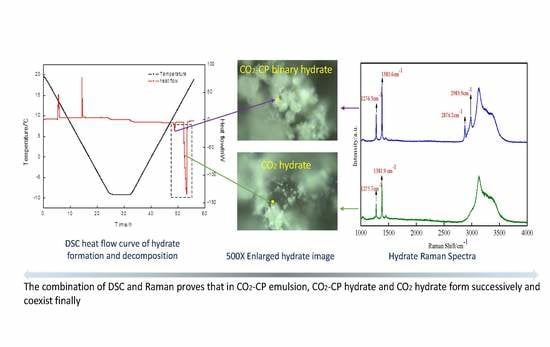
The Relationship between Thermal Characteristics and Microstructure/Composition of Carbon Dioxide Hydrate in the Presence of Cyclopentane
Abstract
1. Introduction
2. Experimental
2.1. Experimental Apparatus and Materials
2.2. Experimental Procedure
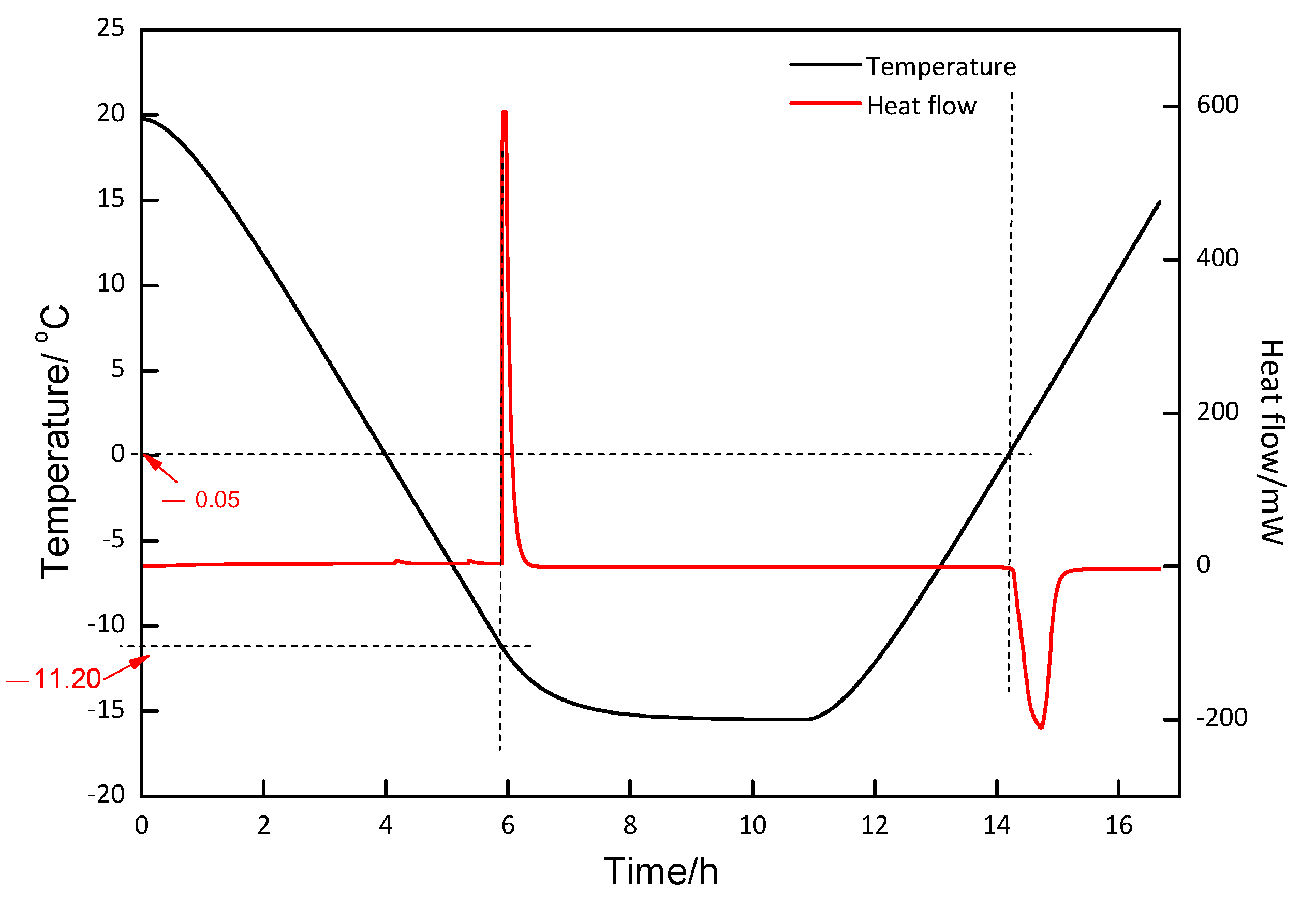
2.2.1. DSC Experiment
2.2.2. Raman Experiment
3. Results and Discussion
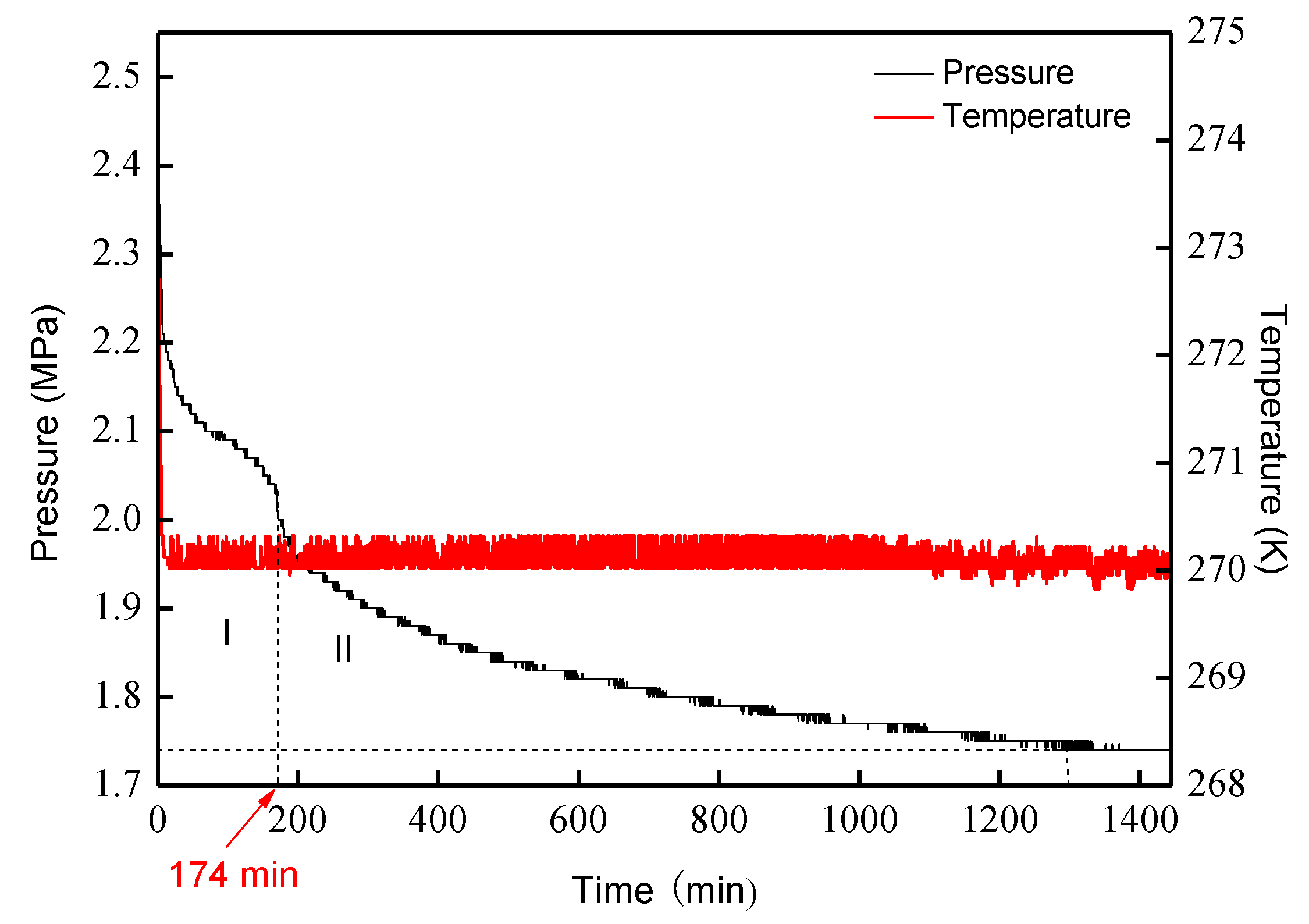
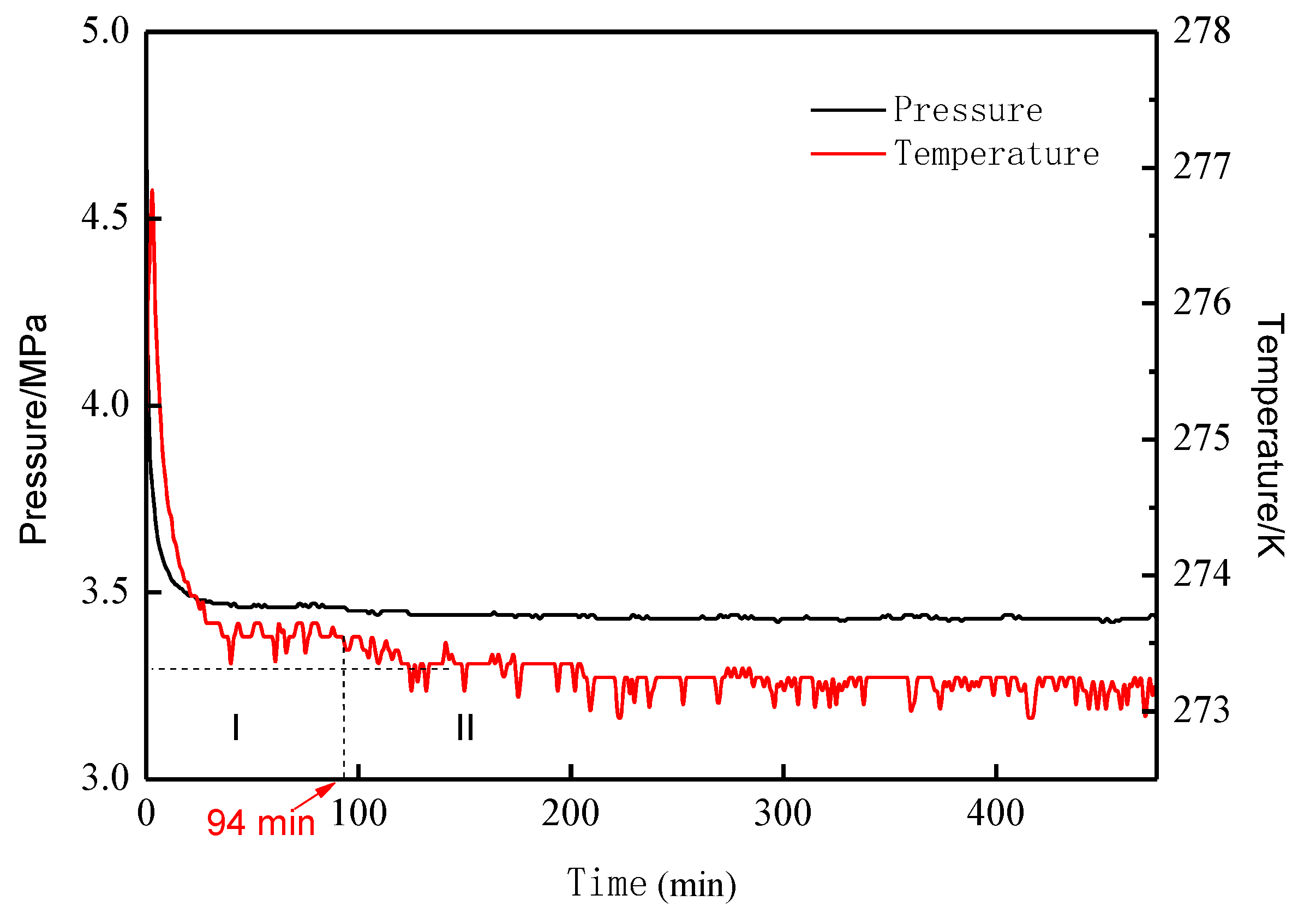
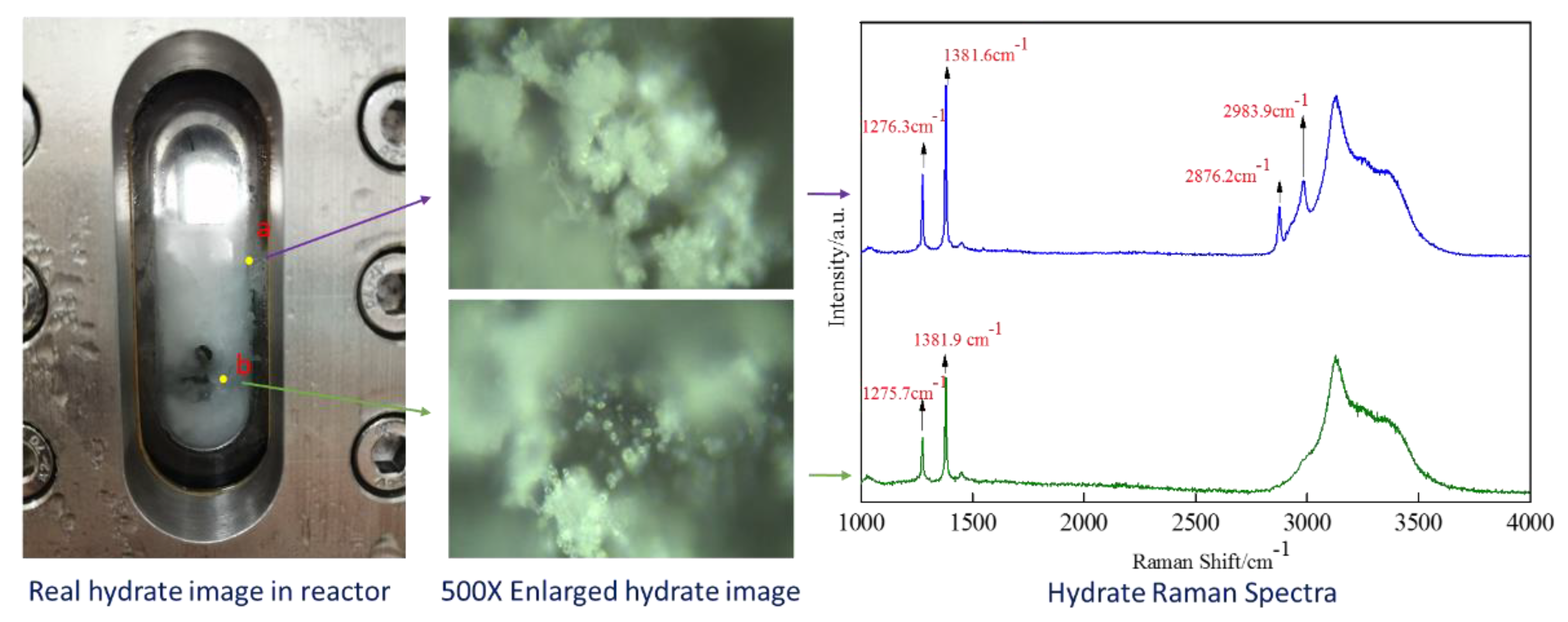
3.1. Analysis of Hydrate Formation Process
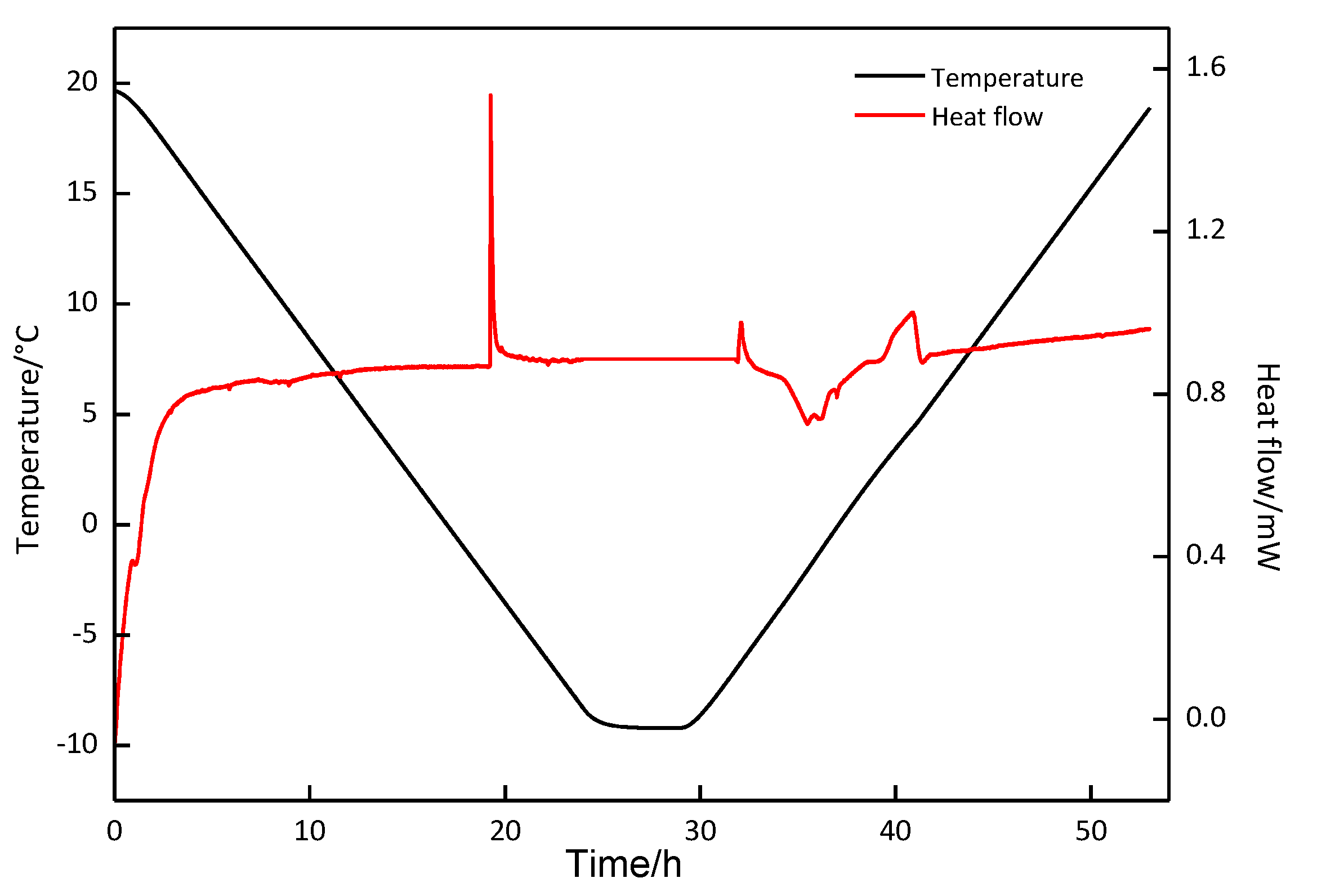
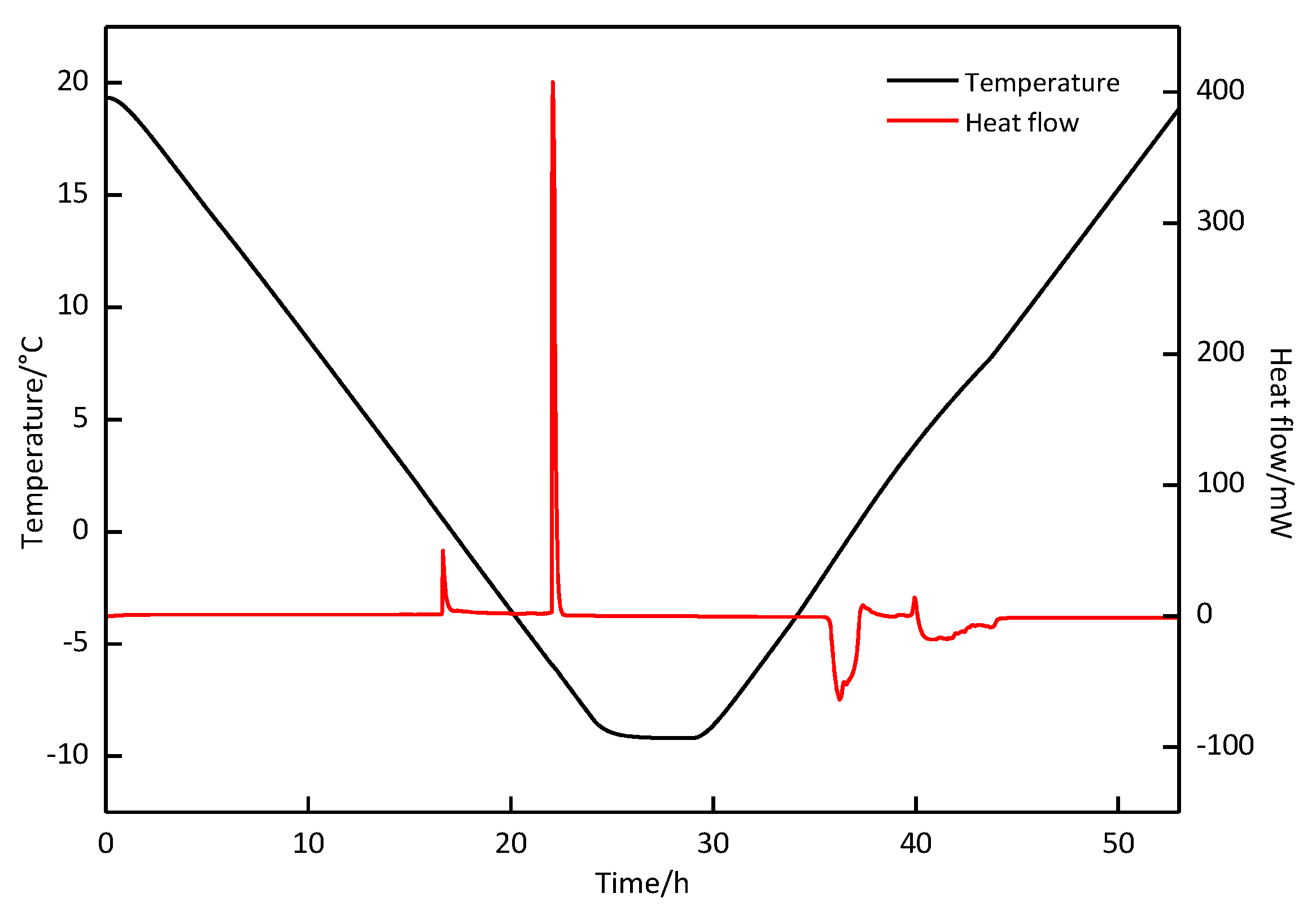
3.2. DSC and Raman Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Hauck, J.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; Le Quere, C.; et al. Global Carbon Budget 2019. Earth Syst. Sci. Data 2019, 11, 1783–1838. [Google Scholar] [CrossRef]
- Yu, B.Y.; Zhao, G.P.; An, R.Y. Framing the picture of energy consumption in China. Nat. Hazards 2019, 99, 1469–1490. [Google Scholar] [CrossRef]
- Xu, C.G.; Cai, J.; Li, X.S.; Lv, Q.N.; Chen, Z.Y.; Deng, H.W. Integrated Process Study on Hydrate-Based Carbon Dioxide Separation from Integrated Gasification Combined Cycle (IGCC) Synthesis Gas in Scaled-Up Equipment. Energ. Fuel 2012, 26, 6442–6448. [Google Scholar] [CrossRef]
- English, N.J.; El-Hendawy, M.M.; Mooney, D.A.; MacElroy, J.M.D. Perspectives on atmospheric CO2 fixation in inorganic and biomimetic structures. Coordin Chem Rev 2014, 269, 85–95. [Google Scholar] [CrossRef]
- Dijkstra, J.W.; Jansen, D. Novel concepts for CO2 capture. Energy 2004, 29, 1249–1257. [Google Scholar] [CrossRef]
- Kotowicz, J.; Chmielniak, T.; Janusz-Szymanska, K. The influence of membrane CO2 separation on the efficiency of a coal-fired power plant. Energy 2010, 35, 841–850. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Chen, Z.Y.; Wu, H.J. Tetra-n-butyl ammonium bromide semi-clathrate hydrate process for post-combustion capture of carbon dioxide in the presence of dodecyl trimethyl ammonium chloride. Energy 2010, 35, 3902–3908. [Google Scholar] [CrossRef]
- Li, A.R.; Wang, J.; Bao, B.P. High-efficiency CO2 capture and separation based on hydrate technology: A review. Greenh. Gases 2019, 9, 175–193. [Google Scholar] [CrossRef]
- English, N.J.; Clarke, E.T. Molecular dynamics study of CO2 hydrate dissociation: Fluctuation-dissipation and non-equilibrium analysis. J. Chem. Phys. 2013, 139. [Google Scholar] [CrossRef]
- Larsen, R.; Knight, C.A.; Sloan, E.D. Clathrate hydrate growth and inhibition. Fluid Phase Equilibria 1998, 150, 353–360. [Google Scholar] [CrossRef]
- Sum, A.K.; Burruss, R.C.; Sloan, E.D. Measurements of clathrate hydrates properties via Raman spectroscopy. In Proceedings of the Ngh 96: 2nd International Conference on Natural Gas Hydrates, Golden, Toulouse, France, 2–6 June 1996; pp. 51–58. [Google Scholar]
- Sum, A.K.; Koh, C.A.; Sloan, E.D. Clathrate Hydrates: From Laboratory Science to Engineering Practice. Ind. Eng. Chem. Res. 2009, 48, 7457–7465. [Google Scholar] [CrossRef]
- Duc, N.H.; Chauvy, F.; Herri, J.M. CO2 capture by hydrate crystallization—A potential solution for gas emission of steelmaking industry. Energy Convers. Manag. 2007, 48, 1313–1322. [Google Scholar] [CrossRef]
- Xu, C.G.; Yu, Y.S.; Ding, Y.L.; Cai, J.; Li, X.S. The effect of hydrate promoters on gas uptake. Phys. Chem. Chem. Phys. 2017, 19, 21769–21776. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, S.P.; Lee, J.W. C-13 NMR analysis of C2H6+C2H4+THF mixed hydrate for an application to separation of C2H4 and C2H6. Korean J. Chem. Eng. 2016, 33, 2186–2190. [Google Scholar] [CrossRef]
- Yang, M.J.; Jing, W.; Zhao, J.F.; Ling, Z.; Song, Y.C. Promotion of hydrate-based CO2 capture from flue gas by additive mixtures (THF (tetrahydrofuran) plus TBAB (tetra-n-butyl ammonium bromide)). Energy 2016, 106, 546–553. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Zhao, Y.S.; Liang, W.G. CH4 Separation from Coal Bed Methane by Hydrate in the SDS and THF Solution. J. Chem.-Ny 2016. [Google Scholar] [CrossRef]
- Zhang, H.; Du, J.W.; Wang, Y.H.; Lang, X.M.; Li, G.; Chen, J.B.; Fan, S.S. Investigation into THF hydrate slurry flow behaviour and inhibition by an anti-agglomerant. Rsc. Adv. 2018, 8, 11946–11956. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.S.; Seo, Y. Thermodynamic, structural, and kinetic studies of cyclopentane + CO2 hydrates: Applications for desalination and CO2 capture. Chem. Eng. J. 2019, 375. [Google Scholar] [CrossRef]
- Mesbah, M.; Soroush, E.; Roham, M.; Shahsavari, S. Phase equilibrium modeling of semi-clathrate hydrates of the CO2+H2/CH4/N2+TBAB aqueous solution system. Petrol. Sci. Technol. 2017, 35, 1588–1594. [Google Scholar] [CrossRef]
- Takeya, S.; Muromachi, S.; Maekawa, T.; Yamamoto, Y.; Mimachi, H.; Kinoshita, T.; Murayama, T.; Umeda, H.; Ahn, D.H.; Iwasaki, Y.; et al. Design of Ecological CO2 Enrichment System for Greenhouse Production using TBAB + CO2 Semi-Clathrate Hydrate. Energies 2017, 10, 927. [Google Scholar] [CrossRef]
- Michel, N.; Clarke, M.A. A short note on modelling the three-phase equilibrium conditions of TBAB semi-clathrates formed in the presence of gas mixtures containing CO2. J. Chem. Thermodyn. 2018, 117, 97–105. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, D.L.; Zheng, W.Y.; Yan, J.; Lu, Y.Y.; Yi, D.T. Morphology and kinetic investigation of TBAB/TBPB semiclathrate hydrates formed with a CO2 + CH4 gas mixture. J. Cryst. Growth 2019, 511, 79–88. [Google Scholar] [CrossRef]
- Kakati, H.; Mandal, A.; Laik, S. Synergistic effect of Polyvinylpyrrolidone (PVP) and L-tyrosine on kinetic inhibition of CH4 + C2H4 + C3H8 hydrate formation. J. Nat. Gas Sci. Eng. 2016, 34, 1361–1368. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, W.; Seo, Y.J.; Lee, J.Y.; Lee, J.; Seo, Y. Structural transition induced by cage-dependent guest exchange in CH4 + C3H8 hydrates with CO2 injection for energy recovery and CO2 sequestration. Appl. Energy 2018, 228, 229–239. [Google Scholar] [CrossRef]
- Khan, M.N.; Warrier, P.; Peters, C.J.; Koh, C.A. Review of vapor-liquid equilibria of gas hydrate formers and phase equilibria of hydrates. J. Nat. Gas Sci. Eng. 2016, 35, 1388–1404. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. A review on the role and impact of various additives as promoters/inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76. [Google Scholar] [CrossRef]
- Ricaurte, M.; Dicharry, C.; Broseta, D.; Renaud, X.; Torre, J.P. CO2 Removal from a CO2-CH4 Gas Mixture by Clathrate Hydrate Formation Using THF and SDS as Water-Soluble Hydrate Promoters. Ind. Eng. Chem. Res. 2013, 52, 899–910. [Google Scholar] [CrossRef]
- Mohammadi, A.; Pakzad, M.; Mohammadi, A.H.; Jahangiri, A. Kinetics of (TBAF + CO2) semi-clathrate hydrate formation in the presence and absence of SDS. Petrol. Sci. 2018, 15, 375–384. [Google Scholar] [CrossRef]
- Yan, K.F.; Li, X.S.; Chen, Z.Y.; Xu, C.G.; Zhang, Y.; Xia, Z.M. The Formation of CH4 Hydrate in the Slit Nanopore between the Smectite Basal Surfaces by Molecular Dynamics Simulation. Energy Fuel 2018, 32, 6467–6474. [Google Scholar] [CrossRef]
- Xia, Z.M.; Li, X.S.; Chen, Z.Y.; Yan, K.F.; Xu, C.G.; Lv, Q.N.; Cai, J. Hydrate-based Capture of Acidic Gases for Clean Fuels with New Synergic Additives. Energy Procedia 2017, 105, 648–653. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Chen, Z.Y.; Wu, H.J. Hydrate-based pre-combustion carbon dioxide capture process in the system with tetra-n-butyl ammonium bromide solution in the presence of cyclopentane. Energy 2011, 36, 1394–1403. [Google Scholar] [CrossRef]
- Xia, Z.M.; Li, X.S.; Chen, Z.Y.; Li, G.; Cai, J.; Wang, Y.; Yan, K.F.; Xu, C.G. Hydrate-based acidic gases capture for clean methane with new synergic additives. Appl. Energy 2017, 207, 584–593. [Google Scholar] [CrossRef]
- Hirai, S.; Okazaki, K.; Tabe, Y.; Kawamura, K. CO2 clathrate-hydrate formation and its mechanism by molecular dynamics simulation. Energy Convers. Manag. 1997, 38, S301–S306. [Google Scholar] [CrossRef]
- Zhang, M.M.; Anderson, B.J.; Warzinski, R.P.; Holder, G.D. Molecular dynamics simulation of hydrate lattice distortion. Abstr. Pap. Am. Chem. S 2009, 237, 1. [Google Scholar]
- Yan, K.F.; Li, X.S.; Chen, Z.Y.; Xu, C.G. Molecular dynamics simulation of CO2 separation from integrated gasification combined cycle syngas via the hydrate formation. Acta Phys. Sin. 2010, 59, 4313–4321. [Google Scholar]
- Qi, Y.X.; Ota, M.; Zhang, H. Molecular dynamics simulation of replacement of CH4 in hydrate with CO2. Energy Convers. Manag. 2011, 52, 2682–2687. [Google Scholar] [CrossRef]
- Nohra, M.; Woo, T.K.; Alavi, S.; Ripmeester, J.A. Molecular dynamics Gibbs free energy calculations for CO2 capture and storage in structure I clathrate hydrates in the presence of SO2, CH4, N2, and H2S impurities. J. Chem. Thermodyn. 2012, 44, 5–12. [Google Scholar] [CrossRef]
- Yan, K.F.; Li, X.S.; Chen, Z.Y.; Xia, Z.M.; Xu, C.G.; Zhang, Z.Q. Molecular Dynamics Simulation of the Crystal Nucleation and Growth Behavior of Methane Hydrate in the Presence of the Surface and Nanopores of Porous Sediment. Langmuir 2016, 32, 7975–7984. [Google Scholar] [CrossRef] [PubMed]
- Handa, Y.P. Calorimetric Determinations of the Compositions, Enthalpies of Dissociation, and Heat-Capacities in the Range 85 to 270-K for Clathrate Hydrates of Xenon and Krypton. J. Chem. Thermodyn. 1986, 18, 891–902. [Google Scholar] [CrossRef]
- Kang, S.P.; Lee, H.; Ryu, B.J. Enthalpies of dissociation of clathrate hydrates of carbon dioxide, nitrogen, (carbon dioxide plus nitrogen), and (carbon dioxide plus nitrogen plus tetrahydrofuran). J. Chem. Thermodyn. 2001, 33, 513–521. [Google Scholar] [CrossRef]
- Koh, C.A.; Westacott, R.E.; Zhang, W.; Hirachand, K.; Creek, J.L.; Soper, A.K. Mechanisms of gas hydrate formation and inhibition. Fluid Phase Equilibria 2002, 194, 143–151. [Google Scholar] [CrossRef]
- Dalmazzone, D.; Hamed, N.; Dalmazzone, C.; Rousseau, L. Application of high pressure DSC to the kinetics of formation of methane hydrate in water-in-oil emulsion. J. Therm. Anal. Calorim. 2006, 85, 361–368. [Google Scholar] [CrossRef]
- Zhang, J.S.; Lee, J.W. Equilibrium of Hydrogen plus Cyclopentane and Carbon Dioxide plus Cyclopentane Binary Hydrates. J Chem. Eng. Data 2009, 54, 659–661. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, W.; Shin, K.; Seo, Y. CH4-CO2 replacement occurring in sII natural gas hydrates for CH4 recovery and CO2 sequestration. Energy Convers. Manag. 2017, 150, 356–364. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, D.; Lee, J.W.; Seo, Y. Enclathration of CO2 as a co-guest of structure H hydrates and its implications for CO2 capture and sequestration. Appl. Energy 2016, 163, 51–59. [Google Scholar] [CrossRef]
- Koh, C.; Savidge, J.; Soper, A.; Wu, X.; Motie, R.; Westacott, R. Spectroscopic studies of natural gas hydrate nucleation, growth and inhibition. In Proceedings of the Ngh 96: 2nd International Conference on Natural Gas Hydrates, Golden, Toulouse, France, 2–6 June 1996; p. 301. [Google Scholar]
- Park, J.; Seo, Y.T.; Lee, J.W.; Lee, H. Spectroscopic analysis of carbon dioxide and nitrogen mixed gas hydrates in silica gel for CO2 separation. Catal. Today 2006, 115, 279–282. [Google Scholar] [CrossRef]
- Rojas, Y.; Lou, X. Instrumental analysis of gas hydrates properties. Asia-Pac J. Chem. Eng. 2010, 5, 310–323. [Google Scholar] [CrossRef]
- Chen, L.T.; Lu, H.L.; Ripmeester, J.A. Raman spectroscopic study of CO2 in hydrate cages. Chem. Eng. Sci. 2015, 138, 706–711. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.S.; Seo, Y. CH4 enclathration in tetra-iso-amyl ammonium bromide (TiAAB) semiclathrate and its significance for natural gas storage. Chem. Eng. J. 2017, 330, 1160–1165. [Google Scholar] [CrossRef]
- Xu, C.G.; Yan, R.; Fu, J.; Zhang, S.H.; Yan, K.F.; Chen, Z.Y.; Xia, Z.M.; Li, X.S. Insight into micro-mechanism of hydrate-based methane recovery and carbon dioxide capture from methane-carbon dioxide gas mixtures with thermal characterization. Appl. Energy 2019, 239, 57–69. [Google Scholar] [CrossRef]
- Li, Z.Y.; Xia, Z.M.; Chen, Z.Y.; Li, X.S.; Xu, C.G.; Yan, R. The plateau effects and crystal transition study in Tetrahydrofuran (THF)/CO2/H2 hydrate formation processes. Appl. Energy 2019, 238, 195–201. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Mayanovic, R.A. An Empirical Study on Raman Peak Fitting and Its Application to Raman Quantitative Research. Appl. Spectrosc. 2017, 71, 2325–2338. [Google Scholar] [CrossRef] [PubMed]

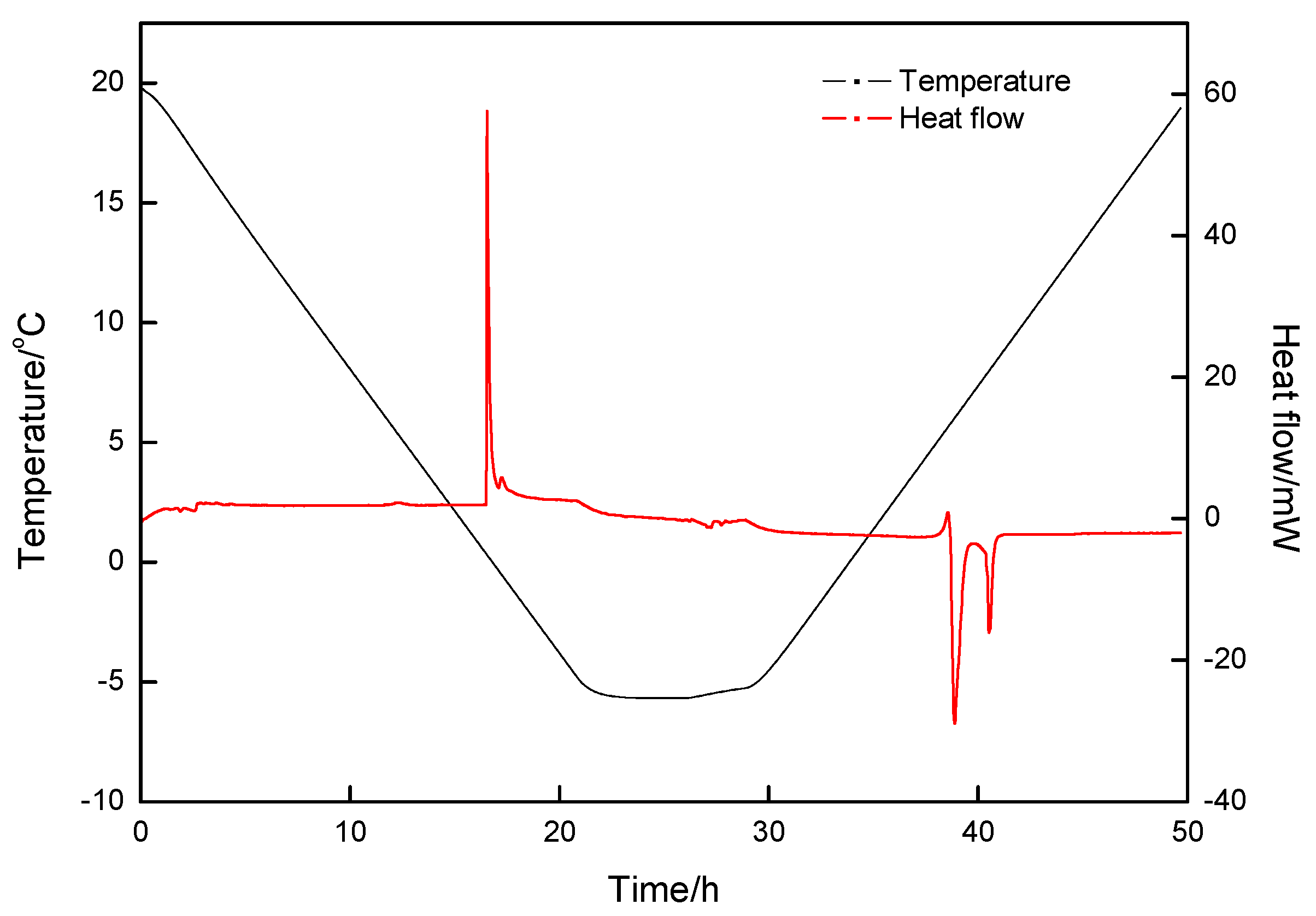
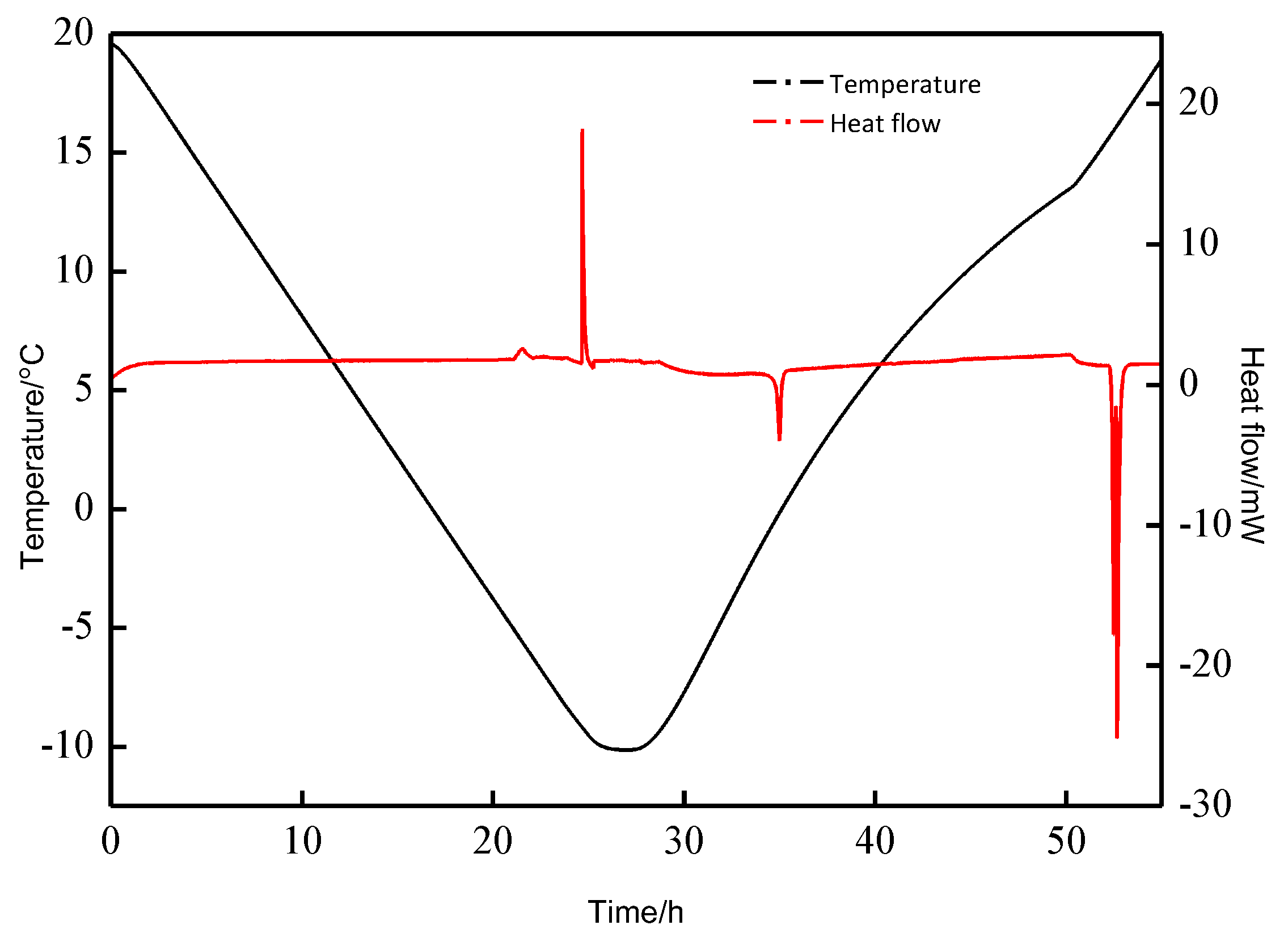
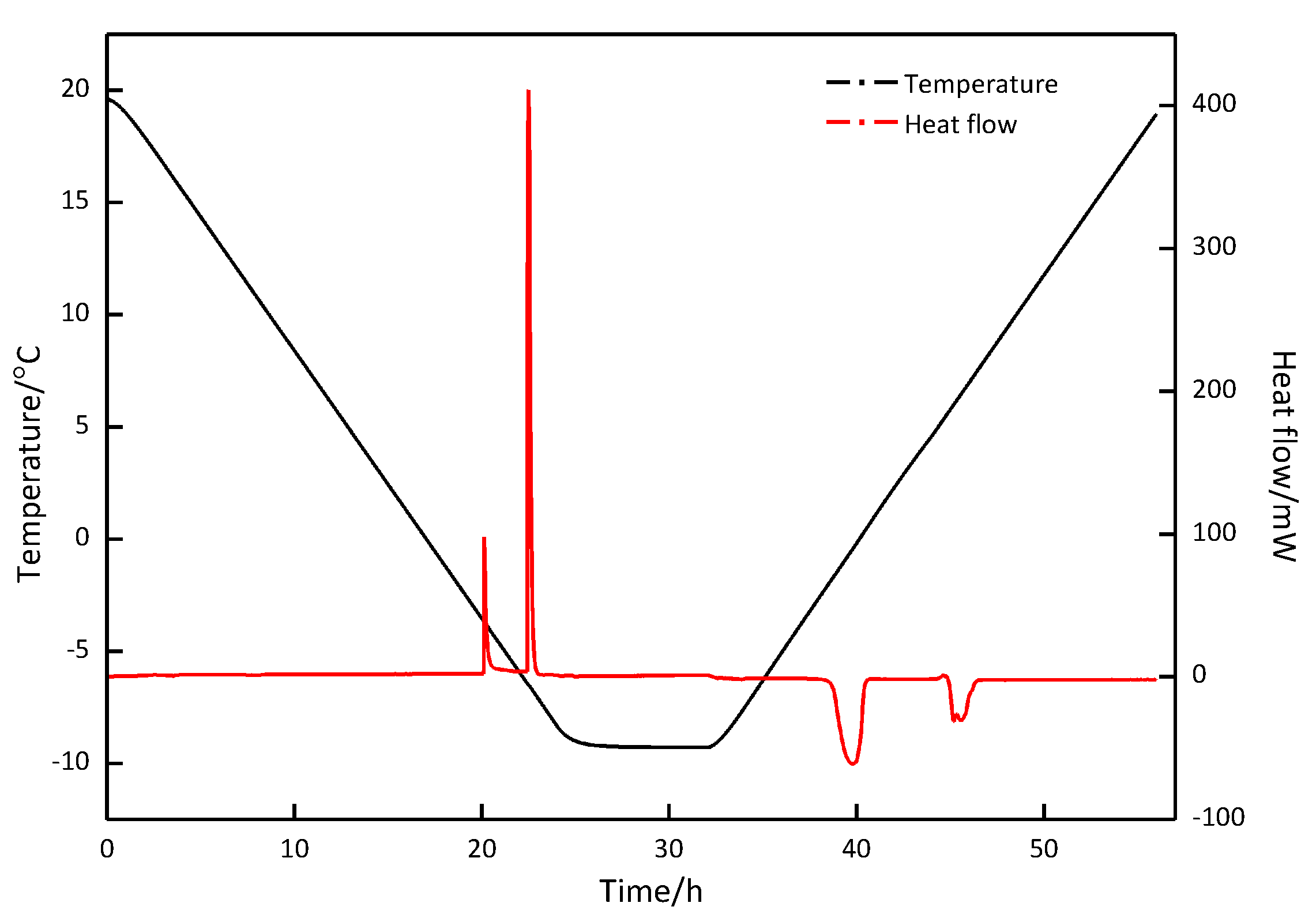
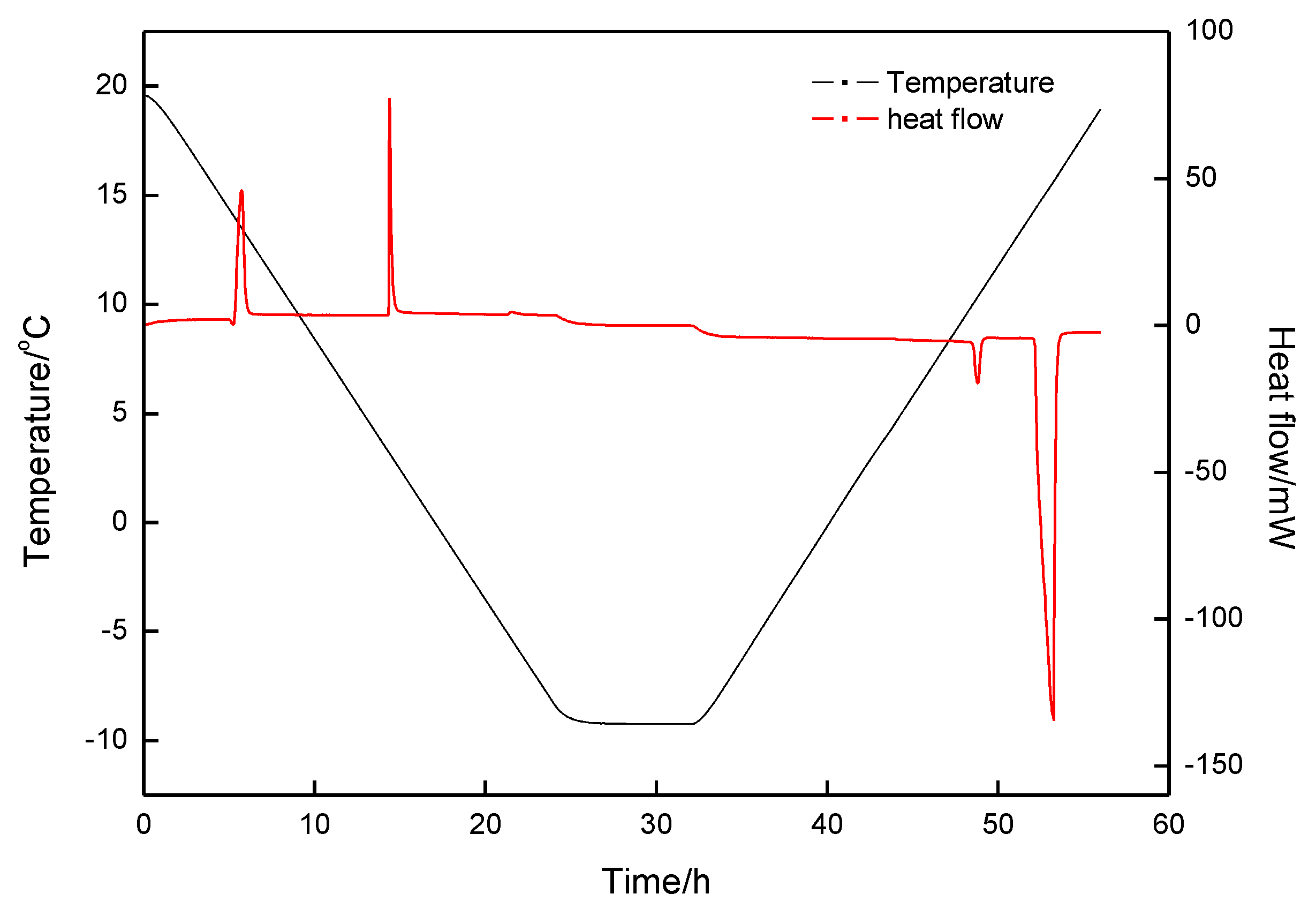



| Exp. Run | P (MPa) | T (°C) | Cooling Rate (K·min−1) | Constant Time (h) | Heating Rate (K·min−1) |
|---|---|---|---|---|---|
| 1 | 2.5 | 20.0~−5.0 | 0.02 | 5 | 0.02 |
| 2 | 2.5 | 20.0~−10.0 | 0.02 | 5 | 0.02 |
| 3 | 2.5 | 20.0~−8.8 | 0.02 | 5 | 0.02 |
| 4 | 5.0 | 20.0~−8.8 | 0.02 | 5 | 0.02 |
| 5 | 0 | 20.0~−8.8 | 0.02 | 5 | 0.02 |
| 6 | 3.5 | 20.0~−8.8 | 0.02 | 5 | 0.02 |
| 7 | 6.0 | 20.0~−8.8 | 0.02 | 5 | 0.02 |
| Exp. Run | P (MPa) | T (°C) | First Formation Peak | Second Formation Peak | First Decomposition Peak | Second Decomposition Peak | (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | (J·g−1) | T (°C) | (J·g−1) | T (°C) | (J·g−1) | T (°C) | (J·g−1) | ||||
| 1 | 2.5 | 20.0~−5.0 | 0.34 | −50.823 | − | − | 5.80 | 37.211 | 6.93 | 13.234 | 99.26 |
| 2 | 2.5 | 20.0~−10.0 | −3.66 | −63.974 | −6.19 | −217.557 | −1.72 | 214.034 | 5.71 | 68.209 | 101.52 |
| 3 | 2.5 | 20.0~−8.8 | −3.65 | −67.769 | −6.50 | −243.679 | −1.72 | 242.622 | 5.70 | 67.212 | 99.48 |
| 4 | 5.0 | 20.0~−8.8 | 13.98 | −77.013 | 3.21 | −39.510 | 10.03 | 39.014 | 14.36 | 77.775 | 100.23 |
| 5 | 0 | 20.0~−8.8 | −2.63 | −0.533 | −2.05 | 0.535 | 100.37 | ||||
| 6 | 3.5 | 20.0~−8.8 | 0.607 | −113.919 | −5.90 | −218.931 | −1.54 | 219.444 | 4.85 | 118.254 | 101.46 |
| 7 | 6.0 | 20.0~−8.8 | T: 6.97 | : −469.133 | −1.62 | 17.020 | 10.216 | 438.734 | 97.15 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.-G.; Wang, M.; Xu, G.; Li, X.-S.; Zhang, W.; Cai, J.; Chen, Z.-Y. The Relationship between Thermal Characteristics and Microstructure/Composition of Carbon Dioxide Hydrate in the Presence of Cyclopentane. Energies 2021, 14, 870. https://doi.org/10.3390/en14040870
Xu C-G, Wang M, Xu G, Li X-S, Zhang W, Cai J, Chen Z-Y. The Relationship between Thermal Characteristics and Microstructure/Composition of Carbon Dioxide Hydrate in the Presence of Cyclopentane. Energies. 2021; 14(4):870. https://doi.org/10.3390/en14040870
Chicago/Turabian StyleXu, Chun-Gang, Min Wang, Gang Xu, Xiao-Sen Li, Wei Zhang, Jing Cai, and Zhao-Yang Chen. 2021. "The Relationship between Thermal Characteristics and Microstructure/Composition of Carbon Dioxide Hydrate in the Presence of Cyclopentane" Energies 14, no. 4: 870. https://doi.org/10.3390/en14040870
APA StyleXu, C.-G., Wang, M., Xu, G., Li, X.-S., Zhang, W., Cai, J., & Chen, Z.-Y. (2021). The Relationship between Thermal Characteristics and Microstructure/Composition of Carbon Dioxide Hydrate in the Presence of Cyclopentane. Energies, 14(4), 870. https://doi.org/10.3390/en14040870