3.1. Physico-Chemical Characterisation of Crude Fibers
The main component in both types of cellulosic fibers from date palm is the cellulose, then lignin and hemicelluloses (
Table 1). In young cellulosic fibers, it was achieved a cellulose content of 57.42%, and 62.31% for old cellulosic fibers. These results were similar to those cited in a previous research [
34]. In fact, old cellulosic fibers were more lignified, at 21.12%, than young ones, at 13.44%.
However, the high percentage of lignin presents a real problem for access of lignocellulolytic enzymes into the interior lignocellulose structure [
35]. Furthermore, the low percentage of mineral material in both types of cellulosic fibers explains that the raw material has a low capacity to produce ash during its combustion. Cellulosic fibers from date palm are the richest lignocellulose biomass in terms of cellulose compared to other biomasses, like: rosemary [
36], olive stone [
37], and olive pomace [
38], which are more lignified than cellulosic fibers from date palm. It is also richer in cellulose content than wheat straw [
39] and sugarcane bagasse [
40].
Thus, this high content of cellulose of the cellulosic fibers from date palm waste makes it a potential source of cellobiose and fermentable monomeric sugars, which could be liberated during pretreatment and enzymatic hydrolysis steps, in order to produce bioethanol. In general, low concentrations of lignin, particularly in the cellulosic fibers from date palm, decrease the liberation inhibitors that block the activities of enzymes and microorganisms, during saccharification and fermentation processes, respectively [
41].
3.2. Structural Characterisation of Cellulosic Fibers from Date Palm Fibers by ATR-FTIR
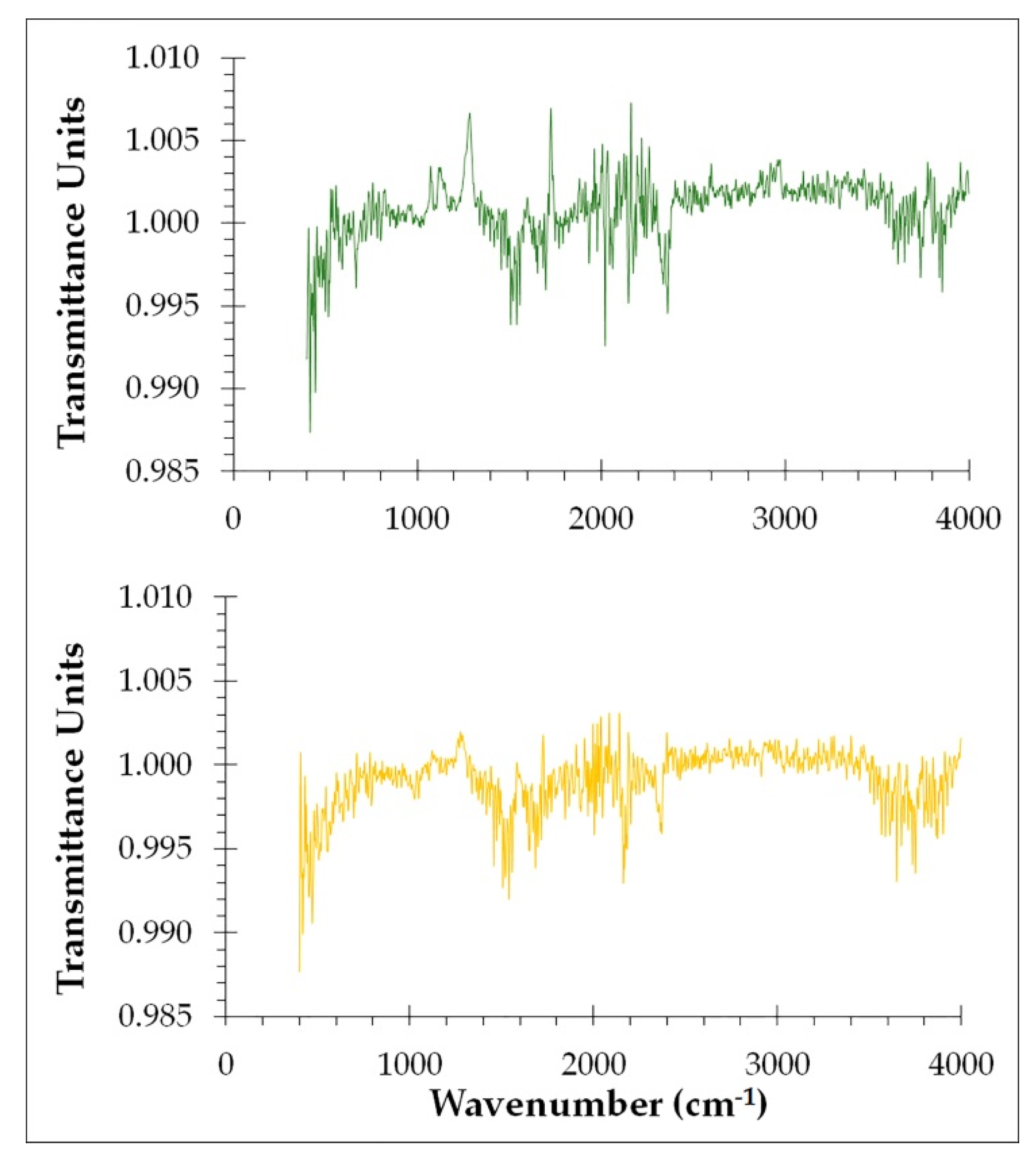
In order to track the structure of both young and old cellulosic fibers of ‘
P. dactylifera L.’ ATR-FTIR spectroscopy was employed to explore functional groups and molecular vibration detection (
Figure 3). This method has the ability to identify or characterise both the organic and inorganic components of a sample. The idea behind this analysis method is that no two molecules that are structurally different will produce the same infrared spectrum, which is useful in positively identifying samples. Different samples have different compositions, resulting in various infrared absorption band intensities at different wavenumbers, which allows for the characterization and comparison of samples. In base of previous research bibliographic review [
21,
22,
42], band identification was performed.
Many bands were associated with lignin for young cellulosic fibers from date palm (
Figure 3, green lines); the bands located in the regions 3518 cm
−1 and 3697 cm
−1 were assigned to O-H stretching vibration. Indeed, another region at 2970 cm
−1 was attributed to C-H stretching vibration of lignin. On the other hand, the presence of extractive compounds in young cellulosic fibers from date palm was proved by stretching vibration bands of the functional groups C-O, C-C or C=C, and C=O observed at 1271 cm
−1, 1529 cm
−1 and 1730 cm
−1, respectively. Furthermore, the functional group associated with the free ester of hemicellulose of young cellulosic fibers from date palm was highlighted through the vibration bands located at 1726 cm
−1. Moreover, C-H bending vibration was shown at 1285 cm
−1, which was an indicator of the crystalline cellulose presence in young cellulosic fibers.
In relation to the spectrum obtained from old cellulosic fibers (
Figure 3, yellow lines), it shows many functional groups of lignin located at different wavenumbers. In fact, bands located at 3578 cm
−1, 1665 cm
−1, 1583 cm
−1, 1513 cm
−1, 1467 cm
−1, 1280 cm
−1 and 835 cm
−1 correspond to O-H stretching vibration, C=O stretching vibration (unconjugated), aromatic ring and C=O stretching vibrations, C-C and C=C stretching vibrations, C-H deformation vibration, aromatic ring vibration of guaiacyl lignin, and O-H or C-H deformation of lignin, respectively. Moreover, the functional groups of extractives of old cellulosic fibers from date palm are shown through the vibration bands located at 1730 cm
−1, 1510 cm
−1 and 1271 cm
−1, which were attributed to C=O, C-C+C=C, and C-O stretching vibrations, respectively. Likewise, hemicellulose and crystalline cellulose of old cellulosic fibers from date palm are highlighted through their C=O stretching vibration bands (ketone/aldehyde) and C-H bending vibration bands, respectively, which were shown at 1726 cm
−1 and 1280 cm
−1, in each case. Thus, the conclusions about chemical structure characterisation obtained from ATR-FTIR spectroscopy confirm the lignocellulose nature of both young and old cellulosic fibers. In fact, these results are similar to those reported from ATR-FTIR spectroscopy for date palm ‘
P. dactylifera L.’ leaflets and rachis in a hydrothermal pretreatment of this feedstock studied in order to enhance enzymatic digestibility and bioethanol potential [
43].
Similarly, in a previous study to improve bioethanol production from corn stover [
44], the results reported from FTIR spectra were similar to those obtained during this study. The same tendency was shown from FTIR spectroscopy when greenhouse residues were used as raw material to improve methane production [
45].
3.3. Thermochemical Pretreatment of Cellulosic Fibers from Date Palms
After thermochemical pretreatment, dry solid residues were characterised by the methods described in ‘Characterisation of solid fraction’, determining percentages in residual solids (
S), ash (
P1), holocelluloses (
P2) and insoluble lignin (
P3) for both young and old cellulosic fibers from date palms (
Table 2 and
Table 3, respectively). In relation to hydrolysates obtained (liquid fraction), they were analysed in
d-glucose (
C1),
TRS (
C2), and acetic acid (
C3) for both types of pretreated cellulosic fibers from date palm (
Table 2 and
Table 3).
In the case of young cellulosic fibers, higher values of severity factor (Log
Ro) were reached due to severe conditions (greater concentrations of sulfuric acid and higher temperatures during pretreatment). Furthermore, as the pretreatment temperature was increased, lower residual solids content
(S) values were determined (
Table 2); therefore, this implied a higher percentage of solids solubilisation. This fact could be attributed to the hemicelluloses hydrolysis. In relation to the percentage of insoluble lignin (
P3), it was higher in severe conditions; in fact, the maximum percentage of
AIL, 82.13%, was achieved when log
Ro = 6.58 (220 °C, H
2SO
4 0.05 M). The minimum percentages in holocelluloses were reached at 185 °C and H
2SO
4 0.0125 M in the central point of experimental design (10.61%, 13.05% and 13.35%),
Table 2; however, a minimum in holocelluloses, close to 10.0%, was reached at 220 °C and H
2SO
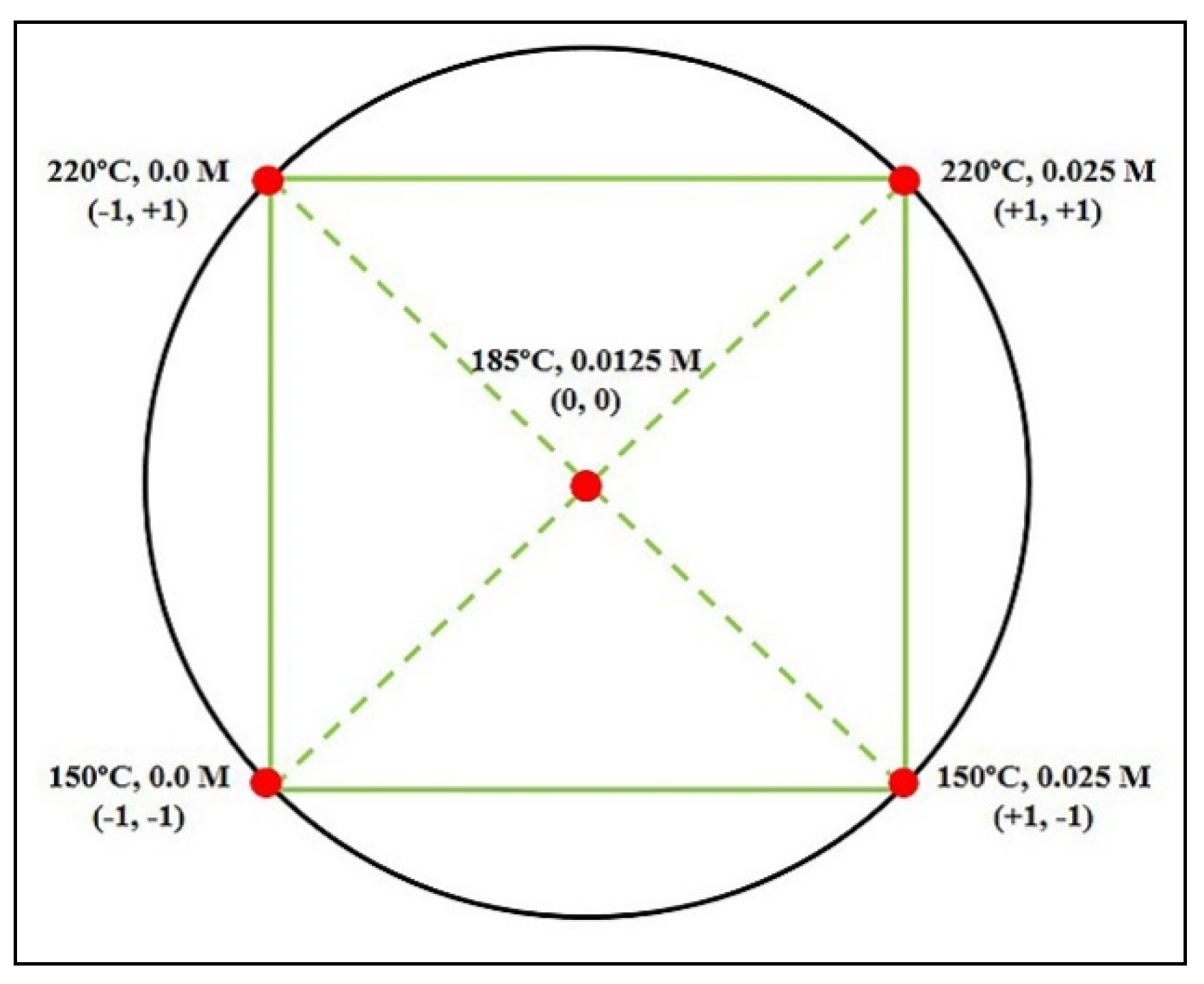
4 0.025 M. According to the full factorial experimental design matrix,
Figure 2, the maximum concentrations of
TRS, 3.17 g/L and 3.98 g/L, were determined at 220 °C and without sulfuric acid, and 150 °C and H
2SO
4 0.025 M, respectively,
Table 2.
During old cellulosic fibers thermochemical pretreatment, the higher values of residual solids content (95.26%, 94.16% and 94.58%) were achieved at 185 °C and concentration of sulfuric acid 0.0125 M,
Table 3; these medium conditions could be considered severe.
In general, when the pretreatment was carried out at fixed of H
2SO
4 concentration, the percentage in holocellulose decreased as the temperature was increased; percentages of holocellulose contents were maximum at 185 °C and H
2SO
4 0.0125 M in the central point (17.80%, 17.52% and 17.35%) (
Table 3). On the other hand, logically, contrary behavior was observed respect to insoluble lignin content; in fact, percentage of
IAL increased at higher temperatures. Moreover, at 220 °C and hydrothermal conditions (0.0 M H
2SO
4), the percentage of holocelluloses and lignin was 61.97% and 37.22%, respectively (
Table 3). These data proved that severe conditions of thermochemical pretreatment enhanced the digestibility of the complex carbohydrates in order to produce monomeric sugars. In fact, the major concentration of reducing sugars, 2.06 g/L, was achieved in the thermochemical pretreatment carried out at 220 °C and H
2SO
4 0.0 M (
Table 3).
Maximum percentages in holocellulose were reached in hydrothermal conditions, at 220 °C for young pretreated cellulosic fibers (45.18%) and 150 °C for old pretreated cellulosic fibers (67.00%) (
Table 2 and
Table 3, respectively). The minimum percentages of insoluble lignin were achieved in hydrothermal conditions, at 220 °C for young pretreated (58.30%), and 150 °C in case of old pretreated cellulosic fibers (27.49%). Fang et al., in at hydrothermal pretreatment of the date palm Phoenix dactylifera L. leaflets and rachis, determined at 210 °C the highest lignin content in pretreated leaflets (64.95%) and in pretreated rachis (45.95%) [
43].
The maximum concentration of acetic acid, 0.56 g/L, was reached for old fibers at 220 °C and H2SO4 0.0 M (severity factor = 6.65), while d-glucose concentration in these conditions was very small, 0.08 g/L.
The relationship between each response (log
Ro,
S,
P1,
P2,
P3,
C1,
C2 and
C3) and each factor (temperature and sulfuric acid concentration) was expressed referring to the coded model described in section ‘Experimental methodology’.
Table 4 and
Table 5 show the empirical equations for each response and the adequate optimal conditions for the thermochemical pretreatment of young and old cellulosic fibers. By way of example, the adjustments made for the fraction of residual solids and holocellulose contents are represented in
Figure 4 and
Figure 5, respectively.
Comparing the results obtained for the pretreatment of young and old cellulosic fibers with other lignocelluloses feedstock, it is possible to conclude that our substrate is the best one to conduct the subsequent enzymatic saccharification. In this sense, the solid fractions of the olive stones pretreated at 220 °C with a solution of sulfuric acid 0.025 M, are composed by 56.8% of lignin and 17.4% of holocelluloses [
37]. On the other hand, rosemary fibers, pretreated at 150 °C and with 0.025 M of sulfuric acid, generated 17.0% of lignin and 81.0% of holocelluloses [
36].
As a result of the previous experiments of both young and old cellulosic fibers, the optimal pretreatment conditions prior to the enzymatic hydrolysis are 220 °C without acid; it is justified due to the significant amount of holocellulose under operation conditions (
Table 2 and
Table 3). These results are similar to those obtained in a previous research refereed to hydrothermal pretreatment of date palm
Phoenix dactylifera L. leaflets and rachis [
42]; in this case, the process was carried out under optimal conditions of hydrothermal pretreatment (210 °C for 10 min), these being the best conditions to enhance enzymatic digestibility and later bioethanol production.
3.4. Enzymatic Hydrolysis
Pretreatment is a step to improve the enzymatic digestibility of highly crystallised lignocellulosic biomass [
46]. Thus, in order to produce
d-glucose, an enzymatic hydrolysis of the solid fractions from the pretreatment step has been carried out, which was performed under optimal conditions at 220 °C and H
2SO
4 0.0 M (maximum
TRS concentrations). During the enzymatic hydrolysis, liquid samples were taken in order to analyse the concentrations of
TRS and
d-glucose, for both young and old cellulosic fibers (
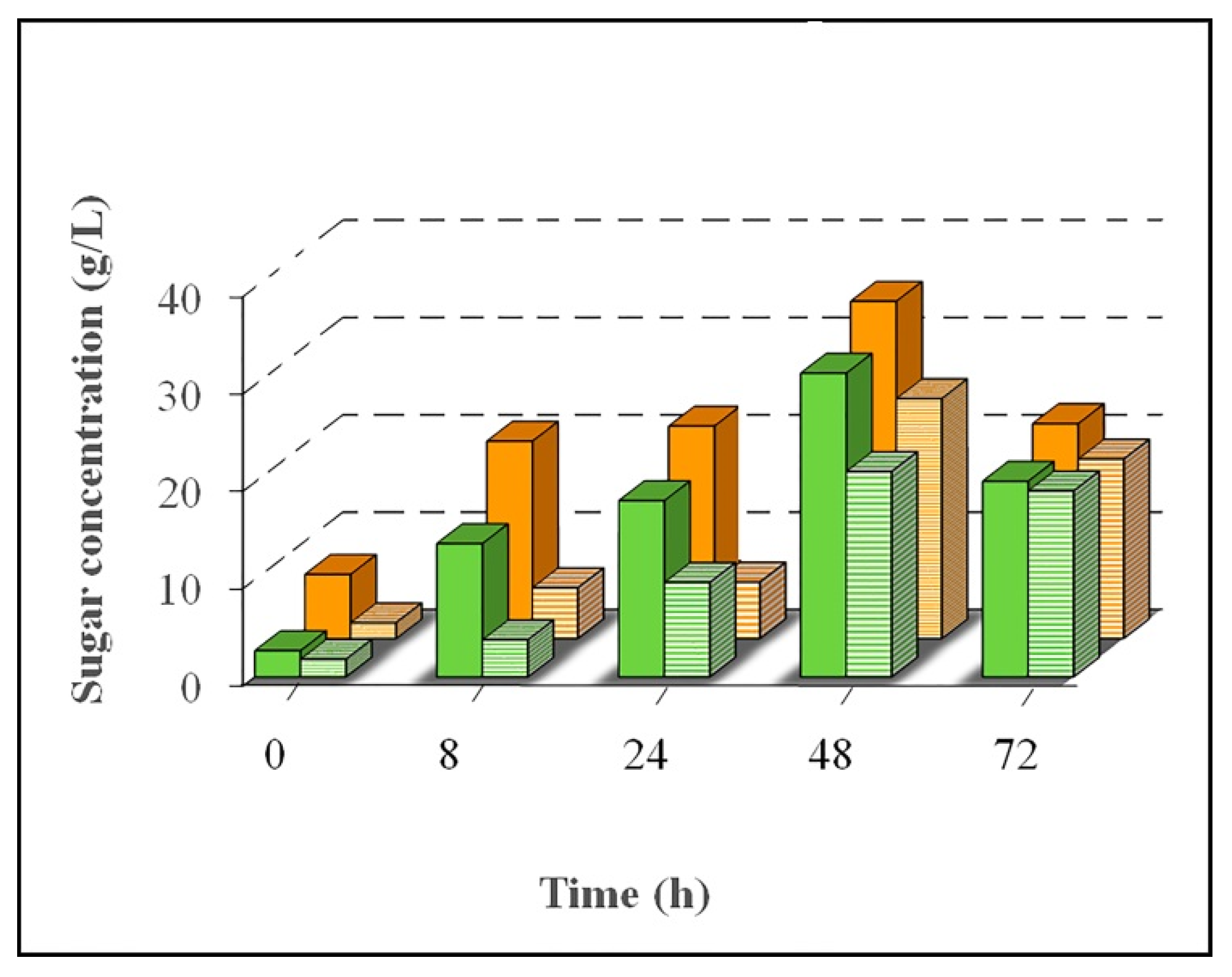
Figure 6); moreover, acetic acid concentration was determined for each type of cellulosic fibers.
At the beginning of enzymatic hydrolysis
TRS concentration increased. After a 48 h process,
TRS concentrations decreased for both pretreated young and old cellulosic fibers (
Figure 6). In fact, in the case of young cellulosic fibers, initial
TRS concentration, 2.7 g/L, increased to 31.2 g/L at 48 h and decreased to 20.1 g/L after 72 h of enzymatic hydrolysis (
Figure 6). The same tendency was observed in
d-glucose concentration for both young and old cellulosic fibers (
Figure 6). During enzymatic hydrolysis of pretreated old cellulosic fibers, initial
d-glucose concentration, 1.6 g/L, increased until reaching 24.6 g/L at 48 h; from this point of the process a decrease of
d-glucose concentration was observed (18.4 g/L at 72 h),
Figure 6.
These diminutions can be due to the inhibitors (among them acetic acid and furfural) released during the pretreatment stage, or could it be due to microbial (contaminant) activity, such as an unwanted fermentation of
d-glucose. The inhibitors presence has a negative influence in the process since it reduces the activity of cellulases and, with it, the production of
d-glucose [
47].
During enzymatic hydrolysis, maximum TRS yields (YTRS) were reached at 48 h for both pretreated cellulosic fibers from date palms, 0.46 g TRS/g dry pretreated young fiber, and 0.49 g TRS/g dry pretreated old fiber. Furthermore, maximum d-glucose yields (YG) were determined at 48 h for both young and old cellulosic fibers (0.31 g d-glucose/g dry pretreated young fiber and 0.35 g d-glucose/g dry pretreated old fiber, respectively).
During enzymatic hydrolysis, acetic acid concentration increased until reaching 2.5 g/L and 2.1 g/L at 72 h for young and old cellulosic fibers, respectively.
3.5. Fermentation Process
Fermentation assays of hydrolysates of pretreated young and old cellulosic fibers, derived from the enzymatic hydrolysis, were carried out by
P. tannophilus yeast. The concentrations of
d-xylose,
l-arabinose, D-mannose and D-galactose were determined at the beginning and end of the fermentation processes. Their concentrations were very small and at the end of the fermentation process it was found that they were completely consumed except for
d-xylose and
l-arabinose (
Table 6).
During the fermentation of the hydrolysates by P. tannophilus yeast, ethanol was produced until reaching a maximum concentration, 12.40 g/L for young fibers and 10.20 g/L for old fibers, after 6 h and 7 h of fermentation. After that, there was a decrease in ethanol concentration, which could be explained by the evaporation of this product as well as its possible consumption by the yeast (metabolic routes).
If the complete process is considered, from the initial raw material to the bioproduct production, ethanol yields (
YE) were 10.64 g ethanol/100 g of dry fiber for young fibers and 10.88 g ethanol/100 g of dry fiber for old fibers (
Figure 7). These values are similar to those obtained for ethanol yields in the fermentation of olive stones by
P. tannophilus [
37].
Ethanol yields referred to
TRS (
YE/TRS) and
d-glucose consumption (
YE/G) were 37.94 g ethanol/100 g
TRS and 39.68 g ethanol/100 g
d-glucose, respectively, for young cellulosic fibers from date palm; in case of old cellulosic fibers, it was reached 35.84 g ethanol/100 g
TRS and 38.90 g ethanol/100 g
d-glucose. It should be noted that these obtained yields are high, and considering the theoretical yield (or Gay-Lussac yield) referred to
d-glucose (0.511 g ethanol/g
d-glucose) the
YE/G for young fiber represents 77.7% of the theoretical yield and for old fiber 76.1%. In previous studies that referred to microbial fermentation of juice extracted from sugarcorn plants in order to produce ethanol [
48], it was reached similar values in ethanol yield. In fact, sugarcorn juice used in the study contained a maximum of 145 g/L carbohydrates, with sucrose,
d-glucose and D-fructose accounting for 80% of the sugars. In the fermentation of this juice by
S. cerevisiae it was determined similar values, 45.6 g/L ethanol in 72 h (ethanol yield = 0.41 g ethanol per g carbohydrates).
Nowadays, there are some studies that improve
d-xylose assimilation by yeasts which is the more abundant component in plant cells, which can lead to higher bioethanol productivity [
49].
This study proves that biofuel yield from date palm waste via fermentation is high, as recent research also shows [
50].