 40% relative to the assay with an initial sugars concentration of 50 g·L−1. Accordingly, it was found that the higher the initial sugars concentrations, the lower the yield of the fermentation process what certainly points to an inhibitory effect. Furthermore, this increase in sugars concentrations leads to a longer time required to consume all the sugars and, consequently, to conclude the fermentation process. The monoculture assay using S. passalidarum achieved a similar ethanol concentration to the co-culture with an initial sugars concentration of 100 g·L−1. However, monoculture resulted in a residual concentration of sucrose and a decrease in productivity was observed [59]. The co-culture using a respiratory deficient mutant of S. cerevisiae and S. passalidarum improved the fermentation performance, achieving a maximum ethanol concentration of about 37 g·L−1. Some possible reasons pointed out by the authors included no occurrence of ethanol reassimilation and all the oxygen availability in the medium to S. passalidarum pentoses fermentation since S. cerevisiae mutant strain had not respiratory ability. However, the authors noticed that xylose was consumed at a slow rate, which seems to be more pronounced in fermentation medium when high mixed sugars concentrations are used [59]. These results are summarized in Table S2 (Supplementary Materials).
3.3.4. Fermentation Using S. passalidarum Monoculture
A monoculture assay of S. passalidarum was carried out to evaluate its fermentation performance using fed-batch hydrolysate. Surprisingly, it was not detected any bioethanol production and the low sugars consumption registered, nearly 13% (data not shown), probably was only used as the carbon source for any biomass cell growth detected over the assay. Biomass concentration increased with a reduced specific growth rate of 0.070 ± 0.007 h−1, attaining a low maximum concentration of 3.83 ± 0.04 g·L−1 at the end of the fermentation process.
At the end of the assay (after about 30 h), glucose concentration was still quite high (up to 100 g·L−1), while xylose remained almost constant. Glycerol was detected at a negligible level.
Considering the weak performance, the fermentability of S. passalidarum was attempted in synthetic media, mimicking the sugars concentration in hydrolysate, to evaluate if inhibition occurred by any bark hydrolysate inhibitor effect or by a high concentration of substrate. Indeed, no studies were found in the literature reporting S. passalidarum fermentation using this range of sugars concentrations (about 115 g·L−1 and 20 g·L−1 of glucose and xylose, respectively). Furthermore, it was found that most published works used feedstocks in which the concentration of xylose was higher than glucose (Table S3, available in the Supplementary Materials) [58,61,62]. In contrast, the hydrolysate used in this work had a glucose concentration of about 5-fold higher than xylose.
An assay with synthetic media SM1 was carried out with an initial concentration similar to the hydrolysate of 114.2 g·L−1 and 21.2 g·L−1 of glucose and xylose, respectively (Figure 4). Only 31% of the initial glucose concentration was consumed, whereas xylose remained unchanged. Although it was detected the presence of some ethanol, its maximum concentration was only 8.4 ± 1.0 g·L−1, which is underperforming. Glycerol was formed, achieving a maximum concentration of about 0.95 g·L−1.
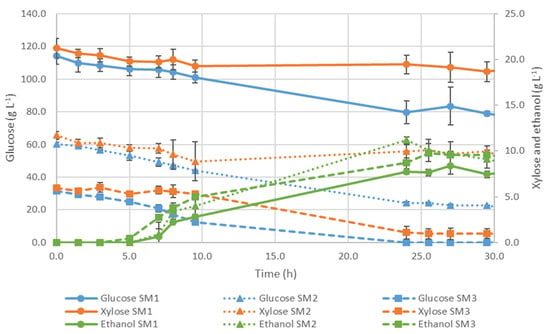
Figure 4.
Evolution of glucose, xylose, and ethanol concentrations for S. passalidarum fermentations using synthetic media SM1, SM2, and SM3 (100 mL, 180 rpm, and 28 °C).
The increase in the biomass concentration was quite significant, attaining its maximum of 9.08 ± 0.47 g·L−1, which was more than 2-fold higher compared to the assay using hydrolysate. According to Souza et al. [63], a high carbon-nitrogen ratio may promote fermentation metabolism instead of cell growth. Nonetheless, this ratio was neither controlled or monitored during these assays. Furthermore, the hydrolysate may contain certain inhibitory compounds that are suppressing both growth and fermentation metabolic activities. Hou and Yao [64] described S. passalidarum as yeast with a low tolerance to inhibitors typically coming from pretreated feedstocks derived from lignocellulosic biomass as furfural and hydroxymethylfurfural (HMF) [64]. Du et al. also reported that S. passalidarum showed a weaker robustness and stress tolerance than K. marxianus CICC 1727-5 after submitting both of them to a saccharification co-fermentation process using non-detoxified acid-pretreated corncob [65]. Acetic acid, traditionally released on lignocellulosic pretreatments, can negatively impact fermentation performance by reducing specific growth rate, sugar consumption uptake, and the extension of the cell adaptation period if it is present in concentrations between 2 and 5 g·L−1 [57,66]. Many authors directed efforts to improve fermentation performance and inhibitor tolerance of this yeast [58,62,64,67,68,69,70].
During this assay, the pH dropped over time from 6.02 to 2.73. The sharp pH drop on the assay using synthetic media supports the hypothesis related to hydrolysate’s buffer capacity. It is important to emphasize that such low pH certainly caused some inhibitory effect on the yeast’s metabolic activities, whose optimal activity generally requires pH, around 5 [59,64]. Farias and Filho [59] emphasized the importance of keeping a pH of around 5.0 to avoid cell growth inhibition and ensured this condition through the addition of molasses.
An additional assay using synthetic media SM2 was carried out with S. passalidarum (Figure 4), which corresponds to about half of the initial concentration of glucose and xylose present in the hydrolysate (60.3 g·L−1 and 11.7 g·L−1, respectively). Glucose was consumed at a rate of 1.22 ± 0.07 g·L−1·h−1, which indicates that its exhaustion would require about 46 h. At the end of the process (after 30 h), around 60% of the initial glucose was consumed, which represents about 2-fold compared to the previous assay. Glycerol minimal production was detected, but its concentration was even lower than the reported in the previous assay. In terms of biomass, its maximum concentration was about 6.68 ± 1.95 g·L−1. This assay achieved a slightly higher bioethanol concentration of 11.2 ± 0.4 g·L−1 and a productivity of 0.47 ± 0.01 g·L−1·h−1.
In the following experiment, a fermentation assay by S. passalidarum strain was performed using synthetic media SM3, mimicking a quarter of the initial concentration of glucose and xylose in the hydrolysate, about 31.6 g·L−1, and 5.9 g·L−1, respectively.
According to Figure 4, the profile of ethanol concentration was similar for the three assays using synthetic media. The maximum ethanol concentration using SM3 (9.7 ± 0.2 g·L−1) was slightly lower than in the previous assay, even though the initial sugar concentration was reduced by about half. Furthermore, it was found that the higher the initial concentration of sugars, the longer is the delay in the fermentation stage. For SM1, a slight decrease in glucose concentration was only observed after 25 h of fermentation. This time was reduced about five-fold in the SM2 assay, whose initial concentration is about half. Comparing both SM2 and SM3 assays, it was noted that there was no need for an adaptation period in the latter, observing a sharp decrease in glucose concentration soon 2 h after the beginning of the process. Accordingly, it is demonstrated that a high initial substrate concentration inhibits S. passalidarum. These results corroborate those reported by Farias and Filho [59].
Among all the assays carried out with S. passalidarum using synthetic media, SM3 was the only one where glucose depletion occurred. Although glucose exhaustion was only detected at 24 h of fermentation, it is estimated that it occurred around 15 h, assuming the constant consumption rate of 2.38 ± 0.25 g·L−1·h−1 calculated from 3 h until 9.5 h. Regarding xylose, its consumption was more evidenced using SM3. According to the xylose concentration profile presented in Figure 4, it remained almost constant until nearly 10 h of the process when there was still about 10 g·L−1 of glucose in the fermentation medium. At 24 h of fermentation, glucose had already been exhausted, and xylose concentration was about 1 g·L−1. Although the xylose present in the media was almost all consumed, it could not considerably improve bioethanol production. Accordingly, it was concluded that glucose repression occurred. When the culture medium contains glucose and xylose, sequential sugars consumption is observed, leading to glucose preference as substrate. A similar effect was already reported, revealing that S. passalidarum showed a significantly higher rate of xylose consumption after the glucose had been fully consumed, identical to the behavior of S. stipitis, the known diauxic effect [58,66,71]. Hou [72] suggested that the behavior of S. passalidarum in terms of the consumption of sugars depends on oxygen availability. Under aerobic conditions, glucose and xylose are consumed simultaneously, while glucose repression seems to be more pronounced under anaerobic conditions [72]. The sequential consumption of sugars requires a prolonged fermentation process and leads to incomplete substrate utilization [57]. Recently, Farias et al. [57] pointed out that maintaining a low concentration of glucose during the fermentation process may stimulate the co-fermentation of sugars. This strategy seems to repress the occurrence of diauxic phenomena.
Some strategies have been adopted to overcome this undesirable effect, namely, through cell recycle, fed-batch operation mode, and ethanol in situ extraction [57,61,66]. Farias and colleagues [57] carried out a sequential fed-batch extractive fermentation of sugarcane bagasse hydrolysate supplemented with molasses, using both cell recycling and ethanol removal in situ strategies [57]. The best results were obtained for the second fed-batch run, achieving an ethanol production of 31.4 g·L−1, corresponding to a yield of 0.482 g·g−1 and a conversion efficiency of 94.3%. Therefore, this approach was successfully employed, achieving the highest productivity reported in the literature (9.5 g·L−1·h−1). This boost in efficiency and productivity proved that removing ethanol in situ is a promising technique to avoid inhibition caused by product accumulation [57]. Higher ethanol concentrations were already reported using synthetic media [61,62] or adapted strains [73]. The fermentation parameters of some studies involving the fermentation of different raw materials using S. passalidarum yeast are summarized in Table S3 (Supplementary Materials).
Concluding, although S. passalidarum was able to consume xylose in synthetic media with a low initial sugar concentration, its performance was much lower than previous strains, namely S. cerevisiae and Ethanol Red®. Furthermore, S. passalidarum seems unsuitable for the range of cellulosic sugars concentration present in this hydrolysate. Another possible reason found in the literature that may justify these unexpected results related to the low ethanol production is related to the oxygen requirements. The present work was performed at the Erlenmeyer scale for obtaining preliminary results regarding the fermentation ability of the tested strains using a hydrolysate from E. globulus bark kraft pulp. However, this bench-scale hinders the control of oxygen availability [74]. Moreover, sampling procedures may also contribute to increasing the dissolved oxygen. Some authors have stated that the aeration rate control is one of the most determining parameters to maximize ethanol production from xylose-fermenting yeasts [58,62]. Long and co-workers [73] observed that ethanol production was induced under oxygen-limiting conditions, whereas its formation was not detected in aerobic conditions. Under fully aerobic conditions, the yeast cells exhibited a very long adaptation phase [73]. In contrast, Hou [72], Nakanishi et al. [66] and Veras et al. [75] reported an excellent fermentation performance of S. passalidarum under anaerobic conditions (no aeration). According to Hou [72], using a defined mixed sugar medium, S. passalidarum showed faster cell growth, higher sugar consumption, and ethanol production under oxygen-limiting conditions. Nakanishi et al. [66] proved that similar behavior could be observed by using a sugarcane hydrolysate. Veras et al. [75] corroborate the previous observations, indicating that this strain could ferment relatively well xylose under anaerobiosis. Therefore, it is concluded that there is no broad consensus in the literature regarding the influence of oxygen availability on the fermentation metabolism capacity of S. passalidarum [76]. Further research should be focused on this topic. More in-depth knowledge is crucial for advances in the optimization of the fermentation step using S. passalidarum.
4. Conclusions
Enzymatic hydrolysis of kraft pulp from E. globulus bark, an abundant industrial residue in the Portuguese pulp and paper industry, produced a hydrolysate with a high concentration of cellulosic sugars (about 160 g·L−1) through a fed-batch strategy.
S. cerevisiae and Ethanol Red® demonstrated a high fermentation performance, despite the negligible consumption of xylose. Nevertheless, no improvement in ethanol production was achieved through a simultaneous co-culture of Ethanol Red® and S. passalidarum, a pentose fermenting yeast. Monoculture of S. passalidarum could not produce any bioethanol during 30 h of the assay. It was suspected that probably this strain was inhibited due to bark inhibitors or the high initial concentration of substrate. Even in synthetic media, this strain showed worse performance than S. cerevisiae and Ethanol Red®, with an ethanol concentration ranging from 8.4 to 11.2 g·L−1. S. passalidarum was inhibited by substrate, seeming to be not suitable for fermenting the range of cellulosic sugars concentration present in this hydrolysate. Among all the assays, the best fermentation performance was accomplished by Ethanol Red® industrial strain, attaining a maximum ethanol concentration of about 50 g·L−1 after 20.5 h, corresponding to high productivity (above 2.4 g·L−1·h−1) and conversion efficiency (80%). Comparing to the works already reported in the literature, these can be considered promising results since it was reached a fermentation performance similar to the assays where noble wood was used as raw material.
Future research should be focused on ethanol production at bioreactor-scale from Ethanol Red® industrial strain. A fed-batch approach and SSF configuration might be promising strategies to achieve even higher ethanol titers and reduce costs by integrating steps [29,36,49,77]. Furthermore, from a commercial scale point of view, it could be interesting to study some low-cost supplementation alternatives [78,79,80,81]. However, the complex and highly heterogeneous nature of the bark requires the process to be flexible to variations in its chemical composition [11,82]. Furthermore, the integration of this process depends on a techno-economic assessment, also considering the modifications to be implemented in the existing process [19]. Co-culture fermentation should also be further investigated through the optimization of aeration conditions for xylose fermentation by S. passalidarum. It was found that there is no general understanding regarding the influence of oxygen availability on the metabolism and fermentative capacity of S. passalidarum. Fully knowledge on this topic is crucial for advances on S. passalidarum fermentation ability that would contribute to pentoses fermentation, namely xylose utilization.
Concluding, the present work proved that kraft pulp from E. globulus bark has a high potential to be converted into cellulosic bioethanol. The integration of this valorisation route in the pulp and paper industry within the biorefinery concept might be highly advantageous.
Supplementary Materials
The following tables are available online at https://www.mdpi.com/article/10.3390/en14082185/s1: Table S1: Fermentation parameters of some studies involving the fermentation of E. globulus bark using Ethanol Red® industrial strain; Table S2: Fermentation parameters of some studies involving the fermentation using co-culture strategy and Table S3: Literature results for S. passalidarum fermentation.
Author Contributions
M.S.T.A. carried out the experiments, collected, analyzed data, and wrote the manuscript; J.M.S.R. and A.M.R.B.X. developed the concept, supervised the work, and reviewed the manuscript. L.S.S. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out under the Project InPaCTus—Innovative Products and Technologies from Eucalyptus, Project N. 21874 funded by Portugal 2020 through European Regional Development Fund (ERDF) in the frame of COMPETE 2020 nº246/AXIS II/2017. This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 & UIDP/50011/2020, financed by national funds through the Portuguese Foundation for Science and Technology/MCTES. Authors would also like to thank the CIEPQPF—Strategic Research Centre Project UIDB/00102/2020, funded by the Fundação para a Ciência e Tecnologia (FCT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to The Navigator Company and RAIZ—Instituto de Investigação da Floresta e do Papel for supplying the pulp, the enzymatic consortium and all the equipment required for the enzymatic hydrolysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Resquin, F.; Barrichelo, L.E.G.; da Silva, F.G.; Brito, J.O.; Sansigolo, C.A. Wood quality for kraft pulping of Eucalyptus globulus origins planted in Uruguay. Sci. For. Sci. 2006, 72, 57–66. [Google Scholar]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2019, 37, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Espí, E.; Ribas, Í.; Diáz, C.; Sastrón, Ó. Feedstocks for Advanced Biofuels. In Sustainable Mobility; Llamas, B., Romero, M.F.O., Sillero, E., Eds.; IntechOpen: London, UK, 2020; pp. 1–22. ISBN 9781626239777. [Google Scholar]
- Liguori, R.; Faraco, V. Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour. Technol. 2016, 215, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Branco, R.; Serafim, L.; Xavier, A. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2019, 5, 4. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Hodge, D.B. Integration of (Hemi)-Cellulosic Biofuels Technologies with Chemical Pulp Production. In Biorefineries: Integrated Biochemical Processes for Liquid Biofuels; Qureshi, N., Hodge, D., Vertes, A., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; pp. 73–100. ISBN 9780444595041. [Google Scholar]
- Celpa: Associação da Indústria Papeleira. Boletim Estatístico 2017: Indústria Papeleira Portuguesa. Available online: http://www.celpa.pt/wp-content/uploads/2018/10/Boletim_WEB-2.pdf (accessed on 26 February 2021).
- Neiva, D.M.; Araújo, S.; Gominho, J.; Carneiro, A.C.; Pereira, H. Potential of Eucalyptus globulus industrial bark as a biorefinery feedstock: Chemical and fuel characterization. Ind. Crop. Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Pinto, P.C.O.R.; Barreiro, M.F.; Costa, C.A.E.; Mota, M.I.F.; Fernandes, I. Chemical Pulp Mills as Biorefineries. In An Integrated Approach for Added-Value Products from Lignocellulosic Biorefineries: Vanillin, Syringaldehyde, Polyphenols, and Polyurethane; Springer Nature Switzerland AG: Cham, Switzerland, 2018; pp. 1–51. ISBN 9783319993126. [Google Scholar]
- Pinto, F.; Gominho, J.; André, R.N.; Gonçalves, D.; Miranda, M.; Varela, F.; Neves, D.; Santos, J.; Lourenço, A.; Pereira, H. Improvement of gasification performance of Eucalyptus globulus stumps with torrefaction and densification pre-treatments. Fuel 2017, 206, 289–299. [Google Scholar] [CrossRef]
- Souza, A.G.; Lima, G.F.; Rodrigues, R.C.L.B.; Cesarino, I.; Leão, A.L.; Rosa, D.S. A New Approach for Conversion of Eucalyptus Lignocellulosic Biomass into Cellulose Nanostructures: A Method that Can Be Applied in Industry. J. Nat. Fibers 2019, 1–11. [Google Scholar] [CrossRef]
- Vea, E.B.; Romeo, D.; Thomsen, M. Biowaste Valorisation in a Future Circular Bioeconomy. In Proceedings of the 25th CIRP Life Cycle Engineering (LCE) Conference, Copenhagen, Denmark, 30 April–2 May 2018; Volume 69, pp. 591–596. [Google Scholar]
- Celpa—Associação da Indústria Papeleira. Boletim Estatístico Indústria Papeleira Portuguesa 2019. Lisboa, 2020. Available online: http://www.celpa.pt/category/boletins-estatisticos/ (accessed on 26 February 2021).
- Faubert, P.; Barnabé, S.; Bouchard, S.; Côté, R.; Villeneuve, C. Pulp and paper mill sludge management practices: What are the challenges to assess the impacts on greenhouse gas emissions? Resour. Conserv. Recycl. 2016, 108, 107–133. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of phenolic components in polar extracts of Eucalyptus globulus labill. Bark by high-performance liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef] [PubMed]
- Romaní, A.; Michelin, M.; Domingues, L.; Teixeira, J.A. Valorization of wastes from agrofood and pulp and paper industries within the biorefinery concept: Southwestern Europe scenario. In Waste Biorefinery: Potential and Perspectives; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; pp. 487–504. ISBN 9780444639936. [Google Scholar]
- Domingues, R.M.A.; Sousa, G.D.A.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Eucalyptus globulus biomass residues from pulping industry as a source of high value triterpenic compounds. Ind. Crop. Prod. 2010, 31, 65–70. [Google Scholar] [CrossRef]
- Ek, M.; Gellerstedt, G. Pulping Chemistry and Technology. In Pulp and Paper Chemistry and Technology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; Walter de Gruyter: Berlin, Germany, 2009; Volume 2. [Google Scholar]
- Ressel, J.B. Wood Yard Operations. In Handbook of Pulp; Sixta, H., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; Volume 1, pp. 69–107. ISBN 3527309993. [Google Scholar]
- Neiva, D.M.; Costa, R.A.; Gominho, J.; Ferreira-Dias, S.; Pereira, H. Fractionation and valorization of industrial bark residues by autohydrolysis and enzymatic saccharification. Bioresour. Technol. Reports 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Guerrero, M. Portugal Biofuels Standing Report 2015; USDA Foreign Agricultural Service: Global Agricultural Information Network: Madrid, Spain, 2015.
- Neiva, D.M.; Luís, Â.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark residues valorization potential regarding antioxidant and antimicrobial extracts. Wood Sci. Technol. 2020, 54, 559–585. [Google Scholar] [CrossRef]
- Kemppainen, K.; Inkinen, J.; Uusitalo, J.; Nakari-Setälä, T.; Siika-aho, M. Hot water extraction and steam explosion as pretreatments for ethanol production from spruce bark. Bioresour. Technol. 2012, 117, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.I.F.; Pinto, P.C.O.R.; Novo, C.C.; Sousa, G.D.A.; Guerreiro, O.; Guerra, Â.; Duarte, M.F.P.; Rodrigues, A.E. Eucalyptus globulus bark as a source of polyphenolic compounds with biological activity. O Pap. 2013, 74, 57–64. [Google Scholar]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef]
- Gomes, D.G.; Michelin, M.; Romaní, A.; Domingues, L.; Teixeira, J.A. Co-production of biofuels and value-added compounds from industrial Eucalyptus globulus bark residues using hydrothermal treatment. Fuel 2021, 285, 119265. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the economy of lignocellulose-based biorefineries with organosolv pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Recent advances in membrane technologies for biorefining and bioenergy production. Biotechnol. Adv. 2012, 30, 817–858. [Google Scholar] [CrossRef]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from lignocellulosic biomass: Current findings determine research priorities. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Guigou, M.; Cabrera, M.N.; Vique, M.; Bariani, M.; Guarino, J.; Ferrari, M.D.; Lareo, C. Combined pretreatments of eucalyptus sawdust for ethanol production within a biorefinery approach. Biomass Convers. Biorefin. 2019, 9, 293–304. [Google Scholar] [CrossRef]
- Chen, H.Z.; Liu, Z.H. Enzymatic hydrolysis of lignocellulosic biomass from low to high solids loading. Eng. Life Sci. 2017, 17, 489–499. [Google Scholar] [CrossRef]
- Gomes, A.C.; Moysés, D.N.; Santa Anna, L.M.M.; Castro, A.M. Fed-batch strategies for saccharification of pilot-scale mild-acid and alkali pretreated sugarcane bagasse: Effects of solid loading and surfactant addition. Ind. Crop. Prod. 2018, 119, 283–289. [Google Scholar] [CrossRef]
- He, L.; Han, Q.; Jameel, H.; Chang, H.M.; Phillips, R.; Wang, Z. Comparison of One-Stage Batch and Fed-Batch Enzymatic Hydrolysis of Pretreated Hardwood for the Production of Biosugar. Appl. Biochem. Biotechnol. 2018, 184, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yunyun, L.; Jingliang, X.; Zhanhong, Y.; Wei, Q.; Xinshu, Z.; MinChao, H. High solid and low enzyme loading based saccharification of agricultural biomass. BioResources 2012, 7, 345–353. [Google Scholar] [CrossRef]
- Branco, R.H.R.; Amândio, M.S.T.; Serafim, L.S.; Xavier, A.M.R.B. Ethanol production from hydrolyzed kraft pulp by mono- and co-cultures of yeasts: The challenge of C6 and C5 sugars consumption. Energies 2020, 13, 744. [Google Scholar] [CrossRef]
- Radler, F.; Schütz, H. Glycerol production of various strains of Saccharomyces. Am. J. Enol. Vitic. 1982, 33, 36–40. [Google Scholar]
- Scanes, K.T.; Hohmann, S.; Prior, B.A. Glycerol Production by the Yeast Saccharomyces cerevisiae and its Relevance to Wine: A Review. S. Afr. J. Enol. Vitic. 1998, 19, 17–24. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Y.; Guo, Z.; Ding, Z.; Shi, G. Improving the ethanol yield by reducing glycerol formation using cofactor regulation in Saccharomyces cerevisiae. Biotechnol. Lett. 2011, 33, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chang, K.S.; Huang, C.W.; Hsu, C.L.; Jang, H. Der Comparison of batch and fed-batch fermentations using corncob hydrolysate for bioethanol production. Fuel 2012, 97, 166–173. [Google Scholar] [CrossRef]
- Coote, N.; Kirsop, B.H. Factors responsible for the decrease in pH during beer fermentations. J. Inst. Brew. 1976, 82, 149–153. [Google Scholar] [CrossRef]
- Monrroy, M.; Garcia, J.-R.; Mendonça, R.T.; Baeza, J.; Freer, J. Kraft pulping of Eucalyptus globulus as a pretreatment for bioethanol production by simultaneous saccharification and fermentation. J. Chil. Chem. Soc. 2012, 57, 1113–1117. [Google Scholar] [CrossRef]
- Wistara, N.J.; Pelawi, R.; Fatriasari, W. The Effect of Lignin Content and Freeness of Pulp on the Bioethanol Productivity of Jabon Wood. Waste Biomass Valorization 2016, 7, 1141–1146. [Google Scholar] [CrossRef]
- Przybysz Buzała, K.; Kalinowska, H.; Małachowska, E.; Przybysz, P. The utility of selected kraft hardwood and softwood pulps for fuel ethanol production. Ind. Crops Prod. 2017, 108, 824–830. [Google Scholar] [CrossRef]
- Frankó, B.; Galbe, M.; Wallberg, O. Bioethanol production from forestry residues: A comparative techno-economic analysis. Appl. Energy 2016, 184, 727–736. [Google Scholar] [CrossRef]
- Mendes, C.V.T.; Rocha, J.M.S.; Menezes, F.F.; Carvalho, M.G.V.S. Batch and fed-batch simultaneous saccharification and fermentation of primary sludge from pulp and paper mills. Environ. Technol. 2017, 38, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Edgardo, A.; Carolina, P.; Manuel, R.; Juanita, F.; Baeza, J. Selection of thermotolerant yeast strains Saccharomyces cerevisiae for bioethanol production. Enzyme Microb. Technol. 2008, 43, 120–123. [Google Scholar] [CrossRef]
- Ko, C.H.; Wang, Y.N.; Chang, F.C.; Chen, J.J.; Chen, W.H.; Hwang, W.S. Potentials of lignocellulosic bioethanols produced from hardwood in Taiwan. Energy 2012, 44, 329–334. [Google Scholar] [CrossRef]
- Kossatz, H.L.; Rose, S.H.; Viljoen-Bloom, M.; van Zyl, W.H. Production of ethanol from steam exploded triticale straw in a simultaneous saccharification and fermentation process. Process. Biochem. 2017, 53, 10–16. [Google Scholar] [CrossRef]
- Demeke, M.M.; Dietz, H.; Li, Y.; Foulquié-Moreno, M.R.; Mutturi, S.; Deprez, S.; Den Abt, T.; Bonini, B.M.; Liden, G.; Dumortier, F.; et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol. Biofuels 2013, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Leaf by Lesaffre Industrial Ethanol. Available online: https://lesaffreadvancedfermentations.com/ethanol_yeast/ (accessed on 2 February 2021).
- Frankó, B.; Galbe, M.; Wallberg, O. Influence of bark on fuel ethanol production from steam-pretreated spruce. Biotechnol. Biofuels 2015, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Collograi, K.C.; da Costa, A.C.; Ienczak, J.L. Effect of contamination with Lactobacillus fermentum I2 on ethanol production by Spathaspora passalidarum. Appl. Microbiol. Biotechnol. 2019, 103, 5039–5050. [Google Scholar] [CrossRef]
- Farias, D.; Maugeri-Filho, F. Sequential fed batch extractive fermentation for enhanced bioethanol production using recycled Spathaspora passalidarum and mixed sugar composition. Fuel 2020, 288, 119673. [Google Scholar] [CrossRef]
- Bonan, C.I.D.G.; Biazi, L.E.; Dionísio, S.R.; Soares, L.B.; Tramontina, R.; Sousa, A.S.; de Oliveira Filho, C.A.; Costa, A.C.; Ienczak, J.L. Redox potential as a key parameter for monitoring and optimization of xylose fermentation with yeast Spathaspora passalidarum under limited-oxygen conditions. Bioprocess. Biosyst. Eng. 2020, 43, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.; Maugeri Filho, F. Co-culture strategy for improved 2G bioethanol production using a mixture of sugarcane molasses and bagasse hydrolysate as substrate. Biochem. Eng. J. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Chen, Y. Development and application of co-culture for ethanol production by co-fermentation of glucose and xylose: A systematic review. J. Ind. Microbiol. Biotechnol. 2011, 38, 581–597. [Google Scholar] [CrossRef]
- Neitzel, T.; Lima, C.S.; Biazi, L.E.; Collograi, K.C.; Carvalho da Costa, A.; Vieira dos Santos, L.; Ienczak, J.L. Impact of the Melle-Boinot process on the enhancement of second-generation ethanol production by Spathaspora passalidarum. Renew. Energy 2020, 160, 1206–1216. [Google Scholar] [CrossRef]
- Su, Y.K.; Willis, L.B.; Jeffries, T.W. Effects of aeration on growth, ethanol and polyol accumulation by Spathaspora passalidarum NRRL Y-27907 and Scheffersomyces stipitis NRRL Y-7124. Biotechnol. Bioeng. 2015, 112, 457–469. [Google Scholar] [CrossRef]
- Fátima Rodrigues Souza, R.; Dutra, E.D.; Leite, F.C.B.; Cadete, R.M.; Rosa, C.A.; Stambuk, B.U.; Stamford, T.L.M.; Morais, M.A. Production of ethanol fuel from enzyme-treated sugarcane bagasse hydrolysate using d-xylose-fermenting wild yeast isolated from Brazilian biomes. 3 Biotech 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Hou, X.; Yao, S. Improved inhibitor tolerance in xylose-fermenting yeast Spathaspora passalidarum by mutagenesis and protoplast fusion. Appl. Microbiol. Biotechnol. 2012, 93, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Li, Y.; Zhao, X.; Pei, X.; Yuan, W.; Bai, F.; Jiang, Y. The production of ethanol from lignocellulosic biomass by Kluyveromyces marxianus CICC 1727-5 and Spathaspora passalidarum ATCC MYA-4345. Appl. Microbiol. Biotechnol. 2019, 103, 2845–2855. [Google Scholar] [CrossRef]
- Nakanishi, S.C.; Soares, L.B.; Biazi, L.E.; Nascimento, V.M.; Costa, A.C.; Rocha, G.J.M.; Ienczak, J.L. Fermentation strategy for second generation ethanol production from sugarcane bagasse hydrolyzate by Spathaspora passalidarum and Scheffersomyces stipitis. Biotechnol. Bioeng. 2017, 114, 2211–2221. [Google Scholar] [CrossRef]
- Morales, P.; Gentina, J.C.; Aroca, G.; Mussatto, S.I. Development of an acetic acid tolerant Spathaspora passalidarum strain through evolutionary engineering with resistance to inhibitors compounds of autohydrolysate of Eucalyptus globulus. Ind. Crop. Prod. 2017, 106, 5–11. [Google Scholar] [CrossRef]
- Soares, L.B.; Bonan, C.I.D.G.; Biazi, L.E.; Dionísio, S.R.; Bonatelli, M.L.; Andrade, A.L.D.; Renzano, E.C.; Costa, A.C.; Ienczak, J.L. Investigation of hemicellulosic hydrolysate inhibitor resistance and fermentation strategies to overcome inhibition in non-saccharomyces species. Biomass Bioenergy 2020, 137, 105549. [Google Scholar] [CrossRef]
- Su, Y.K.; Willis, L.B.; Rehmann, L.; Smith, D.R.; Jeffries, T.W. Spathaspora passalidarum selected for resistance to AFEX hydrolysate shows decreased cell yield. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef]
- Yu, H.; Guo, J.; Chen, Y.; Fu, G.; Li, B.; Guo, X.; Xiao, D. Efficient utilization of hemicellulose and cellulose in alkali liquor-pretreated corncob for bioethanol production at high solid loading by Spathaspora passalidarum U1-58. Bioresour. Technol. 2017, 232, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rodrussamee, N.; Sattayawat, P.; Yamada, M. Highly efficient conversion of xylose to ethanol without glucose repression by newly isolated thermotolerant Spathaspora passalidarum CMUWF1-2. BMC Microbiol. 2018, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Hou, X. Anaerobic xylose fermentation by Spathaspora passalidarum. Appl. Microbiol. Biotechnol. 2012, 94, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Long, T.M.; Su, Y.K.; Headman, J.; Higbee, A.; Willis, L.B.; Jeffries, T.W. Cofermentation of glucose, xylose, and cellobiose by the beetle-associated yeast Spathaspora passalidarum. Appl. Environ. Microbiol. 2012, 78, 5492–5500. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Kostov, Y.; Harms, P.; Rao, G. Noninvasive measurement of dissolved oxygen in shake flasks. Biotechnol. Bioeng. 2002, 80, 594–597. [Google Scholar] [CrossRef]
- Veras, H.C.T.; Parachin, N.S.; Almeida, J.R.M. Comparative assessment of fermentative capacity of different xylose-consuming yeasts. Microb. Cell Fact. 2017, 16, 153. [Google Scholar] [CrossRef]
- Selim, K.A.; Easa, S.M.; El-Diwany, A.I. The xylose metabolizing yeast Spathaspora passalidarum is a promising genetic treasure for improving bioethanol production. Fermentation 2020, 6, 33. [Google Scholar] [CrossRef]
- Mendes, C.V.T.; Carvalho, M.G.V.S.; Rocha, J.M.S. Bioethanol production from cellulosic fibers: Comparison between batch and fed-batch saccharification. Cellul. Chem. Technol. 2017, 51, 291–299. [Google Scholar]
- Raposo, S.; Constantino, A.; Rodrigues, F.; Rodrigues, B.; Lima-Costa, M.E. Nitrogen Sources Screening for Ethanol Production Using Carob Industrial Wastes. Appl. Biochem. Biotechnol. 2017, 181, 827–843. [Google Scholar] [CrossRef]
- Pereira, F.B.; Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Optimization of low-cost medium for very high gravity ethanol fermentations by Saccharomyces cerevisiae using statistical experimental designs. Bioresour. Technol. 2010, 101, 7856–7863. [Google Scholar] [CrossRef] [PubMed]
- Kadam, K.L.; Newman, M.M. Development of a low-cost fermentation medium for ethanol production from biomass. Appl. Microbiol. Biotechnol. 1997, 47, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Pejó, E.; Negro, M.J.; Sáez, F.; Ballesteros, M. Effect of nutrient addition on preinoculum growth of S. cerevisiae for application in SSF processes. Biomass Bioenergy 2012, 45, 168–174. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, S.; Yuan, Z.; Leitch, M.; Xu, C. Valorization of bark for chemicals and materials: A review. Renew. Sustain. Energy Rev. 2013, 26, 560–578. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).