Spectacular Enhancement of the Thermal and Photochemical Stability of MAPbI3 Perovskite Films Using Functionalized Tetraazaadamantane as a Molecular Modifier
Abstract
1. Introduction
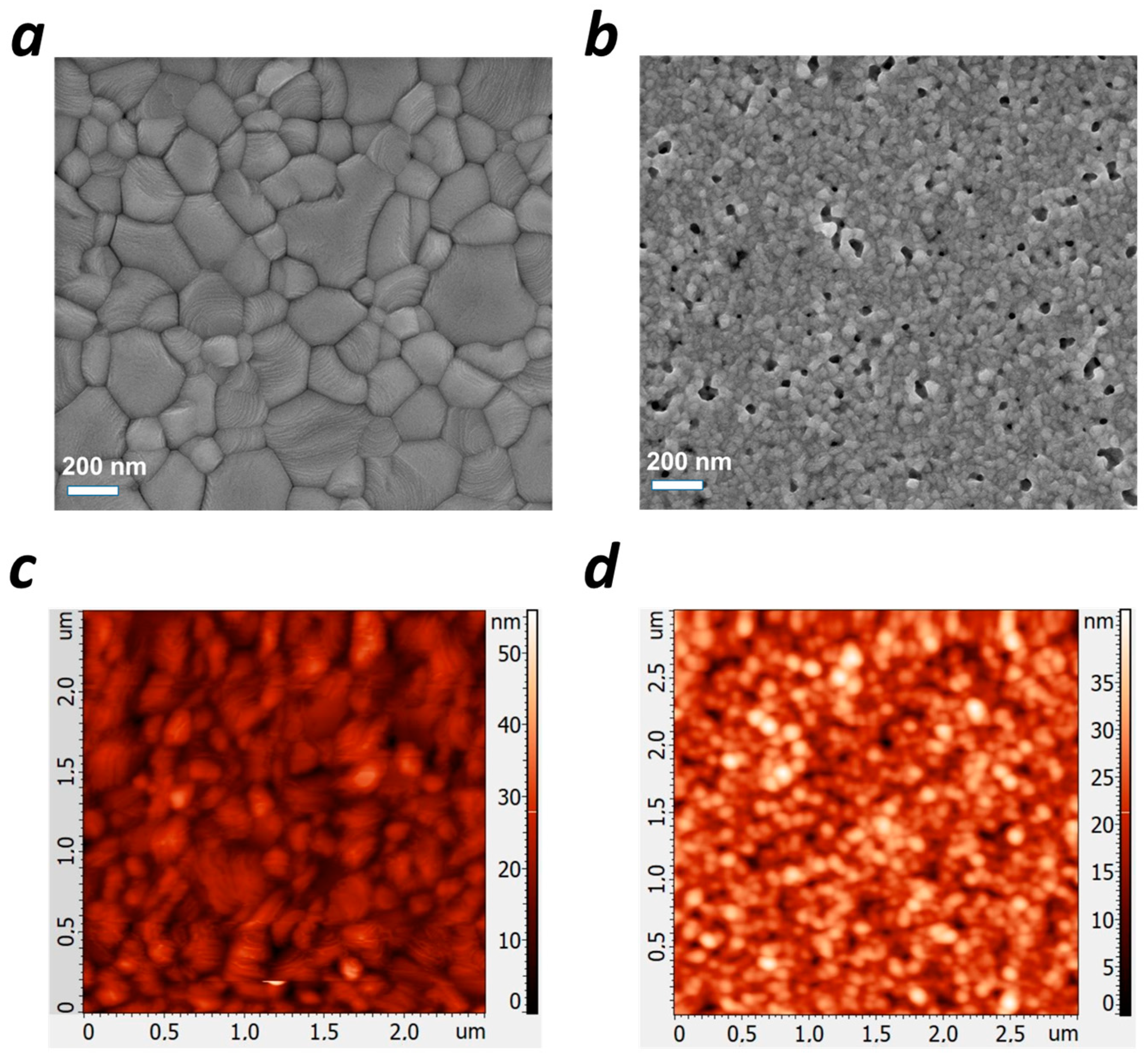
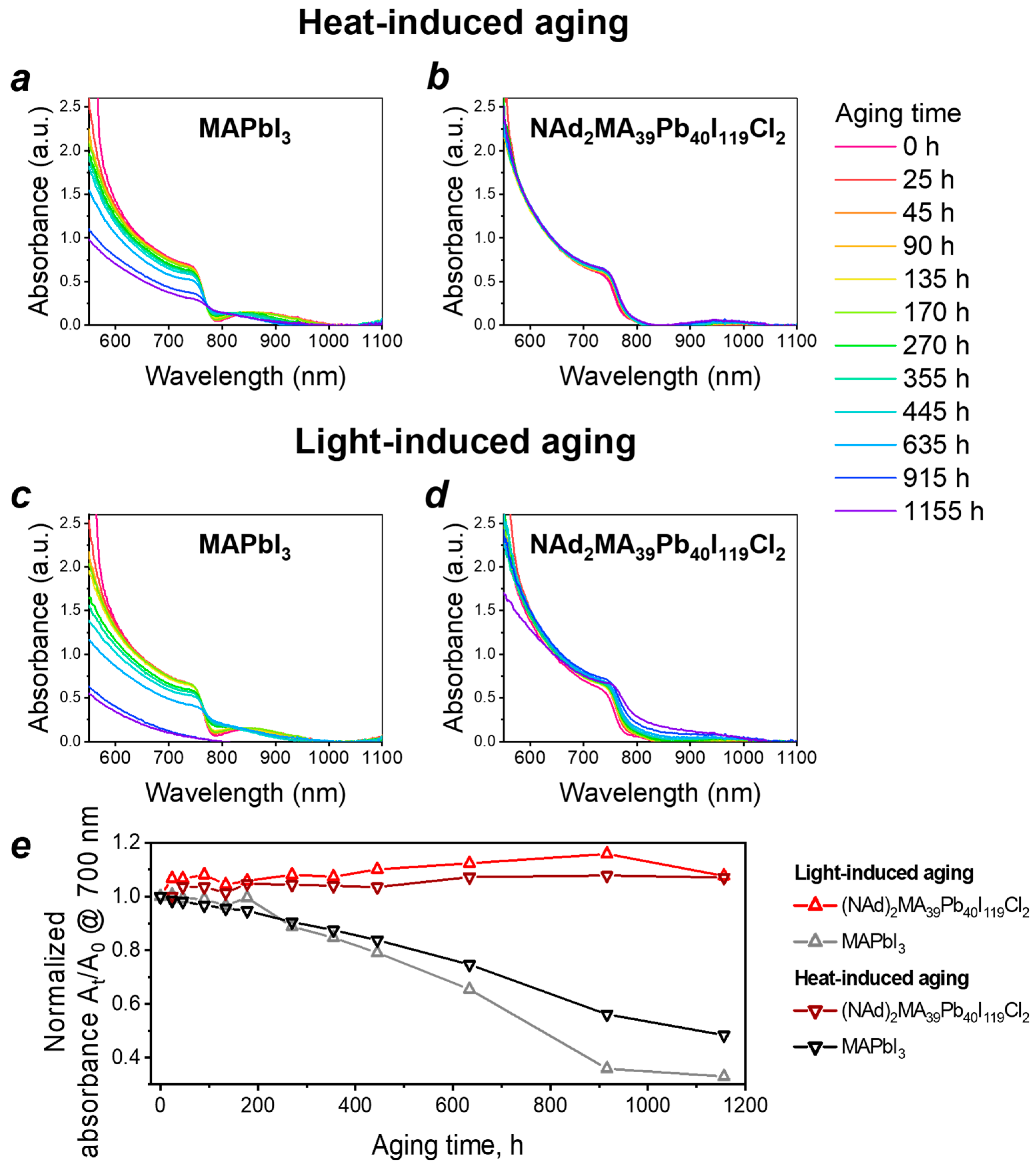
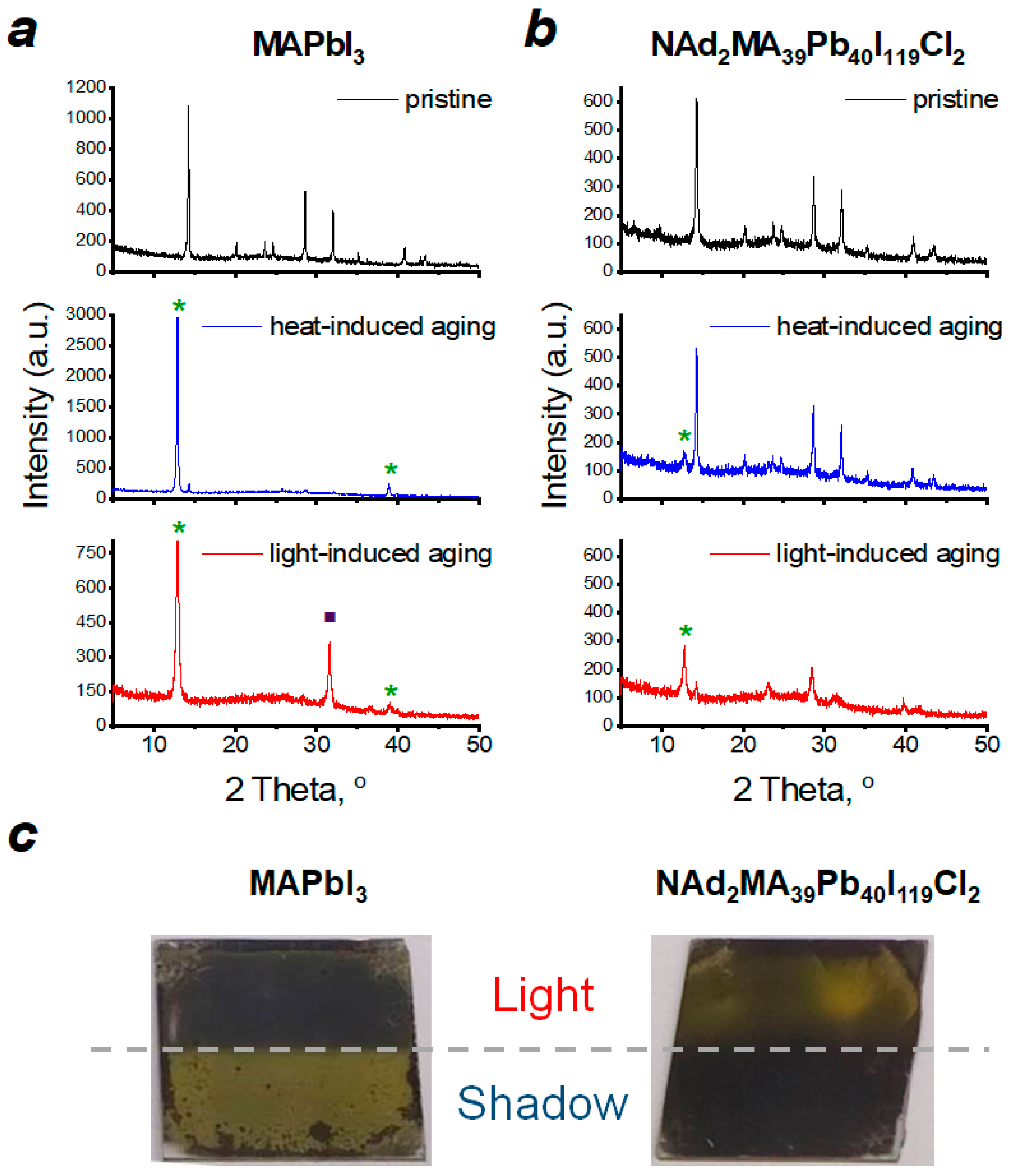
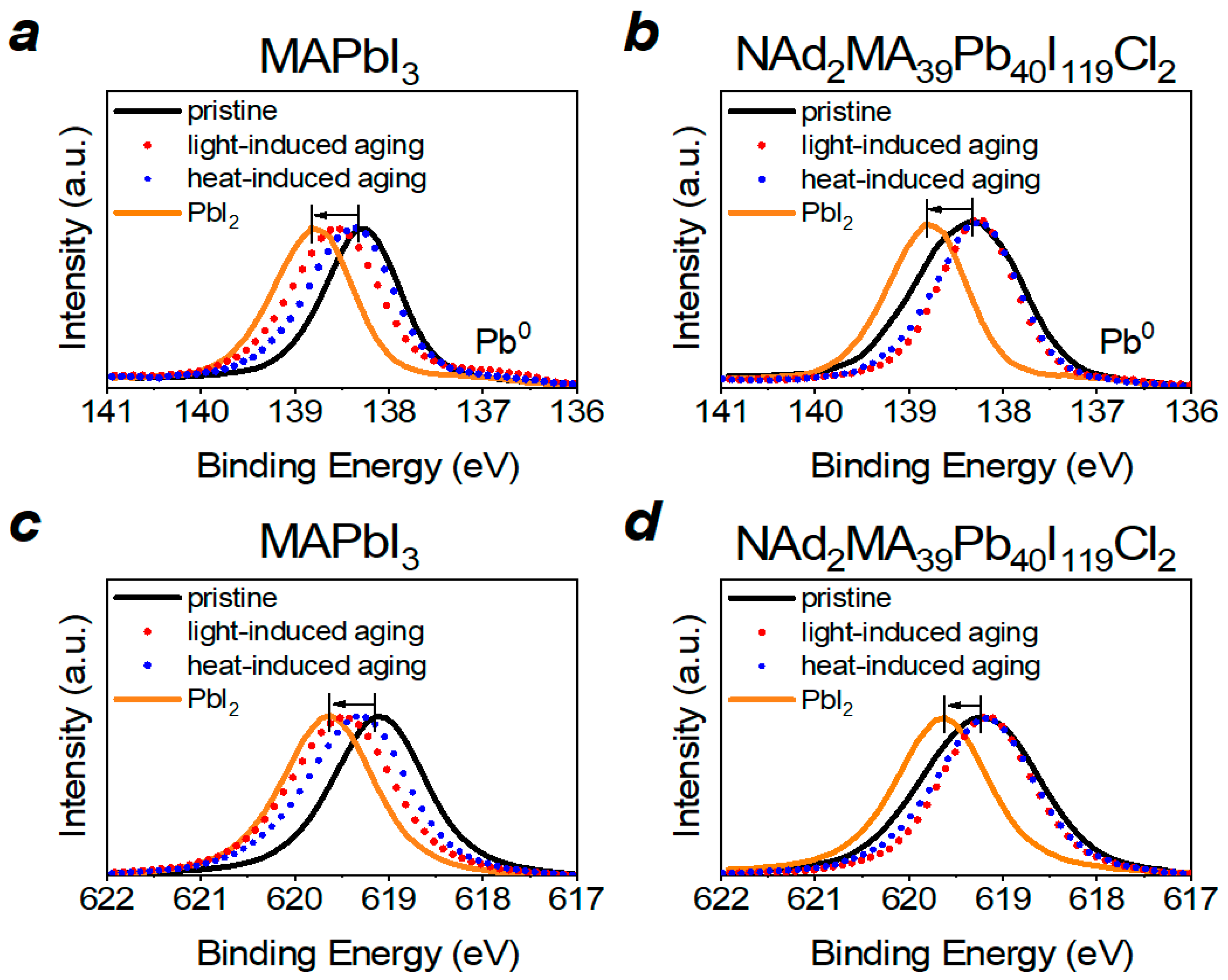
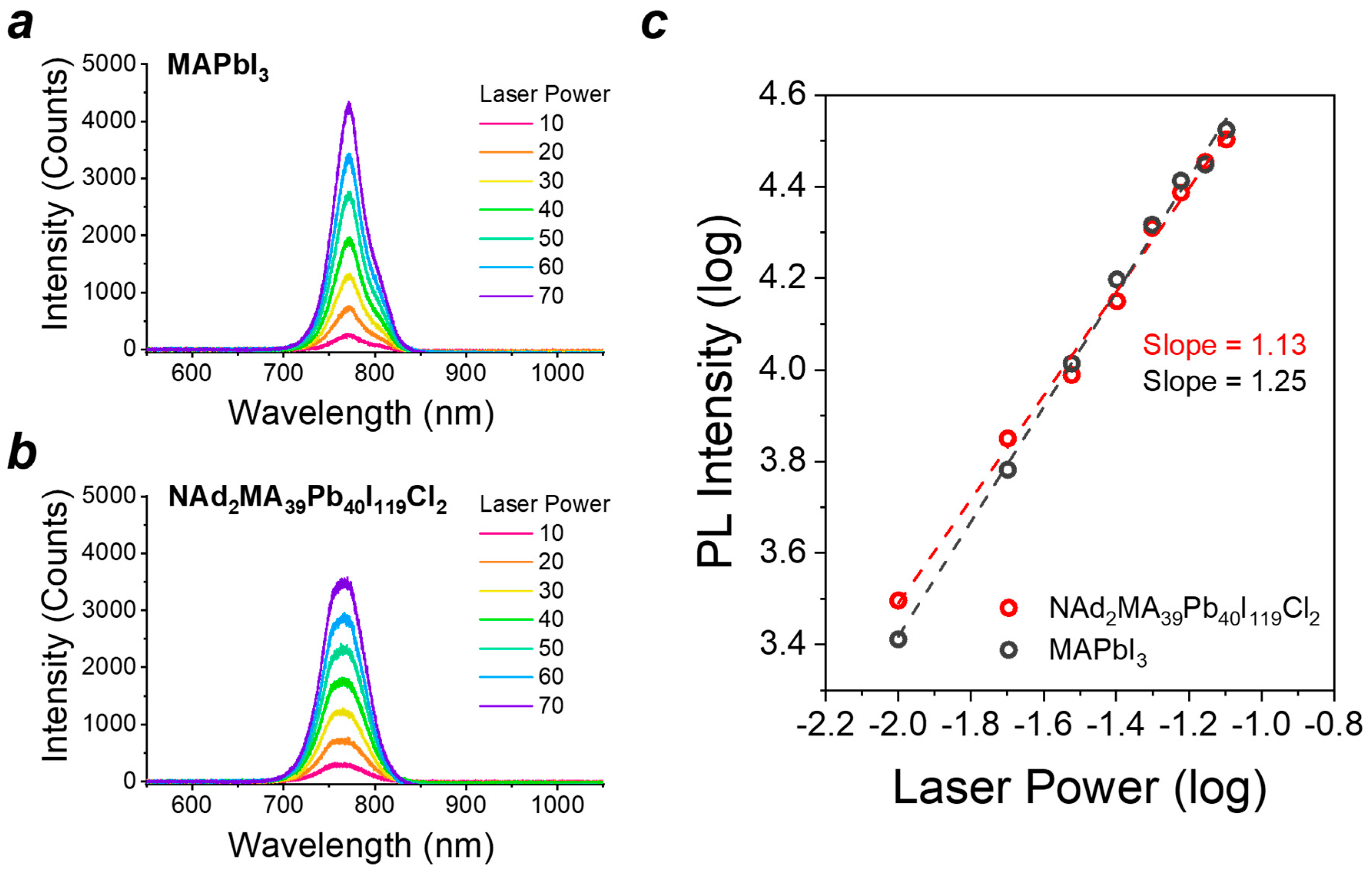
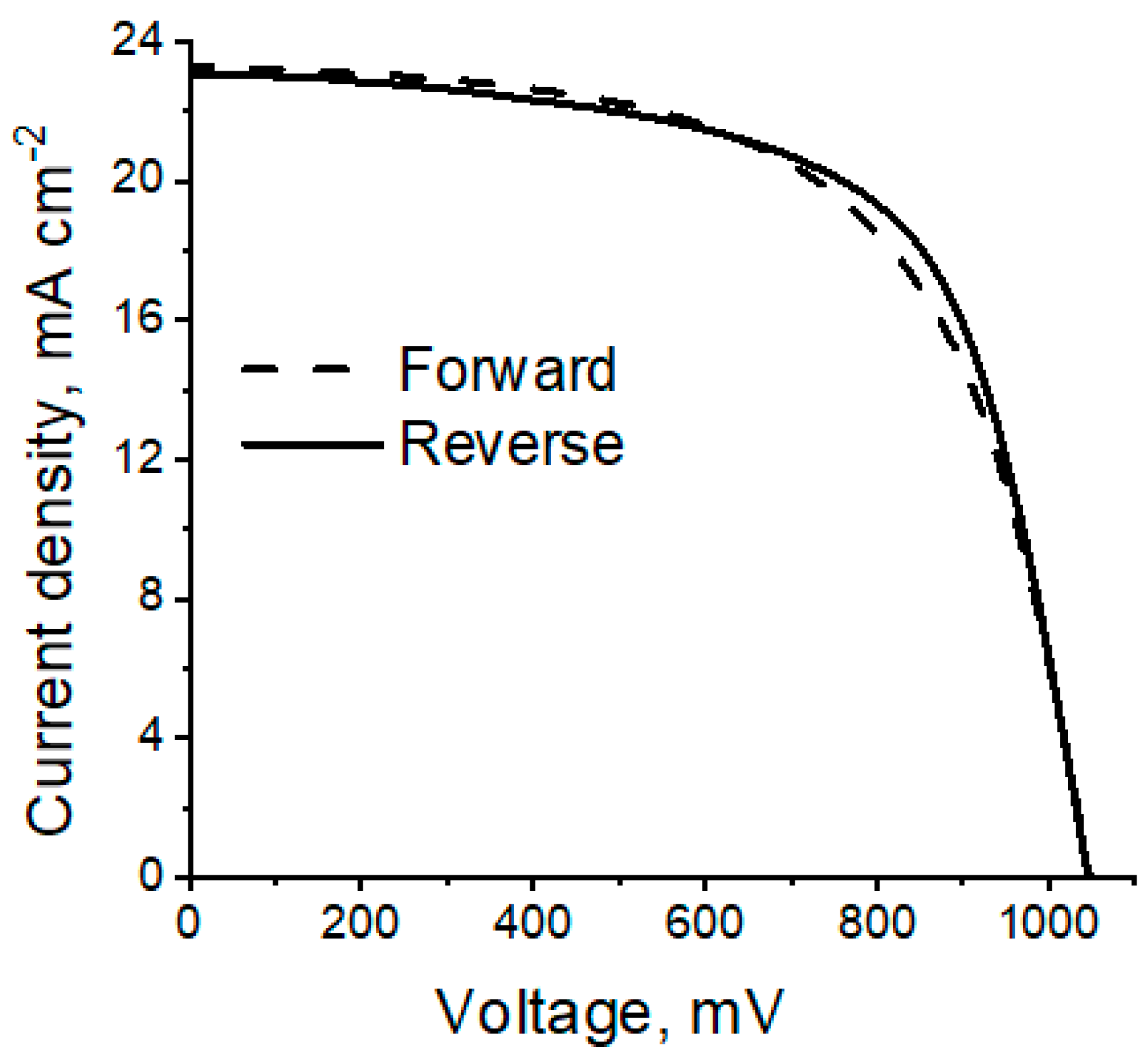
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
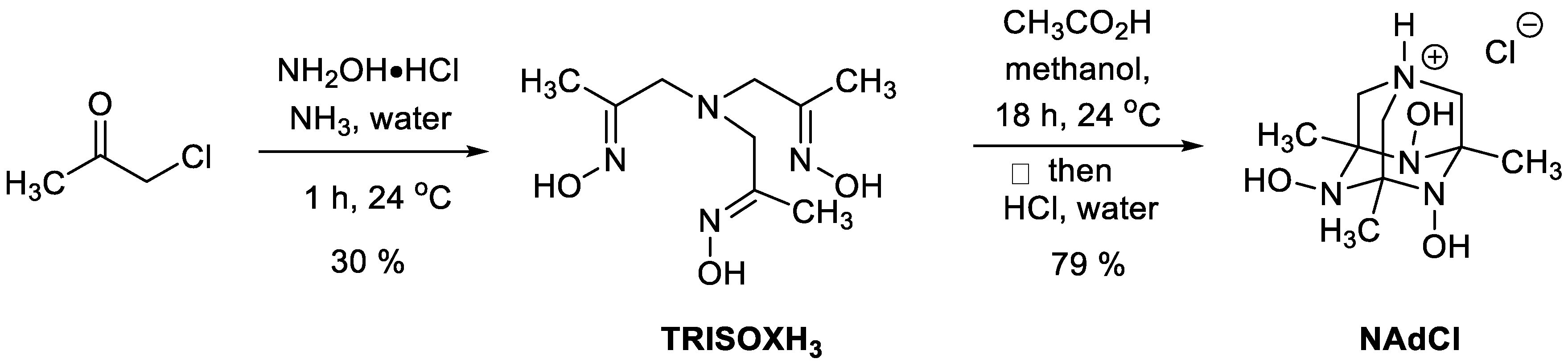
4.2. Synthesis and Characterization of 4,6,10-Trihydroxy-3,5,7-Trimethyl-1,4,6,10-Tetraazaadamantan-1-Ium Chloride (NAdCl)
4.3. Preparation of the MAPbI3 and NAd2MA39Pb40I119Cl2 Films
4.4. Aging Experiments
4.5. Characterization Techniques
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NREL Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 27 January 2021).
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Morales Vilches, A.B.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic Perovskite/Silicon Tandem Solar Cell with >29% Efficiency by Enhanced Hole Extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; You, J.; Yang, Y. Addressing the Stability Issue of Perovskite Solar Cells for Commercial Applications. Nat. Commun. 2018, 9, 5265. [Google Scholar] [CrossRef] [PubMed]
- Leguy AM, A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and Oxygen Induced Degradation Limits the Operational Stability of Methylammonium Lead Triiodide Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef]
- Nayakasinghe, M.T.; Han, Y.; Sivapragasam, N.; Kilin, D.S.; Burghaus, U. Unexpected High Binding Energy of CO2 on CH3NH3PbI3 Lead-Halide Organic–Inorganic Perovskites via Bicarbonate Formation. Chem. Commun. 2018, 54, 9949–9952. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sandhu, S.; Yadav, H.; Lee, J.-J. Stable Triple-Cation (Cs+ –MA+ –FA+) Perovskite Powder Formation under Ambient Conditions for Hysteresis-Free High-Efficiency Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 29941–29949. [Google Scholar] [CrossRef]
- Shekargoftar, M.; Jurmanová, J.; Homola, T. A Study on the Effect of Ambient Air Plasma Treatment on the Properties of Methylammonium Lead Halide Perovskite Films. Metals 2019, 9, 991. [Google Scholar] [CrossRef]
- Brunetti, B.; Cavallo, C.; Ciccioli, A.; Gigli, G.; Latini, A. On the Thermal and Thermodynamic (In)Stability of Methylammonium Lead Halide Perovskites. Sci. Rep. 2016, 6, 31896. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal Degradation of CH3NH3PbI3 Perovskite into NH3 and CH3I Gases Observed by Coupled Thermogravimetry–Mass Spectrometry Analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Martynenko, V.M.; Frolova, L.A.; Dremova, N.N.; Zhidkov, I.; Tsarev, S.A.; Luchkin, S.Y.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; et al. Intrinsic Thermal Decomposition Pathways of Lead Halide Perovskites APbX3. Solar Energy Mater. Solar Cells 2020, 213, 110559. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and Thermal Decomposition in Methylammonium Halide Lead Perovskites and Inferred Design Principles to Increase Photovoltaic Device Stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Luchkin, S.Y.u.; Frolova, L.A.; Dremova, N.N.; Gerasimov, K.L.; Zhidkov, I.S.; Anokhin, D.V.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Probing the Intrinsic Thermal and Photochemical Stability of Hybrid and Inorganic Lead Halide Perovskites. J. Phys. Chem. Lett. 2017, 8, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.K.; Aharon, S.; Li, B.; Mogilyansky, D.; Visoly-Fisher, I.; Etgar, L.; Katz, E.A. Temperature-and Component-Dependent Degradation of Perovskite Photovoltaic Materials under Concentrated Sunlight. J. Phys. Chem. Lett. 2015, 6, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, J.; Li, S.; Liu, S.; Wang, Q.; Mei, A.; Rong, Y.; Han, H.; Hu, Y. Progress in Multifunctional Molecules for Perovskite Solar Cells. Sol. RRL 2020, 4, 1900248. [Google Scholar] [CrossRef]
- Mahapatra, A.; Prochowicz, D.; Tavakoli, M.M.; Trivedi, S.; Kumar, P.; Yadav, P. A Review of Aspects of Additive Engineering in Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 27–54. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Y.; Zhang, X.; You, J. Recent Progresses on Defect Passivation toward Efficient Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1902650. [Google Scholar] [CrossRef]
- Aydin, E.; Bastiani, M.; Wolf, S. Defect and Contact Passivation for Perovskite Solar Cells. Adv. Mater. 2019, 31, 1900428. [Google Scholar] [CrossRef]
- Singh, R.; Ryu, I.; Yadav, H.; Park, J.; Jo, J.W.; Yim, S.; Lee, J.-J. Non-Hydrolytic Sol-Gel Route to Synthesize TiO2 Nanoparticles under Ambient Condition for Highly Efficient and Stable Perovskite Solar Cells. Solar Energy 2019, 185, 307–314. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tien, C.-H.; Jhou, Y.-C.; Lin, W.-C. Co-Solvent Controllable Engineering of MA0.5FA0.5Pb0.8Sn0.2I3 Lead–Tin Mixed Perovskites for Inverted Perovskite Solar Cells with Improved Stability. Energies 2020, 13, 2438. [Google Scholar] [CrossRef]
- Milić, J.V.; Kubicki, D.J.; Emsley, L.; Grätzel, M. Multifunctional Molecular Modulation for Efficient and Stable Hybrid Perovskite Solar Cells. Chimia 2019, 73, 317–323. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Liu, P.; Cheng, Y.-B.; Zhao, H.J.; Yang, H.G. Functionalization of Perovskite Thin Films with Moisture-Tolerant Molecules. Nat. Energy 2016, 1, 15016. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Lee, D.Y.; Paik, M.J.; Na, H.; Lee, J.; Seok, S.I. Optimal Interfacial Engineering with Different Length of Alkylammonium Halide for Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1902740. [Google Scholar] [CrossRef]
- Jung, M.; Shin, T.J.; Seo, J.; Kim, G.; Seok, S.I. Structural Features and Their Functions in Surfactant-Armoured Methylammonium Lead Iodide Perovskites for Highly Efficient and Stable Solar Cells. Energy Environ. Sci. 2018, 11, 2188–2197. [Google Scholar] [CrossRef]
- Guo, P.; Ye, Q.; Yang, X.; Zhang, J.; Xu, F.; Shchukin, D.; Wei, B.; Wang, H. Surface & Grain Boundary Co-Passivation by Fluorocarbon Based Bifunctional Molecules for Perovskite Solar Cells with Efficiency over 21%. J. Mater. Chem. A 2019, 7, 2497–2506. [Google Scholar] [CrossRef]
- Ali, N.; Wang, X.; Rauf, S.; Attique, S.; Khesro, A.; Ali, S.; Mushtaq, N.; Xiao, H.; Yang, C.P.; Wu, H. Enhanced Stability in Cesium Assisted Hybrid 2D/3D-Perovskite Thin Films and Solar Cells Prepared in Ambient Humidity. Solar Energy 2019, 189, 325–332. [Google Scholar] [CrossRef]
- Liu, Y.; Akin, S.; Pan, L.; Uchida, R.; Arora, N.; Milić, J.V.; Hinderhofer, A.; Schreiber, F.; Uhl, A.R.; Zakeeruddin, S.M.; et al. Ultrahydrophobic 3D/2D Fluoroarene Bilayer-Based Water-Resistant Perovskite Solar Cells with Efficiencies Exceeding 22%. Sci. Adv. 2019, 5, eaaw2543. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.S.; Hussain, S.; Zhang, J.; Wang, B.; Yang, C.; Wang, Z.; Wei, Z.; Ahmad, R. Orientationally Engineered 2D/3D Perovskite for High Efficiency Solar Cells. Sustain. Energy Fuels 2020, 4, 324–330. [Google Scholar] [CrossRef]
- Rodríguez-Romero, J.; Hames, B.C.; Mora-Seró, I.; Barea, E.M. Conjugated Organic Cations to Improve the Optoelectronic Properties of 2D/3D Perovskites. ACS Energy Lett. 2017, 2, 1969–1970. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, G.; Zhu, L.; Ye, J.; Zhang, X.; Alsaedi, A.; Hayat, T.; Pan, X.; Dai, S. The Effect of Hydrophobicity of Ammonium Salts on Stability of Quasi-2D Perovskite Materials in Moist Condition. Adv. Energy Mater. 2018, 8, 1800051. [Google Scholar] [CrossRef]
- Li, S.; Hu, L.; Zhang, C.; Wu, Y.; Liu, Y.; Sun, Q.; Cui, Y.; Hao, Y.; Wu, Y. In Situ Growth of a 2D/3D Mixed Perovskite Interface Layer by Seed-Mediated and Solvent-Assisted Ostwald Ripening for Stable and Efficient Photovoltaics. J. Mater. Chem. C 2020, 8, 2425–2435. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Bi, D.; Pan, L.; Hagfeldt, A.; Zakeeruddin, S.M.; Grätzel, M. Adamantanes Enhance the Photovoltaic Performance and Operational Stability of Perovskite Solar Cells by Effective Mitigation of Interfacial Defect States. Adv. Energy Mater. 2018, 8, 1800275. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tress, W.; Milić, J.V.; Kubicki, D.; Emsley, L.; Grätzel, M. Addition of Adamantylammonium Iodide to Hole Transport Layers Enables Highly Efficient and Electroluminescent Perovskite Solar Cells. Energy Environ. Sci. 2018, 11, 3310–3320. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Yadav, P.; Prochowicz, D.; Sponseller, M.; Osherov, A.; Bulović, V.; Kong, J. Controllable Perovskite Crystallization via Antisolvent Technique Using Chloride Additives for Highly Efficient Planar Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803587. [Google Scholar] [CrossRef]
- Milić, J.V.; Im, J.; Kubicki, D.J.; Ummadisingu, A.; Seo, J.; Li, Y.; Ruiz-Preciado, M.A.; Dar, M.I.; Zakeeruddin, S.M.; Emsley, L.; et al. Supramolecular Engineering for Formamidinium-Based Layered 2D Perovskite Solar Cells: Structural Complexity and Dynamics Revealed by Solid-State NMR Spectroscopy. Adv. Energy Mater. 2019, 9, 1900284. [Google Scholar] [CrossRef]
- Martínez-Sarti, L.; Palazon, F.; Sessolo, M.; Bolink, H.J. Dry Mechanochemical Synthesis of Highly Luminescent, Blue and Green Hybrid Perovskite Solids. Adv. Optical Mater. 2020, 8, 1901494. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Li, X.-T.; Zhao, E.-F.; Lv, X.-D.; Meng, F.-L.; Peng, C.; Lai, X.-S.; Huang, M.; Cao, G.; Tao, X.; et al. Hexamethylenetetramine-Mediated Growth of Grain-Boundary-Passivation CH3NH3PbI3 for Highly Reproducible and Stable Perovskite Solar Cells. J. Power Sources 2018, 377, 103–109. [Google Scholar] [CrossRef]
- Semakin, A.N.; Sukhorukov, A.Y.; Lesiv, A.V.; Ioffe, S.L.; Lyssenko, K.A.; Nelyubina, Y.V.; Tartakovsky, V.A. Unusual Intramolecular Cyclization of Tris(β-oximinoalkyl)amines. The First Synthesis of 1,4,6,10-Tetraazaadamantanes. Org. Lett. 2009, 11, 4072–4075. [Google Scholar] [CrossRef]
- Tolbin, A.Y.; Sukhorukov, A.Y.; Ioffe, S.L.; Lobach, O.A.; Nosik, D.N.; Tomilova, L.G. Synthesis of a Phtalocyanine -1,4,6,10-Tetraazaadamantane Conjugate and Its Activity Against the Human Immunodeficiency Virus. Mendeleev Commun. 2010, 20, 24–26. [Google Scholar] [CrossRef]
- Golovanov, I.S.; Mazeina, G.S.; Nelyubina, Y.V.; Novikov, R.A.; Mazur, A.S.; Britvin, S.N.; Tartakovsky, V.A.; Ioffe, S.L.; Sukhorukov, A.Y. Exploiting Coupling of Boronic Acids with Triols for a pH-Dependent “Click-Declick” Chemistry. J. Org. Chem. 2018, 83, 9756–9773. [Google Scholar] [CrossRef]
- Premuzič, D.; Hołynska, M.; Ozarowski, A.; Pietzonka, C.; Roseborough, A.; Stoian, S.A. Model Dimeric Manganese(IV) Complexes Featuring Terminal Trishydroxotetraazaadamantane and Various Bridging Ligands. Inorg. Chem. 2020, 59, 10768–10784. [Google Scholar] [CrossRef]
- Cohen, B.-E.; Wierzbowska, M.; Etgar, L. High Efficiency and High Open Circuit Voltage in Quasi 2D Perovskite Based Solar Cells. Adv. Funct. Mater. 2017, 27, 1604733. [Google Scholar] [CrossRef]
- Quan, L.N.; Yuan, M.; Comin, R.; Voznyy, O.; Beauregard, E.M.; Hoogland, S.; Buin, A.; Kirmani, A.R.; Zhao, K.; Amassian, A.; et al. Ligand-Stabilized Reduced-Dimensionality Perovskites. J. Am. Chem. Soc. 2016, 138, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Koh, T.M.; Febriansyah, B.; Bruno, A.; Mathews, N.; Mhaisalkar, S.G.; Soci, C. Mixed-Dimensional Naphthylmethylammonium-Methylammonium Lead Iodide Perovskites with Improved Thermal Stability. Sci. Rep. 2020, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Yun, J.S.; Kim, J.; Soufiani, A.M.; Chen, S.; Cho, Y.; Deng, X.; Seidel, J.; Lim, S.; Huang, S.; et al. Passivation of Grain Boundaries by Phenethylammonium in Formamidinium-Methylammonium Lead Halide Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 647–654. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Frolova, L.A.; Dremova, N.N.; Zhidkov, I.; Martynenko, V.M.; Tsarev, S.A.; Luchkin, S.Y.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; et al. Light or Heat: What Is Killing Lead Halide Perovskites under Solar Cell Operation Conditions? J. Phys. Chem. Lett. 2020, 11, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, A.G.; Akbulatov, A.F.; Elnaggar, M.; Luchkin, S.Y.; Danilov, A.V.; Zhidkov, I.S.; Yamilova, O.R.; Fedotov, Y.S.; Bredikhin, S.I.; Kurmaev, E.Z.; et al. Impact of Charge Transport Layers on the Photochemical Stability of MAPbI3 in Thin Films and Perovskite Solar Cells. Sustain. Energy Fuels 2019, 3, 2705–2716. [Google Scholar] [CrossRef]
- Boldyreva, A.G.; Zhidkov, I.S.; Tsarev, S.; Akbulatov, A.F.; Tepliakova, M.M.; Fedotov, Y.S.; Bredikhin, S.I.; Postnova, E.Y.; Luchkin, S.Y.; Kurmaev, E.Z.; et al. Unraveling the Impact of Hole Transport Materials on Photostability of Perovskite Films and p–i–n Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 19161–19173. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Motti, S.G.; Mosconi, E.; Barker, A.J.; Ball, J.; Andrea Riccardo Perini, C.; Deschler, F.; Petrozza, A.; De Angelis, F. Iodine Chemistry Determines the Defect Tolerance of Lead-Halide Perovskites. Energy Environ. Sci. 2018, 11, 702–713. [Google Scholar] [CrossRef]
- Draguta, S.; Thakur, S.; Morozov, Y.V.; Wang, Y.; Manser, J.S.; Kamat, P.V.; Kuno, M. Spatially Non-Uniform Trap State Densities in Solution-Processed Hybrid Perovskite Thin Films. J. Phys. Chem. Lett. 2016, 7, 715–721. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Frolova, L.A.; Tsarev, S.A.; Zhidkov, I.; Luchkin, S.Y.; Kurmaev, E.Z.; Stevenson, K.J.; Aldoshin, S.M.; Troshin, P.A. Film Deposition Techniques Impact the Defect Density and Photostability of MAPbI3 Perovskite Films. J. Phys. Chem. C 2020, 124, 21378–21385. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, M.; Liu, Z.; Jamshaid, A.; Ono, L.K.; Qi, Y. Highly Efficient Perovskite Solar Cells Enabled by Multiple Ligand Passivation. Adv. Energy Mater. 2020, 10, 1903696. [Google Scholar] [CrossRef]
- Chen, J.; Kim, S.-G.; Ren, X.; Jung, H.S.; Park, N.-G. Effect of Bidentate and Tridentate Additives on the Photovoltaic Performance and Stability of Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 4977–4987. [Google Scholar] [CrossRef]
- Tsarev, S.; Dubinina, T.S.; Luchkin, S.Y.; Zhidkov, I.S.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Phenyl-C 61 -Butyric Acid as an Interface Passivation Layer for Highly Efficient and Stable Perovskite Solar Cells. J. Phys. Chem. C 2020, 124, 1872–1877. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozerova, V.V.; Zhidkov, I.S.; Boldyreva, A.; Dremova, N.N.; Emelianov, N.A.; Shilov, G.V.; Frolova, L.A.; Kurmaev, E.Z.; Sukhorukov, A.Y.; Aldoshin, S.M.; et al. Spectacular Enhancement of the Thermal and Photochemical Stability of MAPbI3 Perovskite Films Using Functionalized Tetraazaadamantane as a Molecular Modifier. Energies 2021, 14, 669. https://doi.org/10.3390/en14030669
Ozerova VV, Zhidkov IS, Boldyreva A, Dremova NN, Emelianov NA, Shilov GV, Frolova LA, Kurmaev EZ, Sukhorukov AY, Aldoshin SM, et al. Spectacular Enhancement of the Thermal and Photochemical Stability of MAPbI3 Perovskite Films Using Functionalized Tetraazaadamantane as a Molecular Modifier. Energies. 2021; 14(3):669. https://doi.org/10.3390/en14030669
Chicago/Turabian StyleOzerova, Victoria V., Ivan S. Zhidkov, Aleksandra Boldyreva, Nadezhda N. Dremova, Nikita A. Emelianov, Gennady V. Shilov, Lyubov A. Frolova, Ernst Z. Kurmaev, Alexey Y. Sukhorukov, Sergey M. Aldoshin, and et al. 2021. "Spectacular Enhancement of the Thermal and Photochemical Stability of MAPbI3 Perovskite Films Using Functionalized Tetraazaadamantane as a Molecular Modifier" Energies 14, no. 3: 669. https://doi.org/10.3390/en14030669
APA StyleOzerova, V. V., Zhidkov, I. S., Boldyreva, A., Dremova, N. N., Emelianov, N. A., Shilov, G. V., Frolova, L. A., Kurmaev, E. Z., Sukhorukov, A. Y., Aldoshin, S. M., & Troshin, P. A. (2021). Spectacular Enhancement of the Thermal and Photochemical Stability of MAPbI3 Perovskite Films Using Functionalized Tetraazaadamantane as a Molecular Modifier. Energies, 14(3), 669. https://doi.org/10.3390/en14030669







