Leaching via Weak Spots in Photovoltaic Modules
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Experimental Conditions
- Room temperature , no agitation;
- Room temperature , with agitation (orbital shaking with rotational speed );
- Increased temperature , with agitation (orbital shaking with rotational speed ).
2.2. Heavy Metal Analysis and Determination of Initial Metal Content in Module Pieces
2.3. Mass Balancing at the End of the Leaching Experiments
3. Results
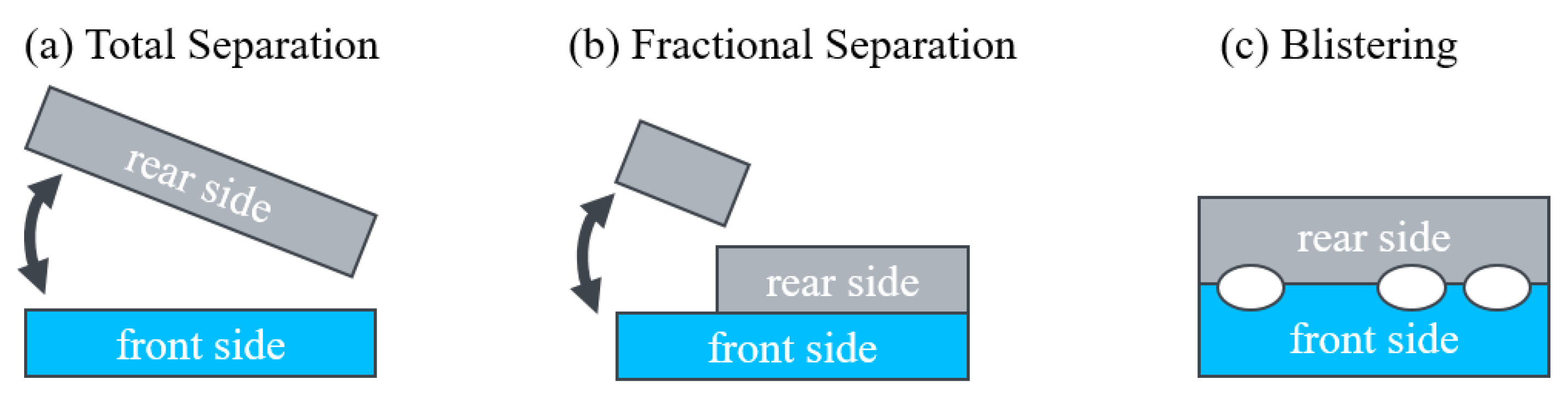
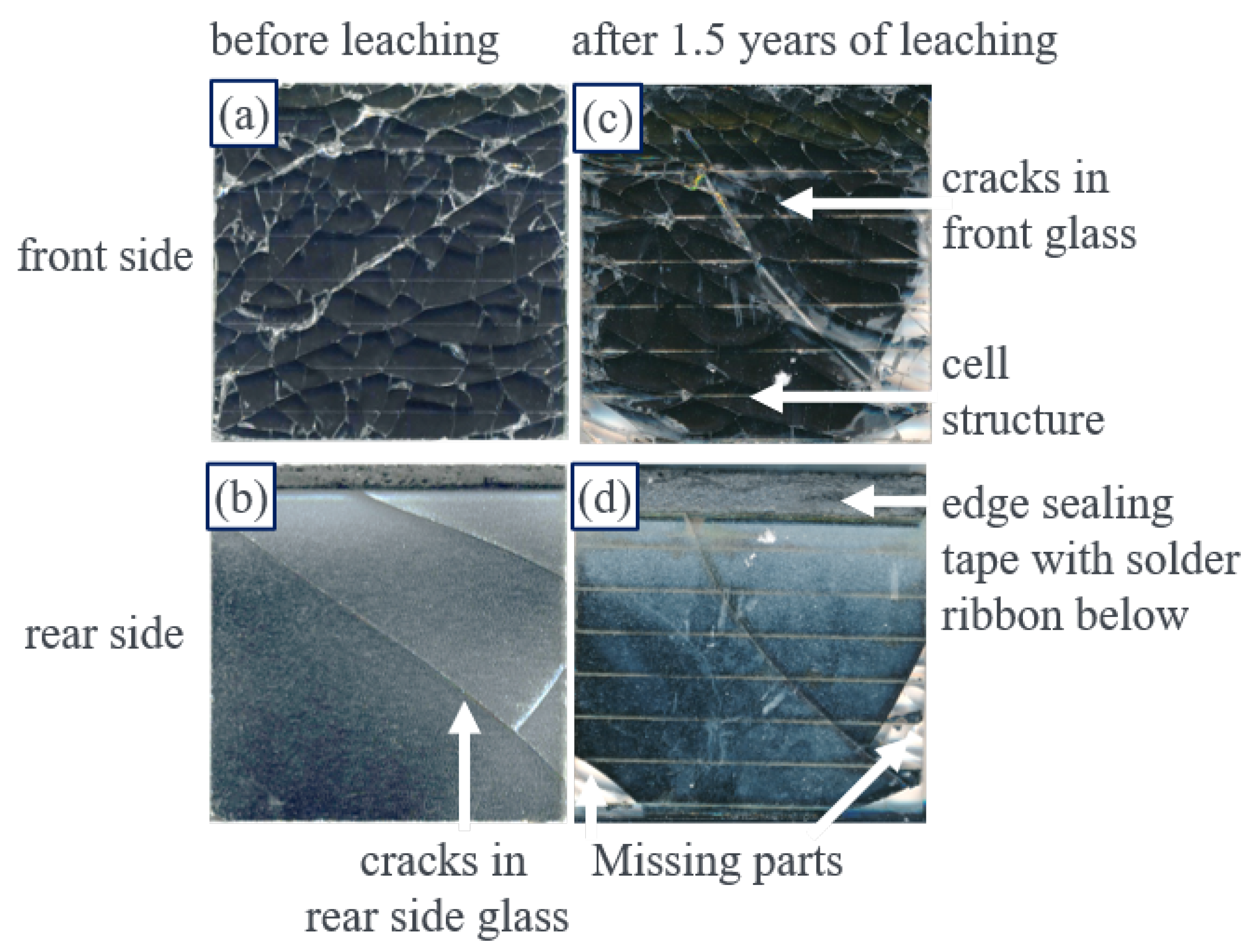
3.1. Delamination of Module Pieces
3.2. Leaching Results
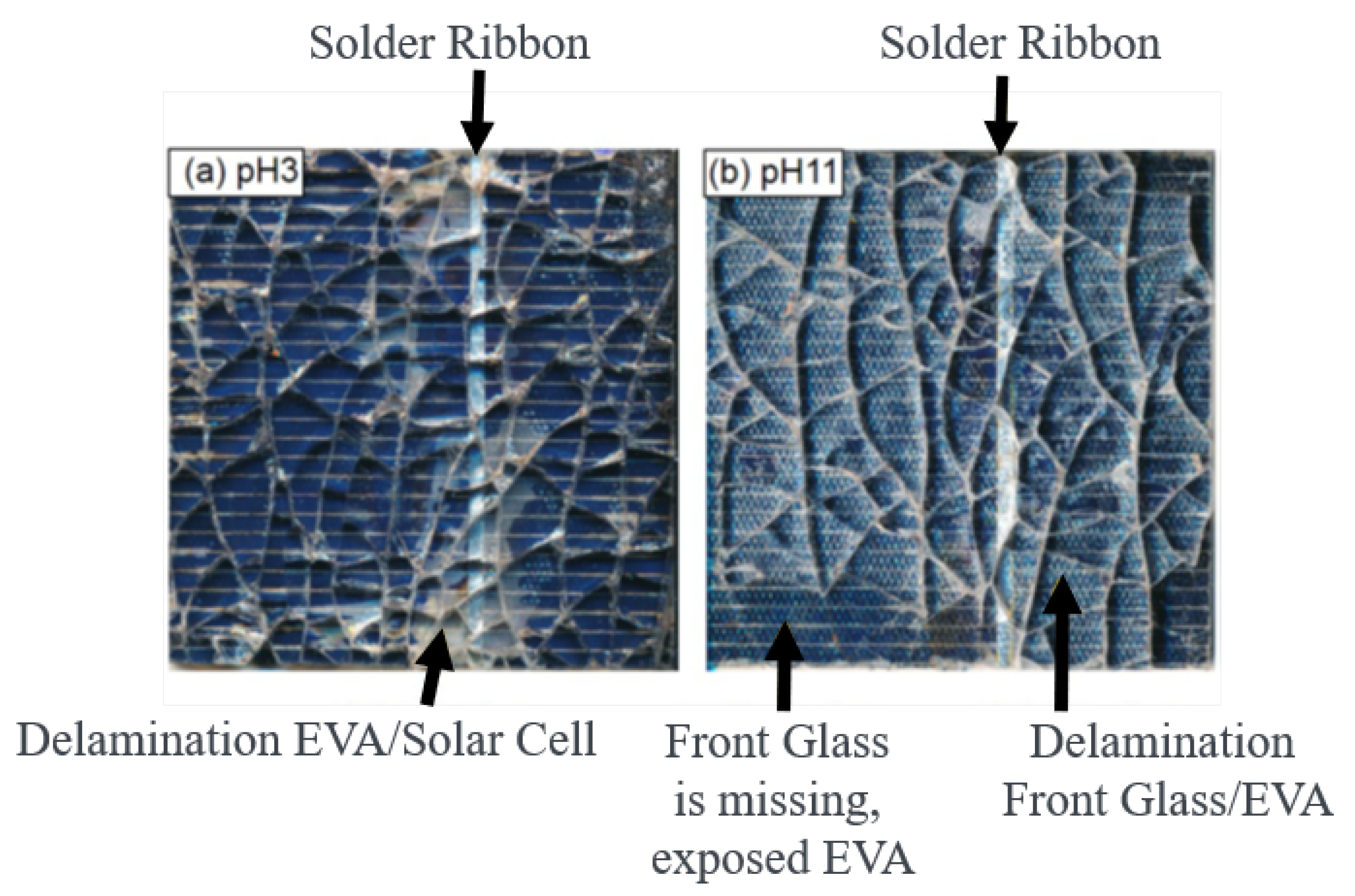
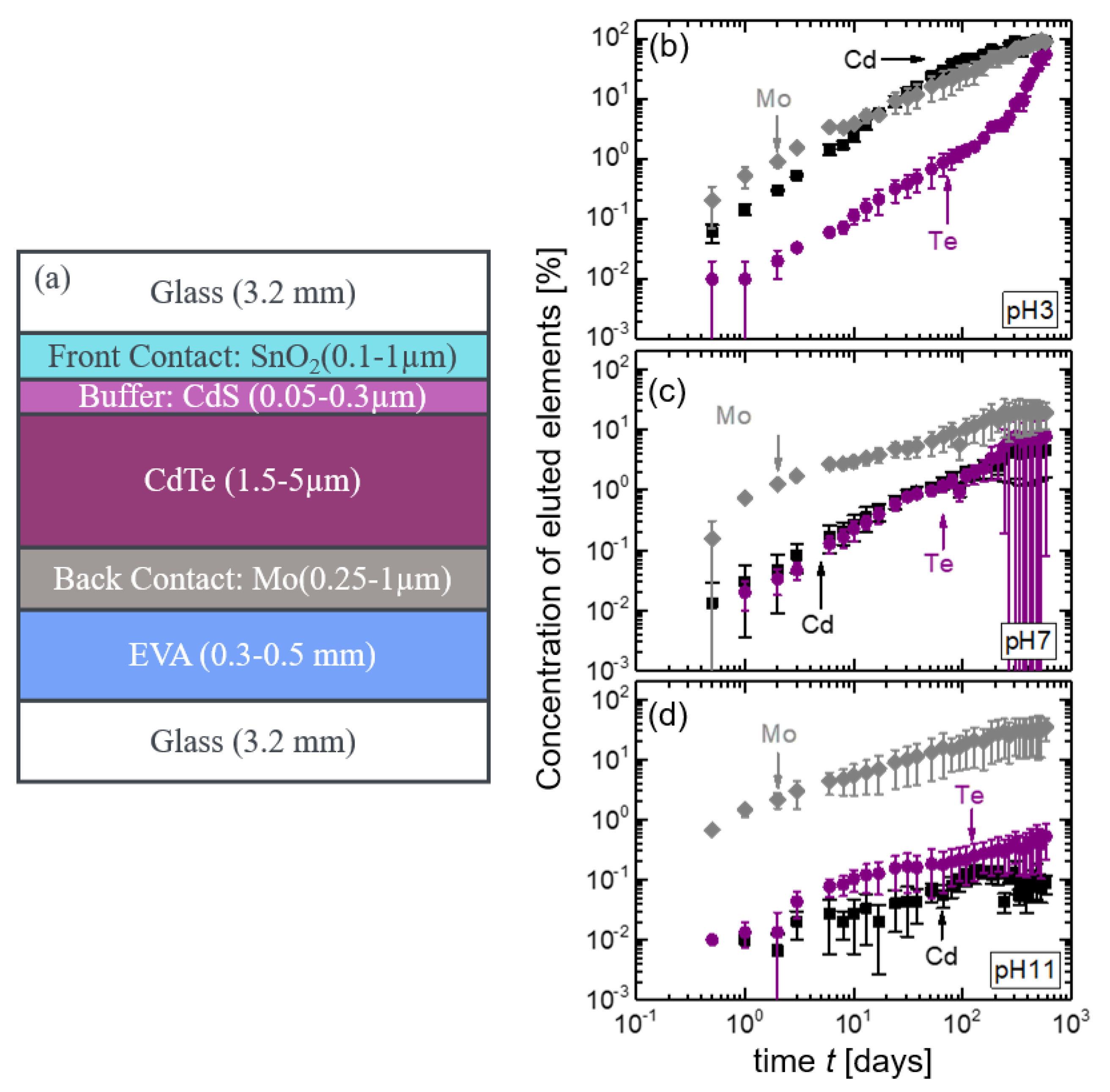
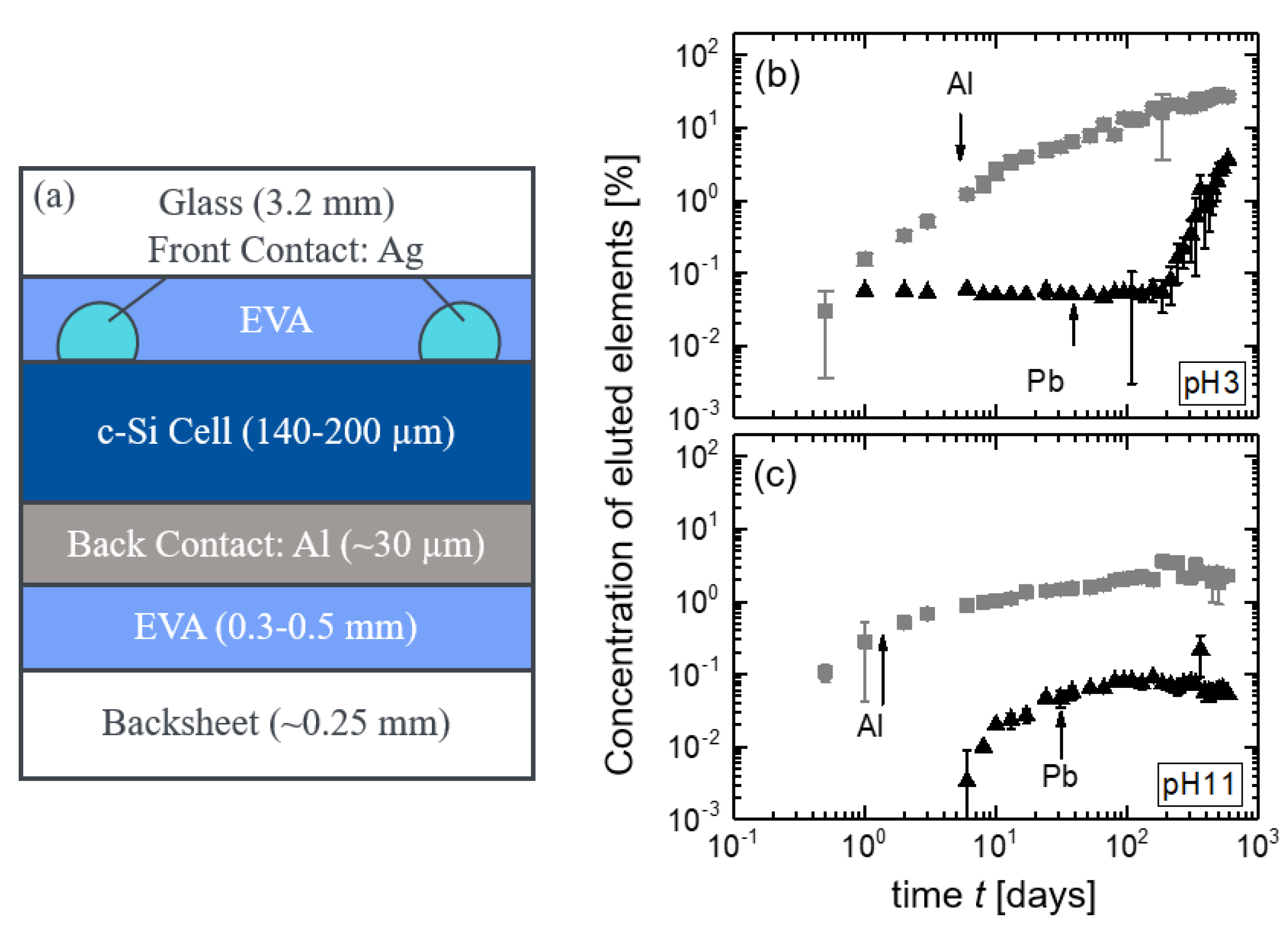
3.2.1. CdTe Module Pieces
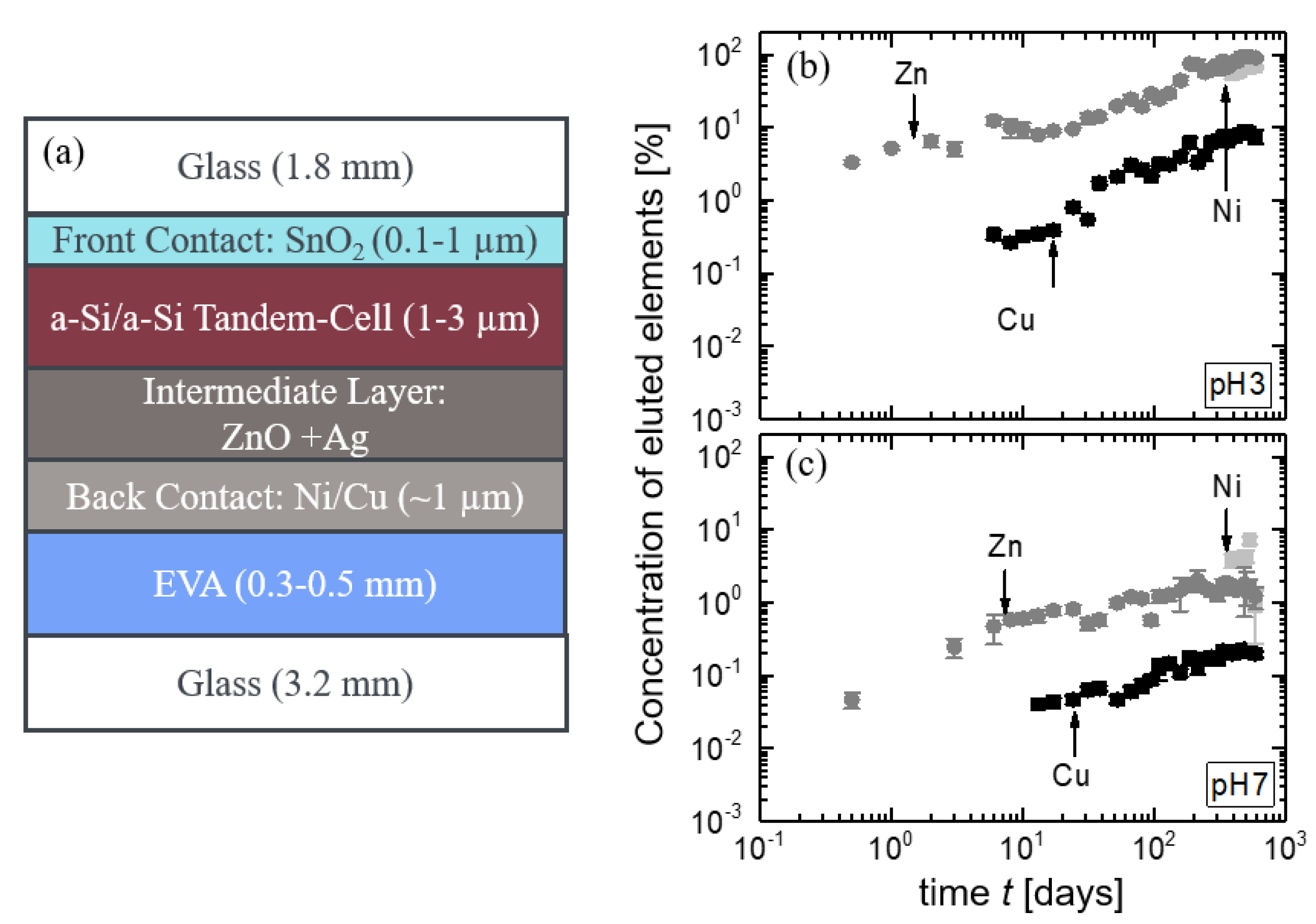
3.2.2. CIGS Module Pieces
3.2.3. c-Si Module Pieces
3.2.4. a-Si Module Pieces
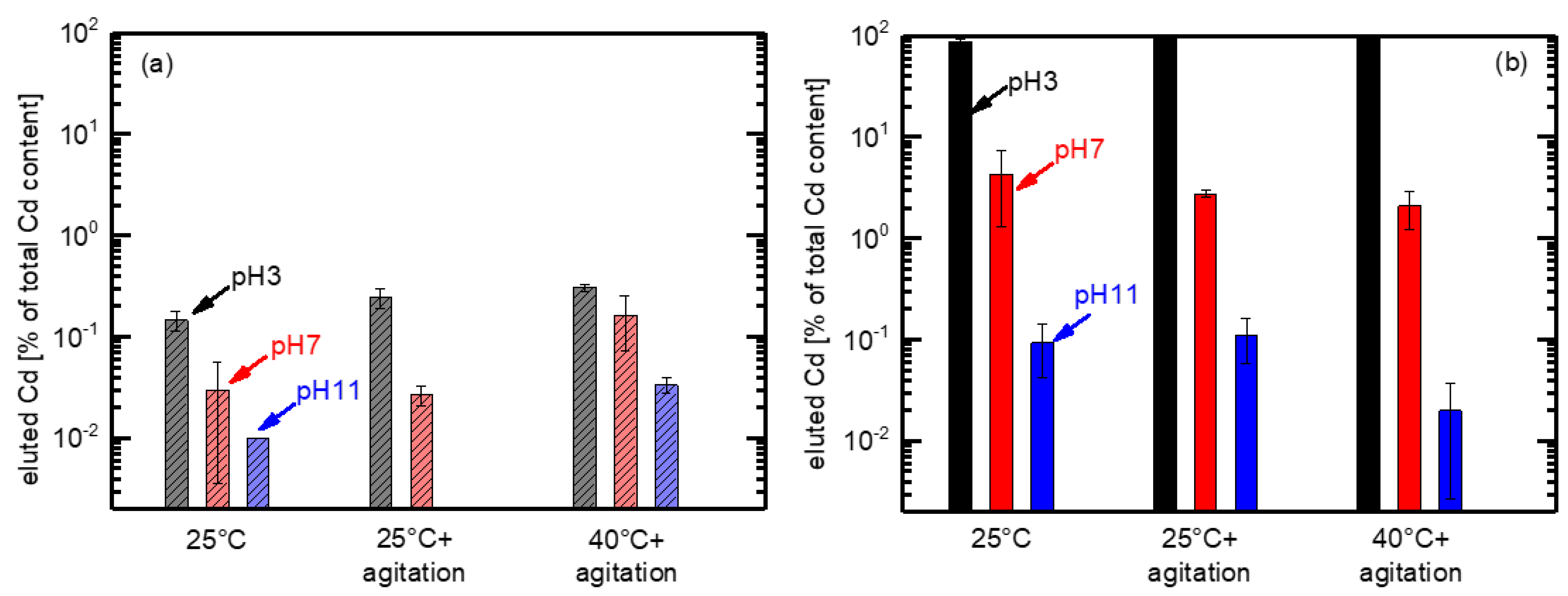
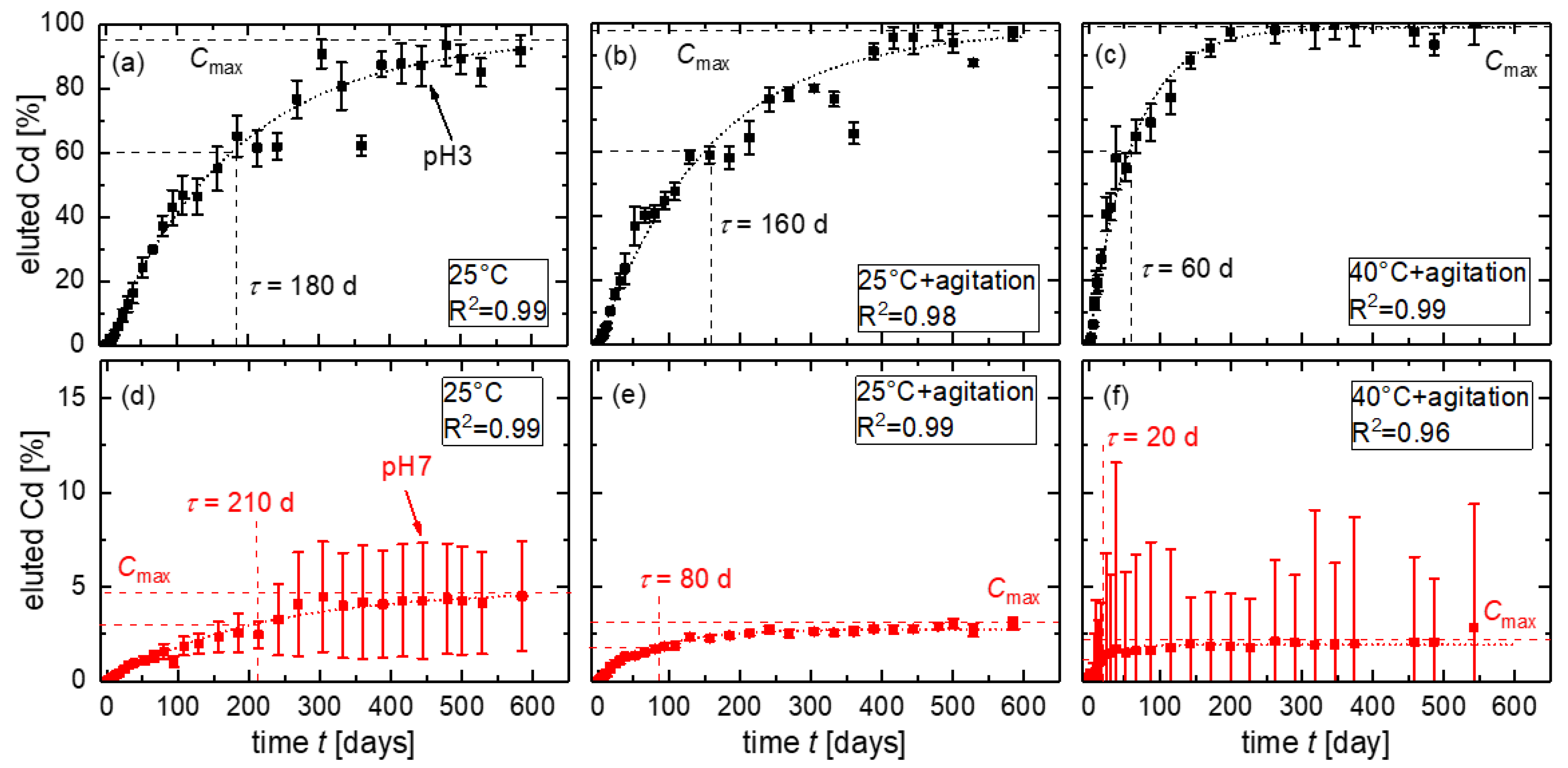
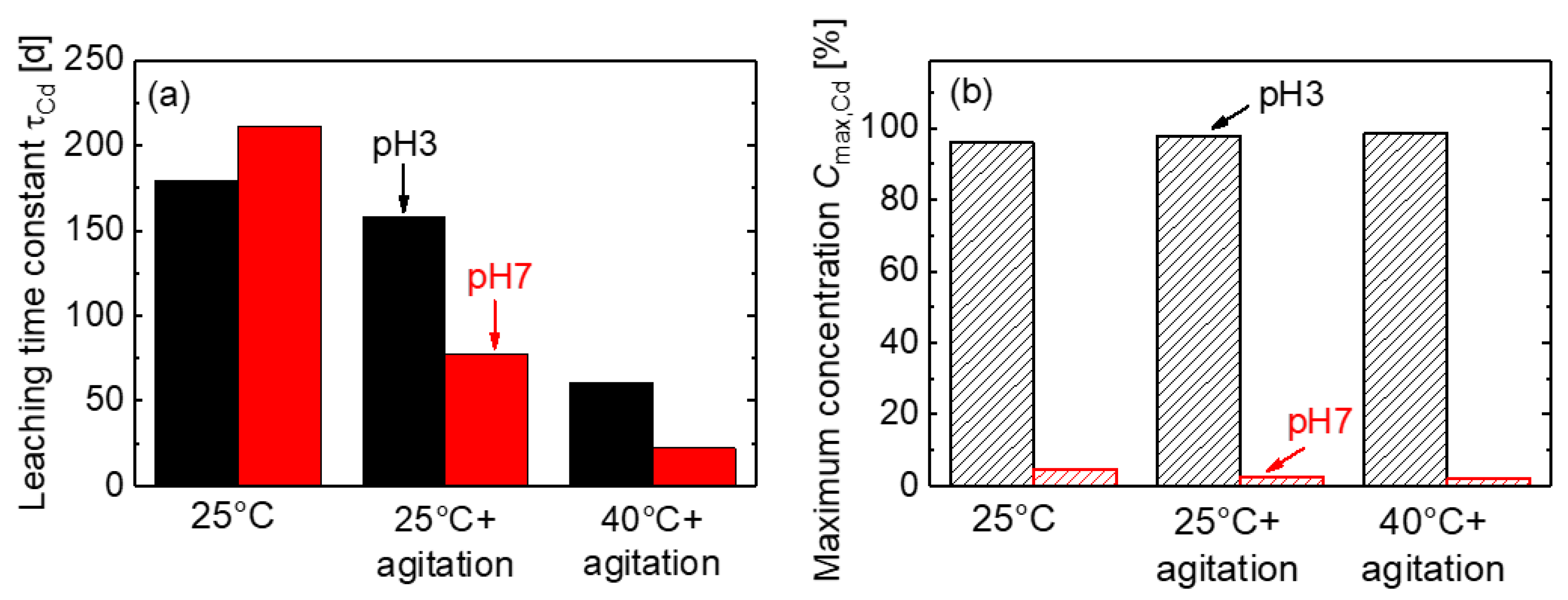
3.3. Accelerating Leaching Parameters for Cd from CdTe Module Pieces
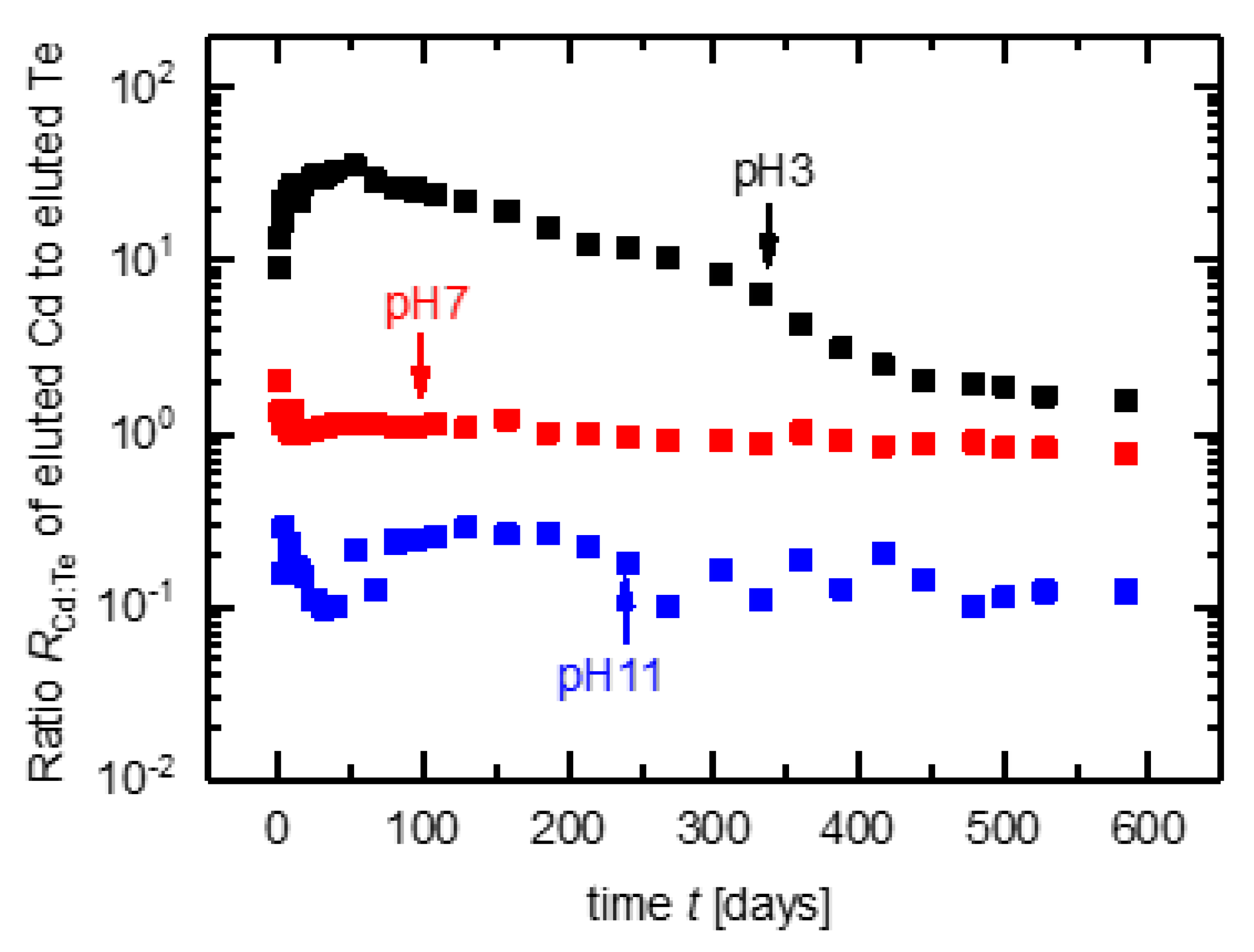
3.4. Analysis of Time Dependence
3.5. Mass Balance for CdTe Module Pieces
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phillips, S.; Warmuth, W. Photovoltaics Report; Fraunhofer ISE: Freiburg, Germany, 2020. [Google Scholar]
- Wade, A.; Heath, G.; Weckend, S.; Wambach, K.; Sinha, P.; Jia, Z.; Komoto, K.; Sander, K. IRENA and IEA PVPS-End-of-Life Management: Solar Photovoltaic Panels; National Renewable Energy Lab.: Golden, CO, USA, 2016.
- Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment Text with EEA Relevance. Available online: http://data.europa.eu/eli/dir/2011/65/oj (accessed on 29 January 2021).
- ITRPV. International Technology Roadmap for Photovoltaic (ITRPV) 2019 Results. Available online: https://itrpv.vdma.org/en/ (accessed on 12 October 2020).
- Ramos-Ruiz, A.; Wilkening, J.; Field, J.; Sierra-Alvarez, R. Leaching of Cadmium and Tellurium From Cadmium Telluride (CdTe) Thin-Film Solar Panels Under Simulated Landfill Conditions. J. Hazard. Mater. 2017, 336, 57. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, Y.S.; Schäffer, A.; Corvini, P.F.X.; Lenz, M. Thin-film photovoltaic cells: Long-term metal (loid) leaching at their end-of-life. Environ. Sci. Technol. 2013, 47, 13151. [Google Scholar] [CrossRef] [PubMed]
- Tammaro, M.; Salluzzi, A.; Rimauro, J.; Schiavo, S.; Manz, S. Experimental investigation to evaluate the potential environmental hazards of photovoltaic panels. J. Hazard. Mater. 2016, 306, 395. [Google Scholar] [CrossRef] [PubMed]
- Zapf-Gottwick, R.; Koch, M.; Fischer, K.; Schwerdt, F.; Hamann, L.; Kranert, M.; Metzger, J.; Werner, J.H. Leaching Hazardous Substances out of Photovoltaic Modules. Int. J. Adv. Appl. Phys. Res. 2015, 2, 7. [Google Scholar] [CrossRef]
- Zeng, C.; Ramos-Ruiz, A.; Field, J.A.; Sierra-Alvarez, R. Cadmium telluride (CdTe) and cadmium selenide (CdSe) leaching behavior and surface chemistry in response to pH and O2. J. Environ. Manag. 2015, 154, 78. [Google Scholar] [CrossRef] [PubMed]
- Nover, J.; Zapf-Gottwick, R.; Feifel, C.; Koch, M.; Metzger, J.; Werner, J.H. Long-term leaching of photovoltaic modules. Jpn. J. Appl. Phys. 2017, 56, 08MD02. [Google Scholar] [CrossRef]
- Nain, P.; Kumar, A. Initial metal contents and leaching rate constants of metals leached from end-of-life solar photovoltaic waste: An integrative literature review and analysis. Renew. Sustain. Energy Rev. 2020, 119, 109592. [Google Scholar] [CrossRef]
- EN 12457-4:2002. Characterization of Waste-Leaching; Compliance Test for Leaching of Granular Waste Materials and Sludges–Part 4: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size below 10 mm (without or with Limited Size Reduction); Swedish Institute for Standards: Stockholm, Sweden, 2002. [Google Scholar]
- United States Environmental Protection Agency. Test Methods for Evaluating Solid Waste: Physical/Chemical Methods; SW-846; United States Environmental Protection Agency: Washington, DC, USA, 1992.
- CCR, California Code of Regulations. Waste Extraction Test (WET) Procedures; Title 22. Division 4.5, Chapter 11, Article 5, Appendix II; 1991. [Google Scholar]
- Japanese Standards Association. JIS K 0102:2016-Testing Methods for Industrial Wastewater; Japanese Standards Association: Tokyo, Japan, 2016. [Google Scholar]
- Nover, J.; Huber, S.; Zapf-Gottwick, R.; Werner, J.H.; Feifel, C.; Koch, M.; Metzger, J. Schadstofffreisetzung aus Photovoltaik-Modulen Abschlussbericht: Laufzeit: 01.09.2014-31.08.2017; Universität Stuttgart, Institut für Photovoltaik: Stuttgart, Germany, 2018. [Google Scholar] [CrossRef]
- DIN 38404-6:1984-05. German Standard Methods for the Examination of Water, Waste Water and Sludge; Physical and Physico-Chemical Parameters (Group C); Determination of the Oxidation Reduction (Redox) Potential (C 6); Swedish Institute for Standards: Stockholm, Sweden, 1984. [Google Scholar]
- ISO 17294-2:2003. Water Quality-Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)-Part 2: Determination of 62 Elements; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Deb, S.K. Recent Advances and Future Opportunities for Thin-Film Solar Cells. In Thin-Film Solar Cells: Next Generation Photovoltaics and Its Applications; Hamakawa, Y., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; p. 27. [Google Scholar]
- Chopra, K.L.; Paulson, P.D.; Dutta, V. Thin-film solar cells: An overview. Prog. Photovolt Res. Appl. 2004, 12, 69. [Google Scholar] [CrossRef]
- Fritsche, J.; Klein, A.; Jaegermann, W. Thin Film Solar Cells: Materials Science at Interfaces. Adv. Eng. Mater. 2005, 7, 914. [Google Scholar] [CrossRef]
- Theelen, M.; Daume, F. Stability of Cu(In,Ga)Se2 solar cells: A literature review. Sol. Energy 2016, 133, 586. [Google Scholar] [CrossRef]
- Gabriel, O.; Kirner, S.; Leendertz, C.; Gerhardt, M.; Heidelberg, A.; Bloeß, H.; Schlatmann, R.; Rech, B. Large area PECVD of a-Si:H/a-Si:H tandem solar cells. Phys. Status Solidi C 2011, 8, 2982. [Google Scholar] [CrossRef]
- Zapf-Gottwick, R.; Zorn, M.; Nover, J.; Koch, M.; Feifel, C.; Werner, J.H. Solubility of Cadmium Telluride in Aqueous Solutions. Energies 2021, 14, 398. [Google Scholar] [CrossRef]
- Collins, M.K.; Anctil, A. Implications for current regulatory waste toxicity characterisation methods from analysing metal and metalloid leaching from photovoltaic modules. Int. J. Sustain. Energy 2017, 36, 531. [Google Scholar] [CrossRef]
- Theelen, M.; Polman, K.; Tomassini, M.; Barreau, N.; Steijvers, H.; Van Berkum, J.; Vroon, Z.; Zeman, M. Influence of deposition pressure and selenisation on damp heat degradation of the Cu(In,Ga)Se2 back contact molybdenum. Surf. Coat. Technol. 2014, 252, 157. [Google Scholar] [CrossRef]
- Pern, F.J.; Noufi, R.; Li, X.; DeHart, C.; To, B. Damp-heat induced degradation of transparent conducting oxides for thin-film solar cells. In Proceedings of the 33rd IEEE Photovoltaic Specialists Conference, San Diego, CA, USA, 11–16 May 2008. [Google Scholar]




| pH | (V) | Chemical Composition |
|---|---|---|
| 3 | 0.62 | 15.4 g/L CHO, 2.8 g/L NaHPO, DI water |
| 7 | 0.56 | 3.7 g/L KHPO, 5 g/L NaHPO, DI water |
| 11 | 0.33 | 0.04 g/L NaOH, DI water |
| Element | c-Si | a-Si | CdTe | CIGS |
|---|---|---|---|---|
| (mg) | (mg) | (mg) | (mg) | |
| Zn | 0.9 ± 0.4 | 16.1 ± 3.1 | ||
| Cd | 13.9 ± 0.9 | 0.2 ± 0.002 | ||
| Te | 15.6 ± 1.1 | |||
| In | 14.1 ± 4.3 | |||
| Ga | 0.7 ± 0.1 | |||
| Se | 6.7 ± 1.3 | |||
| Al | 167 ± 40 | 196 ± 27 | 289 ± 63 | 280 ± 190 |
| Mo | 12.7 ± 1.7 | 5.0 ± 0.2 | ||
| Cu | 254 ± 15 | 130 ± 14 | 80 ± 11 | 146 ± 5.7 |
| Ni | 1.0 ± 0.1 | |||
| Pb | 16.7 ± 0.8 | 2.4 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nover, J.; Zapf-Gottwick, R.; Feifel, C.; Koch, M.; Werner, J.H. Leaching via Weak Spots in Photovoltaic Modules. Energies 2021, 14, 692. https://doi.org/10.3390/en14030692
Nover J, Zapf-Gottwick R, Feifel C, Koch M, Werner JH. Leaching via Weak Spots in Photovoltaic Modules. Energies. 2021; 14(3):692. https://doi.org/10.3390/en14030692
Chicago/Turabian StyleNover, Jessica, Renate Zapf-Gottwick, Carolin Feifel, Michael Koch, and Juergen Heinz Werner. 2021. "Leaching via Weak Spots in Photovoltaic Modules" Energies 14, no. 3: 692. https://doi.org/10.3390/en14030692
APA StyleNover, J., Zapf-Gottwick, R., Feifel, C., Koch, M., & Werner, J. H. (2021). Leaching via Weak Spots in Photovoltaic Modules. Energies, 14(3), 692. https://doi.org/10.3390/en14030692







