Investigation on the Operating Conditions of Proton Exchange Membrane Fuel Cell Based on Constant Voltage Cold Start Mode
Abstract
1. Introduction
2. Experimental
2.1. Single Cell Assembly and Cold Start Experimental Test Platform
2.2. PEMFC Activation Process
2.3. Cold Start Experiment Procedure
2.4. Electrochemical Impedance Spectroscopy Measurement
3. Results and Discussion
3.1. Impact of the Fuel Cell Voltage during Cold Start
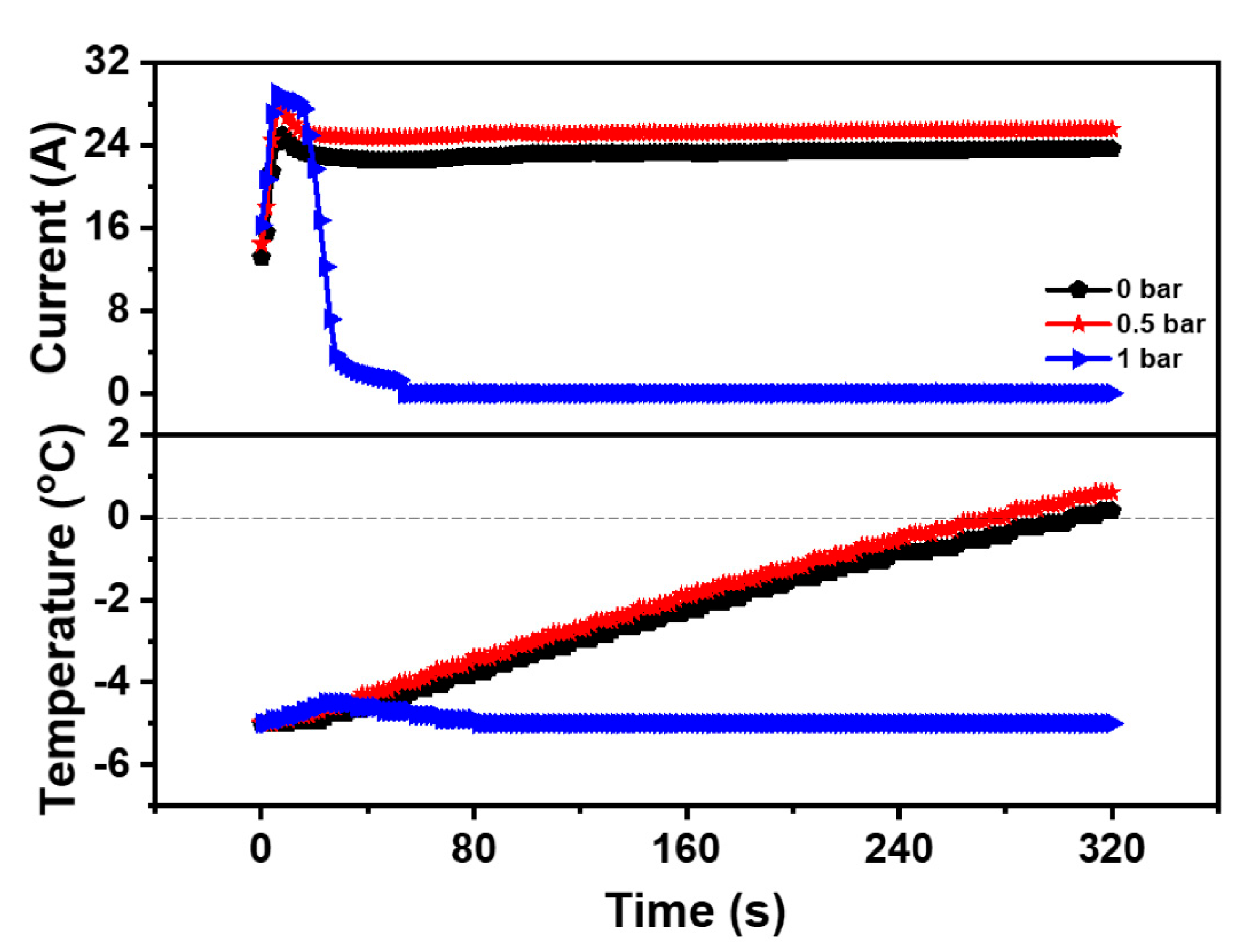
3.2. Impact of the Back-Pressure During Cold Start
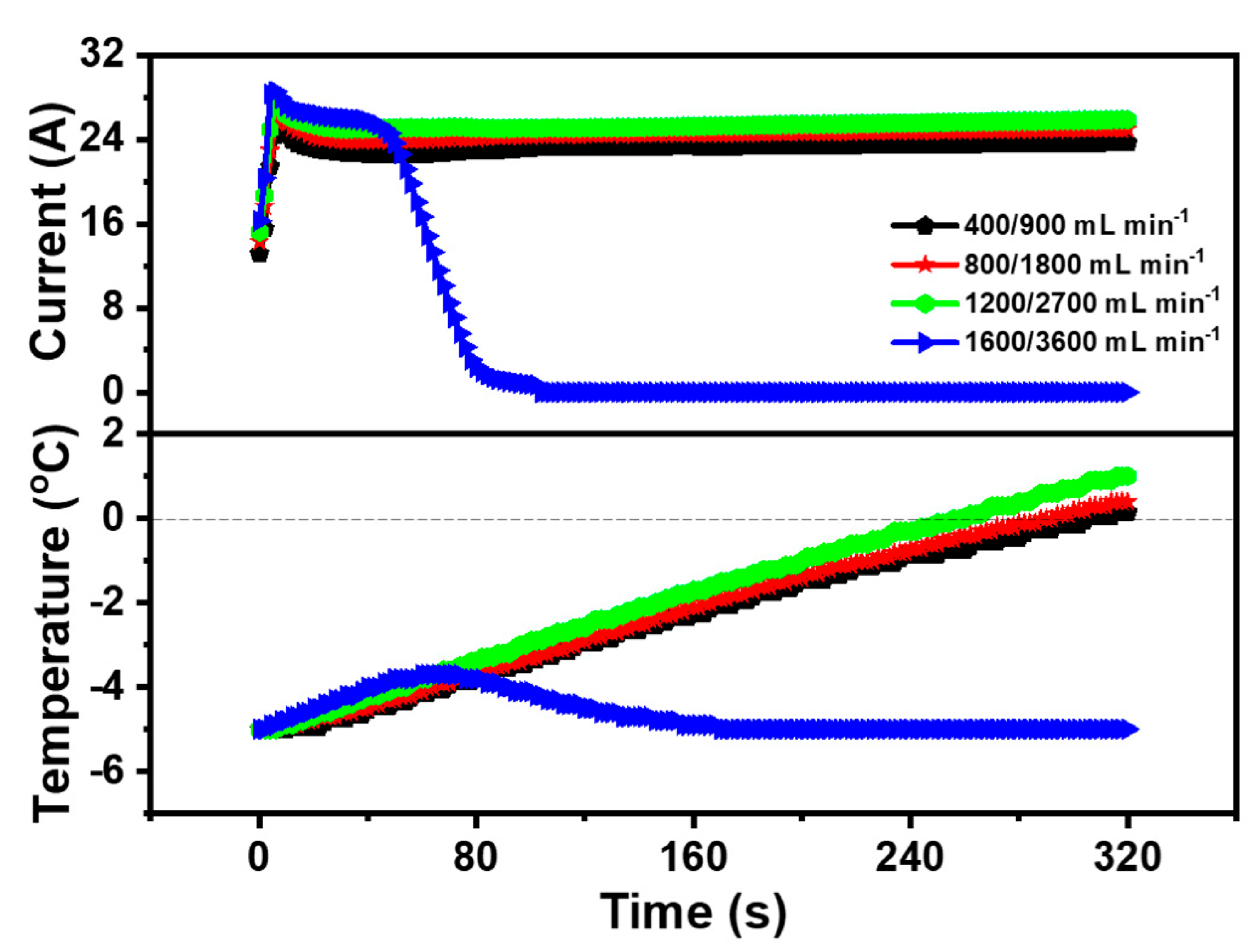
3.3. Impact of the Inlet Gas Flow Rate during Cold Start
4. Conclusions
- (1)
- Based on the constant voltage cold start, the peak current of the PEMFC was the highest when the start-up voltage was 0.1 V. Additionally, the heating rate was faster than that when the start-up voltage was 0.15 V, 0.2 V, 0.25 V, and 0.3 V, indicating that the lower start-up voltage could accelerate the heat production of the PEMFC and develop its cold start property at low temperature. Additionally, the optimized cold start voltage of the PEMFC was 0.1 V.
- (2)
- The increase of the back-pressure could promote the movement of the gas perpendicular to the flow channel, increase the concentration of the reaction gas, accelerate the electrochemical reaction, and enhance the cold start property of the PEMFC at low temperature. However, excessive back-pressure could also reduce the gas flow and made it difficult to drain the generated water, which was detrimental to the cold start property of the PEMFC. Additionally, the optimized cold start back-pressure of the PEMFC was 0.5 bar.
- (3)
- The inlet flow rate was a significant factor affecting the cold start property of the PEMFC. The increase of the inlet gas flow rate could enhance the supply of the reaction gas, augment the current of the PEMFC and accelerate the temperature rise of the PEMFC. However, it is difficult for the PEMFC to maintain the temperature rise due to the high inlet flow rate and rapid heat loss. Thus, the optimized cold start inlet flow rate of the PEMFC in the anode and cathode was 1200 mL·min−1 and 2700 mL·min−1, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Pei, M.; He, Q.; Wu, Q.; Jackson, L.; Mao, L. A novel method for polymer electrolyte membrane fuel cell fault diagnosis using 2D data. J. Power Sources 2021, 482, 228894. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, M.; Wu, S.; Tongsh, C.; Sun, X.; Wang, B.; Park, J.W.; Jiao, K. Investigation of mechanical vibration effect on proton exchange membrane fuel cell cold start. Int. J. Hydrogen Energy 2020, 45, 14528–14538. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Liu, H.; Yan, C.; Hou, Y.; He, Q.; Zhang, J.; Hissel, D. Anode purge management for hydrogen utilization and stack durability improvement of PEM fuel cell systems. Appl. Energy 2020, 275, 115110. [Google Scholar] [CrossRef]
- Sun, L.; Jin, Y.; You, F. Active disturbance rejection temperature control of open-cathode proton exchange membrane fuel cell. Appl. Energy 2020, 261, 114381. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, Y.; Yu, S.; Jiao, K. Modeling of cold start processes and performance optimization for proton exchange membrane fuel cell stacks. J. Power Sources 2014, 247, 738–748. [Google Scholar] [CrossRef]
- Wei, L.; Dafalla, A.M.; Jiang, F. Effects of reactants/coolant non-uniform inflow on the cold start performance of PEMFC stack. Int. J. Hydrogen Energy 2020, 45, 13469–13482. [Google Scholar] [CrossRef]
- Jiao, K.; Alaefour, I.E.; Karimi, G.; Li, X. Cold start characteristics of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 11832–11845. [Google Scholar] [CrossRef]
- Huo, S.; Jiao, K.; Park, J.W. On the water transport behavior and phase transition mechanisms in cold start operation of PEM fuel cell. Appl. Energy 2019, 233–234, 776–788. [Google Scholar] [CrossRef]
- Hirakata, S.; Hara, M.; Kakinuma, K.; Uchida, M.; Tryk, D.A.; Uchida, H.; Watanabe, M. Investigation of the effect of a hydrophilic layer in the gas diffusion layer of a polymer electrolyte membrane fuel cell on the cell performance and cold start behaviour. Electrochim. Acta 2014, 120, 240–247. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, R.; Jiang, Z.; Zhong, D.; Wang, B.; Shangguan, W.; Han, L. Investigation on cold start of polymer electrolyte membrane fuel cells with different cathode serpentine flow fields. Int. J. Hydrogen Energy 2019, 44, 7505–7517. [Google Scholar] [CrossRef]
- Knorr, F.; Sanchez, D.G.; Schirmer, J.; Gazdzicki, P.; Friedrich, K.A. Methanol as antifreeze agent for cold start of automotive polymer electrolyte membrane fuel cells. Appl. Energy 2019, 238, 1–10. [Google Scholar] [CrossRef]
- Yan, Q.; Toghiani, H.; Lee, Y.-W.; Liang, K.; Causey, H. Effect of sub-freezing temperatures on a PEM fuel cell performance, startup and fuel cell components. J. Power Sources 2006, 160, 1242–1250. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Three-dimensional multiphase modeling of cold start processes in polymer electrolyte membrane fuel cells. Electrochim. Acta 2009, 54, 6876–6891. [Google Scholar] [CrossRef]
- Sundaresan, M.; Moore, R.M. Polymer electrolyte fuel cell stack thermal model to evaluate sub-freezing startup. J. Power Sources 2005, 145, 534–545. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X. Rapid self-start of polymer electrolyte fuel cell stacks from subfreezing temperatures. J. Power Sources 2006, 162, 502–512. [Google Scholar] [CrossRef]
- Genorio, B.; Strmcnik, D.; Subbaraman, R.; Tripkovic, D.; Karapetrov, G.; Stamenkovic, V.R.; Pejovnik, S.; Markovic, N.M. Selective catalysts for the hydrogen oxidation and oxygen reduction reactions by patterning of platinum with calix [4] arene molecules. Nat. Mater. 2010, 9, 998–1003. [Google Scholar] [CrossRef]
- Sun, S.; Yu, H.; Hou, J.; Shao, Z.; Yi, B.; Ming, P.; Hou, Z. Catalytic hydrogen/oxygen reaction assisted the proton exchange membrane fuel cell (PEMFC) startup at subzero temperature. J. Power Sources 2008, 177, 137–141. [Google Scholar] [CrossRef]
- Amamou, A.A.; Kelouwani, S.; Boulon, L.; Agbossou, K.A. Comprehensive review of solutions and strategies for cold start of automotive proton exchange membrane fuel cells. IEEE Access 2016, 4, 4989–5002. [Google Scholar] [CrossRef]
- Oberholzer, P.; Boillat, P.; Siegrist, R.; Perego, R.; Kästner, A.; Lehmann, E.; Scherer, G.G.; Wokaun, A. Cold-Start of a PEFC visualized with high resolution dynamic in-plane neutron imaging. J. Electrochem. Soc. 2011, 159, B235–B245. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, C.-Y.; Chen, K.S. Current ramping: A strategy for rapid start-up of PEMFCs from subfreezing environment. J. Electrochem. Soc. 2010, 157, B342–B347. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, Q.; Du, Q.; Yin, Y.; Jiao, K. Analysis of cold start processes in proton exchange membrane fuel cell stacks. J. Power Sources 2013, 224, 99–114. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, C.-Y. Potentiostatic start-up of PEMFCs from subzero temperatures. J. Electrochem. Soc. 2008, 155, B743–B751. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Cold start analysis of polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 5077–5094. [Google Scholar] [CrossRef]
- Thounthong, P.; Raël, S.; Davat, B. Control strategy of fuel cell/supercapacitors hybrid power sources for electric vehicle. J. Power Sources 2006, 158, 806–814. [Google Scholar] [CrossRef]
- Du, Q.; Jia, B.; Luo, Y.; Chen, J.; Zhou, Y.; Jiao, K. Maximum power cold start mode of proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2014, 39, 8390–8400. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Effects of various operating and initial conditions on cold start performance of polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 8171–8184. [Google Scholar] [CrossRef]
- Amamou, A.; Kandidayeni, M.; Boulon, L.; Kelouwani, S. Real time adaptive efficient cold start strategy for proton exchange membrane fuel cells. Appl. Energy 2018, 216, 21–30. [Google Scholar] [CrossRef]
- Kong, I.M.; Jung, A.; Kim, B.J.; Baik, K.D.; Kim, M.S. Experimental study on the start-up with dry gases from normal cell temperatures in self-humidified proton exchange membrane fuel cells. Energy 2015, 93, 57–66. [Google Scholar] [CrossRef]
- Jia, L.; Tan, Z.; Kang, M.; Zhang, Z. Experimental investigation on dynamic characteristics of proton exchange membrane fuel cells at subzero temperatures. Int. J. Hydrogen Energy 2014, 39, 11120–11127. [Google Scholar] [CrossRef]
- Zhang, J.; Song, C.; Zhang, J.; Baker, R.; Zhang, L. Understanding the effects of backpressure on PEM fuel cell reactions and performance. J. Electroanal. Chem. 2013, 688, 130–136. [Google Scholar] [CrossRef]
- Liu, Z.; Mao, Z.; Wu, B.; Wang, L.; Schmidt, V.M. Current density distribution in PEFC. J. Power Sources 2005, 141, 205–210. [Google Scholar] [CrossRef]
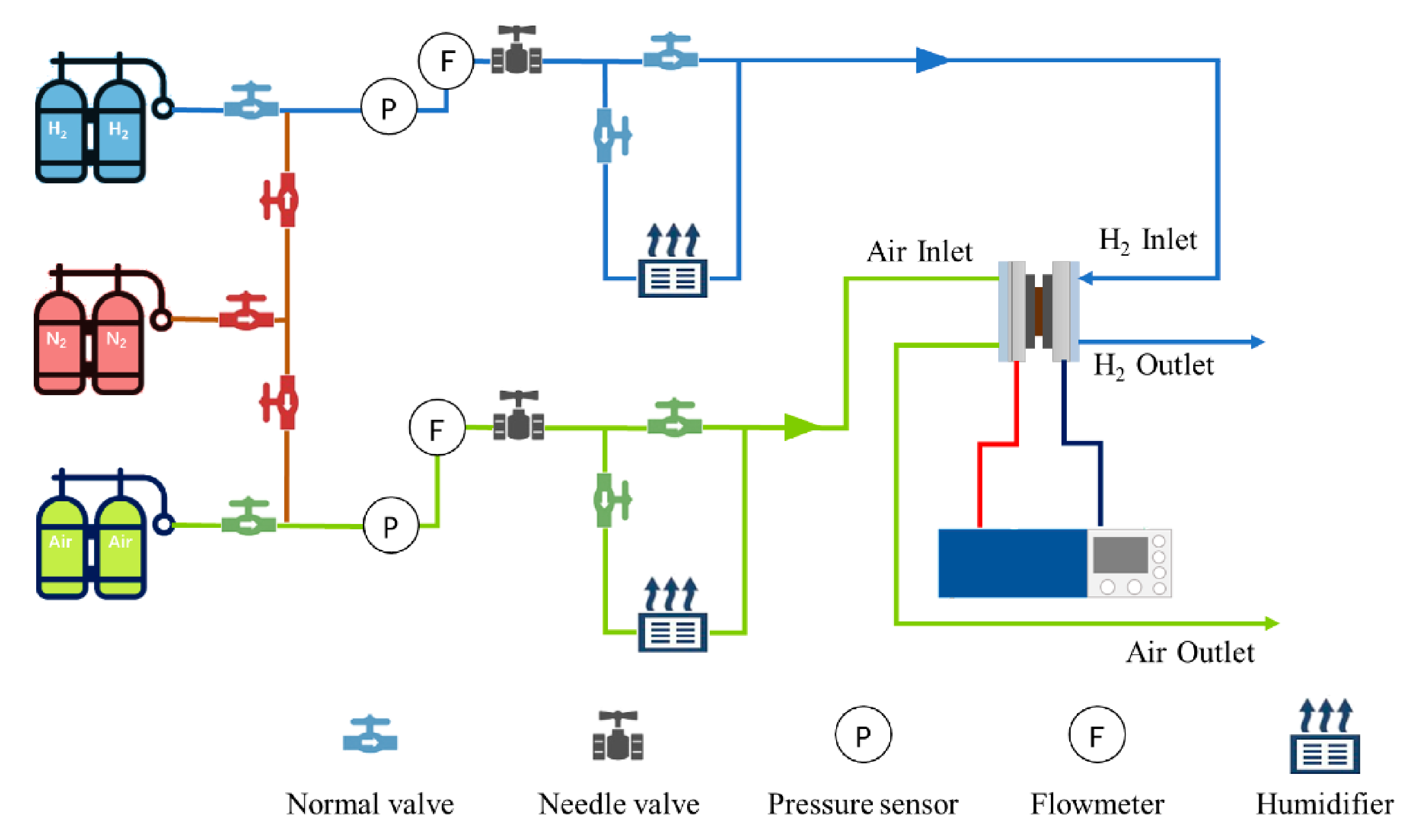


| Name | Parameters |
|---|---|
| MEA | Hyplat |
| Active area | 25 cm2 |
| Gas flow field structure | Serpentine flow field |
| Flow field plate material | Graphite |
| Flow channel width | 1 mm |
| Flow channel depth | 0.8 mm |
| Name | Parameters |
|---|---|
| Pt load at cathode | 0.4 mg cm−2 |
| Pt load at anode | 0.1 mg cm−2 |
| Type of the membrane | Proton exchange membrane |
| Thickness of the membrane | 15 μm |
| Working Parameters | Anode | Cathode |
|---|---|---|
| Gas type | Hydrogen | Air |
| Gas flow rate | 0.8 L min−1 | 1.8 L·min−1 |
| Gas humidity | 100% | 100% |
| Gas temperature | 75 °C | 75 °C |
| Backpressure | 1 bar | 1 bar |
| Case No. | Voltage (V) | Temperature (°C) | Anode Gas Flow Rate (mL·min−1) | Cathode Gas Flow Rate (mL·min−1) | Backpressure (bar) |
|---|---|---|---|---|---|
| 1 | 0.1 | −5 | 400 | 900 | 0 |
| 2 | 0.15 | −5 | 400 | 900 | 0 |
| 3 | 0.2 | −5 | 400 | 900 | 0 |
| 4 | 0.25 | −5 | 400 | 900 | 0 |
| 5 | 0.3 | −5 | 400 | 900 | 0 |
| 6 | 0.1 | −5 | 400 | 900 | 0.5 |
| 7 | 0.1 | −5 | 400 | 900 | 1 |
| 8 | 0.1 | −5 | 800 | 1800 | 0 |
| 9 | 0.1 | −5 | 1200 | 2700 | 0 |
| 10 | 0.1 | −5 | 1600 | 3600 | 0 |
| Working Parameters | Anode | Cathode | Single Cell |
|---|---|---|---|
| Gas type | Hydrogen | Air | - |
| Gas flow rate | 0.2 L min−1 | 0.2 L·min−1 | - |
| Gas humidity | 100% | 100% | - |
| Gas temperature | 25 °C | 25 °C | - |
| Current | - | - | 0.5 mA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Ma, T.; Du, B.; Lin, W.; Yao, N. Investigation on the Operating Conditions of Proton Exchange Membrane Fuel Cell Based on Constant Voltage Cold Start Mode. Energies 2021, 14, 660. https://doi.org/10.3390/en14030660
Yang Y, Ma T, Du B, Lin W, Yao N. Investigation on the Operating Conditions of Proton Exchange Membrane Fuel Cell Based on Constant Voltage Cold Start Mode. Energies. 2021; 14(3):660. https://doi.org/10.3390/en14030660
Chicago/Turabian StyleYang, Yanbo, Tiancai Ma, Boyu Du, Weikang Lin, and Naiyuan Yao. 2021. "Investigation on the Operating Conditions of Proton Exchange Membrane Fuel Cell Based on Constant Voltage Cold Start Mode" Energies 14, no. 3: 660. https://doi.org/10.3390/en14030660
APA StyleYang, Y., Ma, T., Du, B., Lin, W., & Yao, N. (2021). Investigation on the Operating Conditions of Proton Exchange Membrane Fuel Cell Based on Constant Voltage Cold Start Mode. Energies, 14(3), 660. https://doi.org/10.3390/en14030660







